Abstract
Circadian misalignment has been implicated in the development of obesity, diabetes mellitus, and cardiovascular disease. Time-of-day-dependent synchronization of organisms with their environment is mediated by circadian clocks. This cell autonomous mechanism has been identified within all cardiovascular-relevant cell types, including cardiomyocytes. Recent molecular- and genetic- based studies suggest that the cardiomyocyte circadian clock influences multiple myocardial processes, including transcription, signaling, growth, metabolism, and contractile function. Following an appreciation of its physiological roles, the cardiomyocyte circadian clock has recently been linked to the pathogenesis of heart disease in response to adverse stresses, such as ischemia/reperfusion, in animal models. The purpose of this review is therefore to highlight recent advances regarding the roles of the cardiomyocyte circadian clock in both myocardial physiology and pathophysiology (i.e., health and disease).
Keywords: Chronobiology, Hypertrophy, Ischemia, Metabolism, Myocardium
Introduction
Knowing the time of day presents a selective advantage at multiple biological tiers, including cellular, tissue, and whole organism. This information is critical to ensure appropriate and rapid responses are achieved in a temporally appropriate manner. The key to this unique selective advantage is anticipation. Typically, a researcher might consider a cell/system that responds to a stimulus based solely on its initiation (i.e., in the absence of any form of priming). However, the ability to prepare for an event before it occurs is critical for numerous aspects of life, whether it be preparation for a newborn child, the arrival of a food source, or perhaps at the cellular level the binding of a hormone to its receptor. In the latter case, one might envision priming of a distinct branch of a signal transduction pathway in a temporal manner, to ensure an appropriate cellular response to a predictable, recurring stimulus/stress. One cardiovascular-relevant hypothetical example is illustrated by myocardial responsiveness to changes in pressure/sheer stress. Alterations in sheer stress occur on a daily basis within a physiological range, peaking during the active/awake period, a time at which physical activity is typically elevated.1 Increased activity (such as exercise) results in a physiological growth (hypertrophy) of the myocardium. In contrast, a persistent elevation of sheer stress results in a pathological hypertrophic response.2 One potential explanation is that an inappropriate elevation of sheer stress during the inactive/sleep phase (i.e., as observed in non-dipping hypertensives) may contribute to a pathological response, while appropriate anticipation of temporal changes in sheer stress results in a physiological response.
Central to the concept of biological anticipation are two fundamental criteria. First, a stimulus is present/active in a recurring and temporally-distinct period. This might include time-of-day- and/or seasonal- dependence. Second, the cell/organ/organism possesses a molecular timekeeping mechanism. This timekeeping mechanism must retain sufficient plasticity to be entrained by the environment, thereby maintaining a selective advantage (e.g., consider season-and migration- induced alterations in the environment). Indeed, such a timekeeping mechanism has evolved; the circadian clock. Circadian clocks have been identified in both prokaryotes and eukaryotes (although some reports suggest that specific prokaryotes may not possess functional clocks).3–5 Clocks are transcriptionally-based cell autonomous molecular mechanisms, that directly regulate cellular/biological function at multiple temporal levels; daily, seasonal, and lifespan.3–5 Circadian clocks have been identified and characterized within almost all mammalian cell types, including cardiomyocytes, vascular smooth muscle cells, endothelial cells, and fibroblasts.6–9 Over the last decade studies from multiple investigative teams have begun to unravel a number of the roles of circadian clocks within the cardiovascular system. Recent advances with regards to the vasculature include observations that mouse models of circadian clock dysfunction exhibit alterations in endothelial function and blood pressure as well as variations in vascular injury susceptibility.10, 11 In humans, distinct genetic polymorphisms in circadian clock gene components (e.g., BMAL1) are associated with increased risk of hypertension.12 The purpose of this article is to highlight our current knowledge regarding the functions of the cardiomyocyte circadian clock, and to discuss whether disruption of this mechanism plays a potential role in the etiology of cardiovascular disease. Given that several reviews have been published recently on this topic, this article will rapidly focus on distinct areas of novel ongoing research.
The Cardiomyocyte Circadian Clock: Influence on Myocardial Physiology
Heart Clock
Circadian clocks can be defined as a transcriptionally-based molecular mechanism, composed of both positive and negative feedback loops, with a free running period of approximately 24 hours.3 Time-of-day-dependent oscillations in clock component gene expression have been characterized in both rodent and human hearts.13–15 Consistent with the cell autonomous nature of this mechanism, circadian clock component gene expression oscillations observed in the intact heart persist in isolated cultured myocardial tissue and cardiomyocytes.6,16 Given the transcriptional nature of this mechanism, several laboratories have investigated oscillations in the transcriptome of the heart over the course of the day. Hearts collected from mice under light/dark (diurnal) and constant dim light (circadian) conditions suggest that expression of approximately 10–15% of all myocardial genes oscillate in a time-of-day-dependent manner.17, 18 However, the reported gene expression oscillations in normal intact hearts could be mediated by extracellular (i.e., neurohumoral) and/or intracellular (i.e., circadian clock) influences. To determine the contribution of the cardiomyocyte circadian clock, we generated a mouse model wherein this mechanism was selectively disrupted. This mouse is termed cardiomyocyte-specific clock mutant (CCM).19 Distinct from classic models of shift work involving manipulation of the light-dark cycle, CCM mice are a model of temporal suspension of the cardiomyocyte circadian clock at the wake-to-sleep transition.20 In addition to identification of clock-controlled genes (CCGs), phenotypic characterization of CCM mice has been useful for revealing novel roles of the cardiomyocyte circadian clock on myocardial physiology and pathophysiology. The following subsections will highlight some of these roles.
Heart Rate
Accumulating evidence suggests that intrinsic circadian clocks exert some level of control over heart rate. Through maintenance of a controlled environment for a prolonged period of time, Hu et al have reported that circadian rhythms in heart rate variability are driven by an intrinsic mechanism in humans.21 Consistent with these observations, genetic mouse models of ubiquitous altered circadian clock function exhibit varying degrees of abnormalities in heart rate diurnal/circadian rhythms, depending on which distinct circadian clock component was modified.10 Additionally, patients with an extended Per3 tandem repeat exhibit elevated heart rate.22 Furthermore, selective deletion of PPARγ, a putative activator of BMAL1, in the vasculature results in diminished heart rate diurnal variations.23 We have recently interrogated the influence of the cardiomyocyte circadian clock on heart rate, both in vivo and ex vivo, through the use of CCM mice. In vivo radiotelemetry studies were performed on young WT and CCM mice for continuous 24 hour monitoring of physical activity, heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP) over a 2 week period. These studies revealed that CCM mice exhibit a reduction in heart rate. Importantly, heart rate depression is greatest during the awake/active phase, resulting in a significant attenuation of the peak-to-trough ratio, compared to WT hearts (i.e., attenuation in the rhythm). ECG radiotelemetry analysis was performed next, to characterize further the observed bradycardia. Significant differences were observed only for the R-R interval (increased in CCM mice), consistent with sinus bradycardia. This depression in heart rate does not appear to be secondary to differences in physical activity and/or alterations in acute responsiveness to humoral influences, as WT and CCM mice exhibit identical physical activity levels/patterns and bradycardia persists in an ex vivo working heart preparation. Collectively, these data suggest that the cardiomyocyte circadian clock regulates heart rate in a time-of-day-dependent manner. In contrast, no differences in SBP, DBP, or MAP were observed between WT and CCM mice.24
No clear explanation for the sinus bradycardia observed in CCM mice has emerged to date. Bradycardia can result from a large spectrum of perturbations, ranging from signaling and ion homeostasis to morphological alterations influencing conduction.25, 26 In the latter case, it is difficult to envisage significant morphological changes over the course of the normal day. In contrast, alterations in signal transduction and ion homeostasis occur over a shorter timescale, in a reversible manner. Consistent with regulation of the processes by the cardiomyocyte circadian clock, gene expression microarray analysis identified multiple signal transduction cascade components and ion channels as CCGs. The importance for proper ion homeostasis in heart rate development and maintenance is well understood. From the depolarizing phase to reestablishment of the membrane resting potential, precise and coordinated ion movement is required.25 Chandler et al. recently modeled that the human sinus node action potential could be achieved by the expression of 9 ion channels.27 While the temporal expression of these channels was not addressed, work in the rat heart has revealed that the potassium channels Kv1.5 and 4.2 exhibit diurnal variations not only in expression but also in current.28 Additionally the regulation of intracellular calcium plays an essential role in the maintenance of heart rate. Although little is known regarding time-of-day-dependent oscillations in Ca2+ channel function in the heart, the ryanodine receptor as well as multiple T-type calcium channels have been proposed to be controlled by the circadian clock within the brain of mice.29, 30 Indeed, various ion channels have been shown to be circadian clock regulated in the SCN.31 Interestingly, we find that the expression of several ion channels, including cacna2d1, slc9a2, slc9a8, slc22a1, slc23a2, and slco3a1, appear to be influenced by the circadian clock.24 Historically, control of heart rate has been described as being driven by factors extrinsic to the heart, however these data suggest a role for the intrinsic circadian clock. Whether the circadian clock within the sinus-atrial node cardiomyocytes plays a significant role in mediating diurnal variations in heart rate awaits elucidation.
Propagation of electrical signals between adjacent cells is crucial for action potential transduction. One mechanism by which the myocardium achieves this is through the formation of gap junctions using connexin proteins. These gap junctions offer low resistance, low selectivity channels for cell-to-cell communication. Cardiac gap junctions are composed from three connexin isoforms, each with distinct heart localization. Connexin 43 is found in the atrial and ventricular myocytes.32 Connexin 45 is found in the conduction system of the heart as well as the atrial and ventricular myocytes.33 Connexin 40 is found in the atrial myocytes and the His-Purkinje cells.34 The importance of connexin 40 in atrial-ventricular conduction was recently demonstrated in the Connexin 40 null mice. Compared to WT hearts Connexin 40 null hearts exhibit delayed atrioventricular conduction as well as increased R-R interval.35 Interestingly, our gene expression microarray analysis identified Connexin 40 as being regulated by the circadian clock within atrial cell (approximately 2-fold oscillation in WT hearts, that is chronically repressed in CCM hearts).24 These data suggests a possible mechanism through which the circadian clock may contribute to diurnal rhythms in heart rate, and also offers a possible explanation for the bradycardia of CCM hearts.
Heart Metabolism
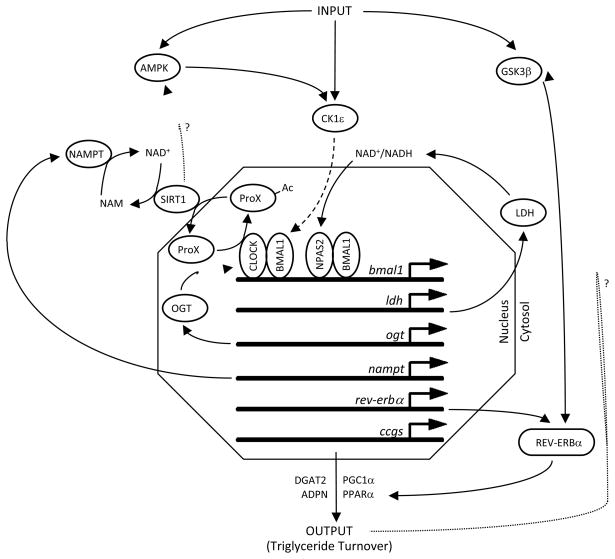
Myocardial metabolism and contractile function are interlinked.36 Imbalances/impairment of energy metabolism adversely affects cardiac function.37 Conversely, periods of increased cardiac output are balanced by increased metabolic fluxes, thereby meeting energetic demands.38 What is becoming increasingly clear is that metabolism is inseparably interlinked with the circadian clock. As illustrated in Figure 1, several feedback loops of the mammalian circadian clock have integrated metabolic functions. Evidence for/against the existence of these metabolic loops in the cardiomyocyte circadian clock will be discussed. Whenever possible, the potential metabolic and functional consequences of these loops on the myocardium will be highlighted.
Figure 1.
Integration of Metabolism within the Mammalian Circadian Clock. Figure summarizes a large number of distinct studies highlighting metabolic loops within the mammalian circadian clock (see section entitled Heart Metabolism). Solid lines represent direct effects/links; dashed lines represent multiple steps; dotted lines (with ‘?’) represent hypothetical links. Ac represents acetyl group on an acetylated protein; ProX represents an undefined protein. Other definitions are included in the List of Abbreviations.
Rutter et al were among the first to describe a metabolic loop within the mammalian circadian clock, at a molecular level. The investigators discovered that the circadian clock transcription factor CLOCK, as well as the CLOCK homolog NPAS2, are redox sensitive. The NAD+/NADH ratio influences the DNA binding affinity of both CLOCK/BMAL1 and NPAS2/BMAL1, wherein a decrease in this ratio promotes binding. Importantly, the investigators also showed that lactate dehydrogenase a (LDHa) was induced by CLOCK/BMAL1 and NPAS2/BMAL1 heterodimers, through binding to E-box elements in the promoter of the ldha gene.39 LDHa-mediated regulation of the intracellular redox status completes the feedback loop (Figure 1). These studies were performed in neuronal cells, raising the question whether this feedback loop is ubiquitous or cell-type specific. This is particularly relevant for a metabolically active organ such as the heart, which possesses a high lactate dehydrogenase maximal activity, thus raising concerns as to whether levels of this enzyme limits cardiomyocyte redox status under physiological conditions. Consistent with this concern, we find that expression of ldha does not significantly oscillate in wild-type hearts in a time-of-day-dependent manner, nor is it altered in CCM hearts (unpublished observations). However, CLOCK and NPAS2 are both highly expressed in the rodent heart (and oscillate in a circadian-like manner).40 Interestingly, NPAS2 may play a particularly significant role in entrainment of the cardiomyocyte circadian clock, as restricted feeding is able to initiate rhythmic expression of clock genes in ClockΔ19 mutant mouse hearts.41, 42 Collectively, these observations led us to hypothesize that the cardiomyocyte circadian clock may be exquisitely sensitive to changes in intracellular redox status, through NPAS2 (and/or CLOCK). Consistent with this hypothesis, ischemia/reperfusion (which induces profound alterations in the cellular redox status) results in a rapid (<24 hours) attenuation of circadian clock component gene expression oscillations within the region of viable ischemic myocardial tissue. In contrast, this insult had no effect on clock gene expression in the non-ischemic region of the myocardium.43
More recently, a second loop of the mammalian circadian clock involving the cofactor NAD+ has been described. NAD+ is an essential cofactor for the deacetylase Sirt1, the proposed molecular target of resveratrol.44 Acetylation/deacetylation is a critical post-translational modification that modulates multiple biological processes, the most highly appreciated being transcription. Interestingly, genome wide acetylation exhibits a time-of-day-dependent oscillation.45 Elegant studies by Doi and colleagues revealed that CLOCK possesses histone acetyltransferse activity, and is capable of acetylating its heterodimerization partner BMAL1, augmenting CLOCK/BMAL1 mediated transcription.46 Analogous to the ldha gene, CLOCK/BMAL1 can bind to the E-box in the nampt gene, resulting in induction. NAMPT is the rate limiting enzyme in the NAD+ salvage pathway. As such, the induction of NAMPT results in increased intracellular levels of NAD+. This results in increased Sirt1 activity.47 Sirt1 in turn closes this metabolism-based feedback loop, through deacetylation of multiple targets, including BMAL1. Consistent with this hypothesis, nampt expression and cellular NAD+ levels oscillate in a time-of-day-dependent manner in multiple tissues, including the heart.18, 24, 48 In the case of myocardial nampt, these oscillations are mediated by the cardiomyocyte circadian clock, consistent with the presence of the NAMPT-NAD+-Sirt1 metabolic loop in the heart.24 These observations are important for multiple reasons. NAD+ is a cofactor for numerous enzymes, raising the possibility that oscillations in intracellular NAD+ levels could affect multiple pathways in the cardiomyocyte. Indicative of a critical role, NAMPT has recently been reported to influence cardiomyocyte survival.49 Sirt1 deacetylates a plethora of protein targets, and has been implicated in the regulation of oxidative metabolism (perhaps through PGC1), insulin signaling (perhaps through Foxo), and myocardial ischemia/reperfusion and oxidative stress tolerance.50–52 Time-of-day-dependent oscillations in Sirt1 activity may therefore modulate responsiveness of the myocardium to multiple extracellular stimuli/stresses, in a circadian-like manner. The implications of this metabolic loop clearly extend far beyond simple oscillations in metabolic fluxes.
Major fuel sources for continued contraction of the myocardium are fatty acids and carbohydrate (e.g. glucose).36 We decided to test the hypothesis that glucose and/or fatty acid metabolism is an integral component of the cardiomyocyte circadian clock. The approach to test this hypothesis was two fold: 1) does the cardiomyocyte circadian clock regulate metabolism of either of these fuels (in a time-of-day-dependent manner); and/or 2) do these fuels influence the timing of the cardiomyocyte circadian clock? Initial studies investigating oxidative metabolism diurnal variations in the rat heart revealed time-of-day-dependent oscillations in glucose, but not fatty acid, oxidation.53, 54 However, glucose oxidation oscillations were not observed in mouse hearts, and neither were significant differences in glucose oxidation between wild-type and CCM hearts. Similarly, fatty acid oxidation did not oscillate in mouse hearts (although fatty acid oxidation rates were higher in CCM hearts in a time-of-day-independent manner).24 These somewhat surprising observations suggested that under basal non-stressed conditions, the cardiomyocyte circadian clock does not directly influence myocardial oxidative metabolism. In addition, initial studies in isolated adult rat cardiomyocytes showed that neither glucose nor fatty acids acutely influenced the expression of circadian clock components (neither amplitude nor phase of oscillation).6, 19
Collectively, the above described experimental observations are not consistent with metabolic loops involving glucose and/or fatty acids being operational within the myocardium. However, several lines of evidence exist suggesting that conclusions based on these observations alone may be premature. Gene expression microarray analysis for hearts isolated from wild-type and CCM littermate hearts over the course of the day identified a large number of genes influencing triglyceride (e.g., dgat2, adpn) and glycogen metabolism (e.g., ppp1cc), the storage forms of fatty acids and glucose respectively, as being cardiomyocyte circadian clock regulated (Figure 1).24 However, changes in gene expression do not always translate to reciprocal changes in protein levels, enzymatic activities, or most importantly, metabolic fluxes. As such, studies were performed to investigate whether the cardiomyocyte circadian clock regulates non-oxidative metabolism of fatty acids and/or glucose. This appears to be the case. For example, total triglyceride levels exhibit a diurnal variation in mouse hearts both in vivo and ex vivo (peaks near the end of the active phase) which is essentially absent in CCM hearts. This appears to be due to circadian clock regulation of lipolysis, which is activated during the sleep/inactive phase.55 In the case of glycogen metabolism, we have previously reported that epinephrine-induced glycogenolysis has a time-of-day-dependence in wild-type hearts, which is attenuated in CCM hearts.24 More recently, we have revealed a diurnal variation in total protein O-GlcNAcylation in both rat and mouse hearts, which is completely abolished in CCM hearts.56 Increased myocardial protein O-GlcNAcylation during the active phase suggests increased flux of glucosyl moieties through the hexosamine biosynthetic pathway at this time. Taken together, these data show that the cardiomyocyte circadian clock regulates non-oxidative fatty acid (i.e., triglyceride) and glucose (i.e., glycogen and O-GlcNAc) metabolism.
Non-oxidative metabolism of nutrients is pivotal in the cycling of carbon between storage, oxidation, and generation of signaling molecules. For example, triglyceride is a rich source of fatty acyl groups that act not only as a metabolic fuel, but also influence the activity of a host of proteins/enzymes through transcriptional, translational, and post-translational mechanisms.57 Similarly, glucose has emerged as an important signaling molecule in mammalian cells, including cardiomycytes. One mechanism by which glucose acts as a signaling molecule is through protein O-GlcNAcylation. This reversible post-translational modification has been shown to target a wide range of proteins (>500), many of which are involved in metabolism, signaling, translation, and transcription.58 This knowledge encouraged us to revisit the issue as to whether non-oxidative metabolism of fatty acids and/or glucose influences the timing of the cardiomyocyte circadian clock. As mentioned above, initial studies challenging synchronized adult rat cardiomyocytes with elevated concentrations of fatty acids or glucose had no direct effect on the timing of circadian clock gene oscillations.6, 19 Similarly, we have previously reported that high fat feeding in rats has no effect on the timing of circadian clock gene expression in the heart.59 However, a recent study has reported that high fat feeding in mice influences the timing of both central and peripheral circadian clocks (although the heart was not investigated).60 The latter suggests that in certain species, altered dietary macronutrient content may influence cell autonomous circadian clocks, perhaps in a tissue-specific manner. The possibility remains that high fat feeding induced alterations in behavior and associated neurohumoral factors (e.g., adipokines) could influence the timing of peripheral circadian clocks, such as that located within the cardiomyocyte. However, currently no evidence is available that support the hypothesis that fatty acids directly influence the cardiomyocyte circadian clock. In contrast, data are immerging in support of the hypothesis that glucose may influence this mechanism. Although initial studies challenging adult rat cardiomyocytes with glucose were negative, these studies were performed in the absence of glutamine. Glutamine is an essential amino acid required for the channeling of glucosyl units through the hexosamine biosynthetic pathway (and subsequent protein O-GlcAcylation). More recently, we have found that conditions which promote protein O-GlcNAcylation are associated with altered gene and protein expression of cardiomyocyte circadian clock components, as well as protein O-GlcNAcylation of CLOCK.56 Non-oxidative glucose therefore appears to be an integral component of the cardiomyocyte circadian clock. Consistent with this concept, uncontrolled streptozotocin-induced hyperglycemia results in a phase shifting of the clock within the heart.61
An additional metabolic link with the cardiomyocyte circadian clock that warrants discussion is regarding regulation of the responsiveness of the heart to fatty acids. When exposed to increased fatty acid levels, the myocardium responds by increasing both oxidative and non-oxidative fatty acid metabolism, in an attempt to maintain intracellular fatty acid concentrations within a physiological range. This is achieved through both post-translational (acute) and transcriptional (chronic) events.57 Initial studies in rat hearts revealed that both the acute and chronic responsiveness of the myocardium has a time-of-day-dependence. For example, challenging the heart with fatty acids during the sleep phase results in an acute depression of cardiac output and efficiency, a phenomenon that is not observed during the active phase.53 In contrast, challenging the rat heart with fatty acids during the active phase results in a greater transcriptional response at this time.62 The latter has been shown to be mediated by the cardiomyocyte circadian clock, and is potentially mediated by clock controlled oscillations in nuclear receptors, such as PPARα, Rev-erbα, and PGC1α (Figure 1).19, 63, 64 Given the temporal-dependence with which humans consume meals, these observations are highly relevant to health and disease. Consumption of dietary lipid at one time of the day (versus another) has profound effects not only on the cardiovascular system, but also on whole body energy homeostasis (and therefore susceptibility to weight gain, adiposity, and insulin resistance).
Heart Growth and Repair
Following generation of CCM mice, phenotypic characterization involved echocardiographic assessment of contractile function. Twelve week old anesthetized WT and CCM mice were utilized. Of the parameters measured, ejection fraction, fractional shortening, and left ventricular mass were all significantly elevated in CCM mice. This was associated with a decreased heart rate (as assessed through radiotelemetric studies; see discussion above), and an increased rate of fatty acid oxidation (as assessed through ex vivo metabolic studies; see discussion above).24 Furthermore, hearts from 22 month old CCM mice exhibit increased mass with no significant depression in left ventricular ejection fraction (unpublished observations). This phenotype of increased ventricular mass, increased contractile function, decreased heart rate, and increased fatty acid oxidation, is reminiscent of exercised trained animals, as opposed to adverse sustained pressure overload. In other words, CCM hearts appear to develop physiological hypertrophy, as opposed to pathological hypertrophy.
Sole and Martino have recently proposed the hypothesis that myocardial pressure overload during the sleep/inactive phase might accelerate the development of pathological hypertrophy.65 Given our recent observations in CCM mice, we wish to extend this hypothesis, to include physiological hypertrophy. Here, we hypothesize that acute elevations in pressure during the active/awake phase (in response to exercise bouts, for example) promotes physiological hypertrophy, whereas persistence of pressure overload into the inactive/sleep phase promotes pathological hypertrophy. Importantly, we hypothesize that the cardiomyocyte circadian clock governs this time-of-day-dependent, physiological versus pathological response of the myocardium to pressure/sheer stress.
The observation that CCM hearts display what appears to be physiological hypertrophy led us to initiate investigations into the molecular mechanisms by which the circadian clock regulates myocardial growth. Physiological hypertrophy can be mediated by the binding of a growth factor, such as IGF-1, to a tyrosine kinase receptor on the surface of the cardiomyocyte. Upon receptor activation, PI3K (composed of p85 and p110α subunits) is recruited to the plasma membrane where it will phosphorylate the membrane phospholipid phosphatidylinositol 4,5 bisphosphate (PIP2). This phosphorylation results in co-localization of Akt and 3-phosphoinositide-dependent protein kinase-1 (PDK1) through their interaction with PIP3. PDK1 will then phosphorylate and activate Akt. Akt exerts it’s trophic effects by modulating several pathways. First, Akt activation of mTOR (and p70S6K) has been demonstrated to upregulate ribosome biogenesis, as well as activate initiation factors required for protein synthesis. Akt is also capable of phosphorylating GSK-3β, inactivating it and thereby releasing it’s repression of translation initiation factors (thereby allowing protein synthesis required for hypertrophic growth).2, 66 Studies within our laboratory suggest that several components of the PI3K-AKT-GSK3β-p70S6K pathway may be regulated by the cardiomyocyte circadian clock. For example, gene expression microarray studies revealed that p85, the regulatory subunit of PI3K, is subject to cardiomyocyte circadian clock control at a transcriptional level, and is chronically repressed in CCM hearts.24 Importantly, the phosphorylation status of Akt, GSK-3β, and p70S6K all oscillate in hearts over the course of the day, and are chronically elevated in CCM hearts (unpublished observations). While targets of this pathway such as Akt or GSK-3β could also be regulated in response to pathophysiologic signals, p85 appears to be unique to physiological hypertrophy signaling.
Data are emerging which suggest that AMPK is potentially involved in myocardial growth regulation.67 AMPK, while commonly thought of as a cellular energy gauge, has been shown to have additional roles involved in repressing protein synthesis and in turn cell growth. This became obvious when transgenic mouse models expressing mutated AMPKγ subunits exhibited hypertrophy which was commonly associated with increased glycogen storage. Furthermore, several human polymorphisms have been identified in the AMPK subunits. Of these, missense mutations in the γ subunit are associated with left ventricular hypertrophy, abnormal glycogen accumulation and conduction disorders.68 We find that AMPK activity levels oscillate in the WT heart, peaking during the middle of the active phase. CCM hearts exhibit a significant depression in AMPK activity during the middle of the active phase (unpublished observations). Importantly this is the time at which we propose physiological hypertrophy would result from increased activity. These data suggest that circadian clock control of AMPK activity may in turn influence heart growth. Interestingly, AMPK has been shown to influence the timing of the mammalian circadian clock by multiple laboratories, suggesting that this kinase may be an integral clock component (Fig. 1).69, 70
Increased protein synthesis required for hypertrophy also involves several translation initiation factors (EIFs) and elongation factors (EFs).71 Microarray analysis revealed four EIF subunits under cardiomyocyte circadian clock regulation (eif2c3, eif3s3, eif4g2, eif1a).24 While we have not measured the activity of these EIFs in WT versus CCM hearts, their enrichment in a pathway required for hypertrophy is intriguing. In addition to its potential role in protein synthesis regulation, the circadian clock is known to influence protein degradation. Indeed, protein ubiquitination and degradation are integral processes in the circadian clock mechanism.72 Protein turnover is important for the removal of damaged proteins from the cell. Elegant studies by Taegtmeyer and colleagues suggest that increased protein turnover is important during both periods of myocardial growth and atrophy.73–75 We have found that multiple components of the ubiquitin/proteosome system (e.g., usp2, ubc, ube3c) are regulated by the cardiomyocyte circadian clock.24 Although protein turnover rates in WT and CCM hearts have not been measured to date, these measures will likely provide important insight into cardiomyocyte circadian clock regulation of myocardial growth.
The Cardiomyocyte Circadian Clock: Role in the Pathogenesis of Cardiovascular Disease
The onset of various pathological events, such as myocardial infarction, stroke, arrythmias, abdominal aortic aneurism rupture, and sudden cardiac death have all been shown to be time-of-day-dependent in humans, peaking near the sleep-to-wake transition (i.e., early morning).76, 77 Many of these diseases, including ischemic stroke and myocardial infarction, have been shown to exhibit both weekly and seasonal variations as well, with increased incidence on Mondays and during the fall.77, 78 Classically, these rhythms have been ascribed to fluctuations in extracardiac factors, such as sympathetic activity, sheer stress and prothrombotic factors.76 The possibility that a molecular mechanism intrinsic to the cardiomyocyte, such as the circadian clock, may contribute to cardiovascular disease is the subject of contemporary investigation. Within this subsection, we will focus on emerging evidence showing that the cardiomyocyte circadian clock is altered during disease states, and that this molecular mechanism may influence the etiology of several cardiovascular disease states.
A principal role of cell autonomous circadian clocks is to allow anticipation of changes in extracellular/environmental stimuli. The presence of such a mechanism enables the cell/organ/organism to react to a stimulus with appropriate timing and response.3 Therefore, impairment of the clock mechanism may lead to responses outside of the normal physiologic range. Interestingly, although essentially all mammalian cell types possess functional circadian clocks, this mechanism appears to be regulated in a cell-type specific manner.79 This raises the possibility that dyssynchronization between distinct cell types (e.g., cardiomyocytes, vascular smooth muscle cells, endothelial cells) could occur within a given system/organ (e.g., heart) during specific physiologic/pathologic situations. Whether prolonged circadian dyssychronization contributes towards the pathogenesis of cardiovascular disease is an interesting hypothesis, which is being investigated by multiple research laboratories.
Alterations in circadian clocks have been demonstrated in various models of cardiovascular disease. In Dahl salt-sensitive rats fed a high salt diet for 6 weeks the development of hypertrophy is associated with decreased amplitude of circadian expression of core clock genes in the heart.80 Similarly, ascending aortic constriction induced pressure overload attenuates rhythmic expression of several circadian clock genes in the rat heart.14, 54 However, pressure overload induced hypertrophy in the murine heart does not appear to affect expression of circadian clock genes.81 In an animal model of uncontrolled insulin-dependent diabetes mellitus, circadian clock genes within the heart exhibited a phase shift (3 hours delay) when compared to control hearts.61, 82
The master clock, located in the suprachiasmatic nucleus (SCN), is entrained by light. This SCN clock is thought to entrain peripheral clocks via modulation of neurohumoral signals.3 As such, alterations in light/dark cycle conditions can have profound effects on both central and peripheral clocks. The rate of resynchronization of cellular clocks following a shift in the light/dark cycle is tissue/organ specific, with a rapid resynchronization of the SCN clock, followed by peripheral clocks. We have demonstrated that re-entrainment of clock gene oscillations within the rat heart requires 5 to 8 days following a reversal (i.e., 12 hour shift) of the light/dark cycle.19 However, rhythms in heart rate and blood pressure have previously been show to reset within 1 to 2 days following light/dark cycle reversal.83–85 These data suggests that the heart is out of synchrony with its environment for 3 to 7 days following this light/dark cycle alteration. The impact that this dyssynchrony may have on the development of heart disease is beginning to emerge. Martino et al recently reported that circadian misalignment through light/dark cycle manipulation augments pressure overload induced myocardial dysfunction.81 Similarly, a hamster model of ubiquitous altered circadian clock periodicity (22 hours) develops accelerated age-onset cardiorenal disease.86 Consistent with these rodent models of circadian misalignment, shift-workers have a significantly greater risk for the development of cardiovascular disease (as well as multiple feature of the Cardiometabolic Syndrome).87–89 Whether changes in the timing of the cardiomyocyte circadian clock during shift work contributes to disease risk is an attractive, and as of yet non-tested, hypothesis.
As mentioned above, Sole and Martino have recently hypothesized that inappropriate elevations in pressure during the sleep/inactive phase may significantly contribute towards the development of pathological hypertrophy, and ultimately contractile dysfunction (i.e., cardiomyopathy).65 Pathological hypertrophy, as opposed to physiological hypertrophy, is associated with a reversion to fetal gene expression (including ANF and BNP), increased fibrosis and apoptosis, as well as concentric growth.90, 91 In response to biomechanical stress or neurohumoral signals, pathological hypertrophy can be induced by activation of the heterotrimeric G-protein Gq; activation of this receptor may in turn activate Akt, in addition to PKC/IP3 induced calcium release, and activation of calcineurin. Activation of the latter has been shown to play a central role in pathological hypertrophy development.66 Diurnal variations in the responsiveness of the myocardium to pressure overload may potentially be mediated by the cardiomyocyte circadian clock, as discussed above. Consistent with the hypothesis that the cardiomyocyte circadian clock attenuates pressure overload-induced pathological hypertrophic growth during the active phase, the potent calcineurin inhibitor, RCAN1, is induced in the heart at this time. The RCAN1 rhythm is driven by the cardiomyocyte circadian clock.24 Clearly additional studies are required to investigate this intriguing hypothesis.
We have recently interrogated the effects of ischemia/reperfusion (I/R) on the expression of circadian clock genes in the heart. Using a rat model of I/R, we observed rapid alterations of the clock within the ischemic (versus non-ischemic) region. All of the genes encoding core clock components (i.e. bmal1, clock, npas2, per1, per2, per3, cry1, cry2, and rev-erbaα) exhibited decreased amplitudes in their oscillations (i.e. peak-to-trough fold change). This was attributed to either decreased peak expression (npas2, per1, per2, per3, cry1, cry2, and rev-erbaα) or a rapid and persistent induction (clock and dec1). Importantly, known clock controlled gene expression patterns were also altered in the ischemic region. The PAR transcription factors dbp and tef were significantly repressed in ischemic versus non-ischemic regions, whereas e4bp4 (an antagonist of PAR transcription factor activity) was rapidly induced.43 These observations suggest that following I/R the heart is not only out of synchrony with it’s environment, but the ischemic region is out of synchrony with the non-ischemic region. We hypothesized that this intra- and inter- organ dyssynchrony contribute to I/R-induced cardiac dysfunction.
Given that the cardiomyocyte circadian clock influences myocardial physiology, and that this mechanism becomes rapidly inactivated following an ischemic event, we hypothesized that the cardiomyocyte circadian clock modulates I/R tolerance. Using the murine closed chest infarct model, I/R was induced at distinct times of the day in WT and CCM mice.92 After 24 hours of reperfusion WT hearts which underwent 45 minutes of ischemia at the sleep-to-wake transition exhibited a 3.5-fold increase in infarct size compared to those at the wake-to-sleep transition. CCM hearts did not exhibit a rhythm in infarct size, and consistent with previous observations, appear to be temporally suspended at the wake-to-sleep transition. These patterns of infarct size persisted after one month of reperfusion, suggesting the smaller infarcts were not simply due to a delay in cell death. Additionally, the largest infarcts exhibited by WT hearts subjected to I/R at the sleep-to-wake transition were associated with a significant increase in fibrosis and left ventricular end diastolic diameter (LVEDD). CCM hearts again did not exhibit a time-of-day-dependence in these parameters. The adverse effects of remodeling by the WT hearts which underwent I/R at the sleep-to-wake transition was evident by the greatest depression in ejection fraction (EF) and fractional shortening (FS).93 These data reveal that the myocardium exhibits tolerance to I/R in a time-of-day dependent fashion, which is mediated by the cardiomyocyte circadian clock.
Previously published studies have revealed the importance of the mitochondrial permeability transition pore (mPTP) in I/R injury. Extensive pre- and post- conditioning research has revealed that increasing the ROS and Ca2+ threshold for mPTP opening enhances the resistance of the cardiomyocyte to oxidant stress and ultimately cell death. Upon opening of the mPTP, the mitochondrial membrane potential is lost leading to mitochondrial swelling and rupture.94 The fraction of mitochondria which undergo mPTP opening following hypoxia is negatively correlated with cardiomyocyte survival.95 While the exact components and structure of the mPTP are not fully understood, three integral components have been proposed: adenine nucleotide translocase (ANT), voltage-dependent anion channel (VDAC), and cyclophilin-D (CypD). Of these, genetic ablation studies suggest that only CypD is essential for mPTP opening.96 CypD deficient mice exhibit significant cardioprotection following I/R.97, 98 Additionally, mitochondria isolated from hearts of CypD deficient mice are resistant to mitochondrial swelling and mPTP opening in vitro. In contrast, CypD overexpressing mice exhibit mitochondrial swelling and spontaneous cell death.97 Interestingly, gene expression microarray analysis of WT and CCM hearts identified cypd as a circadian clock regulated gene.24 RT-PCR analysis confirmed significant time-of-day and genotype effects in the expression level of myocardial cypd. Although cypd mRNA peaks at the sleep-to-wake transition in WT hearts, it is chronically elevated in CCM hearts, inconsistent with cardioprotection (unpublished observations).
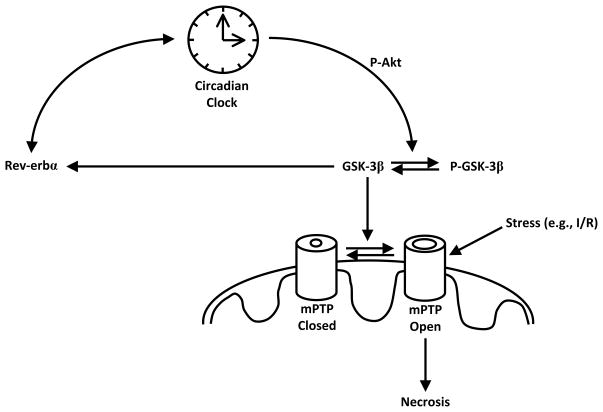
Activation of multiple pathways lends cardioprotection following I/R by attenuating mPTP opening. Interestingly, many of these pathways (involving PKA, PKB, PKC, PKG, AKT, ERK1, ERK2) ultimately converge on glycogen synthase kinase 3 β (GSK-3β).99, 100 When dephosphorylated, GSK-3β is active, and is believed to promote mPTP opening. A less well understood link between GSK-3β and the circadian clock has been proposed in the liver and SCN.101 In these tissues, as well as cultured NIH3T3 cells, the phosphorylation status of GSK-3β displays a robust circadian oscillation.102 Indeed, GSK-3β is likely an integral circadian clock component (Figure 2). Rhythmic clock gene expression is delayed by the GSK-3β inhibitor lithium.101, 103 Conversely, overexpression of GSK-3β advances the phase of clock gene expression. GSK-3β has also been shown to interact with Per2 in vitro and in vivo, and recombinant GSK-3β phosphorylates Per2 in vitro.102 Due to its integral function within the clock, and its known role in mPTP control, GSK-3β was investigated in WT and CCM hearts as a potential explanation for our I/R tolerance observations. Neither gene expression nor total protein levels of GSK-3β were altered in the heart with respect to the time-of-day, or WT versus CCM. However, phospho-GSK-3β in WT hearts tended to be higher at the wake-to-sleep transition, the time of day at which smallest infarcts were observed. Importantly, CCM hearts possess greater levels of phospho-GSK-3β independent of the time of day, consistent with cardioprotection (unpublished observations). These data have lead to the hypothesis that GSK-3β may mediate, at least in part, the effects of the cardiomyocyte circadian clock on myocardial I/R tolerance (Figure 2).
Figure 2.
Hypothetical Model for the Regulation of Myocardial I/R Tolerance by the Cardiomyocyte Circadian Clock. GSK-3β, in its dephosphorylated and active form, promotes mPTP opening in response to stresses such as ischemia/reperfusion, and subsequent cell death. Inhibition of GSK-3β, through Akt-mediated phosphorylation, for example, limits mPTP opening. Akt may also promote cardioprotection through GSK-3β independent mechanisms. The cardiomyocyte circadian clock modulates Akt and GSK3β activity, which potentially mediates time-of-day-dependent oscillations in myocardial ischemia/reperfusion tolerance.
Development of atrial and/or ventricular fibrosis has been implicated in numerous cardiovascular diseases including hypertension, various cardiomyopathies, myocardial infarction and conduction disorders.26, 104 Atrial fibrosis leads to the development of atrial fibrillation, the most common clinical arrhythmia.105 Increasing amounts of fibrosis in the heart have been strongly correlated with an increase in atrial and ventricular tacchyarrythmias and sudden cardiac death, both of which exhibit a strong circadian rhythm in patients.106, 107 Likewise, increased fibrosis has been linked to decoupling of muscle fibers, conduction slowing, and conduction blocks.108, 109 The early stage of fibrosis development involves degradation of the existing extracellular matrix (ECM) by extracellular matrix metalloproteinases (MMPs). Numerous pathological conditions associated with fibrosis such as heart failure, myocardial infarction, and hypertension, have also been reported to be associated with increased levels and activities of MMPs.110–112 The activity of MMPs is normally counter-balanced by the presence of tissue inhibitors of matrix metalloproteinases (TIMPs). During times of ECM breakdown MMP levels have been shown to be elevated and TIMP levels repressed. This is followed by a decrease in MMP activity (by TIMP inhibition) and collagen deposition.113 Following I/R, we observed profoundly lower levels of fibrosis in CCM hearts relative to WT hearts. Gene expression microarray analysis of WT versus CCM hearts revealed an enrichment of genes involved in the turnover of extracellular matrix and deposition of collagen fibers as being regulated by the cardiomyocyte circadian clock. Two MMPs (mmp14 and mmp24) and two TIMPs (timp1 and timp3) were identified as being circadian clock regulated.24 Previous research has demonstrated that activation of the SMAD complex (SMAD2/SMAD3/SMAD4) induces the transcription of several types of collagen, including type I, type III, and type VI.114 Interestingly, we find that expression levels of both smad3 and smad4 appear to be influenced by the cardiomyocyte circadian clock.24 Consistent with alterations in SMAD complex components, a large number of collagen genes were identified as clock regulated (col3a1, col4a1, col4a2, col5a1, and col6a3).24 Collectively these data suggest a possible role for the cardiomyocyte circadian clock in modulating cardiac fibrosis, which may in turn influence the pathogenesis of cardiovascular disease.
Clock Genes: The Ultimate Thrifty Gene?
The circadian clock mechanism evolved to provide the selective advantage of anticipation. In doing so, the clock ensures that biological events occur at a temporally appropriate time of the day.3 It has been suggested that one evolutionary pressure for circadian clock selection includes regulation of the cell cycle; by restricting DNA repair and replication to the dark phase, the circadian clock ensures that light phase induced DNA damage (i.e., UV irradiation) is not inadvertently transmitted to daughter cells.115 As with light, metabolic challenges imposed on the cell also have a strong (and predictable) time-of-day-dependence, and as such it is not surprising that metabolism has integrated into the circadian clock mechanism (see above discussion and Figure 1). Studies investigating the role(s) of the cardiomyocyte circadian clock suggest that this mechanism may allow the heart to anticipate prolongation of the sleep-phase fast, when the animal in the wild is initially unsuccessful in its forage for food.116 The concept that it is evolutionarily advantageous to anticipate periods of prolonged fasting has been suggested previously, and may explain, in part, the increased prevalence of cardiometabolic disease in Western society. In 1962, Neel proposed the thrifty gene hypothesis.117 This hypothesis proposes that humans evolved to store excessive calories (as triglyceride in adipose) during periods of ample nutrient supply (e.g., summer), in anticipation of prolonged periods of minimal nutrient availability (e.g., winter). The combination of this strong genetic selective pressure, and the current lack of seasonal changes in nutrient supply in the industrialized world, potentially contributes to our ongoing obesity epidemic. Several candidate thrifty genes have been identified through numerous human genomic approaches, and include leptin, MCR4, and POMC.118
Several important criteria need to be fulfilled in order for a gene to be designated a thrifty gene, according to the original definition. These include: 1) highly conserved; 2) enable the cell/organism to anticipate periods of prolonged fasting; 3) influence both cellular and whole body metabolism (particularly triglyceride metabolism); 4) significantly influence seasonal changes in metabolism; and 5) exhibit polymorphisms associated with adiposity. Circadian clock genes fulfill all these criteria. As discussed above, circadian clock mechanisms have been identified in both eukaryotes and prokaryotes, and show a relatively high level of conservation across species.3 Studies from our laboratory suggest that this mechanism not only regulates myocardial triglyceride (and glycogen) metabolism, but also allows the heart to anticipate prolonged fasting.19, 24, 55 As reviewed by Bass and colleagues, circadian clocks undoubtedly play a central role in regulating whole body metabolism and adiposity.119 Circadian clock genes also play a critical role in seasonal alterations in physiology. This includes migration, reproduction, and metabolism. Indeed, the circadian clock is also a seasonal clock, enabling the cell to know the time of year through alterations in the amplitude of clock component gene expression and melatonin oscillations.3–5 More recently, Scott et al have shown that polymorphisms in the human clock gene are associated with BMI (a clinical surrogate for adiposity).120 As such, clock genes are undoubtedly strong thrifty gene candidates. Given the close association between obesity, diabetes, and cardiovascular disease, as well as the direct effects that the circadian clock imposes on myocardial function, clock genes likely influence cardiac physiology and pathophysiology both directly (i.e., through the cardiomyocyte circadian clock) and indirectly (e.g., through central and peripheral clock effects on behavior and the neurohumoral milieu).
Summary
The cardiomyocyte circadian clock has emerged as a molecular mechanism influencing multiple critical myocardial processes. This mechanism profoundly influences myocardial gene expression, signaling, metabolism, and contractile function, in a time-of-day-dependent manner. More specifically, the cardiomyocyte circadian clock appears to regulate heart rate, growth, triglyceride and glycogen metabolism, contractility, as well as modulate the responsiveness of the myocardium to extracellular factors/stimuli, such as fatty acids and β-adrenergic signaling. This mechanism is altered in multiple animal models of cardiovascular disease, and modulates the severity of myocardial damage in response to adverse/pathological stresses (e.g., ischemic episodes). It is therefore tempting to speculate that dyssynchrony of the cardiomyocyte circadian clock during shift work, diabetes mellitus, and/or obesity, contributes to the pathogenesis of cardiovascular disease.
Acknowledgments
David J. Durgan is in the Graduate Program in Cardiovascular Sciences.
Sources of Funding
This work was supported by the NIH/NHLBI (HL-074259; MEY), the USDA/ARS (6250-51000-044; MEY), and the NSF (GK-12 Fellowship; DJD).
Non-standard Abbreviations and Acronyms
- ADPN
Adiponutrin
- AKT
v-akt murine thymoma viral oncogene homolog 1
- AMPK
AMP-activated protein kinase
- ANT
Adenine nucleotide translocase
- BMAL1
Brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1
- CCG
Clock controlled gene
- CCM
Cardiomyocyte-specific circadian clock mutant
- CK1ε
Casein kinase 1ε
- CLOCK
Circadian locomotor output cycles kaput
- COL
Collagen
- CRY
Cryptochrome
- CYPD
Cyclophilin D
- DBP
D site of albumin promoter (albumin D-box) binding protein
- DEC1
Stimulated by retinoic acid 13 homolog
- DGAT2
Diacylglycerol O-acyltransferase homolog 2
- E4BP4
E4 promoter binding-protein 4
- EIF
Eukaryotic translation initiation factor
- EF
Ejection fraction
- FOXO
Forkhead box O
- FS
Fractional shortening
- GSK-3β
Glycogen synthase kinase 3β
- I/R
Ischemia/reperfusion
- IGF-1
Insulin-like growth factor 1
- LDHa
Lactate dehydrogenase isoform a
- LVEDD
Left ventricular end diastolic dimensions
- MMP
Matrix metalloprotease
- MPTP
Mitochondrial permeability transition pore
- MTOR
Mammalian target of rapamycin
- NAMPT
Nicotinamide phosphoribosyltransferase
- NPAS2
Neuronal PAS domain protein 2
- O-GlcNAc
O-linked N-acetylglucosamine
- OGT
O-GlcNAc transferase
- PAR
Proline- and acidic- residue rich
- PER
Period
- PGC1α
Peroxisome proliferator-activated receptor gamma, coactivator 1α
- PDK1
3-Phosphoinositide dependent protein kinase-1
- PI3K
Phosphoinositide-3-kinase
- PIP2
Phospholipid phosphatidylinositol 4,5 bisphosphate
- PPARα
Peroxisome proliferator-activated receptor alpha
- PPP1CC
Protein phosphatase 1, catalytic subunit, gamma isoform
- RCAN1
Regulator of calcineurin 1
- REV-ERBα
Nuclear receptor subfamily 1, group D, member 1
- ROS
Reactive oxygen species
- RT-PCR
Reverse transcription polymerase chain reaction
- SCN
Suprachiasmatic nucleus SLC, Solute carrier
- TEF
Thyrotrophic embryonic factor
- TIMP
Tissue inhibitors of matrix metalloproteinases
- UBC
Ubiquitin C
- UBE3C
Ubiquitin protein ligase E3C
- USP2
Ubiquitin specific protease 2
- VDAC
Voltage-dependent anion channel
- WT
Wild-type
Footnotes
Disclosures
None.
References
- 1.Smolensky MH, Portaluppi F. Ambulatory blood pressure monitoring. Application to clinical medicine and antihypertension medication trials. Ann N Y Acad Sci. 1996;783:278–294. doi: 10.1111/j.1749-6632.1996.tb26724.x. [DOI] [PubMed] [Google Scholar]
- 2.McMullen JR, Jennings GL. Differences between pathological and physiological cardiac hypertrophy: novel therapeutic strategies to treat heart failure. Clin Exp Pharmacol Physiol. 2007;34(4):255–262. doi: 10.1111/j.1440-1681.2007.04585.x. [DOI] [PubMed] [Google Scholar]
- 3.Edery I. Circadian rhythms in a nutshell. Physiol Genomics. 2000;3:59–74. doi: 10.1152/physiolgenomics.2000.3.2.59. [DOI] [PubMed] [Google Scholar]
- 4.Wilsbacher LD, Takahashi JS. Circadian rhythms: molecular basis of the clock. Curr Opin Genet Dev. 1998;8(5):595–602. doi: 10.1016/s0959-437x(98)80017-8. [DOI] [PubMed] [Google Scholar]
- 5.Dunlap J. Molecular Basis of Circadian Clocks. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 6.Durgan D, Hotze M, Tomlin T, Egbejimi O, Graveleau C, Abel E, Shaw C, Bray M, Hardin P, Young M. The intrinsic circadian clock within the cardiomyocyte. Am J Physiol Heart Circ Physiol. 2005;289:H1530–H1541. doi: 10.1152/ajpheart.00406.2005. [DOI] [PubMed] [Google Scholar]
- 7.Nonaka H, Emoto N, Ikeda K, Fukuya H, Rohman MS, Raharjo SB, Yagita K, Okamura H, Yokoyama M. Angiotensin II induces circadian gene expression of clock genes in cultured vascular smooth muscle cells. Circulation. 2001;104(15):1746–1748. doi: 10.1161/hc4001.098048. [DOI] [PubMed] [Google Scholar]
- 8.McNamara P, Seo SB, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell. 2001;105(7):877–889. doi: 10.1016/s0092-8674(01)00401-9. [DOI] [PubMed] [Google Scholar]
- 9.Takeda N, Maemura K, Horie S, Oishi K, Imai Y, Harada T, Saito T, Shiga T, Amiya E, Manabe I, Ishida N, Nagai R. Thrombomodulin is a clock-controlled gene in vascular endothelial cells. J Biol Chem. 2007;282(45):32561–32567. doi: 10.1074/jbc.M705692200. [DOI] [PubMed] [Google Scholar]
- 10.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A. 2007;104(9):3450–3455. doi: 10.1073/pnas.0611680104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anea CB, Zhang M, Stepp DW, Simkins GB, Reed G, Fulton DJ, Rudic RD. Vascular disease in mice with a dysfunctional circadian clock. Circulation. 2009;119(11):1510–1517. doi: 10.1161/CIRCULATIONAHA.108.827477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woon PY, Kaisaki PJ, Braganca J, Bihoreau MT, Levy JC, Farrall M, Gauguier D. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci U S A. 2007;104(36):14412–14417. doi: 10.1073/pnas.0703247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakamoto K, Nagase T, Fukui H, Horikawa K, Okada T, Tanaka H, Sato K, Miyake Y, Ohara O, Kako K, Ishida N. Multitissue circadian expression of rat period homolog (rPer2) mRNA is governed by the mammalian circadian clock, the suprachiasmatic nucleus in the brain. J Biol Chem. 1998;273(42):27039–27042. doi: 10.1074/jbc.273.42.27039. [DOI] [PubMed] [Google Scholar]
- 14.Young M, Razeghi P, Taegtmeyer H. Clock genes in the heart: characterization and attenuation with hypertrophy. Circ Res. 2001;88:1142–1150. doi: 10.1161/hh1101.091190. [DOI] [PubMed] [Google Scholar]
- 15.Leibetseder V, Humpeler S, Svoboda M, Schmid D, Thalhammer T, Zuckermann A, Marktl W, Ekmekcioglu C. Clock genes display rhythmic expression in human hearts. Chronobiol Int. 2009;26(4):621–636. doi: 10.1080/07420520902924939. [DOI] [PubMed] [Google Scholar]
- 16.Davidson AJ, London B, Block GD, Menaker M. Cardiovascular tissues contain independent circadian clocks. Clin Exp Hypertens. 2005;27(2–3):307–311. [PubMed] [Google Scholar]
- 17.Martino T, Arab S, Straume M, Belsham DD, Tata N, Cai F, Liu P, Trivieri M, Ralph M, Sole MJ. Day/night rhythms in gene expression of the normal murine heart. J Mol Med. 2004;82(4):256–264. doi: 10.1007/s00109-003-0520-1. [DOI] [PubMed] [Google Scholar]
- 18.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417(6884):78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- 19.Durgan D, Trexler N, Egbejimi O, McElfresh T, Suk H, Petterson L, Shaw C, Hardin P, Bray M, Chandler M, Chow C, Young M. The circadian clock within the cardiomyocyte is essential for responsiveness of the heart to fatty acids. J Biol Chem. 2006;281:24254–24269. doi: 10.1074/jbc.M601704200. [DOI] [PubMed] [Google Scholar]
- 20.Young ME. Anticipating Anticipation: Pursuing Identification of Cardiomyocyte Circadian Clock Function. J Appl Physiol. 2009 doi: 10.1152/japplphysiol.00473.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu K, Ivanov P, Hilton MF, Chen Z, Ayers RT, Stanley HE, Shea SA. Endogenous circadian rhythm in an index of cardiac vulnerability independent of changes in behavior. Proc Natl Acad Sci U S A. 2004;101(52):18223–18227. doi: 10.1073/pnas.0408243101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viola AU, James LM, Archer SN, Dijk DJ. PER3 polymorphism and cardiac autonomic control: effects of sleep debt and circadian phase. Am J Physiol Heart Circ Physiol. 2008;295(5):H2156–2163. doi: 10.1152/ajpheart.00662.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang N, Yang G, Jia Z, Zhang H, Aoyagi T, Soodvilai S, Symons JD, Schnermann JB, Gonzalez FJ, Litwin SE, Yang T. Vascular PPARgamma controls circadian variation in blood pressure and heart rate through Bmal1. Cell Metab. 2008;8(6):482–491. doi: 10.1016/j.cmet.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bray M, Shaw C, Moore M, Garcia R, Zanquetta M, Durgan D, Jeong W, Tsai J, Bugger H, Zhang D, Rohrwasser A, Rennison J, Dyck J, Litwin S, Hardin P, Chow C, Chandler M, Abel E, Young M. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function; metabolism; and gene expression. Am J Physiol Heart Circ Physiol. 2008;294:H1036–H1047. doi: 10.1152/ajpheart.01291.2007. [DOI] [PubMed] [Google Scholar]
- 25.Barbuti A, DiFrancesco D. Control of cardiac rate by “funny” channels in health and disease. Ann N Y Acad Sci. 2008;1123:213–223. doi: 10.1196/annals.1420.024. [DOI] [PubMed] [Google Scholar]
- 26.Ten Tusscher KH, Panfilov AV. Influence of diffuse fibrosis on wave propagation in human ventricular tissue. Europace. 2007;9(Suppl 6):vi38–45. doi: 10.1093/europace/eum206. [DOI] [PubMed] [Google Scholar]
- 27.Chandler NJ, Greener ID, Tellez JO, Inada S, Musa H, Molenaar P, Difrancesco D, Baruscotti M, Longhi R, Anderson RH, Billeter R, Sharma V, Sigg DC, Boyett MR, Dobrzynski H. Molecular architecture of the human sinus node: insights into the function of the cardiac pacemaker. Circulation. 2009;119(12):1562–1575. doi: 10.1161/CIRCULATIONAHA.108.804369. [DOI] [PubMed] [Google Scholar]
- 28.Yamashita T, Sekiguchi A, Iwasaki YK, Sagara K, Iinuma H, Hatano S, Fu LT, Watanabe H. Circadian variation of cardiac K+ channel gene expression. Circulation. 2003;107(14):1917–1922. doi: 10.1161/01.CIR.0000058752.79734.F0. [DOI] [PubMed] [Google Scholar]
- 29.Pfeffer M, Muller CM, Mordel J, Meissl H, Ansari N, Deller T, Korf HW, von Gall C. The mammalian molecular clockwork controls rhythmic expression of its own input pathway components. J Neurosci. 2009;29(19):6114–6123. doi: 10.1523/JNEUROSCI.0275-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nordskog BK, Hammarback JA, Godwin DW. Diurnal gene expression patterns of T-type calcium channels and their modulation by ethanol. Neuroscience. 2006;141(3):1365–1373. doi: 10.1016/j.neuroscience.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 31.Ko GY, Shi L, Ko ML. Circadian regulation of ion channels and their functions. J Neurochem. 2009;110(4):1150–1169. doi: 10.1111/j.1471-4159.2009.06223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas SA, Schuessler RB, Berul CI, Beardslee MA, Beyer EC, Mendelsohn ME, Saffitz JE. Disparate effects of deficient expression of connexin43 on atrial and ventricular conduction: evidence for chamber-specific molecular determinants of conduction. Circulation. 1998;97(7):686–691. doi: 10.1161/01.cir.97.7.686. [DOI] [PubMed] [Google Scholar]
- 33.Coppen SR, Severs NJ, Gourdie RG. Connexin45 (alpha 6) expression delineates an extended conduction system in the embryonic and mature rodent heart. Dev Genet. 1999;24(1–2):82–90. doi: 10.1002/(SICI)1520-6408(1999)24:1/2<82::AID-DVG9>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 34.Gros D, Jarry-Guichard T, Ten Velde I, de Maziere A, van Kempen MJ, Davoust J, Briand JP, Moorman AF, Jongsma HJ. Restricted distribution of connexin40, a gap junctional protein, in mammalian heart. Circ Res. 1994;74(5):839–851. doi: 10.1161/01.res.74.5.839. [DOI] [PubMed] [Google Scholar]
- 35.Bevilacqua LM, Simon AM, Maguire CT, Gehrmann J, Wakimoto H, Paul DL, Berul CI. A targeted disruption in connexin40 leads to distinct atrioventricular conduction defects. J Interv Card Electrophysiol. 2000;4(3):459–467. doi: 10.1023/a:1009800328836. [DOI] [PubMed] [Google Scholar]
- 36.Taegtmeyer H. Metabolism--the lost child of cardiology. J Am Coll Cardiol. 2000;36:1386–1388. doi: 10.1016/s0735-1097(00)00870-6. [DOI] [PubMed] [Google Scholar]
- 37.Neubauer S. The failing heart--an engine out of fuel. N Engl J Med. 2007;356(11):1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 38.Goodwin G, Taylor C, Taegtmeyer H. Regulation of energy metabolism of the heart during acute increase in heart work. J Biol Chem. 1998;273:29530–29539. doi: 10.1074/jbc.273.45.29530. [DOI] [PubMed] [Google Scholar]
- 39.Rutter J, Reick M, Wu L, McKnight S. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science. 2001;293:510–514. doi: 10.1126/science.1060698. [DOI] [PubMed] [Google Scholar]
- 40.Young M. The circadian clock within the heart: potential influence on myocardial gene expression; metabolism; and function. Am J Physiol Heart Circ Physiol. 2006;290:H1–H16. doi: 10.1152/ajpheart.00582.2005. [DOI] [PubMed] [Google Scholar]
- 41.Minami Y, Horikawa K, Akiyama M, Shibata S. Restricted feeding induces daily expression of clock genes and Pai-1 mRNA in the heart of Clock mutant mice. FEBS Lett. 2002;526(1–3):115–118. doi: 10.1016/s0014-5793(02)03159-9. [DOI] [PubMed] [Google Scholar]
- 42.Oishi K, Miyazaki K, Ishida N. Functional CLOCK is not involved in the entrainment of peripheral clocks to the restricted feeding: entrainable expression of mPer2 and BMAL1 mRNAs in the heart of Clock mutant mice on Jcl:ICR background. Biochem Biophys Res Commun. 2002;298(2):198–202. doi: 10.1016/s0006-291x(02)02427-0. [DOI] [PubMed] [Google Scholar]
- 43.Kung T, Egbejimi O, Cui J, Ha N, Durgan D, Essop M, Bray M, Shaw C, Hardin P, Stanley W, Young M. Rapid attenuation of circadian clock gene oscillations in the rat heart following ischemia-reperfusion. J Mol Cell Cardiol. 2007;43:744–753. doi: 10.1016/j.yjmcc.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403(6771):795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 45.Grimaldi B, Nakahata Y, Kaluzova M, Masubuchi S, Sassone-Corsi P. Chromatin remodeling, metabolism and circadian clocks: the interplay of CLOCK and SIRT1. Int J Biochem Cell Biol. 2009;41(1):81–86. doi: 10.1016/j.biocel.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 46.Doi M, Hirayama J, Sassone-Corsi P. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125(3):497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 47.Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR, Milbrandt J, Kiess W, Imai S. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6(5):363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Powanda MC, Wannemacher RW., Jr Evidence for a linear correlation between the level of dietary tryptophan and hepatic NAD concentration and for a systematic variation in tissue NAD concentration in the mouse and the rat. J Nutr. 1970;100(12):1471–1478. doi: 10.1093/jn/100.12.1471. [DOI] [PubMed] [Google Scholar]
- 49.Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res. 2009;105(5):481–491. doi: 10.1161/CIRCRESAHA.109.203703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shinmura K, Tamaki K, Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am J Physiol Heart Circ Physiol. 2008;295(6):H2348–2355. doi: 10.1152/ajpheart.00602.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100(10):1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 52.Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett. 2008;582(1):46–53. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Durgan D, Moore M, Ha N, Egbejimi O, Fields A, Mbawuike U, Egbejimi A, Shaw C, Bray M, Nannegari V, Hickson-Bick D, Heird W, Dyck J, Chandler M, Young M. Circadian rhythms in myocardial metabolism and contractile function: influence of workload and oleate. Am J Physiol Heart Circ Physiol. 2007;293:H2385–H2393. doi: 10.1152/ajpheart.01361.2006. [DOI] [PubMed] [Google Scholar]
- 54.Young M, Razeghi P, Cedars A, Guthrie P, Taegtmeyer H. Intrinsic diurnal variations in cardiac metabolism and contractile function. Circ Res. 2001;89:1199–1208. doi: 10.1161/hh2401.100741. [DOI] [PubMed] [Google Scholar]
- 55.Tsai J-Y, Nannegari V, Heird WC, Young ME. Abstract 5383: The Circadian Clock within the Cardiomyocyte is a Novel Mechanism Regulating Myocardial Triglyceride Metabolism. Circulation. 2008;118:S_539-b. 18_MeetingAbstracts. [Google Scholar]
- 56.Chatham J, Laczy B, Durgan D, Young M. Direct Interrelationship Between Protein O-GlcNAcation and the Cardiomyocyte Circadian Clock. Cir Res. 2009;105:e50. [Google Scholar]
- 57.Young M, McNulty P, Taegtmeyer H. Adaptation and maladaptation of the heart in diabetes: Part II: Potential Mechanisms. Circulation. 2002;105:1861–1870. doi: 10.1161/01.cir.0000012467.61045.87. [DOI] [PubMed] [Google Scholar]
- 58.Fulop N, Marchase RB, Chatham JC. Role of protein O-linked N-acetyl-glucosamine in mediating cell function and survival in the cardiovascular system. Cardiovasc Res. 2007;73(2):288–297. doi: 10.1016/j.cardiores.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bray M, Young M. Diurnal variations in myocardial metabolism. Cardiovasc Res. 2008 doi: 10.1093/cvr/cvn054. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 60.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6(5):414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 61.Young M, Wilson C, Razeghi P, Guthrie P, Taegtmeyer H. Alterations of the Circadian Clock in the Heart by Streptozotocin-induced Diabetes. J Mol Cell Cardiol. 2002;34:223–231. doi: 10.1006/jmcc.2001.1504. [DOI] [PubMed] [Google Scholar]
- 62.Stavinoha M, RaySpellicy J, Hart-Sailors M, Mersmann H, Bray M, Young M. Diurnal variations in the responsiveness of cardiac and skeletal muscle to fatty acids. Am J Physiol. 2004;287:E878–E887. doi: 10.1152/ajpendo.00189.2004. [DOI] [PubMed] [Google Scholar]
- 63.Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447(7143):477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- 64.Sonoda J, Mehl IR, Chong LW, Nofsinger RR, Evans RM. PGC-1beta controls mitochondrial metabolism to modulate circadian activity, adaptive thermogenesis, and hepatic steatosis. Proc Natl Acad Sci U S A. 2007;104(12):5223–5228. doi: 10.1073/pnas.0611623104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sole MJ, Martino TA. Diurnal Physiology: Core Principles with Application to the Pathogenesis, Diagnosis, Prevention and Treatment of Myocardial Hypertrophy and Failure. J Appl Physiol. 2009 doi: 10.1152/japplphysiol.00426.2009. [DOI] [PubMed] [Google Scholar]
- 66.Dorn GW, 2nd, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest. 2005;115(3):527–537. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem. 2004;279(31):32771–32779. doi: 10.1074/jbc.M403528200. [DOI] [PubMed] [Google Scholar]
- 68.Arad M, Seidman CE, Seidman JG. AMP-activated protein kinase in the heart: role during health and disease. Circ Res. 2007;100(4):474–488. doi: 10.1161/01.RES.0000258446.23525.37. [DOI] [PubMed] [Google Scholar]
- 69.Um JH, Yang S, Yamazaki S, Kang H, Viollet B, Foretz M, Chung JH. Activation of 5′-AMP-activated kinase with diabetes drug metformin induces casein kinase Iepsilon (CKIepsilon)-dependent degradation of clock protein mPer2. J Biol Chem. 2007;282(29):20794–20798. doi: 10.1074/jbc.C700070200. [DOI] [PubMed] [Google Scholar]
- 70.Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, Thompson CB, Evans RM. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326(5951):437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Favier FB, Benoit H, Freyssenet D. Cellular and molecular events controlling skeletal muscle mass in response to altered use. Pflugers Arch. 2008;456(3):587–600. doi: 10.1007/s00424-007-0423-z. [DOI] [PubMed] [Google Scholar]
- 72.Dardente H, Cermakian N. Molecular circadian rhythms in central and peripheral clocks in mammals. Chronobiol Int. 2007;24(2):195–213. doi: 10.1080/07420520701283693. [DOI] [PubMed] [Google Scholar]
- 73.Razeghi P, Baskin KK, Sharma S, Young ME, Stepkowski S, Essop MF, Taegtmeyer H. Atrophy, hypertrophy, and hypoxemia induce transcriptional regulators of the ubiquitin proteasome system in the rat heart. Biochem Biophys Res Commun. 2006;342(2):361–364. doi: 10.1016/j.bbrc.2006.01.163. [DOI] [PubMed] [Google Scholar]
- 74.Razeghi P, Sharma S, Ying J, Li YP, Stepkowski S, Reid MB, Taegtmeyer H. Atrophic remodeling of the heart in vivo simultaneously activates pathways of protein synthesis and degradation. Circulation. 2003;108(20):2536–2541. doi: 10.1161/01.CIR.0000096481.45105.13. [DOI] [PubMed] [Google Scholar]
- 75.Razeghi P, Taegtmeyer H. Hypertrophy and atrophy of the heart: the other side of remodeling. Ann N Y Acad Sci. 2006;1080:110–119. doi: 10.1196/annals.1380.011. [DOI] [PubMed] [Google Scholar]
- 76.Muller J, Tofler G, Stone P. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation. 1989;79:733–743. doi: 10.1161/01.cir.79.4.733. [DOI] [PubMed] [Google Scholar]
- 77.Manfredini R, Boari B, Gallerani M, Salmi R, Bossone E, Distante A, Eagle KA, Mehta RH. Chronobiology of rupture and dissection of aortic aneurysms. J Vasc Surg. 2004;40(2):382–388. doi: 10.1016/j.jvs.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 78.Manfredini R, Casetta I, Paolino E, la Cecilia O, Boari B, Fallica E, Granieri E. Monday preference in onset of ischemic stroke. Am J Med. 2001;111(5):401–403. doi: 10.1016/s0002-9343(01)00836-1. [DOI] [PubMed] [Google Scholar]
- 79.Hirota T, Fukada Y. Resetting mechanism of central and peripheral circadian clocks in mammals. Zoological Sci. 2004;21:359–368. doi: 10.2108/zsj.21.359. [DOI] [PubMed] [Google Scholar]
- 80.Mohri T, Emoto N, Nonaka H, Fukuya H, Yagita K, Okamura H, Yokoyama M. Alterations of circadian expressions of clock genes in dahl salt-sensitive rats fed a high-salt diet. Hypertension. 2003;42:189–194. doi: 10.1161/01.HYP.0000082766.63952.49. [DOI] [PubMed] [Google Scholar]
- 81.Martino TA, Tata N, Belsham DD, Chalmers J, Straume M, Lee P, Pribiag H, Khaper N, Liu PP, Dawood F, Backx PH, Ralph MR, Sole MJ. Disturbed diurnal rhythm alters gene expression and exacerbates cardiovascular disease with rescue by resynchronization. Hypertension. 2007;49(5):1104–1113. doi: 10.1161/HYPERTENSIONAHA.106.083568. [DOI] [PubMed] [Google Scholar]
- 82.Oishi K, Kasamatsu M, Ishida N. Gene- and tissue-specific alterations of circadian clock gene expression in streptozotocin-induced diabetic mice under restricted feeding. Biochem Biophys Res Commun. 2004;317:330–334. doi: 10.1016/j.bbrc.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 83.Chau N, Mallion J, de GR, Ruche E, Siche J, Pelen O, Mathern G. Twenty-four-hour ambulatory blood pressure in shift workers. Circulation. 1989;80:341–347. doi: 10.1161/01.cir.80.2.341. [DOI] [PubMed] [Google Scholar]
- 84.van den Buuse M. Circadian rhythms of blood pressure and heart rate in conscious rats: effects of light cycle shift and timed feeding. Physiol Behav. 1999;68:9–15. doi: 10.1016/s0031-9384(99)00148-1. [DOI] [PubMed] [Google Scholar]
- 85.Sundberg S, Kohvakka A, Gordin A. Rapid reversal of circadian blood pressure rhythm in shift workers. J Hypertens. 1988;6:393–396. [PubMed] [Google Scholar]
- 86.Martino T, Oudit G, Herzenberg A, Tata N, Koletar M, Kabir G, Belsham D, Backx P, Ralph M, Sole M. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1675–R1683. doi: 10.1152/ajpregu.00829.2007. [DOI] [PubMed] [Google Scholar]
- 87.Harma M, Ilmarinen J. Towards the 24-hour society--new approaches for aging shift workers? Scand J Work Envion Health. 1999;25:610–615. doi: 10.5271/sjweh.488. [DOI] [PubMed] [Google Scholar]
- 88.Knutsson A, Akerstedt T, Jonsson B, Orth-Gomer K. Increased risk of ischaemic heart disease in shift workers. Lancet. 1986;12:89–92. doi: 10.1016/s0140-6736(86)91619-3. [DOI] [PubMed] [Google Scholar]
- 89.Koller M. Health risks related to shift work: An example of time-contingent effects of long-term stress. Int Arch Occup Environ Health. 1983;53:59–75. doi: 10.1007/BF00406178. [DOI] [PubMed] [Google Scholar]
- 90.Oka T, Xu J, Molkentin JD. Re-employment of developmental transcription factors in adult heart disease. Semin Cell Dev Biol. 2007;18(1):117–131. doi: 10.1016/j.semcdb.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Molkentin JD. Calcineurin and beyond: cardiac hypertrophic signaling. Circ Res. 2000;87(9):731–738. doi: 10.1161/01.res.87.9.731. [DOI] [PubMed] [Google Scholar]
- 92.Nossuli TO, Lakshminarayanan V, Baumgarten G, Taffet GE, Ballantyne CM, Michael LH, Entman ML. A chronic mouse model of myocardial ischemia-reperfusion: essential in cytokine studies. Am J Physiol Heart Circ Physiol. 2000;278(4):H1049–1055. doi: 10.1152/ajpheart.2000.278.4.H1049. [DOI] [PubMed] [Google Scholar]
- 93.Durgan DJ, Young ME. Abstract 3876: Diurnal Variation in Myocardial Ischemia/Reperfusion Tolerance: Mediation by the Circadian Clock within the Cardiomyocyte. Circulation. 2008;118:S_496. 18_MeetingAbstracts. [Google Scholar]
- 94.Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995;307 ( Pt 1):93–98. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113(11):1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9(5):550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434(7033):658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 98.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434(7033):652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 99.Juhaszova M, Zorov DB, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Role of glycogen synthase kinase-3beta in cardioprotection. Circ Res. 2009;104(11):1240–1252. doi: 10.1161/CIRCRESAHA.109.197996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miura T, Miki T. GSK-3beta, a Therapeutic Target for Cardiomyocyte Protection. Circ J. 2009;73(7):1184–1192. doi: 10.1253/circj.cj-09-0284. [DOI] [PubMed] [Google Scholar]
- 101.Yin L, Wang J, Klein PS, Lazar MA. Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science. 2006;311(5763):1002–1005. doi: 10.1126/science.1121613. [DOI] [PubMed] [Google Scholar]
- 102.Iitaka C, Miyazaki K, Akaike T, Ishida N. A role for glycogen synthase kinase-3beta in the mammalian circadian clock. J Biol Chem. 2005;280(33):29397–29402. doi: 10.1074/jbc.M503526200. [DOI] [PubMed] [Google Scholar]
- 103.Kaladchibachi SA, Doble B, Anthopoulos N, Woodgett JR, Manoukian AS. Glycogen synthase kinase 3, circadian rhythms, and bipolar disorder: a molecular link in the therapeutic action of lithium. J Circadian Rhythms. 2007;5:3. doi: 10.1186/1740-3391-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999;79(1):215–262. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- 105.Lin CS, Pan CH. Regulatory mechanisms of atrial fibrotic remodeling in atrial fibrillation. Cell Mol Life Sci. 2008;65(10):1489–1508. doi: 10.1007/s00018-008-7408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Everett THt, Olgin JE. Atrial fibrosis and the mechanisms of atrial fibrillation. Heart Rhythm. 2007;4(3 Suppl):S24–27. doi: 10.1016/j.hrthm.2006.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Strain JE, Grose RM, Factor SM, Fisher JD. Results of endomyocardial biopsy in patients with spontaneous ventricular tachycardia but without apparent structural heart disease. Circulation. 1983;68(6):1171–1181. doi: 10.1161/01.cir.68.6.1171. [DOI] [PubMed] [Google Scholar]
- 108.de Bakker JM, van Rijen HM. Continuous and discontinuous propagation in heart muscle. J Cardiovasc Electrophysiol. 2006;17(5):567–573. doi: 10.1111/j.1540-8167.2006.00367.x. [DOI] [PubMed] [Google Scholar]
- 109.Kawara T, Derksen R, de Groot JR, Coronel R, Tasseron S, Linnenbank AC, Hauer RN, Kirkels H, Janse MJ, de Bakker JM. Activation delay after premature stimulation in chronically diseased human myocardium relates to the architecture of interstitial fibrosis. Circulation. 2001;104(25):3069–3075. doi: 10.1161/hc5001.100833. [DOI] [PubMed] [Google Scholar]
- 110.Spinale FG, Coker ML, Bond BR, Zellner JL. Myocardial matrix degradation and metalloproteinase activation in the failing heart: a potential therapeutic target. Cardiovasc Res. 2000;46(2):225–238. doi: 10.1016/s0008-6363(99)00431-9. [DOI] [PubMed] [Google Scholar]
- 111.Weber KT, Sun Y, Guarda E, Katwa LC, Ratajska A, Cleutjens JP, Zhou G. Myocardial fibrosis in hypertensive heart disease: an overview of potential regulatory mechanisms. Eur Heart J. 1995;16 (Suppl C):24–28. doi: 10.1093/eurheartj/16.suppl_c.24. [DOI] [PubMed] [Google Scholar]
- 112.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101(25):2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 113.Pauschinger M, Chandrasekharan K, Li J, Schwimmbeck PL, Noutsias M, Schultheiss HP. Mechanisms of extracellular matrix remodeling in dilated cardiomyopathy. Herz. 2002;27(7):677–682. doi: 10.1007/s00059-002-2413-4. [DOI] [PubMed] [Google Scholar]
- 114.Verrecchia F, Chu ML, Mauviel A. Identification of novel TGF-beta/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem. 2001;276(20):17058–17062. doi: 10.1074/jbc.M100754200. [DOI] [PubMed] [Google Scholar]
- 115.Pittendrigh CS. Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993;55:16–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- 116.Durgan DJ, Young ME. Linking the cardiomyocyte circadian clock to myocardial metabolism. Cardiovasc Drugs Ther. 2008;22(2):115–124. doi: 10.1007/s10557-008-6086-y. [DOI] [PubMed] [Google Scholar]
- 117.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet. 1962;14:353–362. [PMC free article] [PubMed] [Google Scholar]
- 118.Arner P. Obesity--a genetic disease of adipose tissue? Br J Nutr. 2000;83 (Suppl 1):S9–16. doi: 10.1017/s0007114500000891. [DOI] [PubMed] [Google Scholar]
- 119.Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134(5):728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Scott EM, Carter AM, Grant PJ. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes (Lond) 2008;32(4):658–662. doi: 10.1038/sj.ijo.0803778. [DOI] [PubMed] [Google Scholar]