Abstract
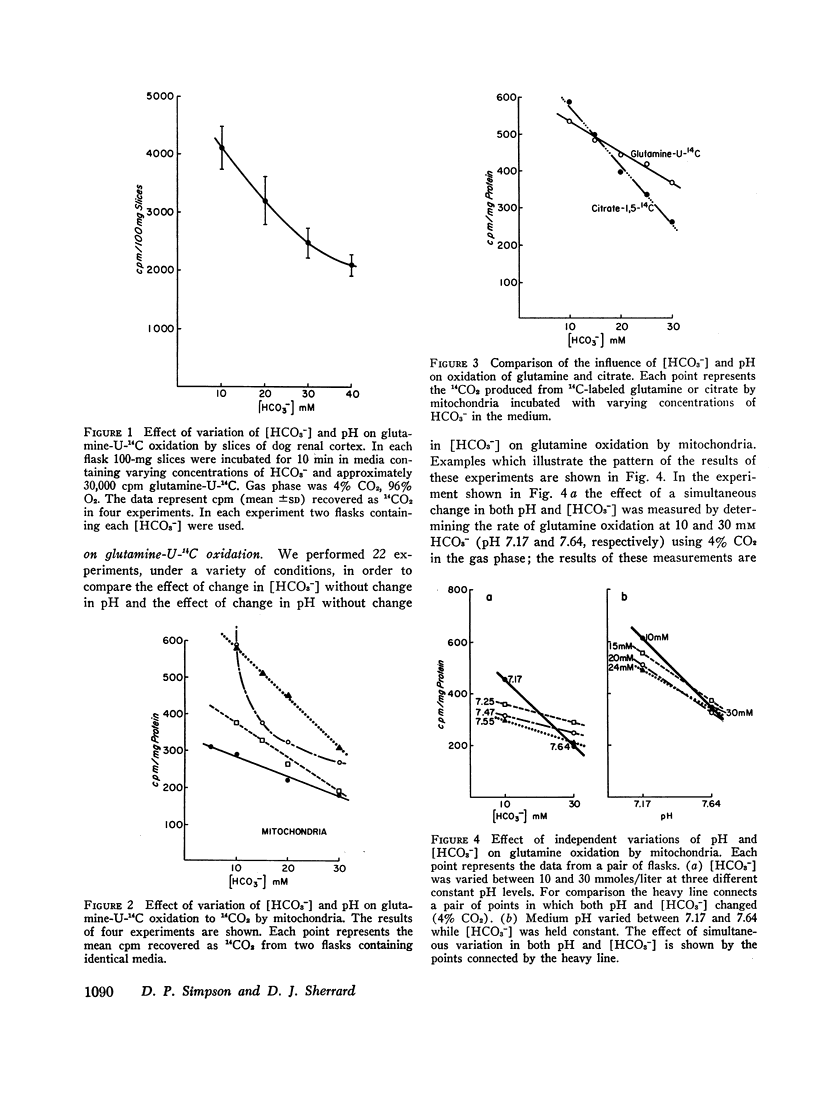
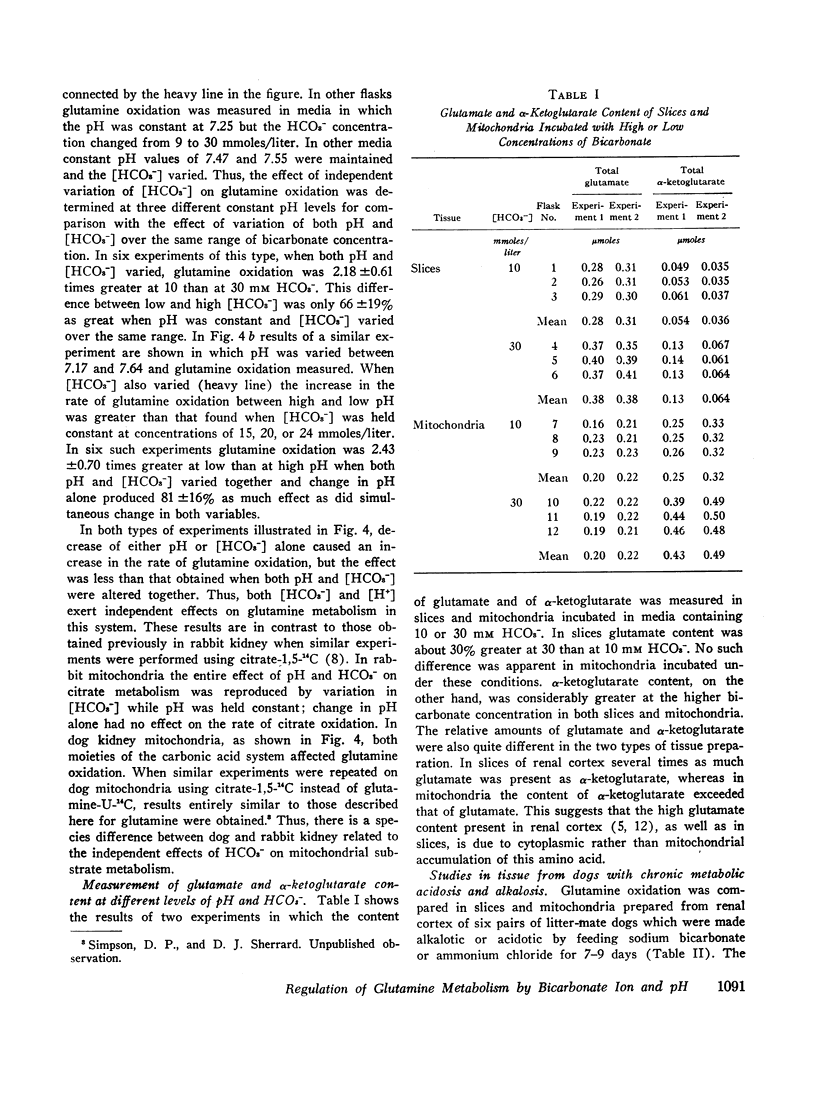
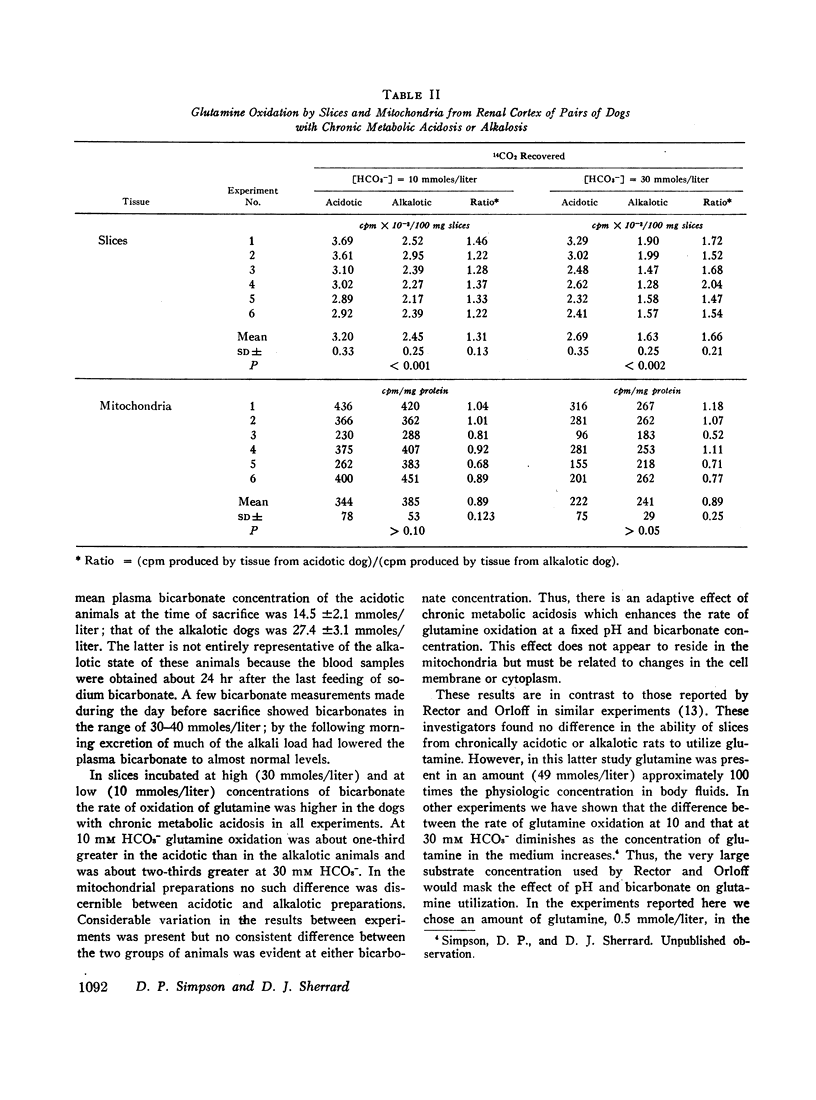
The effect of variations of medium pH and bicarbonate concentration on glutamine oxidation was studied in slices and mitochondria from dog renal cortex. Decreasing pH and bicarbonate concentration increased the rate of oxidation of glutamine-U-14C to 14CO2 in both slices and mitochondria, an effect comparable to the acute stimulation of glutamine utilization produced by metabolic acidosis. Decreases in the concentration of glutamate and α-ketoglutarate, which accompany metabolic acidosis in the intact animal, also occurred in tissue slices when pH and [HCO3-] were lowered; decrease in α-ketoglutarate but not in glutamate content occurred in mitochondria under these conditions. Study of independent variations of medium pH and [HCO3-] showed that simultaneous changes in both pH and [HCO3-] produced a greater effect on glutamine metabolism than did change in either of these parameters alone.
The rate of glutamine oxidation was also compared in tissue preparations from pairs of litter-mate dogs with chronic metabolic acidosis and alkalosis. No significant difference in the rate of glutamine oxidation was present in mitochondria from the two sets of animals. Slices from animals with chronic metabolic acidosis consistently oxidized glutamine at a more rapid rate than slices from alkalotic dogs both at high and at low concentrations of bicarbonate in the medium. We believe this difference is a result of the same mechanism which leads to the delayed increase in ammonium excretion during induction of metabolic acidosis.
The close parallel between the effects demonstrated here and the changes in ammonium production and glutamine utilization in the intact animal with metabolic acidosis suggest that the observed in vitro changes accurately represent the operation of the physiologic mechanism by which acid-base changes regulate ammonium excretion. The similarity between the changes in glutamine oxidation observed in this study and those described previously for citrate suggests that one control mechanism affects the metabolism of both citrate and glutamine. Thus, we believe that the increase in citrate clearance in metabolic alkalosis and the increase in glutamine utilization and ammonium production in metabolic acidosis reflect the operation of the same underlying biochemical mechanism. This mechanism permits changes in pH and [HCO3-] in the cellular environment to regulate the rate of mitochondrial uptake and oxidation of several physiologically important substrates.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addae S. K., Lotspeich W. D. Relation between glutamine utilization and production in metabolic acidosis. Am J Physiol. 1968 Aug;215(2):269–277. doi: 10.1152/ajplegacy.1968.215.2.269. [DOI] [PubMed] [Google Scholar]
- Alleyne G. A. Concentrations of metabolic intermediates in kidneys of rats with metabolic acidosis. Nature. 1968 Mar 2;217(5131):847–848. doi: 10.1038/217847a0. [DOI] [PubMed] [Google Scholar]
- CARTER N. W., SELDIN D. W., TENG H. C. Tissue and renal response to chronic respiratory acidosis. J Clin Invest. 1959 Jun;38(6):949–960. doi: 10.1172/JCI103878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAWFORD M. A., MILNE M. D., SCRIBNER B. H. The effects of changes in acid-base balance on urinary citrate in the rat. J Physiol. 1959 Dec;149:413–423. doi: 10.1113/jphysiol.1959.sp006348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES B. M. A., YUDKIN J. Studies in biochemical adaptation; the origin or urinary ammonia as indicated by the effect of chronic acidosis and alkalosis on some renal enzymes in the rat. Biochem J. 1952 Nov;52(3):407–412. doi: 10.1042/bj0520407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., WATTIAUX R., BAUDHUIN P. Distribution of enzymes between subcellular fractions in animal tissues. Adv Enzymol Relat Subj Biochem. 1962;24:291–358. doi: 10.1002/9780470124888.ch6. [DOI] [PubMed] [Google Scholar]
- Goldberg N. D., Passonneau J. V., Lowry O. H. Effects of changes in brain metabolism on the levels of citric acid cycle intermediates. J Biol Chem. 1966 Sep 10;241(17):3997–4003. [PubMed] [Google Scholar]
- Goldstein L. Relation of glutamate to ammonia production in the rat kidney. Am J Physiol. 1966 Mar;210(3):661–666. doi: 10.1152/ajplegacy.1966.210.3.661. [DOI] [PubMed] [Google Scholar]
- Goodman A. D., Fuisz R. E., Cahill G. F., Jr Renal gluconeogenesis in acidosis, alkalosis, and potassium deficiency: its possible role in regulation of renal ammonia production. J Clin Invest. 1966 Apr;45(4):612–619. doi: 10.1172/JCI105375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorno W. E., Rector F. C., Jr, Seldin D. W. Relation of renal gluconeogenesis to ammonia production in the dog and rat. Am J Physiol. 1967 Oct;213(4):969–974. doi: 10.1152/ajplegacy.1967.213.4.969. [DOI] [PubMed] [Google Scholar]
- Graham L. T., Jr, Aprison M. H. Fluorometric determination of aspartate, glutamate, and gamma-aminobutyrate in nerve tissue using enzymic methods. Anal Biochem. 1966 Jun;15(3):487–497. doi: 10.1016/0003-2697(66)90110-2. [DOI] [PubMed] [Google Scholar]
- HERRIN R. C., LARDINOIS C. C. Renal clearance of citric acid in the dog. Proc Soc Exp Biol Med. 1958 Feb;97(2):294–297. doi: 10.3181/00379727-97-23720. [DOI] [PubMed] [Google Scholar]
- Krebs H. A. Metabolism of amino-acids: The synthesis of glutamine from glutamic acid and ammonia, and the enzymic hydrolysis of glutamine in animal tissues. Biochem J. 1935 Aug;29(8):1951–1969. doi: 10.1042/bj0291951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEONARD E., ORLOFF J. Regulation of ammonia excretion in the rat. Am J Physiol. 1955 Jul;182(1):131–138. doi: 10.1152/ajplegacy.1955.182.1.131. [DOI] [PubMed] [Google Scholar]
- OWEN E. E., ROBINSON R. R. Amino acid extraction and ammonia metabolism by the human kidney during the prolonged administration of ammonium chloride. J Clin Invest. 1963 Feb;42:263–276. doi: 10.1172/JCI104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTS R. F., DEHAAS J., KLEIN J. Relation of renal amino and amide nitrogen extraction to ammonia production. Am J Physiol. 1963 Feb;204:187–191. doi: 10.1152/ajplegacy.1963.204.2.187. [DOI] [PubMed] [Google Scholar]
- PITTS R. F., PILKINGTON L. A., DEHAAS J. C. N15 TRACER STUDIES ON THE ORIGIN OF URINARY AMMONIA IN THE ACIDOTIC DOG, WITH NOTES ON THE ENZYMATIC SYNTHESIS OF LABELED CLUTAMIC ACID AND GLUTAMINES. J Clin Invest. 1965 May;44:731–745. doi: 10.1172/JCI105186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLAK A., HAYNIE G. D., HAYS R. M., SCHWARTZ W. B. Effects of chronic hypercapnia on electrolyte and acid-base equilibrium. I. Adaptation. J Clin Invest. 1961 Jul;40:1223–1237. doi: 10.1172/JCI104353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts R. F. The renal metabolism of ammonia. Physiologist. 1966 May;9(2):97–109. [PubMed] [Google Scholar]
- RECTOR F. C., Jr, ORLOFF J. The effect of the administration of sodium bicarbonate and ammonium chloride on the excretion and production of ammonia; the absence of alterations in the activity of renal ammonia-producing enzymes in the dog. J Clin Invest. 1959 Feb;38(2):366–372. doi: 10.1172/JCI103810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECTOR F. C., Jr, SELDIN D. W., COPENHAVER J. H. The mechanism of ammonia excretion during ammonium chloride acidosis. J Clin Invest. 1955 Jan;34(1):20–26. doi: 10.1172/JCI103058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHTERICH R. W., GOLDSTEIN L. Distribution of glutamine metabolizing enzymes and production of urinary ammonia in the mammalian kidney. Am J Physiol. 1958 Nov;195(2):316–320. doi: 10.1152/ajplegacy.1958.195.2.316. [DOI] [PubMed] [Google Scholar]
- SIMPSON D. P. INFLUENCE OF PLASMA BICARBONATE CONCENTRATION AND PH ON CITRATE EXCRETION. Am J Physiol. 1964 Apr;206:875–882. doi: 10.1152/ajplegacy.1964.206.4.875. [DOI] [PubMed] [Google Scholar]
- SIMPSON D. P. TISSUE CITRATE LEVELS AND CITRATE UTILIZATION AFTER SODIUM BICARBONATE ADMINISTRATION. Proc Soc Exp Biol Med. 1963 Nov;114:263–265. doi: 10.3181/00379727-114-28647. [DOI] [PubMed] [Google Scholar]
- STERN J. R., OCHOA S., LYNEN F. Enzymatic synthesis of citric acid. V. Reaction of acetyl coenzyme A. J Biol Chem. 1952 Sep;198(1):313–321. [PubMed] [Google Scholar]
- Sartorius O. W., Roemmelt J. C., Pitts R. F., Calhoon D., Miner P. THE RENAL REGULATION OF ACID-BASE BALANCE IN MAN. IV. THE NATURE OF THE RENAL COMPENSATIONS IN AMMONIUM CHLORIDE ACIDOSIS. J Clin Invest. 1949 May;28(3):423–439. doi: 10.1172/JCI102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. P. Regulation of renal citrate metabolism by bicarbonate ion and pH: observations in tissue slices and mitochondria. J Clin Invest. 1967 Feb;46(2):225–238. doi: 10.1172/JCI105525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. L., Goodman A. D., Treble D. H. Effect of metabolic acidosis on renal gluconeogenesis in vivo. Am J Physiol. 1968 Jul;215(1):211–217. doi: 10.1152/ajplegacy.1968.215.1.211. [DOI] [PubMed] [Google Scholar]
- Stone W. J., Balagura S., Pitts R. F. Diffusion equilibrium for ammonia in the kidney of the acidotic dog. J Clin Invest. 1967 Oct;46(10):1603–1608. doi: 10.1172/JCI105652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone W. J., Pitts R. F. Pathways of ammonia metabolism in the intact functioning kidney of the dog. J Clin Invest. 1967 Jul;46(7):1141–1150. doi: 10.1172/JCI105607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD F. J. Ammonium chloride acidosis. Clin Sci. 1955 Feb;14(1):81–89. [PubMed] [Google Scholar]


