Abstract
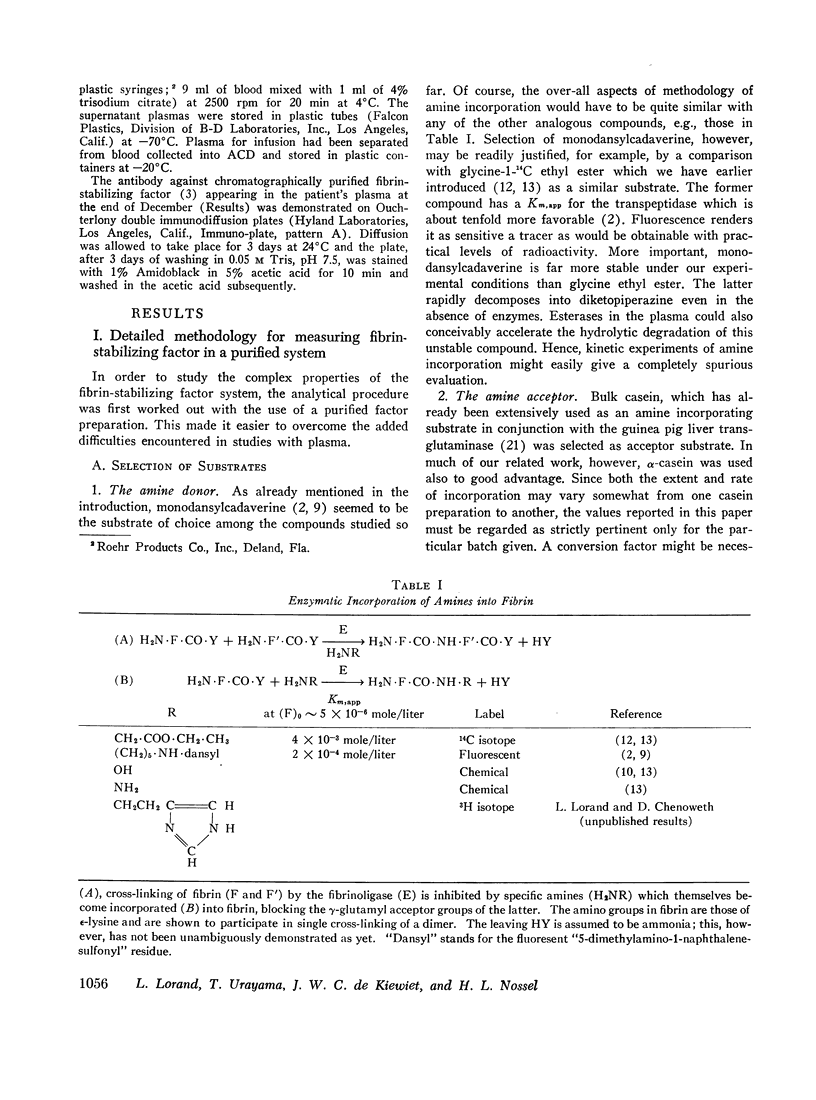
Fibrinoligase, the fibrin cross-linking enzyme, transiently appearing during the course of coagulation in normal blood, was shown to catalyze the incorporation of a fluorescent amine, monodansylcadaverine [or N-(5-aminopentyl)-5-dimethylamino-1-naphthalene-sulfonamide] into casein. The reaction provided the basis of a sensitive fluorimetric method for measuring the activity of the enzyme (and also of similar other transpeptidases, such as transglutaminase).
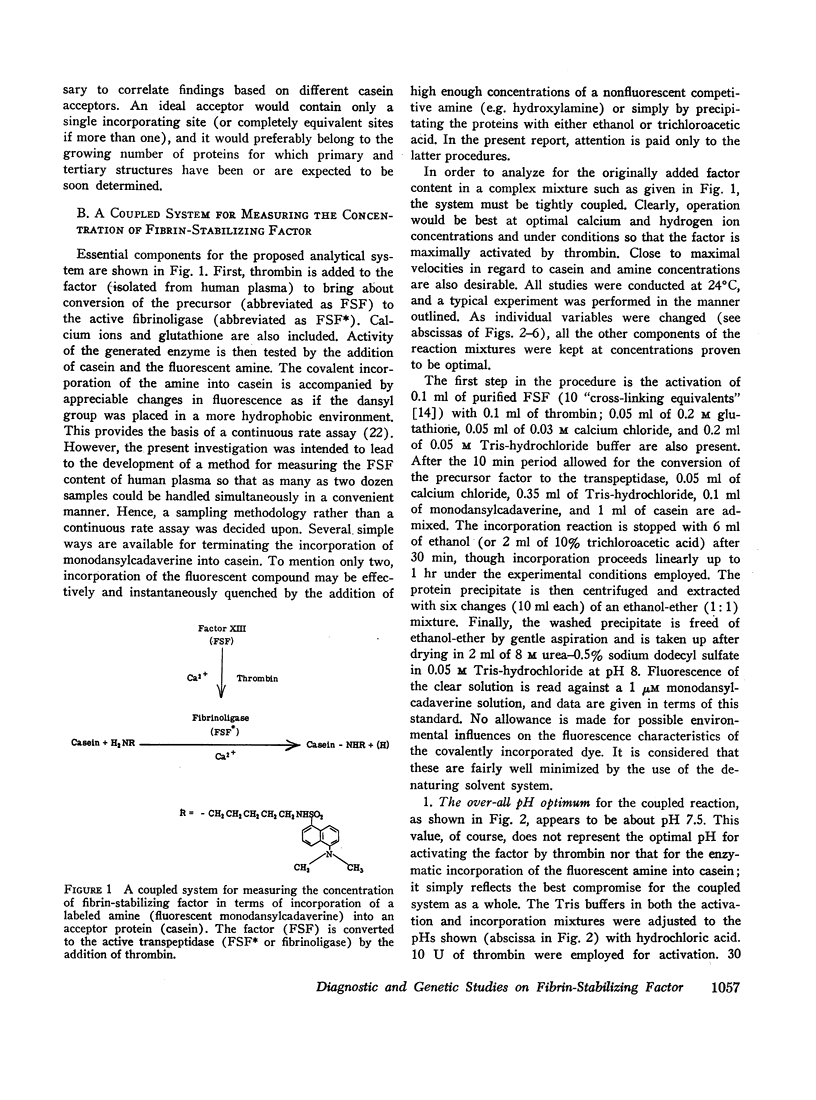
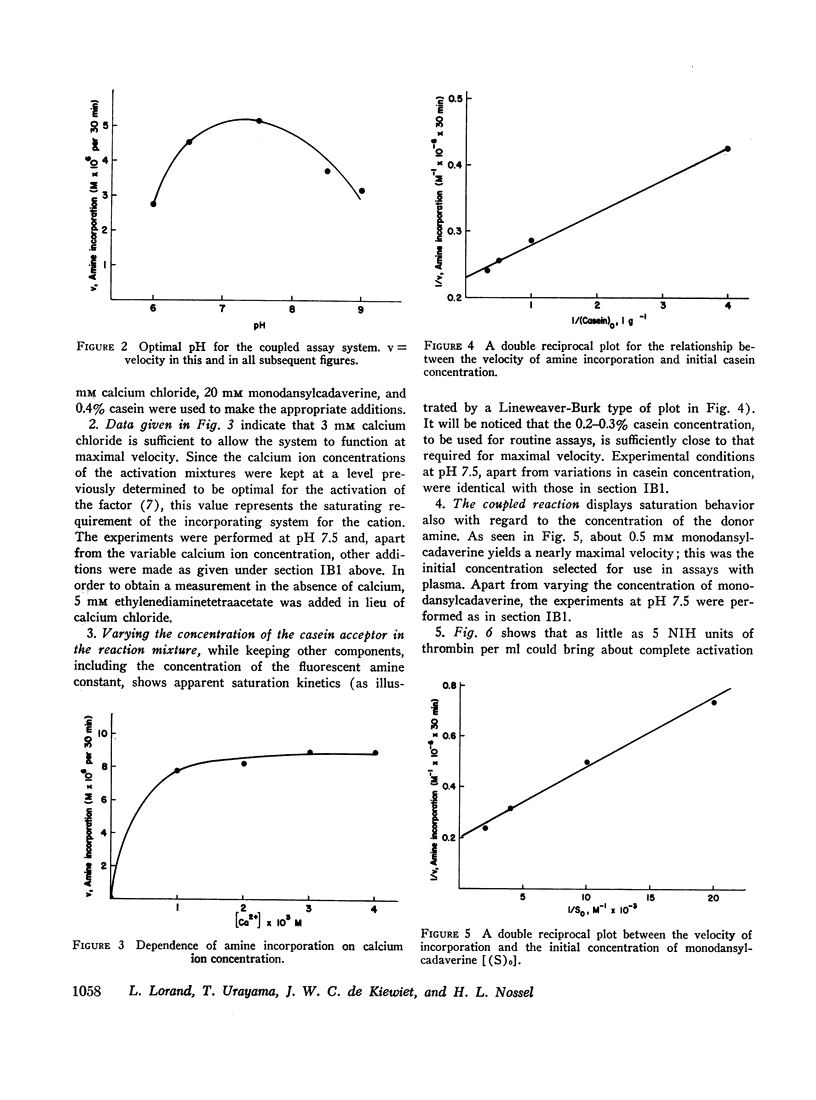
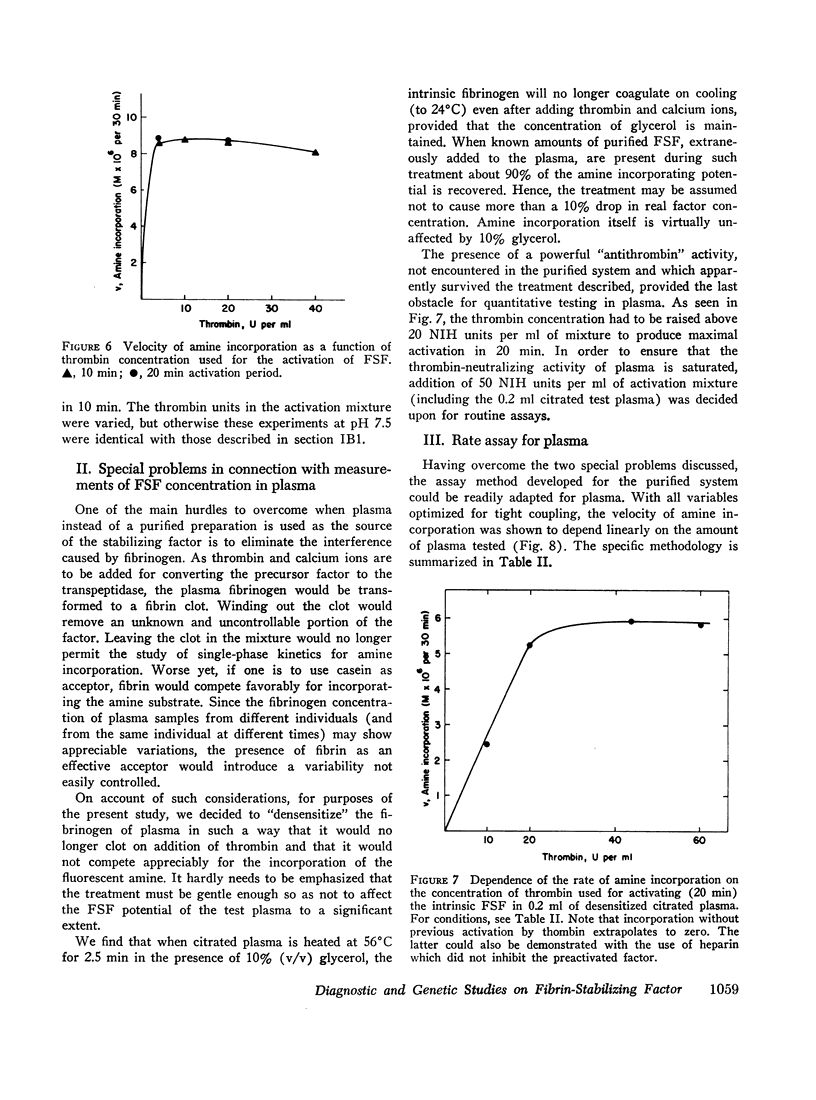
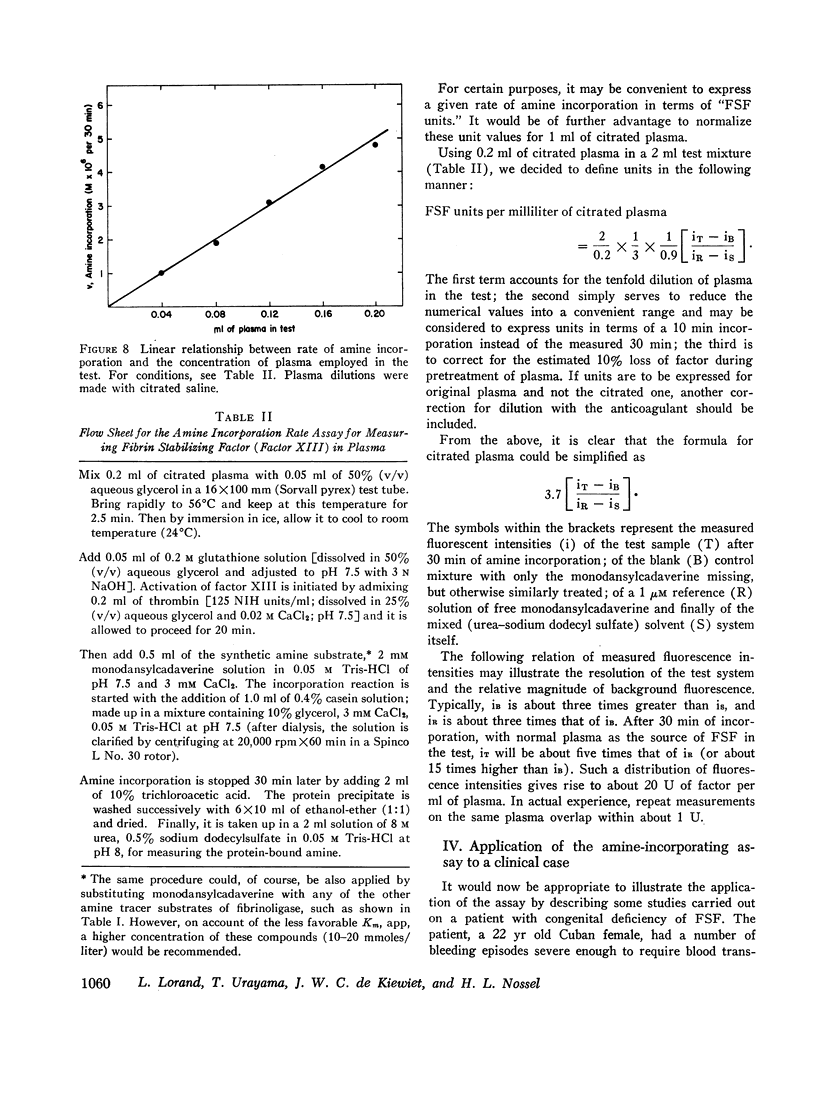
In tests involving plasma, certain difficulties had to be overcome which were mainly due to the fact that the enzyme itself does not occur in citrated plasma. Only its precursor (fibrin-stabilizing factor or factor XIII) is present, still requiring limited proteolytic activation by thrombin. Thus, in order to measure amine incorporation with plasma as a source of the factor, thrombin must be added. This necessitated a differential desensitization of the intrinsic fibrinogen so that the latter could not clot and could not thereby interfere with amine incorporation. Also, the thrombin-inactivating capacity of plasma had to be saturated to enable full conversion of the factor to the transpeptidase. Concentrations of casein, monodansylcadaverine, calcium, and hydrogen ions were chosen to permit almost maximal velocity of amine incorporation. A linear relationship with regard to plasma concentration could be obtained only under such conditions. No similar assay is presently available for quantitatively evaluating fibrin-stabilizing factor levels in plasma.
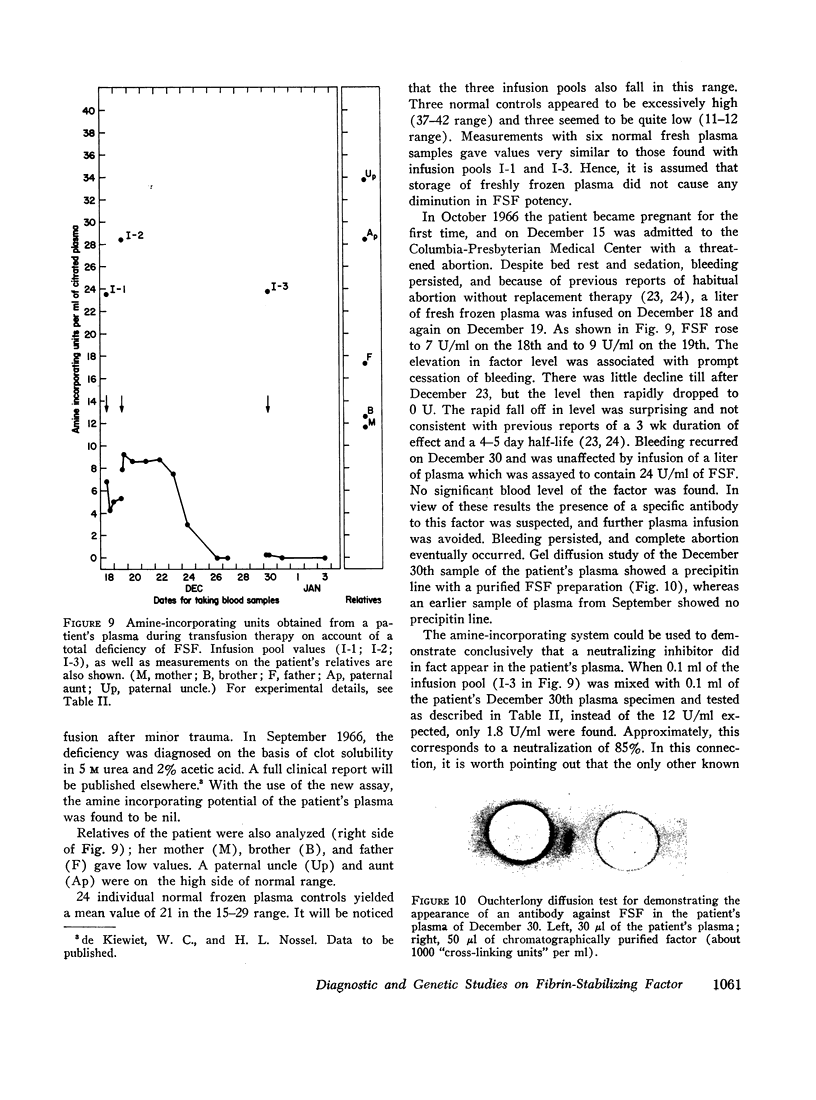
The amine incorporation test was applied to a clinical case of hereditary total fibrin-stabilizing factor deficiency. The effect of transfusion therapy was studied, and some of the patient's relatives were examined. Whereas a paternal aunt and uncle gave values well within the normal range, a brother and the mother proved to be partially deficient and could be considered as heterozygous carriers. The father appeared to have a reduced level of fibrin-stabilizing factor, though not quite as low as the other two relatives.
Two infusions (1 liter each) of fresh normal plasma, administered about 26 hr apart, brought levels in the patient's plasma close to those found in the mother and brother. The corrective power of the transfusions, however, rapidly declined within 5-6 days. Futility of the last transfusion could be ascribed to the appearance of a neutralizing antibody directed against the precursor stabilizing factor, a serious complication.
General diagnostic versatility and potential of the quantitative amine incorporation assay with plasma is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DUCKERT F., JUNG E., SHMERLING D. H. A hitherto undescribed congenital haemorrhagic diathesis probably due to fibrin stabilizing factor deficiency. Thromb Diath Haemorrh. 1960 Dec 15;5:179–186. [PubMed] [Google Scholar]
- Deranleau D. A., Neurath H. The combination of chymotrypsin and chymotrypsinogen with fluorescent substrates and inhibitors for chymotrypsin. Biochemistry. 1966 Apr;5(4):1413–1425. doi: 10.1021/bi00868a040. [DOI] [PubMed] [Google Scholar]
- Hampton J. W., Cunningham G. R., Bird R. M. The pattern of inheritance of defective fibrinase (Factor 13). J Lab Clin Med. 1966 Jun;67(6):914–921. [PubMed] [Google Scholar]
- IKKALA E., MYLLYLAE G., NEVANLINNA H. R. TRANSFUSION THERAPY IN FACTOR 13 (F.S.F.) DEFICIENCY. Scand J Haematol. 1964;1:308–312. doi: 10.1111/j.1600-0609.1964.tb00028.x. [DOI] [PubMed] [Google Scholar]
- LOEWY A. G., VENEZIALE C., FORMAN M. Purification of the factor involved in the formation of urea-insoluble fibrin. Biochim Biophys Acta. 1957 Dec;26(3):670–671. doi: 10.1016/0006-3002(57)90133-6. [DOI] [PubMed] [Google Scholar]
- LORAND L., DICKENMAN R. C. Assay method for the fibrin-stabilizing factor. Proc Soc Exp Biol Med. 1955 May;89(1):45–48. doi: 10.3181/00379727-89-21711. [DOI] [PubMed] [Google Scholar]
- LORAND L. Fibrin clots. Nature. 1950 Oct 21;166(4225):694–695. doi: 10.1038/166694a0. [DOI] [PubMed] [Google Scholar]
- LORAND L., JACOBSEN A. SPECIFIC INHIBITORS AND THE CHEMISTRY OF FIBRIN POLYMERIZATION. Biochemistry. 1964 Dec;3:1939–1943. doi: 10.1021/bi00900a026. [DOI] [PubMed] [Google Scholar]
- LORAND L., KONISHI K. ACTIVATION OF THE FIBRIN STABILIZING FACTOR OF PLASMA BY THROMBIN. Arch Biochem Biophys. 1964 Apr;105:58–67. doi: 10.1016/0003-9861(64)90235-8. [DOI] [PubMed] [Google Scholar]
- LORAND L., KONISHI K., JACOBSEN A. Transpeptidation mechanism in blood clotting. Nature. 1962 Jun 23;194:1148–1149. doi: 10.1038/1941148a0. [DOI] [PubMed] [Google Scholar]
- LUSCHER E. F. Ein fibrinstabilisierender Faktor aus Thrombocyten. Schweiz Med Wochenschr. 1957 Oct 2;87(39-40):1220–1221. [PubMed] [Google Scholar]
- Lewis J. H., Szeto I. L., Ellis L. D., Bayer W. L. An acquired inhibitor to coagulation factor 13. Johns Hopkins Med J. 1967 Jun;120(6):401–407. [PubMed] [Google Scholar]
- Lorand J. B., Urayama T., Lorand L. Transglutaminase as a blood clotting enzyme. Biochem Biophys Res Commun. 1966 Jun 21;23(6):828–834. doi: 10.1016/0006-291x(66)90562-6. [DOI] [PubMed] [Google Scholar]
- Lorand L., Downey J., Gotoh T., Jacobsen A., Tokura S. The transpeptidase system which crosslinks fibrin by gamma-glutamyle-episilon-lysine bonds. Biochem Biophys Res Commun. 1968 Apr 19;31(2):222–230. doi: 10.1016/0006-291x(68)90734-1. [DOI] [PubMed] [Google Scholar]
- Lorand L., Jacobsen A., Bruner-Lorand J. A pathological inhibitor of fibrin cross-linking. J Clin Invest. 1968 Feb;47(2):268–273. doi: 10.1172/JCI105723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L., Jacobsen A. Isonicotinic acid hydrazide as an inhibitor of transpeptidation: relevance for blood coagulation. Nature. 1967 Nov 4;216(5114):508–509. doi: 10.1038/216508b0. [DOI] [PubMed] [Google Scholar]
- Lorand L., Ong H. H. Labeling of amine-acceptor cross-linking sites of fibrin by transpeptidation. Biochemistry. 1966 May;5(5):1747–1753. doi: 10.1021/bi00869a043. [DOI] [PubMed] [Google Scholar]
- Lorand L., Ong H. H. Studies on fibrin crosslinking. Nature of the acceptor groups in transpeptidation. Biochem Biophys Res Commun. 1966 Apr 19;23(2):188–193. doi: 10.1016/0006-291x(66)90526-2. [DOI] [PubMed] [Google Scholar]
- Lorand L. Physiological roles of fibrinogen and fibrin. Fed Proc. 1965 Jul-Aug;24(4):784–793. [PubMed] [Google Scholar]
- Lorand L., Rule N. G., Ong H. H., Furlanetto R., Jacobsen A., Downey J., Oner N., Bruner-Lorand J. Amine specificity in transpeptidation. Inhibition of fibrin cross-linking. Biochemistry. 1968 Mar;7(3):1214–1223. doi: 10.1021/bi00843a043. [DOI] [PubMed] [Google Scholar]
- Matacić S., Loewy A. G. The identification of isopeptide crosslinks in insoluble fibrin. Biochem Biophys Res Commun. 1968 Feb 26;30(4):356–362. doi: 10.1016/0006-291x(68)90750-x. [DOI] [PubMed] [Google Scholar]
- NUSSBAUM M., MORSE B. S. PLASMA FIBRIN STABILIZING FACTOR ACTIVITY IN VARIOUS DISEASES. Blood. 1964 May;23:669–678. [PubMed] [Google Scholar]
- RASMUSSEN P. S. Purification of thrombin by chromatography. Biochim Biophys Acta. 1955 Jan;16(1):157–158. doi: 10.1016/0006-3002(55)90194-3. [DOI] [PubMed] [Google Scholar]
- Sigg P. The monoiodoacetate (MIA) tolerance test, a new quantitative method for the fibrin stabilizing factor (factor 13) assay. Thromb Diath Haemorrh. 1966 Jan 31;15(1):238–251. [PubMed] [Google Scholar]
- Wajda I. J., Lee J. M., Waelsch H. Transglutaminase and experimental allergic encephalomyelitis. The effects of encephalitogenic components in mice and guinea pigs. J Neurochem. 1967 Apr;14(4):389–399. doi: 10.1111/j.1471-4159.1967.tb09536.x. [DOI] [PubMed] [Google Scholar]



