Abstract
Aims/hypothesis
Lower adiponectin levels are associated with higher risk of incident type 2 diabetes (T2D). Most analyses have been adjusted for confounding factors, but few have taken into account insulin resistance per se. We tested the hypothesis that the association of adiponectin levels with incident T2D differs in insulin resistant (IR) vs sensitive (IS) individuals.
Methods
We studied two prospective cohorts: the Framingham Offspring (n=2023) and KORA S4/F4 Studies (n=887). Insulin resistance was estimated by homeostasis model assessment (HOMA-IR). We used logistic regression analysis to test the association between adiponectin and incident T2D overall and in IR vs IS individuals (defined by ≥ vs <75th percentile of HOMA-IR).
Results
At baseline, Framigham’s participants were 60±9 years old and 56% were women; KORA’s participants were 63±5 years old and 49% were women. T2D incidence was 5.4% over 6.5 years (n=109) in Framingham and 10.5% over 8 years (n=93) in KORA. Lower adiponectin levels were associated with T2D incidence in both cohorts. In IR individuals, lower adiponectin levels were associated with higher risk of T2D incidence (OR=1.60 [95%CI: 1.10–2.31] per SD decrease in Framingham; p=0.01, and OR= 2.34 [95%CI: 1.16–4.73] in KORA; P=0.02); while this was not observed in IS individuals (OR=1.10 [95%CI: 0.73–1.67] in Framingham; p=0.64, and OR=1.34 [95%CI: 0.88–2.03] in KORA; P=0.18).
Conclusions/interpretation
We conclude that lower adiponectin levels are associated with higher risk of T2D in IR but not in IS individuals. This suggests that some level of insulin resistance is needed to see deleterious effects of low adiponectin.
Introduction
Many adipokines produced by the adipose tissue are hypothesized to relate to type 2 diabetes physiopathology, linking excess weight and development of the disease. One of the adipokines of interest is adiponectin. In contrast to other adipokines, adiponectin levels are inversely correlated with adiposity and insulin resistance in most studies, in animals and humans. Moreover, prospective human studies have demonstrated that low adiponectin levels are associated with an increased risk of developing diabetes [1]. Genetic variants in the gene that encodes adiponectin (ADIPOQ) are associated with hyperglycemia [2]. Based on those reports, some have suggested that low adiponectin levels are a primary defect leading to insulin resistance and the development of diabetes, but this needs further investigation in humans.
We tested the hypothesis that low adiponectin is associated with increased risk of type 2 diabetes incidence in two well-characterized prospective cohorts: Framingham Offspring Study and KORA (Cooperative Health Research in the Region of Augsburg) S4/F4 study. We explored the influence of insulin resistance on the association between adiponectin and diabetes incidence by adjusting for levels of insulin resistance and by stratifying the population into insulin resistant vs insulin sensitive individuals.
Methods
Framingham Offspring Study
The Framingham Offspring Study is a community-based, prospective, observational study that has followed the children of the original Framingham Heart Study cohort, and their spouses [3]. At baseline in 1971, 5,124 participants of European ancestry were recruited. For this analysis, we used the follow-up from examination cycle 7 (1998–2001) to cycle 8 (2003–2007): a total of 2023 participants free of diabetes at baseline and with adipokines measurements completed were included in the present report. The study protocol was approved by the Institutional Review Boards of the Boston University Medical Center and of the Massachusetts General Hospital; all participants provided written informed consent.
At every examination cycle, participants had a detailed medical history and physical examination, standardized anthropometric measurements, and fasting blood samples. Body mass index (BMI) was calculated as the weight in kilograms divided by the square of height in meters (kg/m2). Diabetes was defined as use of diabetes medication or a fasting plasma glucose (FPG) ≥7.0 mmol/L at the index exam. Insulin resistance was defined by the homeostasis model (HOMA-IR), calculated as (fasting glucose x fasting insulin)/22.5 [4]. FPG was measured immediately with a hexokinase reagent kit (A-gent glucose test, Abott, South Pasadena, CA), and other plasma samples were frozen at −80°C until assay. Fasting plasma insulin was measured with a human specific insulin assay having essentially no cross-reactivity to proinsulin or insulin split-products (Linco Inc., St Louis, MO). Total adiponectin levels were measured by ELISA (R&D Systems, Minneapolis, MN); other adipokine levels (resistin and TNFα), available at exam 7, were also measured by ELISA (R&D Systems, Minneapolis, MN). Intra-assay coefficients of variation were <3% for glucose, 6.1% for insulin, 5.8% for adiponectin, 9.0% for resistin and 6.6% for TNFα.
KORA S4/F4 cohort study
The study sample involved 2,656 subjects aged 55 to 74 years living in the city of Augsburg (Germany) and in 16 towns and villages from the surrounding districts in 1999 (600,000 inhabitants) [5]. Informed consent was obtained from the participants. Overall, 1,653 (62%) subjects participated. After excluding those with known diabetes and further drop-outs, 1,353 subjects had an OGTT at baseline. This cohort was re-investigated in 2006 to 2008 [6]. The present study includes only participants without known or newly diagnosed diabetes at baseline, who successfully completed a baseline OGTT, and who lived within the study region at the time of the follow-up examination (n=1,202). Among those individuals, 98 (8%) subjects deceased until 2006, and 887 (74%) participated in the follow-up (OGTT).
Cases of incident diabetes (self-reports) and the date of diagnoses were validated by contacting the general practitioners, who treated the participants. Among all other subjects, OGTT were performed during the morning hours after overnight fasting. Fasting venous blood glucose was sampled and 75 g of anhydrous glucose were given (Dextro OGT, Boehringer Mannheim). Incident diabetes was defined based on (1) validated physician diagnoses, or (2) newly diagnosed diabetes (≥7.0 mmol/l fasting or ≥11.1 mmol/l 2-h glucose).
Body weight was measured in light clothing by trained investigators to the nearest 0.1 kg, and height to the nearest 0.5 cm. Waist circumference was measured at the minimum abdominal girth to the nearest 0.1 cm. Blood pressure was measured by quality-certified study investigators three times at the right arm in a sitting position after a fifteen-minute rest using a validated automatic device (OMRON HEM 705-CP). The mean of the second and third measurement was used for the analysis. Family diabetes history was assessed in a structured interview.
Blood was collected with minimal stasis, refrigerated to 4–8 °C and shipped on refrigerant packaging within 2 to 4 h to the laboratory of Augsburg Central Hospital. Blood glucose was measured using a hexokinase method (Gluco-quant; Roche Diagnostics, Mannheim, Germany). At baseline, the interassay coefficients of variation for glucose were 2.4% at 98 mg/dl and 2.1% at 235 mg/dl. Serum adiponectin concentration was measured using the radioimmunoassay from Linco Research (St. Charles, MS, USA). Mean intra- and inter-assay variations in control sera were 5.5% and 9.2%, respectively [7]. Fasting insulin was determined using a microparticle enzyme immunoassay (Abbott Laboratories, Germany). The insulin resistance score (HOMA-IR) was calculated (fasting plasma glucose (mmol/l)*fasting serum insulin (mU/l)/22.5 [4].
Statistical analysis
Baseline characteristics are presented as means (±SD) for continuous variables, or percentage (%) for categorical variables. Spearman correlation was used to test the association between HOMA-IR and adiponectin levels. Multivariable logistic regression analysis was used to analyze the association between diabetes incidence and variables, including the primary exposure of interest: adiponectin levels. Because of its significant association with adiponectin levels and the putative insulin-sensitizing function of adiponectin, we stratified our population according to insulin resistance state measured by HOMA-IR. By convention, we defined insulin resistant as HOMA-IR ≥ 75th percentile and insulin sensitive as HOMA-IR < 75th percentile (the 75th percentile within each cohort was used to define insulin resistant vs insulin sensitive participants). Using Framingham Offspring Study data, we conducted secondary analyses: we adjusted for other adipokines levels at baseline (resistin or TNFα); for waist circumference or visceral adipose tissue (VAT; measured by computerized tomography, n=921) instead of BMI to assess the impact of central adiposity. Finally, we used metabolic syndrome as a proxy for insulin resistance instead of HOMA-IR in the main and stratified analyses. Metabolic syndrome was defined according to NCEP-ATP3 definition [8]. We considered P values less than 0.05 to indicate statistical significance. We performed all analyses using SAS software (version 8.1, SAS Institute, Cary, NC in FOS; version 9.2, SAS Institute, Cary, NC, USA, in KORA).
Results
Baseline characteristics of participants are presented in Table 1. Despite being clinically similar to each other, participants from KORA were statistically older (p<0.0001), had a slightly higher BMI (p=0.04), and were more likely to have a family history of T2D (p=0.001).
Table 1.
Baseline characteristics of participants free of diabetes in the Framingham Offspring Study (1998–2001) and in KORA S4/F4 Study (1999 – 2001)
| Characteristics | Framingham Offspring Study | KORA |
|---|---|---|
| Mean (SD) or N (%) n=2023 |
Mean (SD) or N (%) n=887 |
|
| Age, yr | 59.6 (9.1) | 63.2 (5.4) |
| Sex, %Male | 893 (44.1%) | 449 (50.6%) |
| Family History of Diabetes | 342 (16.9%) | 203 (22.9%) |
| BMI, kg/m2 | 27.7 (5.0) | 28.1 (4.0) |
| Waist circumference, cm | 98.3 (13.5) | 94.5 (11.1) |
| HDL cholesterol, mmol/L | 1.43 (0.44) | 1.53 (0.42) |
| Triacylglycerols, mmol/L | 1.46 (0.88) | 1.44 (0.80) |
| Fasting Glucose, mml/L | 5.39 (0.53) | 5.47 (0.52) |
| SBP, mmHG | 125.1 (18.2) | 133.1 (18.8) |
| DBP, mmHG | 74.3 (9.6) | 79.8 (10.2) |
| Fasting Plasma Insulin, pmol/La | 82.9 (47.9) | 91.7 (121.6) |
| HOMA-IRa | 3.7 (2.5) | 3.3 (4.2) |
| Adiponectin, mg/mLa | 8.8 (1.8) | 10.0 (5.5) |
In KORA, median (IQR) values for Fasting Insulin= 67.2 (47.9; 97.9); for HOMA-IR =2.4 (1.6, 3.5); for adiponectin = 9.0 (6.2, 12.3) BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; HOMA-IR: insulin resistance based on homeostasis model
Over 6.5-year follow-up in Framingham Offspring Study, 5.4% of participants (n=109) developed type 2 diabetes. Lower adiponectin levels were associated with increased risk of diabetes, even after adjustment for age, sex and BMI (OR= 1.87 [1.36–2.57] per SD decrease in adiponectin levels; p=0.0001, see Table 2). Consistent with our previous report [9], baseline adiponectin levels were inversely associated with insulin resistance measured by HOMA-IR (Spearman r= −0.40; p<0.0001). Further adjustment for HOMA-IR in multivariable models predicting diabetes incidence reduced the effect of adiponectin, but the association remained significant (OR=1.44 [1.04–1.97]; p=0.03, see Table 1). Over 8 years of follow-up in KORA, incidence of diabetes was 10.5% (93 cases). After adjustment for age, sex, BMI, and HOMA-IR, lower adiponectin levels were associated with increased risk of diabetes in KORA participants, (OR= 1.71 [1.19–2.45] per SD decrease in adiponectin levels; p=0.004, see Table 2).
Table 2.
Multivariable logistic regression models predicting diabetes incidence in participants of the Framingham Offspring Study and KORA S4/F4 study
| Framingham Offspring Study | KORA | |||||
|---|---|---|---|---|---|---|
| OR for T2D | 95% CI | p-value | OR for T2D | 95% CI | p-value | |
| Adiponectin (per SD decrease) | ||||||
| 1) Adjusted for age, sex | 2.27 | 1.66 – 3.11 | <0.0001 | 1.87 | 1.32 – 2.67 | 0.0005 |
| 2) Adjusted for age, sex, BMI | 1.87 | 1.36 – 2.57 | 0.0001 | 1.76 | 1.23 – 2.52 | 0.002 |
| 3) Adjusted for age, sex, BMI, and HOMA-IR | 1.44 | 1.04 – 1.97 | 0.03 | 1.71 | 1.19 – 2.45 | 0.004 |
| Stratified analyses (adjusted for age, sex, BMI) | ||||||
| Insulin sensitive (HOMA-IR <75th percentile) | 1.10 | 0.73 – 1.67 | 0.64 | 1.34 | 0.88 – 2.03 | 0.18 |
| Insulin resistant (HOMA-IR ≥75th percentile) | 1.60 | 1.10 – 2.31 | 0.01 | 2.34 | 1.16 – 4.73 | 0.02 |
OR: Odds Ratio; T2D: type 2 diabetes; CI: confidence interval; BMI: body mass index; HOMA-IR: insulin resistance by homeostasis model; insulin resistance was defined above study-specific 75th percentile
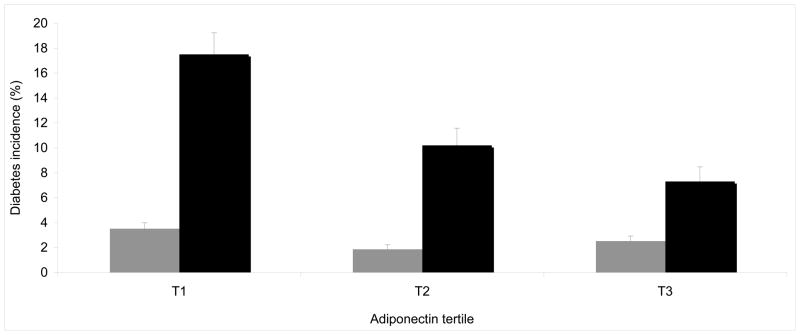
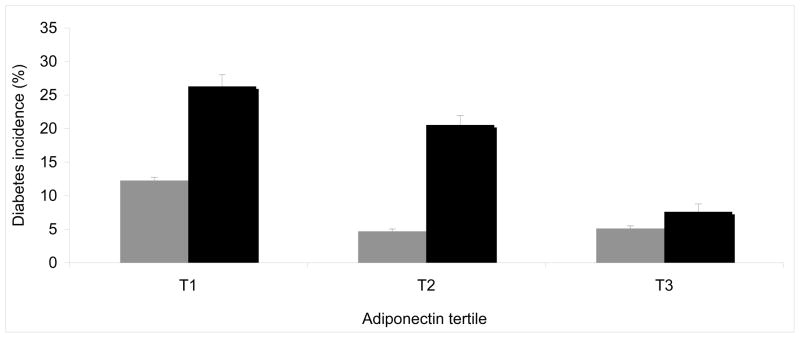
Next, we tested the association of adiponectin levels and diabetes incidence after stratification of participants in insulin resistant (HOMA-IR ≥ 75th percentile) and insulin sensitive (HOMA-IR < 75th percentile; see Table 2). In insulin resistant participants, low adiponectin levels were associated with higher risk of diabetes (OR=1.60 [1.10–2.31] per SD decrease in Framingham, p=0.01; and OR =2.34 [1.16–4.73] in KORA. p=0.02), while this was not observed in insulin sensitive participants (OR= 1.10 [0.73–1.67] in Framingham, p=0.64; OR=1.34 [0.88–2.03] in KORA; p=0.18). The interaction between adiponectin levels and insulin-resistant state was statistically significant in KORA (p=0.045) but not in Framingham Offspring Study (p=0.41). We also tested the interaction between adiponectin and HOMA-IR treated as continuous variables in Framingham (see Supplementary Table 1); both lower adiponectin levels and higher HOMA-IR were significantly associated with higher risk of diabetes incidence, the interaction was not significant (p=0.25, adjusted for age, sex, BMI). Diabetes incidence in each tertile of baseline adiponectin levels in insulin-resistant vs insulin-sensitive participants is illustrated for Framingham in Figure 1 and for KORA in Figure 2.
Figure 1. Type 2 diabetes incidence over 6.5-year follow-up in each tertile of adiponectin, stratified by insulin resistance (HOMA-IR < vs ≥ 75th percentile) in participants of the Framingham Offspring Study.
Legend: Individuals classified as insulin sensitive (HOMA-IR < 75th percentile) are represented in grey bars; individuals classified as insulin-resistant (HOMA-IR ≥75th percentile) are represented in black bars. Diabetes incidence in each tertile was adjusted for age and sex. Adiponectin level (mg/mL) ranges in each tertile: T1= 0.90–6.70; T2= 6.80–11.50; T3= 11.60–59.88
Figure 2. Type 2 diabetes incidence over 8-year follow-up in each tertile of adiponectin, stratified by insulin resistance (HOMA-IR < vs ≥ 75th percentile) in participants of the KORA S4/F4 Study.
Legend: Individuals classified as insulin sensitive (HOMA-IR < 75th percentile) are represented in grey bars; individuals classified as insulin-resistant (HOMA-IR ≥75th percentile) are represented in black bars. Diabetes incidence in each tertile was adjusted for age and sex. Adiponectin level (mg/mL) ranges in each tertile: T1=1.80 – 7.30, T2= 7.40 – 11.00, T3=11.10 – 89.90
Subsidiary analyses in Framingham Offspring Study
Further adjustment of our main analyses with resistin or TNFα levels measured at baseline did not modify the association between adiponectin levels and type 2 diabetes incidence (OR=1.43 [1.04–1.97]; p=0.03), indicating that the association is independent of other adipokines previously shown to be associated with insulin resistance in FOS [9]. Adjusting for waist circumference (OR=1.42 [1.03–1.95]; p=0.03) or VAT (OR=1.38 [0.88–2.15]; p=0.15) instead of BMI did not substantially influence the ORs (but the p-value after adjustment for VAT was no longer significant, driven by smaller sample size n=921). Finally, as a sensitivity analysis, we used metabolic syndrome to classify individuals at higher metabolic risk associated with insulin resistance: the association between adiponectin levels and type 2 diabetes incidence remained significant even when adjusting for metabolic syndrome instead of HOMA-IR. In the group with metabolic syndrome there was a significant association between lower adiponectin and higher risk of type 2 diabetes (OR=1.49 [1.10–2.03] p=0.01), while in the group without metabolic syndrome, the association was not significant (OR= 1.22 [0.70–2.13] p=0.47), similarly to analyses in stratified analyses of insulin resistance vs sensitive individuals.
Discussion
We have demonstrated that lower adiponectin levels are associated with increased risk of type 2 diabetes in participants of two population-based cohorts: the Framingham Offspring Study and the KORA Study. This finding is in accordance with many previous reports in other prospective cohorts of various ethnic background as reported in a recent meta-analysis [1]. A few studies have adjusted for insulin resistance measured by fasting insulin [10, 11] or HOMA-IR [12, 13]; as in our analyses, the strength of associations was reduced by adjustment for insulin resistance but remained significant in most studies. We have further added to the understanding of the complex inter-relationships of insulin resistance and adiponectin on the risk of T2D incidence by demonstrating that low adiponectin was associated with increased risk of developing T2D only in insulin resistant individuals and not in insulin sensitive participants in two independent samples, even with a larger sample size in insulin-sensitive sub-groups. This observation suggests that some degree of insulin resistance needs to be present to observe the association between low adiponectin and progression to diabetes. This is concordant with other reports where associations with adiponectin levels were stronger in individuals with higher metabolic risk profile. For example, Wannamethee et al. have reported that the association between adiponectin levels and type 2 diabetes is stronger in individuals with higher BMI [13]. Moreover, we have previously shown that in Framingham Offspring Study participants, adiponectin levels are more strongly associated with insulin resistance in individuals having the metabolic syndrome [9].
Low adiponectin levels have been suggested to be on causal pathway leading to diabetes. In rhesus monkeys, adiponectin levels decrease in parallel with decreased insulin sensitivity, before the increase in glycemic levels and diabetes incidence [14]. This does not preclude that some events early in the development of insulin resistance have to occur at the adipocyte level to decrease adiponectin secretion, which cannot then have its full beneficial action on peripheral tissues. In fact in our analysis of ADIPOQ variants in the Framingham Offspring Study [2], we found that single nucleotide polymorphisms associated with low adiponectin levels were not associated with hyperglycemia, once again suggesting that it is not necessarily a primary defect of adiponectin production or secretion that leads to type 2 diabetes.
Strengths and limitations
Our study’s strengths include the inclusion of two independents samples from well-established cohorts with a large sample size, prospective design, and standardized measurements, including fasting blood samples for diagnosis of type 2 diabetes incidence. We observed a significant interaction in KORA analyses, but not in Framingham; this may be due to a lower incidence rate in Framingham. KORA participants were more at risk of diabetes at baseline (older, slightly heavier, more likely to have a positive family history) and were followed for a longer period, which results in higher cumulative incidence than in Framingham. The lower incidence in the later might also be related to the fact that Framingham did not perform OGTT at follow-up, leading to potential misclassification. HOMA-IR is a surrogate of insulin resistance, yet it was shown to be a fair way to classify participants in large cohorts such as here. However, HOMA-IR did not allow us to further investigate the contributions of hepatic and muscular insulin resistance on the interaction. Finally, both cohorts are composed of individuals who are mainly of white European descent and were at least of middle age when adiponectin levels were measured; our findings might not be generalizable to younger populations or those from different ancestral backgrounds.
Conclusions
We have demonstrated that low adiponectin levels are associated with an increased risk of developing diabetes in the Framingham Offspring Study and the KORA study, but only in individuals who are relatively insulin resistant. We believe that adiponectin has an important role in the pathophysiology of type 2 diabetes but our observation suggests that some event(s) prior to a decrease in adiponectin levels is necessary to induce the insulin resistant cascade leading to development of diabetes that is associated with low adiponectin. Longitudinal and repeated measures of both adiponectin levels and insulin resistance in humans will be helpful to clarify the sequence of events leading to type 2 diabetes.
Supplementary Material
Acknowledgments
The study was supported by the National Heart, Lung and Blood Institute’s Framingham Heart Study (Contract No. N01-HC-25195) the National Institutes of Health, National Center for Research Resources, NIH grants RO1 HL064753; RO1 HL076784; R01 AG028321 (EJB) General Clinical Research Centers Program (Grant Number M01-RR-01066), and by a Career Development Award from the American Diabetes Association and NIDDK K24 DK080140 (JBM). DMN is supported in part by the Earle Charlton Fund for Innovative Diabetes Research. JBM currently has research grants from GlaxoSmithKline, and has a consulting agreement with Interleukin Genetics.
The KORA S4/F4 Diabetes Cohort Study was funded by a German Research Foundation project grant to W.R. (DFG; RA 459/2-1). The German Diabetes Center is funded by the German Federal Ministry of Health, and the Ministry of School, Science and Research of the State of North-Rhine-Westfalia. The KORA research platform and the KORA Augsburg studies are financed by the Helmholtz Zentrum München, German Research Center for Environmental Health, which is funded by the German Federal Ministry of Education, Science, Research and Technology and by the State of Bavaria. We thank the field staff in Augsburg who were involved in the conduct of the studies.
Footnotes
Author Contributions: MFH interpreted data, wrote manuscript. LMS performed statistical review. PS performed statistical analyses for Framingham Offspring Study. WR, BK and CH performed analyses for KORA Study. CSF, DMN, CM, BT and JBM contributed to discussion. All authors reviewed, edited and made intellectual contributions to the manuscript.
Disclosures: The authors report no conflict of interest with the content of this manuscript
References
- 1.Li S, Shin JJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302:179–188. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 2.Hivert MF, Manning AK, McAteer JB, et al. Common variants in the adiponectin gene (ADIPOQ) associated with plasma adiponectin levels, type 2 diabetes, and diabetes-related quantitative traits: the Framingham Offspring Study. Diabetes. 2008;57:3353–3359. doi: 10.2337/db08-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kannel WB, Gordon T, Castelli WP. Obesity, lipids, and glucose intolerance. The Framingham Study. Am J Clin Nutr. 1979;32:1238–1245. doi: 10.1093/ajcn/32.6.1238. [DOI] [PubMed] [Google Scholar]
- 4.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 5.Rathmann W, Haastert B, Icks A, et al. High prevalence of undiagnosed diabetes mellitus in Southern Germany: target populations for efficient screening. The KORA survey 2000. Diabetologia. 2003;46:182–189. doi: 10.1007/s00125-002-1025-0. [DOI] [PubMed] [Google Scholar]
- 6.Rathmann W, Strassburger K, Heier M, et al. Incidence of Type 2 diabetes in the elderly German population and the effect of clinical and lifestyle risk factors: KORA S4/F4 cohort study. Diabet Med. 2009;26:1212–1219. doi: 10.1111/j.1464-5491.2009.02863.x. [DOI] [PubMed] [Google Scholar]
- 7.Herder C, Hauner H, Haastert B, et al. Hypoadiponectinemia and proinflammatory state: two sides of the same coin?: results from the Cooperative Health Research in the Region of Augsburg Survey 4 (KORA S4) Diabetes Care. 2006;29:1626–1631. doi: 10.2337/dc05-1900. [DOI] [PubMed] [Google Scholar]
- 8.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). see comment. JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 9.Hivert MF, Sullivan LM, Fox CS, et al. Associations of adiponectin, resistin, and tumor necrosis factor-alpha with insulin resistance. Journal of Clinical Endocrinology & Metabolism. 2008;93:3165–3172. doi: 10.1210/jc.2008-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindsay RS, Funahashi T, Hanson RL, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–58. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- 11.Duncan BB, Schmidt MI, Pankow JS, et al. Adiponectin and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2004;53:2473–2478. doi: 10.2337/diabetes.53.9.2473. [DOI] [PubMed] [Google Scholar]
- 12.Snehalatha C, Mukesh B, Simon M, Viswanathan V, Haffner SM, Ramachandran A. Plasma adiponectin is an independent predictor of type 2 diabetes in Asian indians. Diabetes Care. 2003;26:3226–3229. doi: 10.2337/diacare.26.12.3226. [DOI] [PubMed] [Google Scholar]
- 13.Wannamethee SG, Lowe GD, Rumley A, Cherry L, Whincup PH, Sattar N. Adipokines and risk of type 2 diabetes in older men. Diabetes Care. 2007;30:1200–1205. doi: 10.2337/dc06-2416. [DOI] [PubMed] [Google Scholar]
- 14.Hotta K, Funahashi T, Bodkin NL, et al. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes. 2001;50:1126–1133. doi: 10.2337/diabetes.50.5.1126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.