Abstract
Tritium-labeled digitoxin, digitoxigenin, digoxin, and digoxigenin of established purity and chemcal authenticity were used to study the binding of these compounds to human plasma proteins. 97% of digitoxin in plasma was nondialyzable. Continuous flow paper electrophoresis of plasma containing digitoxin and dialysis experiments in which human serum albumin competed for the glycoside with plasma or plasma protein fractions demonstrated that digitoxin was almost exclusively bound by albumin. Equilibrium dialyses revealed that the interaction was characterized by a single binding site on the albumin molecule and an association constant of 9.62 × 104 liter/mole at 37°C. At 1°C the association constant was 4.64 × 104 liter/mole. The interaction therefore was endothermic; the gain in enthalpy of 3.5 kcal/mole and the free energy change of - 7.06 kcal/mole was derived from a large change in entropy of 33.8 cal/mole per °K. The direction of these thermodynamic changes suggested the formation of a hydrophobic bond between digitoxin and albumin. Quenching of the fluorescence of albumin by digitoxin indicated that the conformation of albumin was altered by the binding process.
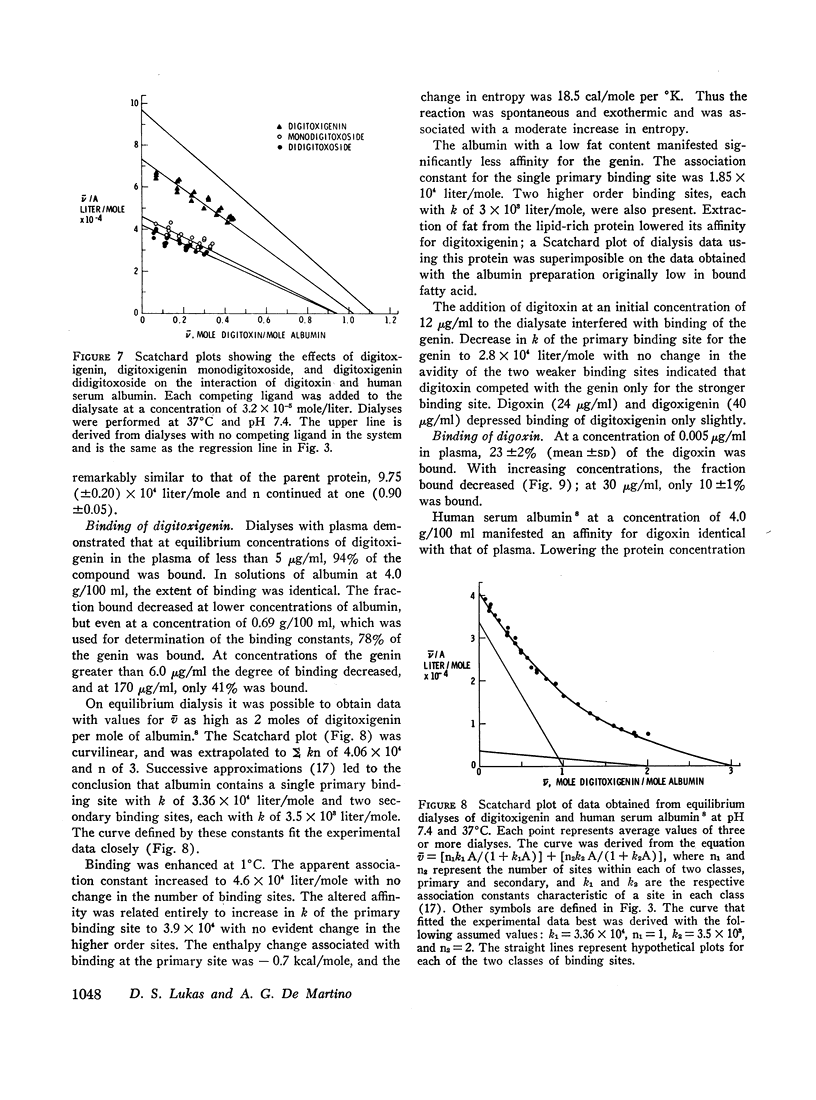
Digitoxigenin, its mono- and didigitoxosides, digoxin, and digoxigenin competed with digitoxn for its binding site on albumin. The affinity of the mono- and didigitoxosides for the site was equal to that of digitoxin, but that of digitoxigenin was only one-third as great. The ability of the digitoxose residues of the glycosides to enhance binding to albumin was also observed with digoxin, which was more extensively bound by the protein than digoxigenin.
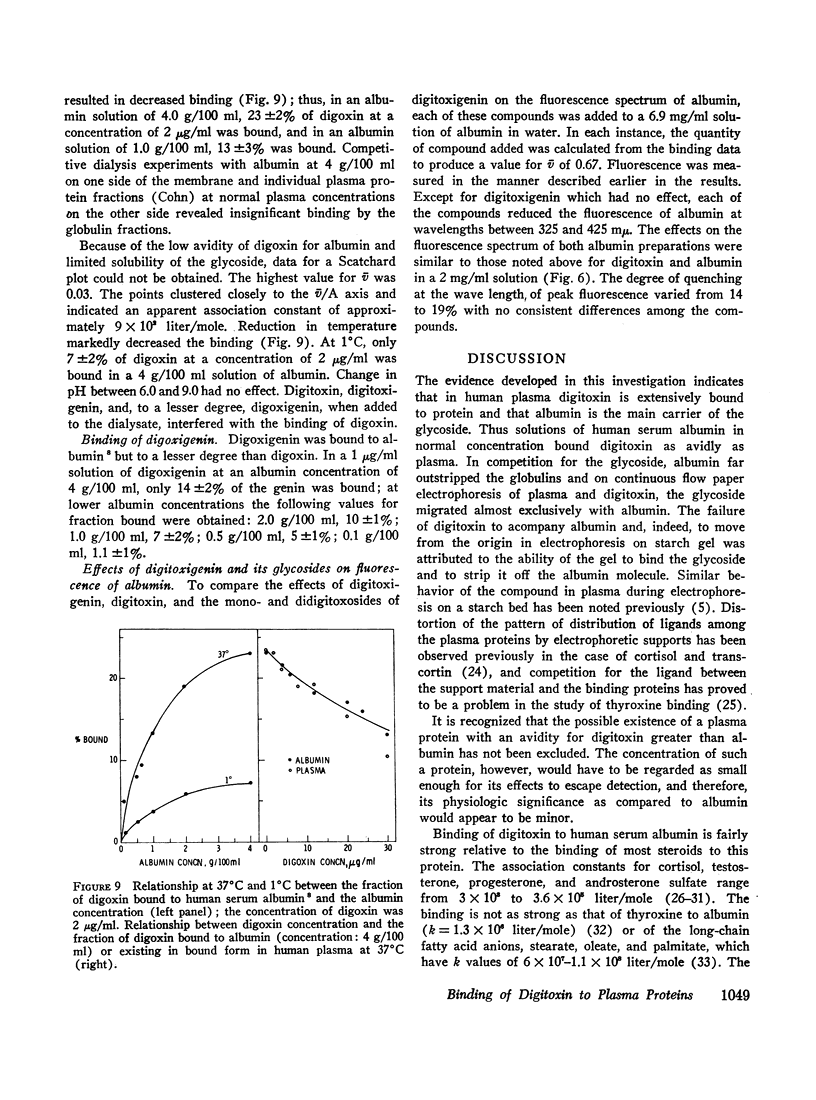
At concentrations of 2 μg/ml or less in plasma, only 23% of digoxin was bound. Albumin, which interacted with digoxin with an apparent association constant of 9 × 102 liter/mole at 37°C, was entirely responsible for the binding. Lowering the temperature from 37° to 1°C decreased the fraction of digoxin bound to albumin by two-thirds.
The marked difference in avidity of digitoxin and digoxin for serum albumin is reflected by the higher plasma concentrations, lower rate of urinary excretion, and longer half-time of digitoxin as compared to those of digoxin when these compounds are administered to man.
Full text
PDF


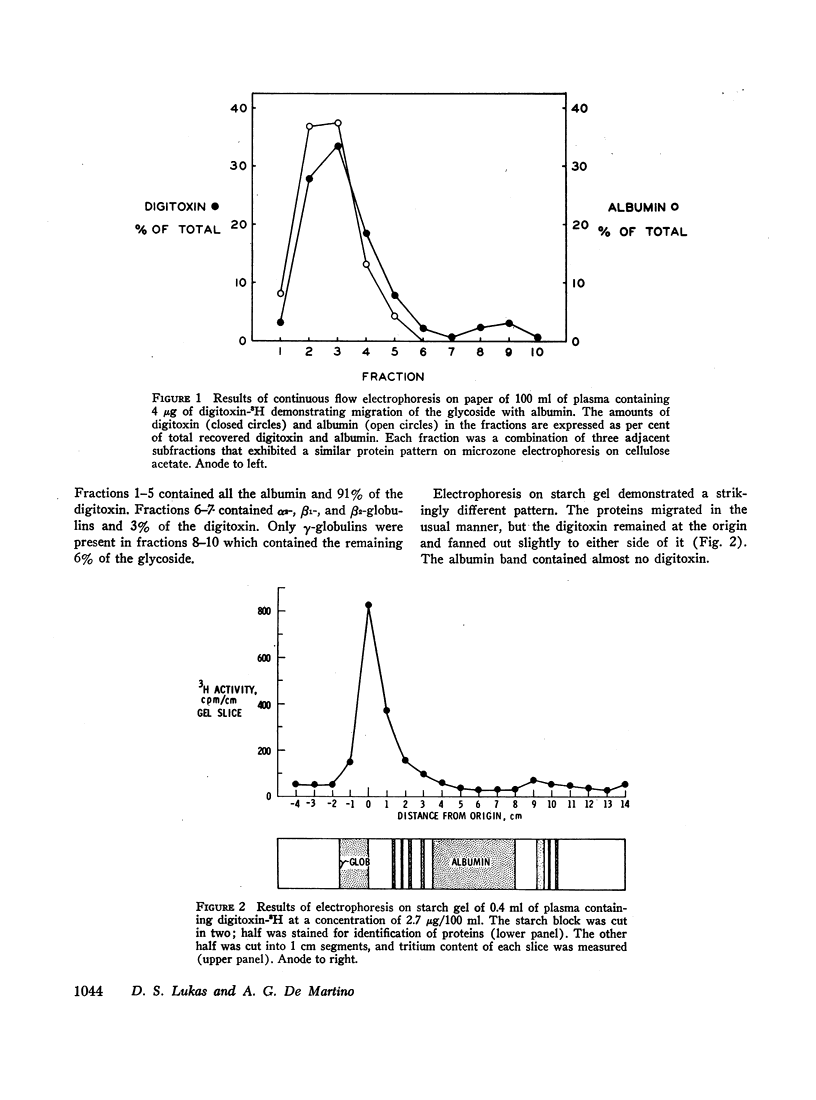
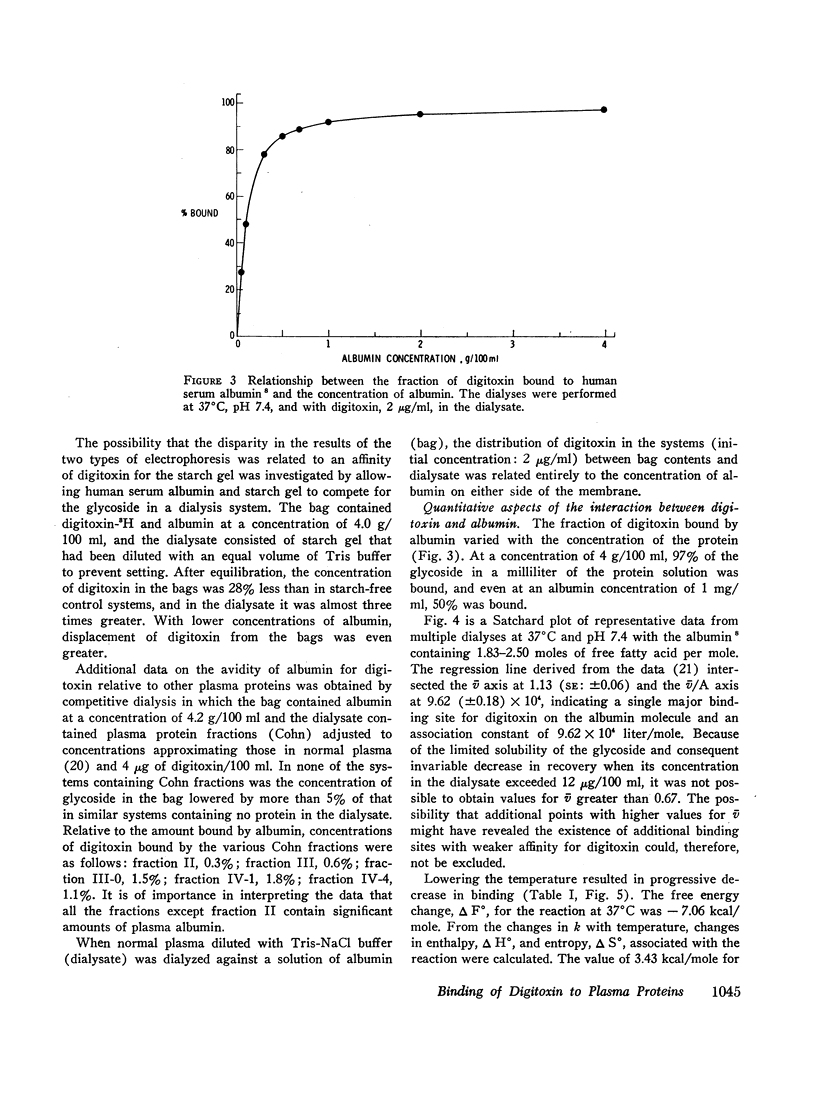
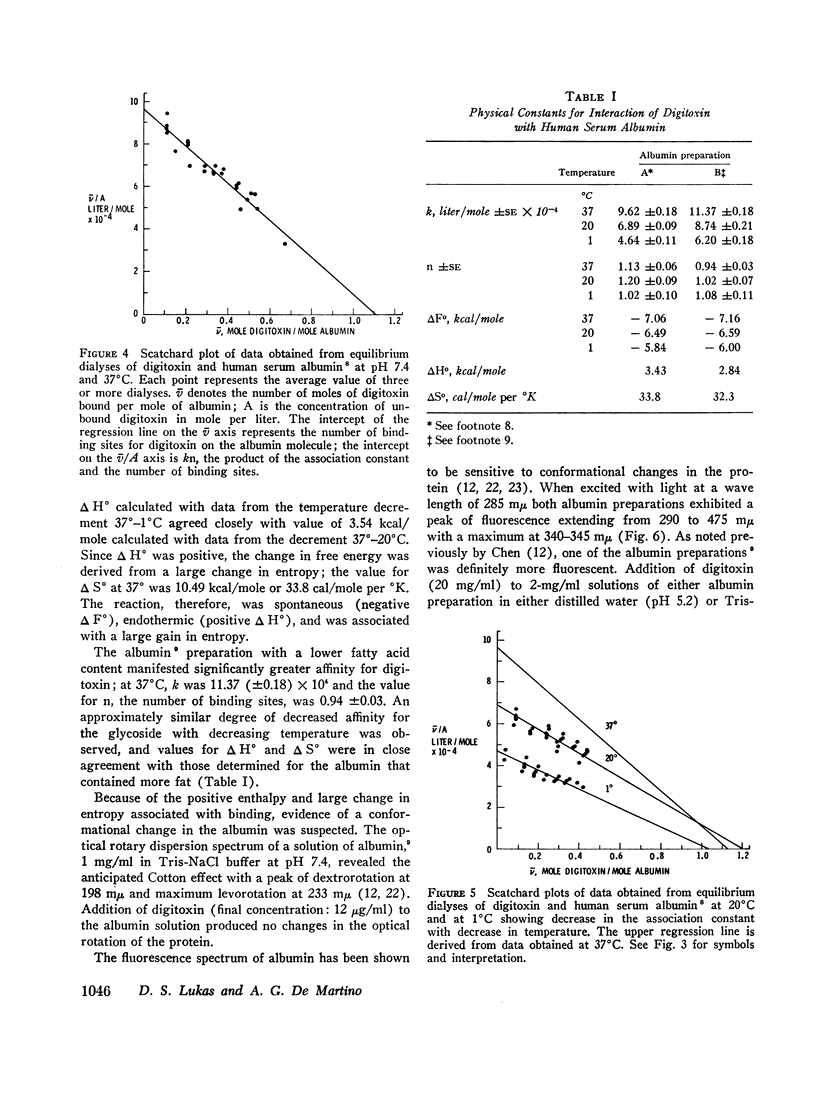
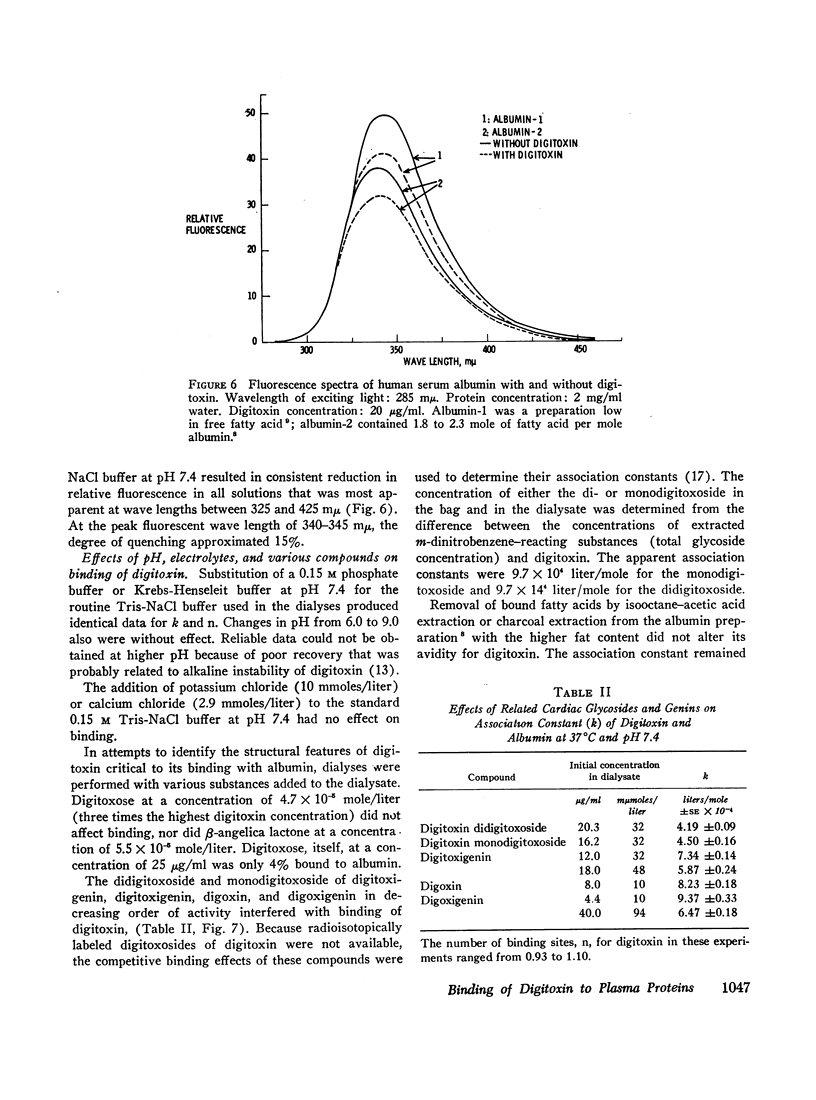




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRODIE B. B., HOGBEN C. A. Some physico-chemical factors in drug action. J Pharm Pharmacol. 1957 Jun;9(6):345–380. doi: 10.1111/j.2042-7158.1957.tb12289.x. [DOI] [PubMed] [Google Scholar]
- CONN H. L., Jr, LUCHI R. J. Some quantitative aspects of the binding of quinidine and related quinoline compounds by human serum albumin. J Clin Invest. 1961 Mar;40:509–516. doi: 10.1172/JCI104278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. F. Fluorescence spectra of human serum albumin in the pH region of the N-F transition. Biochim Biophys Acta. 1966 May 12;120(1):169–171. doi: 10.1016/0926-6585(66)90291-3. [DOI] [PubMed] [Google Scholar]
- DAUGHADAY W. H. Binding of corticosteroids by plasma proteins. IV. The electrophoretic demonstration of corticosteroid binding globulin. J Clin Invest. 1958 Apr;37(4):519–523. doi: 10.1172/JCI103633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOHERTY J. E., PERKINS W. H., MITCHELL G. K. Tritiated digoxin studies in human subjects. Arch Intern Med. 1961 Oct;108:531–539. doi: 10.1001/archinte.1961.03620100023004. [DOI] [PubMed] [Google Scholar]
- EIK-NES K., SCHELLMAN J. A., LUMRY R., SAMUELS L. T. The binding of steroids to protein. I. Solubility determinations. J Biol Chem. 1954 Jan;206(1):411–419. [PubMed] [Google Scholar]
- GOODMAN D. S. Preparation of human serum albumin free of long-chain fatty acids. Science. 1957 Jun 28;125(3261):1296–1297. doi: 10.1126/science.125.3261.1296. [DOI] [PubMed] [Google Scholar]
- JIRGENSONS B. THE COTTON EFFECTS IN THE OPTICAL ROTATORY DISPERSION OF PROTEINS AS NEW CRITERIA OF CONFORMATION. J Biol Chem. 1965 Mar;240:1064–1071. [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lubash G. D., Stenzel K. H., Rubin A. L. Methodology for study of nitrogenous compounds in hemodialysate. Trans Am Soc Artif Intern Organs. 1964;10:337–339. [PubMed] [Google Scholar]
- Lukas D. A., Peterson R. E. Double isotope dilution derivative assay of digitoxin in plasma, urine, and stool of patients maintained on the drug. J Clin Invest. 1966 May;45(5):782–795. doi: 10.1172/JCI105393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCUS F. I., KAPADIA G. J., KAPADIA G. G. THE METABOLISM OF DIGOXIN IN NORMAL SUBJECTS. J Pharmacol Exp Ther. 1964 Aug;145:203–209. [PubMed] [Google Scholar]
- MEYER C. J., LAYNE D. S., TAIT J. F., PINCUS G. The binding of aldosterone to plasma proteins in normal, pregnant, and steroid-treated women. J Clin Invest. 1961 Sep;40:1663–1671. doi: 10.1172/JCI104389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus F. I., Burkhalter L., Cuccia C., Pavlovich J., Kapadia G. G. Administration of tritiated digoxin with and without a loading dose. A metabolic study. Circulation. 1966 Nov;34(5):865–874. doi: 10.1161/01.cir.34.5.865. [DOI] [PubMed] [Google Scholar]
- O'Reilly R. A. Studies on the coumarin anticoagulant drugs: interaction of human plasma albumin and warfarin sodium. J Clin Invest. 1967 May;46(5):829–837. doi: 10.1172/JCI105582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OYAKAWA E. K., LEVEDAHL B. H. Testosterone binding to bovine and human serum albumin: the role of tyrosine groups. Arch Biochem Biophys. 1958 Mar;74(1):17–23. doi: 10.1016/0003-9861(58)90195-4. [DOI] [PubMed] [Google Scholar]
- PERT J. H., ENGLE R. E., Jr, WOODS K. R., SLEISENGER M. H. Preliminary studies on quantitative zone electrophoresis in starch gel. J Lab Clin Med. 1959 Oct;54:572–584. [PubMed] [Google Scholar]
- PLAGER J. E. THE BINDING OF ANDROSTERONE SULFATE, ETHIOCHOLANOLONE SULFATE, AND DEHYDROISOANDROSTERONE SULFATE BY HUMAN PLASMA PROTEIN. J Clin Invest. 1965 Jul;44:1234–1239. doi: 10.1172/JCI105229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman W. H., Crépy O. Steroid-protein interaction with particular reference to testosterone binding by human serum. J Biol Chem. 1967 Jan 25;242(2):182–189. [PubMed] [Google Scholar]
- REPKE K., SAMUELS L. T. ENZYMATIC BASIS FOR EPIMERIZATION OF CARDIOTONIC STEROIDS AT CARBON 3 IN RAT LIVER. Biochemistry. 1964 May;3:689–695. doi: 10.1021/bi00893a016. [DOI] [PubMed] [Google Scholar]
- ROBBINS J. Reverse-flow zone electrophoresis; a method for determining the thyroxine-binding capacity of serum protein. Arch Biochem Biophys. 1956 Aug;63(2):461–469. doi: 10.1016/0003-9861(56)90062-5. [DOI] [PubMed] [Google Scholar]
- SANDBERG A. A., SLAUNWHITE W. R., Jr, ANTONIADES H. N. The binding of steroids and steroid conjugates to human plasma proteins. Recent Prog Horm Res. 1957;13:209–267. [PubMed] [Google Scholar]
- SPRATT J. L., OKITA G. T. Protein binding radioactive digitoxin. J Pharmacol Exp Ther. 1958 Oct;124(2):109–114. [PubMed] [Google Scholar]
- Steiner R. F., Roth J., Robbins J. The binding of thyroxine by serum albumin as measured by fluorescence quenching. J Biol Chem. 1966 Feb 10;241(3):560–567. [PubMed] [Google Scholar]
- TERESI J. D., LUCK J. M. The combination of organic anions with serum albumin. VIII. Fatty acid salts. J Biol Chem. 1952 Feb;194(2):823–834. [PubMed] [Google Scholar]
- WESTPHAL U., ASHLEY B. D. Steroidprotein interactions. IV. Influence of functional groups in delta 4-3-Ketosteroids on interaction with serum albumin and beta-lactoglobulin. J Biol Chem. 1958 Jul;233(1):57–62. [PubMed] [Google Scholar]


