SUMMARY
The walking paths of male cockroaches, Periplaneta americana, tracking point-source plumes of female pheromone often appear similar in structure to those observed from flying male moths. Flying moths use visual-flow-field feedback of their movements to control steering and speed over the ground and to detect the wind speed and direction while tracking plumes of odors. Walking insects are also known to use flow field cues to steer their trajectories. Can the upwind steering we observe in plume-tracking walking male cockroaches be explained by visual-flow-field feedback, as in flying moths? To answer this question, we experimentally occluded the compound eyes and ocelli of virgin P. americana males, separately and in combination, and challenged them with different wind and odor environments in our laboratory wind tunnel. They were observed responding to: (1) still air and no odor, (2) wind and no odor, (3) a wind-borne point-source pheromone plume and (4) a wide pheromone plume in wind. If walking cockroaches require visual cues to control their steering with respect to their environment, we would expect their tracks to be less directed and more variable if they cannot see. Instead, we found few statistically significant differences among behaviors exhibited by intact control cockroaches or those with their eyes occluded, under any of our environmental conditions. Working towards our goal of a comprehensive understanding of chemo-orientation in insects, we then challenged flying and walking male moths to track pheromone plumes with and without visual feedback. Neither walking nor flying moths performed as well as walking cockroaches when there was no visual information available.
KEY WORDS: orientation, pheromone, vision, cockroach, behavior
INTRODUCTION
It is well known that flying insects control their steering and flight speed using visual-flow fields caused by their movements through the environment (Srinivasan et al., 1999; Warrant and Dacke, 2011). When tracking wind-borne plumes of odor, these visual control systems also provide information on wind speed and direction (Kennedy, 1939; Kennedy and Marsh, 1974) and are modulated by olfactory information (Bhandawat et al., 2010; Chow and Frye, 2008; Duistermars and Frye, 2008; Olberg and Willis, 1990; Willis and Avondet, 2005). However, relatively fewer studies have addressed the role of visual cues in the control of maneuvering while walking, and even fewer have addressed the role of vision in odor tracking while walking (Bell and Tobin, 1981; Götz and Wenking, 1973; Tobin, 1981; Zanker and Collett, 1984).
Flying male moths tracking pheromone plumes are not in physical contact with the ground and are thought to use wind-induced drifting of the visual-flow-field feedback caused by their movement through the environment to determine the wind speed and direction (David, 1986; Kennedy and Marsh, 1974; Marsh et al., 1978). Mechanosensors on their bodies (e.g. antennae and wind-sensitive hairs) are thought to be able to provide only information about the movement of the animals with respect to the air (David, 1986; Marsh et al., 1978). Recent studies suggest that the antennae might also serve as Coriolis force detectors for rapid flight stabilization (Sane et al., 2007). Steering with respect to the wind using visual feedback from the environment is known as optomotor anemotaxis (Kennedy, 1939). For walking plume-tracking insects, constant contact with a stationary reference (i.e. the ground) means that wind-deflected mechanosensors can provide continuous information on wind speed and direction (Bell and Kramer, 1979).
Relative to flight, much less is known about the role of visual information in the control of walking in insects, especially in those that track wind-borne odor. Most observations and experiments have shown that, unlike flying insects tracking odor, the translatory component of optic flow has little or no effect on the speed of walking insects (Götz and Wenking, 1973; Zanker and Collett, 1984). However, these same, and other (Weber, 1990; Weber et al., 1981), studies demonstrated that rotational optic flow can be used to compensate for steering errors (Götz and Wenking, 1973; Strauss et al., 2001; Strauss et al., 1997; Zanker and Collett, 1984). Recent results show that the same wide-field motion-sensitive cells thought to support flight orientation in other flying insects are involved in processing rotational image flow in Drosophila melanogaster flies when they are walking (Chiappe et al., 2010) and flying (Maimon et al., 2010).
Optic flow can also be used to judge the distance traveled in walking and flying social insects, which must return to their nest after foraging (Ronacher and Wehner, 1994; Srinivasan et al., 2000). In walking ants, for example, the optic flow below the animal is important (Ronacher and Wehner, 1994), whereas that to the sides appears to have no impact on speed control or distance estimation (Ronacher et al., 2000). By contrast, honey bees use optic flow in their lateral fields of view to judge how far they fly from foraging sites to the hive (Srinivasan et al., 2000).
Recent studies have shown that cockroaches can use visual information to control many aspects of their behaviors. Individuals of the American cockroach, Periplaneta americana, are less likely to collide with a visible target than a transparent target (Baba et al., 2010), and light levels detected by the visual system of the tropical cockroach Blaberus discoidalis influence the decision of whether to climb under or over an obstacle (Harley et al., 2009). In addition, darkened hiding places are located using visual cues by P. americana (Okada and Toh, 1998). Finally, the positions of fixed visual cues have been shown to be learned and then used as navigational landmarks by P. americana to locate visually undetectable resources (Mizunami et al., 1998). We are unaware of any published observations or experiments specifically addressing the optomotor responses of P. americana or any other cockroach species. However, a study of neural regeneration in P. americana used the response to a rotating striped drum as an assay of post-lesion recovery in the brain (Drescher, 1960). Likewise, in an ongoing study of brain activity in another cockroach species, B. discoidalis, neural and behavioral responses to moving striped patterns show clear directionally selective neural responses and associated turning behavior (Kathman et al., 2011).
Our working hypothesis is that P. americana males tracking odor plumes through their environment use visual cues (e.g. optic flow or landscape cues) to steer more precisely to the odor source. Rather than removing visible cues from our experimental environment, we physically occluded the two visual systems of our P. americana males. These included the large multifaceted compound eyes that detect motion and patterns, and a pair of ocelli (simple eyes) that seem to be incapable of image formation and are thought to function mainly in detecting changes in light intensity (Mizunami, 1994). If visual cues are important to the control of steering maneuvers while tracking plumes of attractive odors, we predicted that individuals with occluded eyes should show increased errors in their steering, possibly resulting in them turning more often, steering less directly towards the source or failing to locate the source.
To determine the importance of visual information to odor-plume tracking in walking P. americana males, we compared the plume-tracking performances of intact control cockroaches (uncovered eyes) with those of individuals for which the eyes had been covered by painting: (1) the compound eyes only, (2) ocelli only and (3) both sets of eyes. We first aimed to determine whether vision was important to wind orientation by observing the steering response of the cockroaches when exposed individually to: (1) still air and no odor, (2) wind and no odor or (3) a wind-borne odor plume. Two additional experiments were aimed at determining the importance of visual input on upwind steering during sustained plume tracking. In the first, we introduced intact control cockroaches and those with their eyes painted into a wind-borne plume of female sex-pheromone narrow enough for their antennae to span. In the second, we challenged another group of similarly manipulated cockroaches to track a pheromone plume issuing from a source ∼20 times the width of the narrow plume. This second plume is at least twice as wide as the span of the antennae of a male cockroach. The aim of this treatment was to make it difficult for the cockroaches to use the high-contrast olfactory ‘landmark’ provided by the edge of the plume. We reasoned that, if vision is important to steering control in walking plume-tracking cockroaches, but they can compensate for its loss by using the edges of the plume as olfactory cues for steering, then we expect the steering precision of eye-painted males to be worse when walking far from the edges of our wide plume. By contrast, if they can perform odor-gated anemotaxis without visual inputs (i.e. directional cues provided solely by mechanosensory inputs), then their odor-activated orientation to the wind direction should cause them to orient and walk directly upwind in the plume.
Because of the importance of vision for detecting the speed and direction of the wind during plume tracking, it has been assumed (but never tested) that removing the visual input from a flying moth will make it unable to track an odor plume. To gain a more complete understanding of the importance of visual information to odor tracking in moths, we compared the ability of intact controls and moths, with their eyes painted as in the cockroach experiments, to track a pheromone plume while walking and flying. For these studies, we used males of the tobacco hornworm moth, Manduca sexta, from our laboratory colony. As in our cockroach experiments, the male moths were either controls with intact visual systems or had their eyes covered with paint to eliminate their possibility of detecting visual information while attempting to track an odor plume.
MATERIALS AND METHODS
Insects
Cockroaches
We collected penultimate-instar male P. americana (L.) from our laboratory colony and allowed them to molt to adults isolated from females. They were housed in plastic containers with water and chicken feed ad libitum until sexual maturation (at least 3 weeks old). The containers were held in an environmental chamber on a 12 h:12 h light:dark cycle, at 27°C and 50% relative humidity, until the animals were used in experiments.
Moths
The male moths, M. sexta (L.), used in this study came from the Willis laboratory colony. Larvae were reared on an artificial diet (Bell and Joachim, 1976) ad libitum and maintained at ∼27°C on a 14 h:10 h light:dark cycle. Mature pupae were removed from the colony, separated according to sex, and the males held in an environmental chamber at ∼27°C on a 14 h:10 h light:dark cycle. Three-to-four-day-old virgin male moths were used in our experiments.
Wind tunnel
Cockroaches
Male cockroaches were individually released onto our 0.92×1.52 m flat aluminum experimental arena, which was held 25.4 cm above the floor of the 1×1×2.5 m working section of our laboratory wind tunnel. In all experiments with an odor stimulus, the source was held 1 cm above the upwind end of the experimental arena, with the wind speed set at 25 cm s–1. The pheromone plume was removed from the room and the building by means of an exhaust system positioned at the downwind end of the wind tunnel. In all experiments using a point-source plume, 0.1 ng of (–)-periplanone-B (Kitahara et al., 1987; Kuwahara and Mori, 1990) was applied to a 0.7 cm diameter disk of filter paper (Whatmans No.1). Solutions of (–)-periplanone-B were made with n-hexane (Acros Organics, Geel, Belgium). To generate a turbulent plume, the plane of the filter paper disk odor source was oriented perpendicular to the air flow in the wind tunnel. The wide plume treatment was generated by increasing the source to a strip of filter paper (14.3×0.7 cm). This increase in width increased the surface area of the source ∼25 times, which we compensated for by increasing the dosage of pheromone solution proportionately. This resulted in the point and wide source both bearing ∼0.05–0.1 ng cm–2 of (–)-periplanone-B. We did not control the visual environment during these experiments. The working section of the wind tunnel is transparent Plexiglas®. The white-painted walls and ceiling of the room as well as the camera mounts and infrared light sources would have been visible to animals in the wind tunnel. Aside from the elevated aluminum arena used in the walking studies, all aspects of the wind tunnel were the same for cockroach and moths trials. We video-recorded the walking tracks of individual cockroaches at 30 frames s–1 with a Burle TC355AC black-and-white camera positioned overhead. This provided an overhead view of the entire experimental arena.
Moths
Walking male moths were released into the same wind tunnel used for the cockroach experiments. When walking moths were the subject of the experiment, their performance was observed on the same arena as that used by the walking cockroaches. When flying moths were the subjects, the walking arena was removed. The same camera and wind tunnel used in the cockroach experiments were used to record both the walking and flying moth performances. We used freshly made extract of virgin female pheromone glands as our attractant odor source (Willis and Arbas, 1991) and applied it to the same type of filter paper disk used in the point-source plume cockroach experiments. Wind speed for the walking moths was held at 25 cm s–1, as in the cockroach studies. The flying moths tracked plumes in 100 cm s–1 wind, the standard wind speed used in our studies of M. sexta plume-tracking flight (Rutkowski et al., 2009; Willis and Arbas, 1991; Willis and Arbas, 1998).
Visualization of odor plumes
To visualize the odor plume, we video-recorded titanium tetrachloride smoke plumes issuing from filter paper sources of the same size and shape as used to distribute pheromone in our behavior experiments. This is a standard procedure for visualizing odor plumes in insect pheromone studies (Baker et al., 1984; Charlton et al., 1993; Willis and Avondet, 2005). We then determined the time-averaged plume boundaries by using our motion-analysis system to digitize smoke packets at the lateral margins of the plume. As in previous studies (Willis and Avondet, 2005), overlaying digitized cockroach tracks on these time-averaged plumes enabled us to view the turns in the walking paths of the cockroaches with respect to the time-averaged plume boundaries.
Experimental design
Cockroaches
We covered the eyes of cockroaches by painting: (1) compound eyes only (N=27 for wind and odor orientation experiment, N=20 for the point-source plume experiment and N=9 for the wide plume experiment), (2) ocelli only (N=30 for the wind and odor orientation experiment, N=20 for the point-source plume experiment and N=8 for the wide plume experiment) and (3) both sets of eyes (N=27 for the wind and odor orientation experiment, N=22 for the point-source plume experiment and N=9 for the wide plume experiment). Intact cockroaches with unpainted eyes were used as controls (N=29 for the wind and odor orientation experiment, N=19 for the point-source plume experiment and N=10 for the wide plume experiment).
We painted the eyes of our experimental animals using methods previously used for P. americana behavior experiments (Ye et al., 2003). First, we removed the layer of oil on the cuticle of the eyes and ocelli of the cockroaches with an acetone-dampened (and almost dried) cotton applicator. Then, to ensure that the eyes and/or ocelli were covered, we applied a layer of red enamel paint (Sharpie paint pen) until the black compound eyes (especially along the edges) could no longer be seen. Finally, when the red paint dried (∼30 s), a layer of black acrylic paint was applied over the red to completely block the light. After each cockroach was painted, it was placed individually in a labeled aluminum window-screen release cage corresponding to the specific treatment.
This method was shown to eliminate almost completely the antennal orientation response of P. americana to a visual target (Ye et al., 2003). Furthermore, our own observations show that males in treatment groups with painted compound eyes show no obvious response to shining a red-filtered flashlight in their eyes, whereas unpainted control males turn to face away from the light. Males in our treatment groups with painted compound eyes also showed no response when we approached to recapture them after their trials in the wind tunnel. Unpainted control males always oriented and walked away from our approaches.
The cockroaches in release cages were then placed into the wind tunnel room, under our standard low-light conditions (∼3 lx), until experiments began ∼2 h into their scotophase, the beginning of their period of peak pheromone response. To ensure that the paint had not been groomed off the eyes of the cockroaches, we examined them under a dissecting microscope after all painting was completed and before they were placed in the wind-tunnel room. We also verified the presence of paint on the cockroaches by checking their eyes again under the dissecting microscope after experiments each day. If any of the eyes was not completely covered after the trials, we removed the responses of such individuals from our sample. This occurred in ∼10% of the cockroaches with both sets of eyes painted, 10% of the individuals with their compound eyes only painted and 50% of the cockroaches with only the ocelli painted.
In each of the three experiments presented here, we used ∼16 cockroaches each day; four unpainted controls, four with painted compound eyes, four with painted ocelli, and four with both painted compound eyes and ocelli. In the initial experiment aimed at understanding the effect of the loss of vision on the ability to orient to wind, and odor plus wind, we placed each cockroach in the center of the arena to allow them freedom to walk in any direction. For the two experiments focused on plume tracking, each cockroach was placed into the odor plume, at the center of the downwind end of the experimental arena after the pheromone source was positioned and the wind and exhaust were turned on. Each cockroach was randomly selected for placement into the arena. To acclimate the cockroach to the experimental environment, it was held in its release cage on the arena in the wind tunnel for 1 min. The behaviors of the cockroach were video-recorded after it was released. A cockroach was scored as successful when it touched the pheromone source. Trials in which pheromone was not used were stopped when the cockroach walked to one of the edges of the arena. When a cockroach did not leave the release point within 5 min of being given access to the arena, it was scored as non-responding.
We performed a pilot study to verify that olfactory inputs from the antennae were mediating the orientation behavior that we were studying. The behavioral responses of intact control cockroaches were compared with those that had both antennae surgically removed at their bases. None of the males (0%) that had their antennae removed before introduction into a wind-borne pheromone plume left the release point within the 5 min observation period, whereas all of the intact controls (100%) immediately tracked the pheromone plume to its source upon release into the arena (N=10 in both cases). Thus, male cockroaches with their antennae removed do not respond to female pheromone or the ambient wind direction. A similar lack of behavioral responses was observed when both antennae were coated with paint.
Moths
The ocelli of M. sexta are inside the head, well below the surface of the cuticle (Eaton, 1971). For this reason, we were only able to paint the compound eyes, using the methods detailed above for cockroaches. It is known that M. sexta males fly upwind to female pheromone readily and flight appears to be their preferred mode of locomotion. It is difficult to make them walk once they have taken flight. Therefore, to assess their ability to orient to a pheromone plume while walking, we surgically removed approximately one-half to two-thirds of their wings. These individuals were cold anesthetized by placing them in a clear plastic cup on crushed ice until they stopped moving (∼15–20 min). Once anesthetized, both sets of wings were removed using surgical scissors. Once the experimental moths were prepared, either by having their wings removed, or eyes painted, or both, they were placed into individual screen cages and placed in the wind-tunnel room, where they experienced ‘lights off’ according to their normal light–dark cycle. They were challenged to track pheromone plumes, either walking or flying, two hours after lights off during their peak responsiveness to pheromone (Sasaki and Riddiford, 1984). These introductory studies of flying and walking plume tracking in moths serve the long-term goal of our laboratory to develop a comprehensive understanding of chemo-orientation in insects by making explicit comparisons between walking and flying odor trackers.
Data analysis
Cockroaches
We used the computerized motion-analysis system called Motus (Vicon Peak, Englewood, CO, USA) to digitize and measure the walking trajectories of the male cockroaches. Motus splits each 1/30 s video frame into two video fields, yielding a 1/60 s temporal resolution. For all experiments, we digitized the walking trajectories of the cockroach by marking its position every fifth time-point (i.e. every 83 ms). At each digitized cockroach position, we marked the center of the head and the distal tip of the abdomen.
A line drawn from the cockroach release point in the center of the arena through the point where each individual encountered the edge of our experimental arena defined their ‘vanishing direction’. We then generated a mean vanishing direction for each treatment group. These mean directions (i.e. angle θ) were compared with a circular random distribution using Rayleigh's test (Cabrera et al., 1991). In addition to determining whether this mean vanishing direction is significantly different from random, this test provides the relative length for a mean vector (r) distributed between 0 (i.e. no movement in the mean direction) and 1.0 (i.e. all individuals vanished in the mean direction). These angles were measured with respect to the wind direction (0 deg), which is parallel to the longitudinal axis of the wind tunnel. We also compared the mean responses (i.e. r and θ) of cockroaches with those of visual-system manipulations to our experimental environments with a Watson–Williams F-test using commercially available circular statistics software called Oriana (Rockware, Golden, CO, USA), to determine whether they were statistically different.
In addition to the orientation of the vanishing direction, we measured eight different response variables from the movement vectors defined by successive positions of the heads of the cockroaches digitized from the video-recorded tracks: the ‘net velocity’ (the speed of the cockroach, if it had traveled in a straight line, from the beginning to the end of the track), ‘ground-speed’ (the speed of the cockroach along its track), ‘track angle’ (the angle of each movement vector with respect to the wind direction), ‘body-axis angle’ (the angle of the body of the cockroach with respect to the wind direction), ‘track width’ (the distance between the apex of each turn perpendicular to the wind direction), ‘inter-turn duration’ (the time between the apex of each turn) and the ‘number of turns per second’ for each track. Potential turns were identified as the local extremes in lateral position (i.e. positions left or right of their immediately adjacent digitized points in the walking tracks). We then calculated a ‘turn identification distance’, which was the product of a variable ‘turn threshold value’ we set and the ‘overall width’ of each track. The overall width was the distance, perpendicular to the wind, between the leftmost and rightmost position of the cockroach over its entire track. If the distance, perpendicular to the wind, between the candidate turn and the next adjacent turn in the opposite direction was longer than the turn identification distance, it was accepted as a turn. If shorter, it was rejected. The turn threshold value was set according to visual comparison between the computer-identified turns and the cockroach tracks. A turn threshold value of 0.5 worked for all of the tracks. All of the above track parameters were calculated with a custom-written script in MATLAB (The MathWorks, Natick, MA, USA) (Rutkowski et al., 2009). We also measured the ‘number’ and ‘duration of stops’ made by each individual during a plume-tracking performance. The cockroach was said to be stopped if at least two successive digitized positions were the same. For the experiment with the wide odor plume, we also calculated a ‘linearity index’ (degree of straightness of the track) and ‘angular velocity’ (degrees turned through per second). We calculated our ‘linearity index’ by dividing the total length of each walking track into the straight-line distance between its first and last digitized position.
During a study of the experimental designs used in olfactory orientation studies, including those in our laboratory, it was shown that our design met all of the assumptions of analyses of variance (Pilla et al., 2005). This study also demonstrated a uniquely appropriate ANOVA procedure for this design (Pilla et al., 2005), which we have used here. First, we averaged the measurements for each individual cockroach and then calculated a grand mean of all of the individuals in a treatment group. We then conducted an analysis of variance (ANOVA) to identify any statistical differences among the grand means. When the ANOVA revealed significant differences, we performed a post hoc Tukey's test to determine which treatments and parameters differed significantly (P<0.05). In the point-source plume experiment, there were a small number of individuals in each treatment group (no paint, N=6; compound only, N=3; ocelli only, N=5; both sets, N=5) that generated long looping tracks before they ‘locked on’ to the plume and tracked it to the source. In all cases, we analyzed only the sections of the plume-tracking performance after each individual had initiated a plume-tracking response that led to the source.
We conducted post hoc power analyses with the commercially available software SigmaStat (Aspire Software International, Ashburn, VA, USA) on our ANOVAs at a 0.80 level (the accepted convention for this sort of analysis) to determine the sample sizes necessary to minimize the likelihood of our analyses producing false negatives (i.e. type II errors). In all cases, the sample sizes in the experiments we conducted were in the range that would reveal statistically significant differences if any existed and would minimize the probability of rejecting real differences as not significant.
Moths
For the purpose of the experiments presented here, our focus was primarily on whether male moths would track an odor plume while walking or flying with their eyes painted. We compared the proportion of intact control individuals with unpainted eyes (N=20) with those with painted eyes (N=19) that were able to track the plume to the source. The same comparison was performed on flying males with unpainted eyes (N=23), comparing them with those with painted eyes (N=16). We then applied Ryan's multiple comparison test for proportions (Ryan, 1960) to determine whether our response proportions were statistically different. We also video recorded the tracking performances of the moths to visualize their movement trajectories, but a complete track analysis is beyond the scope of the study presented here.
RESULTS
Cockroach orientation to wind and odor
Whether intact or with their eyes painted, P. americana males responded to the conditions in our experimental arena in a broadly similar manner. Of the 126 cockroaches tested, 113 (90%) left the release cage and either walked to an edge of the experimental arena or tracked the odor plume, depending on the experimental treatment (Fig. 1). Of the 13 individuals that did not move from the release point, eight were in the no wind and no odor treatment, three in the wind and no odor and two in the wind and odor treatment. All individuals remaining on the release point had their compound eyes painted over. Half of these were from the group that had only their compound eyes painted, and the other half had both sets of eyes painted.
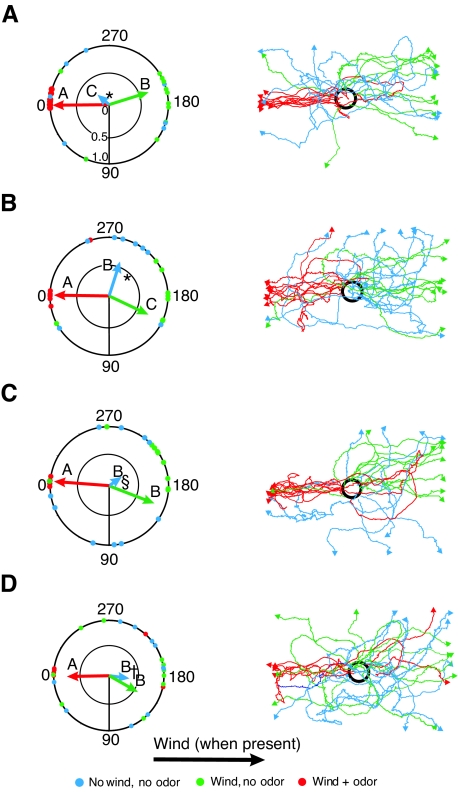
Fig. 1.
Walking orientation of Periplaneta americana males in wind plus odor (red), wind and no odor (green) and no wind and no odor (blue) for cockroaches with (A) unpainted eyes (control), (B) only compound eyes painted, (C) only ocelli painted or (D) both sets of eyes painted. Each dot on the outer circumference of the circle plot represents the direction at which an individual cockroach encountered the edge of the arena. Note that, for each plot, dots representing several of the wind-plus-odor trials are plotted on top of each other near ‘0’. The length of each colored arrow indicates the mean resultant length (r) associated with the mean direction of the vanishing angle (θ) as determined by Raleigh's test. Please refer to the text for values for θ and r for each combination of eye-painting treatment and environmental manipulation. The lengths of the mean vectors (r) are an index of how closely the vanishing directions of the individuals in the population are clumped. This index ranges from 0 to 1.0, with 0 being equivalent to a circular random distribution, and 1.0 indicating that all individuals encountered the edge of the arena in the same direction. The radial axis in the circular plot in (A) has been marked to represent this range, and all circular plots in this figure are consistent. Results of a Watson–Williams F-test comparing: (1) the responses to the three environmental treatments (i.e. wind plus odor, wind plus no odor, etc.) by individuals with each visual-system manipulation are depicted with uppercase letters and (2) the effects of visual-system manipulations (i.e. intact controls, painted compound eyes, etc.) on the responses of individuals to each environment are depicted in typographic symbols (i.e. asterisk, section symbol and dagger). Mean vectors with uppercase letters or typographic symbols in common are not significantly different from each other. There were no statistically significant differences in responses of individuals with any of the visual-system manipulations to the wind-plus-odor or wind-plus-no-odor environments. For the plots of the individual walking tracks on the righthand side of the figure, the arrowheads indicate the orientation of the cockroach at the end of each track in the laboratory wind tunnel. The bold black circle represents the release cage.
Our analysis of these results was stepwise. First we applied Rayleigh's test to determine whether the vanishing directions of the cockroaches were statistically different from a random circular distribution. Then we applied the Watson–Williams F-test to determine whether there were any statistical differences in the average responses to our experimental environments or visual-system manipulations.
Consistent with previous results (Willis and Avondet, 2005), the vanishing directions of the control cockroaches with unpainted eyes were significantly upwind (i.e. 0 deg) when in wind and odor (θ=0.5 deg, r=1.0, P≤0.05), and downwind when exposed to wind only (θ=166.7 deg, r=0.59, P≤0.05). Control cockroaches walking in still air with no pheromone present left the experimental arena in so many different directions that there was no statistical difference from random (θ=50.7 deg, r=0.19, P≥0.05; Fig. 1A). On average, cockroaches with experimental visual-system manipulations generated mean vanishing directions similar to those of the controls, leaving the arena in upwind directions when in wind and odor (compound eyes painted, θ=6.2 deg, r=0.91; ocelli painted, θ=355.8 deg, r=0.99; both sets of eyes painted, θ=2.2 deg, r=0.78; P≤0.05), downwind in wind only (compound eyes painted, θ=174.5 deg, r=0.71; ocelli painted, θ=154.9 deg, r=0.72; both sets of eyes painted, θ=143.3 deg, r=0.55, P≤0.05) and in many different directions in the absence of wind or odor (compound eyes painted, θ=108.9 deg, r=0.58; ocelli painted, θ=233.8 deg, r=0.15; both sets of eyes painted, θ=173.0 deg, r=0.28; P≥0.05; Fig. 1B–D).
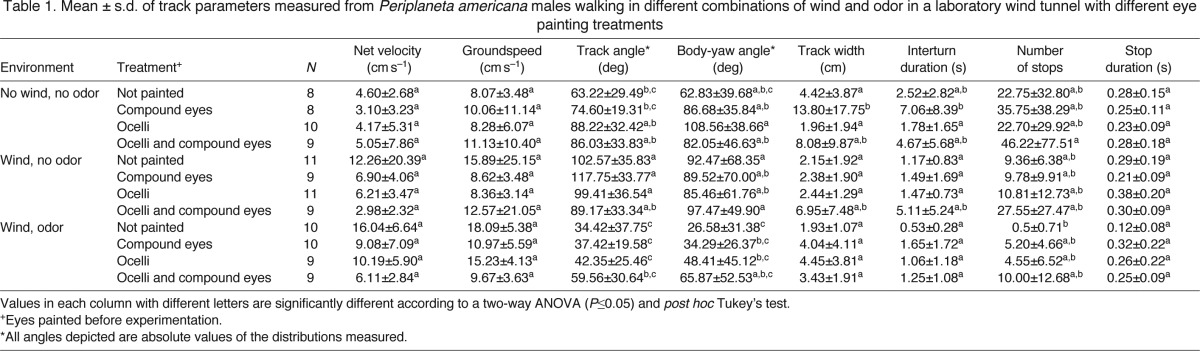
The Watson–Williams F-test uses the mean direction (θ) and vector lengths (r) calculated above to test for statistical differences between treatment groups (Fisher, 2000). The results of our analysis confirm the visual impression of the circular plots in Fig. 1. First, we compared the responses of each of the visual-system manipulations with the three experimental environments we used. Individuals in each of our four visual-system manipulations groups generated mean vanishing directions in the wind-plus-odor environment that were significantly different (P≤0.05) from those in the wind-plus-no-odor environment (Fig. 1). The mean vanishing directions in the no-wind-plus-no-odor environment were less consistent. Even though their low r values indicated a general lack of ‘orientation’ in the mean direction, if the mean direction was sufficiently distinct from the wind-plus-odor or wind-plus-no-odor treatments, the analysis indicated it was significantly different (Fig. 1A,B). However, if the value for mean direction was close to those for one of the other treatments, they were not significantly different (P≥0.05) (Fig. 1C,D). As none of the mean vanishing directions generated in response to the no-wind-plus-no-odor environment were significantly different from a circular random distribution according to the Rayleigh's test, we put little weight in the statistical significance or lack thereof in the no wind-no-odor environment.
There were no statistically significant differences (P≥0.05) among the mean vanishing directions of the four visual-system manipulations in the wind-plus-odor and wind-plus-no-odor environments (Fig. 1). There were statistically significant differences (P≤0.05) in the mean vanishing directions generated by the four visual-system manipulations in the no-wind-plus-no-odor environment (Fig. 1). But, as with the environment-specific comparisons above, we put little weight in these results because the mean vanishing directions generated by each of the four visual-system manipulations in the no-wind-plus-no-odor environment were not significantly different (P≥0.05) from a circular random distribution according to Rayleigh's test.
Analysis of the walking tracks of these individuals revealed broad similarities among the cockroaches with the four visual-system manipulations as they responded to the three experimental environments. Intact and eye-painted cockroaches generated, on average, similar net velocities, similar walking speeds along their tracks and similar mean stopping durations when they stopped, regardless of the wind and odor environment (P>0.05; Table 1). Our analysis did reveal statistically significant differences in the mean track angles, yaw angles, track widths and numbers of stops executed by the cockroaches in this experiment, but there were few obvious trends of increasing or decreasing mean values across the eye-painting treatments or environmental manipulations (Table 1). We did observe consistent trends of increasing track and yaw angles as the visual systems were occluded in cockroaches in the wind-plus-odor environment (Table 1), suggesting some effect of removing visual input on steering behavior, but these differences were not statistically significant. However, this response is noteworthy because steering more off of the wind direction with loss of visual inputs matches our predictions for how steering might change if visual information were important during odor tracking.
Table 1.
Mean ± s.d. of track parameters measured from Periplaneta americana males walking in different combinations of wind and odor in a laboratory wind tunnel with different eye painting treatments
When wind was introduced to the arena, most males oriented with respect to it and left the arena in the downwind direction, whether their eyes were painted or not (Fig. 1). In all cases, except cockroaches with both sets of eyes painted, individuals orienting to odor plumes in wind steered their tracks more directly upwind, as evidenced by their track and body-yaw angles (Fig. 1; Table 1). Cockroaches responding to a wind-borne pheromone plume also had significantly higher rates of turning (turns s–1), regardless of whether their eyes were painted, than those orienting in the absence of attractive odors (Table 1). Few statistically significant differences were revealed in our analysis of this experiment, indicating that the mean responses of our experimental populations were similar. Importantly, it should be noted that, in most cases, the standard deviations are also similar across the experiment (Table 1). Thus, the variation of the cockroaches about their average performances does not increase when the eyes are painted, indicating that variability in the behavior does not increase significantly with loss of visual inputs.
The tracks generated by cockroaches with experimentally manipulated visual systems responding to no-wind-plus-no-odor and wind-plus-no-odor environments have a somewhat different appearance than those of intact controls or any of the cockroaches tracking odor plumes (Fig. 1D). It is probable that the interaction of walking speeds somewhat slower than those in intact control cockroaches, or those in the wind-plus-odor treatment, together with similar turning rates, generated tracks that looked different but yielded measured movement parameters that were not statistically different.
Point-source plume
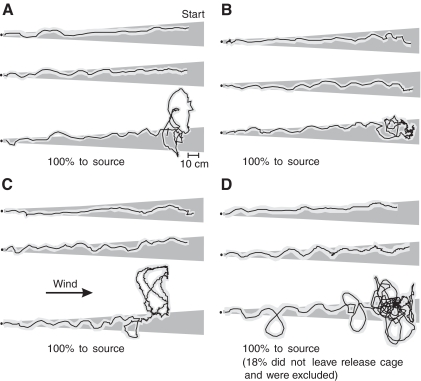
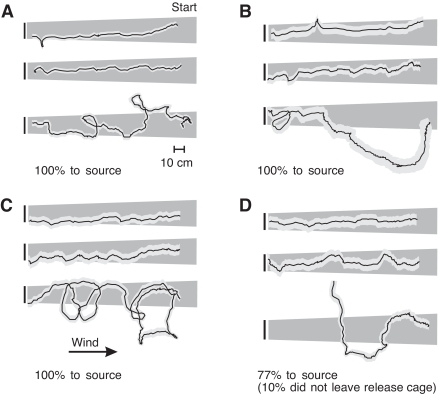
All but four of the 81 individuals challenged to track the point-source plume were successful in reaching the source (i.e. 95% were successful). The four males that did not track the plume had both sets of eyes painted. Visual inspection of the range of tracking performances exhibited by our experimental population reveals no obvious consistent differences caused by occluding either of the visual systems (Fig. 2). Our track analysis showed that, on average, intact males walked along their upwind track the fastest, whereas both treatments that included painting the ocelli walked significantly slower (P>0.05; Table 2). The mean walking speed of males with only their compound eyes painted was not significantly different from that of the other treatments.
Fig. 2.
Examples of typical (center) and extreme (upper and lower) P. americana males tracking a plume from a point-source of pheromone upwind (right to left) in a wind tunnel with: (A) unpainted eyes (control), (B) painted compound eyes, (C) painted ocelli and (D) painted ocelli and compound eyes. To illustrate the variability in the tracking responses, we have plotted three tracks from each experimental group. In each panel, the three tracks plotted represent an example of a track with few turns (top), a track with an intermediate number of turns (middle) and a track with many turns. These tracks were sorted from each experimental group and selected by counting the turns in each track by eye. The percentage of cockroaches successfully tracking the pheromone to the source is stated in the lower-left corner of each panel. The time-averaged plume boundaries of a titanium tetrachloride smoke visualization, as viewed from above, are illustrated in dark gray. The pale-gray envelope around the black line representing the walking track is an approximation of the area swept by the antennae on either side of the body. This area was generated by determining the average distance an antenna projects to the side of a plume-tracking cockroach (Willis and Avondet, 2005) and adding it to, or subtracting it from, each cockroach position in the track. In this figure, wind blows from left to right at 25 cm s–1.
Table 2.
Mean (±s.d.) track parameters measured from P. americana males tracking pheromone upwind with painted eyes
Other than the ground-speed, our analysis revealed no statistically significant differences associated with occluding the eyes. However, there are observable trends. Specifically, individuals in treatment groups with their ocelli painted (including those with both sets of eyes painted) tended to generate slower net velocities and steer greater track angles, on average, than those with only compound eyes painted and non-painted controls. Those with both sets of eyes painted also stopped more and for longer durations than the other three treatments.
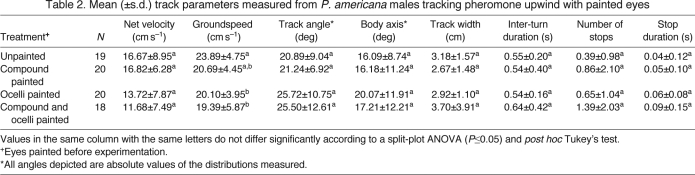
Wide plume
Thirty-three of the 36 cockroaches that initiated walking in response to the wide plume located the source. The few that did not locate the source behaved in a way that suggested that they did not detect the pheromone or responded to it only transiently. They left the time-averaged plume area, did not initiate the search behavior typically performed when contact with pheromone is lost (Willis et al., 2008) and walked until they left the arena (Fig. 3D). As in the previous experiment, visual inspection of the tracks showed broad similarity in the movement tracks across the experiment, with a similar range of performances across all the treatments and the intact controls (Fig. 3). Most individuals appeared to exhibit odor-gated anemotaxis, with their performance characterized by facing into the wind and walking upwind embedded in the plume. A few individuals did wander more widely and ‘locked on’ to the lateral boundary of the plume when they encountered it, tracking the high-contrast pheromone–clean-air edge to the source. Our analysis revealed no statistically significant differences across all of the treatments (Table 3). Like the point-source plume experiment, there was a trend for the treatments with occluded ocelli to be the most different from the intact controls.
Fig. 3.
Examples of typical (center) and extreme (upper and lower) P. americana males tracking a wide (14.3 cm wide source) pheromone plume upwind (right to left) in a wind tunnel with (A) unpainted eyes (control), (B) painted compound eyes, (C) painted ocelli and (D) painted ocelli and compound eyes. Details for this figure are the same as those of Fig. 2.
Table 3.
Mean ± s.d. (coefficient of variation) of track parameters measured from P. americana males walking in a wide plume in a laboratory wind tunnel
Moth orientation to wind and odor
Flying plume tracking
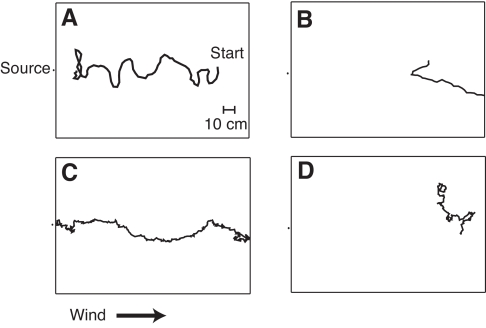
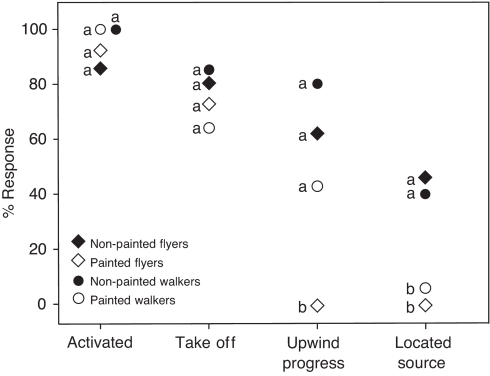
Intact moths with unpainted eyes initiated wing fanning, took flight, made upwind progress in the plume and eventually located the source in a manner similar to that reported previously (Willis and Arbas, 1991; Willis et al., 1995). The males that tracked the plume generated upwind zigzagging flight tracks characteristic of pheromone-modulated plume tracking in many species of moths (Fig. 4A). However, the individuals with painted compound eyes demonstrated very different behavior (Fig. 4B). Of the 23 intact moths we tested, 61% made upwind progress in the plume, with 43% locating the source (Fig. 5). The response of the moths with painted eyes was very different. They often initiated wing fanning upon introduction to the pheromone plume, like a normal intact moth, but showed a much more variable duration of response than the males with unoccluded eyes. The mean duration of wing fanning was longer in eye-painted males (55.8±68.1 s) than in intact controls (18.7±15.3 s), but not significantly so (P>0.05). When moths with their eyes painted did take flight, they immediately lost altitude, drifted downwind and landed on the floor of the wind tunnel, rapidly ceasing wing fanning. None of these males took flight again. Of the 16 moths tested, none with painted compound eyes tracked an odor plume to the source in flight (Fig. 5).
Fig. 4.
Examples of male Manduca sexta moths tracking a pheromone plume from a point-source while walking or flying, with and without visual input. (A) Flight path of a male tracking a plume of female pheromone with an intact visual system; (B) flight track of a male placed in a female pheromone plume with an occluded visual system; (C) walking path of a male with its wings surgically removed tracking a plume of female pheromone with an intact visual system; and (D) walking track of a male released into a plume of female pheromone with an occluded visual system. Wind speed was 100 cm s–1 for flight and 25 cm s–1 for walking experiments. Moth positions were digitized each 1/30 s while flying, and each 12 ms while walking.
Fig. 5.
Comparison of flying and walking M. sexta with painted eyes with intact controls while tracking female pheromone upwind in a wind tunnel. Behaviors with letters that differ are significantly different from one another according to Ryan's multiple comparison test for proportions (P<0.05).
Walking plume tracking
All moths with surgically shortened wings and intact visual systems initiated wing fanning, and many subsequently left the release cage and walked upwind and located the source. The walking plume-tracking males appeared to move in a less-coordinated manner than the walking cockroaches and generated tracks with fewer counterturns (see Fig. 2A, Fig. 4B). Of the 20 moths with unpainted eyes whose wings we surgically removed, 80% made upwind progress, with 40% locating the source (Fig. 5). The moths with wings removed and painted eyes all responded like the intact control moths by moving their antennae forward and initiating wing fanning behavior upon introduction to the plume, but the proportion making the transition to the next sequential behavior decreased at each transition until only 5% located the source (Fig. 5). Thus, although walking moths with painted eyes made more upwind progress than flyers, there was no statistically significant difference in their ability to locate the source (Fig. 5).
DISCUSSION
In most cases, P. americana males with all or part of their visual inputs removed continued to orient to wind and wind plus pheromone in a manner similar to that of intact control males and our earlier observations (Willis and Avondet, 2005). The plume-tracking behavior of males with their eyes painted and placed in wind-borne point-source plumes of pheromone was also, for the most part, statistically similar to that of intact controls and our earlier studies (Willis and Avondet, 2005). When we attempted to make the task more difficult by moving the high-contrast edges of the plume further than antennal width apart, P. americana males with their eyes painted continued to perform as well as intact controls (Fig. 3; Table 3). In each of these experiments, a small number of individuals behaved according to our predictions and showed less-precise steering (Figs 2, 3). A few of these males even demonstrated a lack of pheromone-modulated behaviors. Such behavior is rarely, if ever, observed in intact male cockroaches, where 100% of the population typically tracks the plume to the source (Willis and Avondet, 2005; Willis et al., 2008). Intact walking and flying M. sexta males tracked pheromone plumes to their source with almost equal success, but removing visual input in either of these cases renders these animals relatively incapable of performing plume-tracking behavior (Figs 3, 5).
Before these experiments, we thought that walking cockroaches might steer their paths while tracking an odor plume more directly upwind using visual information based on known examples of similar behavior associated with orientation to distant sources of sound (Böhm et al., 1991; Weber, 1990; Weber et al., 1981). In these previous studies, the addition of fixed visual cues enabled crickets walking on a servosphere locomotion compensator to steer and walk more directly towards a fixed source of their auditory mating call (Weber, 1990; Böhm et al., 1991). Even when the visual cues were present, but not in the same position as the sound source, crickets were able to orient their walking more directly towards the speaker (Böhm et al., 1991). In a manner similar to these crickets, we thought that our cockroaches might steer their plume-tracking paths more directly upwind because of visual references to fixed visible cues in their environments. However, under the conditions of our experiments, lack of visual information seems to have little effect on steering behavior during upwind orientation to an odor plume in walking P. americana males.
It is possible that the directional information available from the wind, probably detected by the antennae (Bell and Kramer, 1979), together with their innate response to female pheromone, could provide sufficient information to direct steering upwind and maintain contact with the odor plume in the absence of visual information. If this is the case, then it is possible that we simply have not challenged our cockroaches with a task difficult enough to require a visual reference for success. Our next series of experiments will be designed to remove directional wind cues while the cockroaches are tracking a plume. It is possible that sudden loss of the primary directional cues used to locate the source, together with the inability to use visual inputs could cause an increase in the variability of their steering responses and result in longer tracking times or an inability to locate the source. In this case, the cockroaches would be forced to track the plume using odor information alone. However, if the cockroaches with occluded visual systems continued to perform as well as unpainted controls, it would suggest that the quality of olfactory information available to these animals is rich enough to support the behavior we observe, with no visual references to the environment and no directional wind information. We have already determined that P. americana males with normal visual systems can continue to locate successfully a source of female pheromone after the loss, or in the absence, of directional information from wind (Willis et al., 2008).
It is also possible that the complete removal of visual input caused by covering the eyes is not equivalent to walking in total darkness to the brains of our cockroaches. By covering the eyes, we have completely removed all inputs to the visual system. This could cause the brains of these cockroaches to ignore, or decrease the sensitivity of, circuitry involved in visual processing and upregulate or enhance the sensitivity of circuitry involved in odor and wind information processing. Thus, it is possible that, by removing all visual input from our cockroaches, we made them more sensitive to the rest of their sensory inputs. Two easy ways to test these ideas are to drop the light levels in our experimental arena to zero or as close to that as we can. Alternatively, we could enrich the visual environment for intact cockroaches or use projected flow-field stimuli to test whether plume-tracking cockroaches with normal visual systems use visual cues to control their steering and locomotion. At this point in our analyses of the role of vision in walking cockroach odor orientation, we have shown only that any differences in the steering behavior of males with and without visual inputs are too subtle to discriminate with the approaches we have taken so far.
Our approach to walking orientation while plume tracking might be too inclusive, and we might need to focus on specific components of this behavior to discriminate effects of the loss of vision. One indication of this is the difference between the mean track and body-yaw angles in our experiments ‘1’ and ‘2’ (Tables 1, 2). In the wind-plus-odor environment in experiment 1, we observed a trend for cockroaches to steer their track and body-yaw angles off the wind direction upon loss of visual inputs (Table 1). We see no such trend in the track and body-yaw angles measured from the responses of cockroaches tracking a point-source in our second experiment (Table 2). It is possible that the initial orientation to the environment upon leaving the release cage is where the effects of loss of visual inputs are most easily discriminated. Once an individual has a ‘global’ orientation to their environment, the high-contrast odor–clean-air boundary at the edge of the plume and direction information from the wind are sufficient to permit successful plume tracking. Further experiments using the ideas above should show whether, and to what extent, lack of visual input affects steering in walking odor-tracking cockroaches.
We found it particularly interesting that, when significant effects of removing visual inputs were revealed, and the tracking performance of an individual failed, it was in treatment groups in which the ocelli had been painted. Studies of the neurophysiology and anatomy of the ocelli in P. americana show that the projection neurons leaving the primary processing plexus under each ocellus lead to sensory processing areas in the protocerebrum underlying the compound eyes and antennae (i.e. the optic lobes and antennal lobes) (Mizunami, 1994). They also project to neuropils in the brain associated with multimodal integration, such as the mushroom bodies (Mizunami, 1994), and send projections through the brain directly to the thoracic ganglion. Therefore, the projections from the ocelli could be modulating the primary processing of other sensory inputs, affecting how those inputs are integrated to affect ongoing behaviors, or directly influencing locomotor circuitry, or all of the above simultaneously. Thus, removing ocellar inputs could have impacts on the sensory-motor control of cockroach behavior on multiple levels simultaneously. In fact, given the broad and diverse projections from the ocelli, it is remarkable that removal of their input seemed to have such a subtle, difficult to observe, effect on most cockroaches.
Recent studies of the ocelli in the blow fly Calliphora vicina have shown a clear physiological interaction between the ocelli and compound eyes consistent with a role in flight stabilization (Parsons et al., 2006; Parsons et al., 2010). The ocelli are more sensitive to changes in light level and have very few synapses before the ocellar retinal responses can be sent to higher brain centers. Consistent with the anatomical studies of cockroaches (Mizunami, 1994), information has been shown to travel from the ocelli directly to the wide-field motion-detecting neurons in the optic lobes of the brain (Parsons et al., 2006; Parsons et al., 2010). In this case, the influence of ocellar inputs to higher-order processing is thought to enhance the speed of response of the fly to its own rotations in space during flight (Parsons et al., 2010).
Previous studies of the effects of eye-painting on the orientation to dark areas of the environment, and the cessation of escape behavior, suggest that information from the ocelli might modulate the processing of visual information by the compound eyes and effect the response to dark hiding places in walking cockroaches (Okada and Toh, 1998). More recently, it has been shown that covering the ocelli caused individuals of the cockroach B. discoidalis to bias their behavioral choices as if it were night (Harley et al., 2009). These animals choose to crawl under or climb over obstacles, depending on ambient light levels. When their ocelli were covered, they behaved as if it were dark, no matter what the ambient light levels were or whether their compound eyes were covered (Harley et al., 2009). We need to know more about the function of ocelli in the control of the behaviors of P. americana and other cockroaches before we can determine how loss of ocellar input might have caused the effects we observed.
Given what is known about the importance of visual information to stabilization and control in flying insects (Frye et al., 2003; Srinivasan et al., 1999), it is perhaps not surprising that M. sexta males with painted compound eyes were unable to generate prolonged bouts of stable flight or track the plume upwind while flying. However, after watching male cockroaches with painted eyes track plumes in a manner nearly indistinguishable from that of intact males, we were surprised to observe male moths with painted eyes fail to show prolonged plume-tracking behavior or successful source location while walking. There are a number of possible explanations for this result. First, surgically removing the wings and painting the eyes was so disruptive to these individuals that they ceased responding to the pheromone plume. However, the ability of moths with unpainted eyes and similarly cropped wings to track the plume and locate the source suggests that removing visual inputs might have a direct effect on the plume-tracking response. If this is a specific effect on the pheromone response, one possible interpretation is that the walking and flying moths use the same control algorithm to track odors, and removal of one of the sensory inputs causes cessation of the behavior. Experiments aimed at understanding the control algorithms underlying odor tracking and how they might change during the transition between flight and walking are ongoing in our laboratory. Another possibility is that M. sexta moths are so specifically adapted for flight and the visual inputs associated with flight control that removing visual information from self-movement feedback suppresses their motivation for any locomotory behavior.
Initially, it might seem surprising, given the size and complexity of the compound eyes, that their functional removal seems to have such a limited effect on walking orientation in P. americana males. However, the compelling nature of the female sex pheromone stimulus, together with wind- and odor-sensing systems specialized to support the behavior of a nocturnal, primarily walking animal, might account for much of the behavior we observed. Further experiments, aimed at resolving the role of vision in nocturnally active walking and flying odor trackers, are ongoing in our laboratory.
ACKNOWLEDGEMENTS
We are very grateful to K. Mori and S. Kuwahara for their kind donation of the synthetic (–)-periplanone-B. Without this contribution, these experiments would have been nearly impossible. We thank Roy Ritzmann, Jim Belanger, Cindy Harley and Jennifer Talley for contributing to the quality of the final paper. Anonymous reviewers also made significant contributions to the quality of the final paper. We also thank Adam Rutkowski for his contribution to the data-analysis capabilities of the laboratory.
FOOTNOTES
FUNDING
The Howard Hughes Medical Institute provided support for E.Z. Further support was provided by ONR-MURI N00014-01-1-0676 to M.A.W.
REFERENCES
- Baba Y., Tsukada A., Comer C. M. (2010). Collision avoidance by running insects: antennal guidance in cockroaches. J. Exp. Biol. 213, 2294-2302 [DOI] [PubMed] [Google Scholar]
- Baker T. C., Willis M. A., Phelan P. L. (1984). Optomotor anemotaxis polarizes self-steered zigzagging in flying moths. Physiol. Entomol. 9, 365-376 [Google Scholar]
- Bell R., Joachim F. (1976). Techniques for rearing laboratory colonies of tobacco hornworms and pink bollworms. Ann. Entomol. Soc. Am. 69, 365-373 [Google Scholar]
- Bell W. J., Kramer E. (1979). Search and anemotactic orientation of cockroaches. J. Insect Physiol. 25, 631-640 [Google Scholar]
- Bell W. J., Tobin T. R. (1981). Orientation to sex pheromone in the American cockroach: Analysis of chemo-orientation mechanisms. J. Insect Physiol. 27, 501-508 [Google Scholar]
- Bhandawat V., Maimon G., Dickinson M. H., Wilson R. I. (2010). Olfactory modulation of flight in Drosophila is sensitive, selective and rapid. J. Exp. Biol. 213, 3625-3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm H., Schildberger K., Huber F. (1991). Visual and acoustic course control in the cricket Gryllus bimaculatus. J. Exp. Biol. 159, 235-248 [Google Scholar]
- Cabrera J., Schmidt-Koenig K., Watsin G. S. (1991). The statistical analysis of circular data. In Perspectives in Ethology: Human Understanding and Animal Awareness, Vol. 9 (ed. Bateson P. P. G., Klopfer P. H.), pp. 285-306 New York and London: Plenum Press; [Google Scholar]
- Charlton R. E., Kanno H., Collins R. D., Cardé R. T. (1993). Influence of pheromone concentration and ambient temperature on the flight of the gypsy moth, Lymantria dispar (L.), in a sustained-flight wind-tunnel. Physiol. Entomol. 18, 349-362 [Google Scholar]
- Chiappe M. E., Seelig J. D., Reisner M. B., Jayaraman V. (2010). Walking modulates speed sensitivity in Drosophila motion vision. Curr. Biol. 20, 1470-1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow D. M., Frye M. A. (2008). Context-dependent olfactory enhancement of optomotor flight control in Drosophila. J. Exp. Biol. 211, 2478-2485 [DOI] [PubMed] [Google Scholar]
- David C. T. (1986). Mechanisms of directional flight in wind. In Mechanisms in Insect Olfaction (ed. Payne T. L., Birch M. C., Kennedy C. E. J.), pp. 49-57 Oxford: Clarendon Press; [Google Scholar]
- Drescher W. (1960). Regenerationversuche am gehirn von Periplaneta americana unter beirucksichtigung von verhaltensanderung und neurosekretion. Z. Morph. Okol. Tiere 48, 576-649 [Google Scholar]
- Duistermars B. J., Frye M. A. (2008). Crossmodal visual input for odor tracking during fly flight. Curr. Biol. 18, 270-275 [DOI] [PubMed] [Google Scholar]
- Eaton J. L. (1971). Insect photoreceptor: an internal ocellus is present in sphinx moths. Science 173, 822-823 [DOI] [PubMed] [Google Scholar]
- Fisher N. I. (2000). Statistical Analysis of Circular Data. Cambridge, UK: Cambridge University Press; [Google Scholar]
- Frye M. A., Tarsitano M., Dickinson M. H. (2003). Odor localization requires visual feedback during free flight in Drosophila melanogaster. J. Exp. Biol. 206, 843-855 [DOI] [PubMed] [Google Scholar]
- Götz K. G., Wenking H. (1973). Visual control of locomotion in the walking fruitfly Drosophila. J. Comp. Physiol. 85, 235-266 [Google Scholar]
- Harley C. M., English B. A., Ritzmann R. E. (2009). Characterization of obstacle negotiation behaviors in the cockroach, Blaberus discoidalis. J. Exp. Biol. 212, 1463-1476 [DOI] [PubMed] [Google Scholar]
- Kathman N. D., Horomanski A. L., Pollack A. J., Ritzmann R. E. (2011). The role of the central complex in optomotor behavior of the cockroach. Program no. 944.06. 2011 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; [Google Scholar]
- Kennedy J. S. (1939). The visual responses of flying mosquitoes. Proc. Zool. Soc. Lond. A 109, 221-242 [Google Scholar]
- Kennedy J. S., Marsh D. (1974). Pheromone regulated anemotaxis in flying moths. Science 184, 999-1001 [DOI] [PubMed] [Google Scholar]
- Kitahara T., Mori M., Mori K. (1987). Pheromone synthesis, Part 122. Total synthesis of (-)-periplanone-B, natural major sex-excitant pheromone of the American cockroach (Periplaneta americana). Tetrahedron 43, 2689-2699 [Google Scholar]
- Kuwahara S., Mori K. (1990). Pheromone synthesis, Part 123. Synthesis of (-)-periplanone-B, a sex pheromone of the American cockroach (Periplaneta americana). Tetrahedron 46, 8075-8082 [Google Scholar]
- Maimon G., Straw A. D., Dickinson M. H. (2010). Active flight increases the gain of visual motion processing in Drosophila. Nat. Neurosci. 13, 393-399 [DOI] [PubMed] [Google Scholar]
- Marsh D., Kennedy J. S., Ludlow A. R. (1978). An analysis of anemotactic zigzagging flight in male moths stimulated by pheromone. Physiol. Entomol. 3, 221-240 [Google Scholar]
- Mizunami M. (1994). Functional diversity of neural organization in the insect ocellar systems. Vision Res. 35, 443-452 [DOI] [PubMed] [Google Scholar]
- Mizunami M., Weibrecht J. M., Strausfeld N. J. (1998). Mushroom bodies of the cockroach: Their participation in place memory. J. Comp. Neurol. 402, 520-537 [PubMed] [Google Scholar]
- Okada J., Toh Y. (1998). Shade response in the escape behavior of the cockroach, Periplaneta americana. Zool. Sci. 15, 831-835 [Google Scholar]
- Olberg R. M., Willis M. A. (1990). Pheromone-modulated optomotor response in male gypsy moths, Lymantria dispar L.: directionally selective visual interneurons in the ventral nerve cord. J. Comp. Physiol. A 167, 707-714 [Google Scholar]
- Parsons M. M., Krapp H. G., Laughlin S. B. (2006). A motion-sensitive neurone responds to signals from the two visual systems of the blowfly, the compound eyes and ocelli. J. Exp. Biol. 209, 4464-4474 [DOI] [PubMed] [Google Scholar]
- Parsons M. M., Krapp H. G., Laughlin S. B. (2010). Sensor fusion in identified visual interneurons. Curr. Biol. 20, 624-628 [DOI] [PubMed] [Google Scholar]
- Pilla R. S., Kitska D. J., Loader C. (2005). Statistical analysis of modified complete randomized designs: applications to chemo-orientation studies. J. Exp.Biol. 208, 1267-1276 [DOI] [PubMed] [Google Scholar]
- Ronacher B., Wehner R. (1994). Desert ants, Cataglyphis fortis, use self-induced optic flow to measure distance traveled. J. Comp. Physiol. A 177, 21-27 [Google Scholar]
- Ronacher B., Gallizzi K., Wohlgemuth S., Wehner R. (2000). Lateral optic flow does not influence distance estimation in the desert ant Cataglyphis fortis. J. Exp. Biol. 203, 1113-1121 [DOI] [PubMed] [Google Scholar]
- Rutkowski A. J., Quinn R. D., Willis M. A. (2009). Three-dimensional characterization of the wind-borne pheromone tracking behavior of male hawkmoths, Manduca sexta. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 195, 39-54 [DOI] [PubMed] [Google Scholar]
- Ryan T. A. (1960). Significance tests for multiple comparison of proportions, variances and other statistics. Psychol. Bull. 57, 318-328 [DOI] [PubMed] [Google Scholar]
- Sane S. P., Dieudonné A., Willis M. A., Daniel T. L. (2007). Antennal mechanosensors mediate flight control in moths. Science 315, 863-866 [DOI] [PubMed] [Google Scholar]
- Sasaki M., Riddiford L. M. (1984). Regulation of reproductive behavior and egg maturation in the tobacco hawk moth, Manduca sexta. Physiol. Entomol. 9, 315-327 [Google Scholar]
- Srinivasan M. V., Poteser M., Kral K. (1999). Motion detection in insect orientation and navigation. Vision Res. 39, 2749-2766 [DOI] [PubMed] [Google Scholar]
- Srinivasan M. V., Zhang S., Altwein M., Tautz J. (2000). Honeybee navigation: nature and calibration of the odometer. Science 287, 851-853 [DOI] [PubMed] [Google Scholar]
- Strauss R., Schuster S., Gotz K. G. (1997). Processing of artificial visual feedback in the walking fruit fly Drosophila melanogaster. J. Exp. Biol. 200, 1281-1296 [DOI] [PubMed] [Google Scholar]
- Strauss R., Renner M., Gotz K. (2001). Task-specific association of photoreceptor systems and steering parameters in Drosophila. J. Comp. Physiol. A 187, 617-632 [DOI] [PubMed] [Google Scholar]
- Tobin T. R. (1981). Pheromone orientation: role of internal control mechanisms. Science 214, 1147-1149 [DOI] [PubMed] [Google Scholar]
- Warrant E. J., Dacke M. (2011). Vision and visual navigation in nocturnal insects. Annu. Rev. Entomol. 56, 239-254 [DOI] [PubMed] [Google Scholar]
- Weber T. (1990). Phonotaxis and visual orientation in Gryllus campestris L.: behavioural experiments. In Sensory Systems and Communication in Arthropods (ed. Gribakin F. G., Wiese K., Popov A. V.), pp. 377-386 Basel: Birkhäuser Verlag; [Google Scholar]
- Weber T., Thorson J., Huber F. (1981). Auditory behavior of the cricket. I. Dynamics of compensated walking and discrimination paradigms on the Kramer treadmill. J. Comp. Physiol. A 165, 165-177 [Google Scholar]
- Willis M. A., Arbas E. A. (1991). Odor-modulated upwind flight of the sphinx moth, Manduca sexta L. J. Comp. Physiol. A 169, 427-440 [DOI] [PubMed] [Google Scholar]
- Willis M. A., Arbas E. A. (1998). Variability in odor-modulated flight by moths. J. Comp. Physiol. A 182, 191-202 [DOI] [PubMed] [Google Scholar]
- Willis M. A., Avondet J. L. (2005). Odor-modulated orientation in walking male cockroaches, Periplaneta americana (L.), and the effects of odor plumes of different structure. J. Exp. Biol. 208, 721-735 [DOI] [PubMed] [Google Scholar]
- Willis M. A., Butler M. A., Tolbert L. P. (1995). Normal glomerular organization of the antennal lobes is not necessary for odor-modulated flight in female moths. J. Comp. Physiol. A 176, 205-216 [DOI] [PubMed] [Google Scholar]
- Willis M. A., Avondet J. L., Finnell A. S. (2008). Effects of altering flow and odor information on plume tracking behavior in walking cockroaches, Periplaneta americana (L.). J. Exp. Biol. 211, 2317-2326 [DOI] [PubMed] [Google Scholar]
- Ye S., Leung A., Khan A., Baba Y., Comer C. M. (2003). The antennal system and cockroach evasive behavior. I. Roles for visual and mechanosensory cues in the response. J. Comp. Physiol. A 189, 89-96 [DOI] [PubMed] [Google Scholar]
- Zanker J. M., Collett T. S. (1984). The optomotor systems on the ground: On the absence of visual control of speed in walking ladybirds. J. Comp. Physiol. A 156, 395-402 [Google Scholar]