Abstract
Intraguild predation (IGP) occurs when one predator species attacks another predator species with which it competes for a shared prey species. Despite the apparent omnipresence of intraguild interactions in natural and managed ecosystems, very few studies have quantified rates of IGP in various taxa under field conditions. We used molecular analyses of gut contents to assess the nature and incidence of IGP among four species of coccinellid predators in soybean fields. Over half of the 368 predator individuals collected in soybean contained the DNA of other coccinellid species indicating that IGP was very common at our field site. Furthermore, 13.2% of the sampled individuals contained two and even three other coccinellid species in their gut. The interaction was reciprocal, as each of the four coccinellid species has the capacity to feed on the others. To our knowledge, this study represents the most convincing field evidence of a high prevalence of IGP among predatory arthropods. The finding has important implications for conservation biology and biological control.
Introduction
Contemporary ecologists struggle with complexity. Communities involve thousands of species interacting in many diverse ways within the spatial and temporal variability of natural ecosystems [1]. In the late 1980's it became apparent that models based on functional trophic levels were not sufficiently universal to understand the dynamics and structure of communities [2]. The necessity of integrating non-trophic and indirect relationships has prompted theoretical and empirical work aimed at examining the role of omnivores. One form of omnivory is intraguild predation (IGP), where one predator species attacks another predator species with which it also competes for a shared prey species [3].
Following the pioneering field study of Polis and McCormick [4] on species of desert scorpions that feed on each other, a fertile and rapidly growing literature on IGP has led to a reconsideration of several classical topics in ecology such as stability and diversity of communities, trophic cascades in food webs, niche shift and species exclusion, as well as the effects of ecosystem productivity on species interactions [3], [5]–[8]. IGP also rapidly became relevant to aspects of applied ecology such as biological control, management of endangered species and the establishment of exotic invasive predators [9]–[11]. IGP is now considered to be ubiquitous in aquatic and terrestrial ecosystems, occurring in a great diversity of taxa from bacteria to mammals [3]. According to an analysis conducted by Arim and Marquet [12] using 113 food webs, 58–87% of animal species are involved in IGP interactions.
Despite this apparent ubiquity of intraguild interactions in both natural and managed ecosystems, and despite the importance of these interactions in structuring communities, very few studies have quantified rates of IGP in various taxa under field conditions. This is especially true for predatory arthropods, most likely because of the perceived difficulty of performing field observations of predation events [13]. Intraguild interactions among arthropod species have traditionally been studied in Petri dishes [14], or in field cage experiments [15]–[18]. Although important for identifying potential functional trophic and guild links among species, these approaches are inadequate for predicting the full complexity of both direct and indirect interactions [13], [19]. Consequently, results from experiments conducted in experimental arenas that have a limited number of interacting species and are conducted for short periods of time have led to skepticism about the actual occurrence and significance of IGP in nature [20].
Some studies have examined IGP in more natural settings using different methodological techniques and are important in complementing the less natural enclosure-based experiments. First, a number of semi-quantitative food-web studies documenting the existence (presence/absence) of trophic linkages between omnivores have shown that predators also include predatory species in their diet [4]. Second, purely observational field studies have quantified predator-predator interactions [21]. Third, experimental studies have been conducted in which the full, natural community of predators and prey were retained, and there was little if any constraint imposed on predator foraging [22]. Finally, a range of biochemical and molecular techniques have been developed to analyze gut contents and assess the diet of predatory arthropods under field conditions [23].
In this study we assess the nature and incidence of IGP among four species of coccinellid predators (Coleoptera: Coccinellidae) in soybean fields under natural conditions. This system has several favourable attributes for the study of IGP. Coccinellids are generalist predators, voracious both during their larval and adult stages. In soybean fields of Québec, Canada, they can be abundant and naturally play a role in aphid control [24]. They show an aggregative response to prey density [25]–[27], thereby increasing encounter rates with conspecific or heterospecific coccinellids. Furthermore, a number of laboratory or exclusion cage experiments have shown that IGP is potentially a common interaction among coccinellids [14], [28] and have identified major ecological determinants of IGP such as relative size of the protagonists, mobility and aggressiveness, feeding specificity and aphid density [14], [29].
A second advantage for using coccinellids as a model system is that we have developed molecular gut-content analyses to assess levels of IGP [30]. This approach uncovers predation events without interfering with the behavior of predators and prey and without disrupting ecosystem processes [31], [32]. Gut-contents analysis using the polymerase chain reaction (PCR) has recently been applied to the study of IGP between predator species [33] and between predators and parasitoids [17], [34].
Methods
Ethics statements
No specific permits were required for the described field studies and it did not involve endangered or protected species. Permission to sample invertebrates in the fields was obtained by each grower.
The study system
We studied the community of coccinellids associated with the soybean aphid, Aphis glycines Matsumura (Homoptera: Aphididae), a recent invasive pest in North America [35]. The four dominant species in soybean fields in the province of Québec are: Coccinella septempunctata Linnaeus, Propylea quatuordecimpunctata Linnaeus, Harmonia axyridis (Pallas) and Coleomegilla maculata lengi Timberlake, the only native species in this system [36]. These four coccinellid species are sympatric and present throughout the season, with H. axyridis arriving later than the others. Their abundance in soybean is mostly correlated with aphid densities, as commonly observed in agroecosystems [37].
Our primary objective was to estimate IGP levels within coccinellid assemblages in soybean fields. For the purposes of this paper, we define the IGP level as the proportion of a sample of a given predator species that contains measurable amounts of DNA of at least one different predator species in their guts. We do not attempt to examine the multitude of ecological factors that can promote the occurrence of IGP (predator and prey densities, predator:prey ratio, predator stage structure, etc) across fields or sampling dates; these analyses will be presented elsewhere. However, to place the present study in context we provide general information about aphid and coccinellid populations. Aphis glycines populations were relatively high with seasonal means of 266 and 371 aphids per plant in 2004 and 2005, respectively (A.E. Gagnon, unpublished data). The coccinellid community in 2004 was dominated by H. axyridis and C. septempunctata (representing 48 % and 41 %, respectively, of all four species) with a small proportion of C. maculata (5 %) and P. quatuordecimpunctata (6 %). In 2005, the proportions of each species were as followed: H. axyridis (59 %), C. septempunctata (18 %), C. maculata (14 %) and P. quatuordecimpunctata (9 %).
Coccinellids were sampled in soybean fields in 2004 and 2005 with sweep netting, put in an electric icebox at 4°C, and brought to the laboratory. Specimens were frozen (−20°C) and then washed in 70% ethanol to prevent possible contamination stemming from the time that predators had been held together in the collecting bag [38], [39]. In experiments done by Greenstone et al. [40], vigorous beating of plants followed by aspiration of insects into a common dry beaker led to incorrect assignment of gut contents – presumably due to regurgitant or feces from non-prey species contaminating the integument of predators. Contamination in our case is expected to be much lower because insects were immediately chilled rather than aspirated into a common beaker [38], [39]. Also, contamination in the Greenstone et al. study was likely particularly high because the prey species they used (larvae of the Colorado potato beetle, Leptotinarsa decemlineata) is known to regurgitate readily and in large amounts, and is often covered with secretions and feces that may be particularly prone to generate contamination [40]. Finally, a substantial fraction of the control animals in the Greenstone et al. experiment showed contamination, which brings into question the validity of the study (as the authors themselves noted). Samples were preserved in vials with 70% ethanol at 4°C until DNA extraction. Coccinellids were sampled in four different fields, located within the municipalities of Maskinongé (46°12′39″, -73°02′02″), Hérouxville (46°39′59″, -72°37′27″), Nicolet-Sud (46°12′04″, -72°36′47″) and Saint-Augustin-de-Desmaures (46°44′19″, -71°28′43″) in the province of Québec. A total of 188 and 180 coccinellid individuals were sampled in 2004 and 2005, respectively (Figure 1 provides details per species). Insects were sampled from mid-July to mid-September. We only used fourth larval instars in our analyses because they are more likely to be engaged in IGP than are other stages [28].
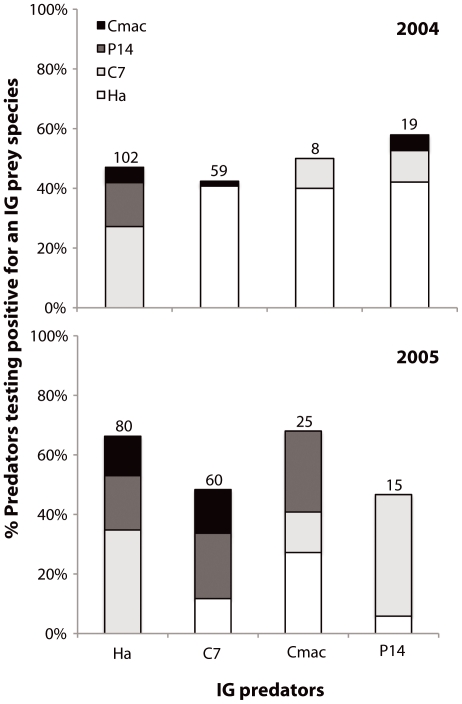
Figure 1. Levels of intraguild predation among four species of coccinellids measured by molecular gut content analysis in soybean fields in Québec, Canada, in 2004 and 2005.
Results are expressed as the proportion of each species of intraguild prey detected in the gut of intraguild predators. Ha = Harmonia axyridis, C7 = Coccinella septempunctata, Cmac = Coleomegilla maculata, P14 = Propylea quatuordecimpunctata. Numbers above histogram bars represent the number of individuals tested.
DNA extraction and PCR cycles
DNA extraction and PCR protocols were modified from Hoogendoorn and Heimpel [41]. DNA was extracted from whole coccinellid larvae. Each insect was ground in a 1.5 ml microcentrifuge tube using sterile plastic pestles (Ultident Scientific inc.) with 100 µl of grinding buffer [42]. PCR amplifications were done separately for each primer pair (H. axyridis; C. septempunctata; C. maculata; P. quatuordecimpunctata). Details of development and cross-reactivity tests of PCR markers are presented in Gagnon et al. [30]. All predators were screened against the primers of all three potential intraguild prey and against a universal primer (12Sai and 12Sbi, [43]). The screening against the universal primer pairs was done to ensure that DNA could be successfully detected in all specimens. Amplifications were performed in total volumes of 25 µl, composed of 20.25 µl of 1× buffer (0.25 mM of each dNTP and 1.5 mM of MgCl2), 2.5 µl of primer mix (20 µM), 0.25 µl of Taq (i.e. 1.75 units) (Promega), and 2 µl DNA sample. The thermocycling program consisted of an initial step of 30s at 94°C (for H. axyridis, we used a hot start, i.e. addition of the Taq after the first step), followed by 30 s at 94°C, 30 s at 52°C (H. axyridis = 55°C), and 30 s at 72°C. The three last steps were repeated 30 times and were followed by a step of 5 min at 72°C. All PCR products (10 µL) were electrophoresed at 120V in 2% agarose gels for approximately 1 h and then stained in ethidium bromide solution for 20 min and then visualized using a UV light-transilluminator. DNA is detectable at very low concentrations (from 35.5 ng × 10−4 to 35.5 ng × 10−6 depending on species primers) under optimal conditions (without heterospecific DNA).
Prey DNA detection success over time (DS50, the time after which 50% of the predators of a cohort that fed at the same time test positive for the presence of a species of prey using the PCR assay) ranged between 5.2 h and 19.3 h among combinations of interacting coccinellid species [30]. For this reason, corrected data using DS50 values for each predator-prey combination need to be used when comparing intensity of IGP between different coccinellid species. Such a correction confers more importance to a “rapidly digesting” species combination where probability of detecting an intraguild prey is lower than for a “slowly digesting:” species combination [44]–[46]. DS50 values for each predator-prey combination were weighted to obtain the DS50 weighted as follows: the shortest DS50 was assigned a value of 1.0 and other weighted DS50 values were obtained by placing this benchmark DS50 in the numerator and each other DS50 value in the denominator. The corrected predation value is calculated by multiplying the proportion of field-collected predators found to contain prey remains by their specific DS50 weighted. We did not attempt to estimate amount eaten per predator because no strong relationship had been found between the number of prey eaten and the duration of DNA in gut-contents of coccinellids [29], [41].
Results
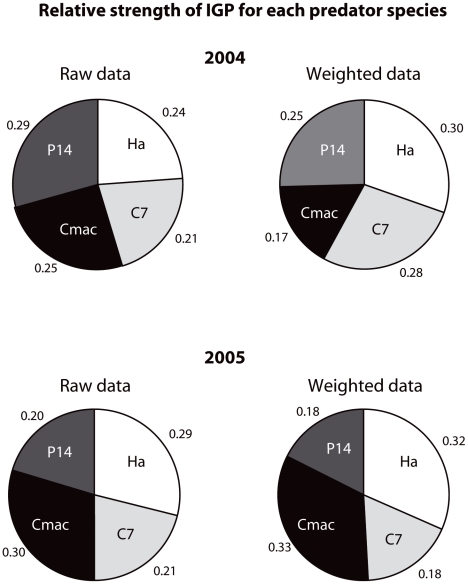
Three novel results emerge from our study. First, levels of IGP were extremely high with averages of 46.8% and 58.9% (non-weighted data) of all coccinellids containing DNA of other coccinellids in their gut in 2004 and 2005, respectively (Table 1). The intensity of IGP for each coccinellid-coccinellid interaction, expressed as the proportion of each species of IG prey detected in the gut of IG predators is shown in Figure 1. Using the weighted DS50 values changed the ranking of predators in terms of IGP strength quite drastically in 2004 but only slightly in 2005. In 2004, the ranking using raw data was as follows: P. quatuordecimpunctata > C. maculata > H. axyridis > C. septempunctata (Figure 2). Using weighted DS50 values revealed the following ranking: H. axyridis > C. septempunctata > P. quatuordecimpunctata > C. maculata (Figure 2). Thus, using raw data leads to an underestimation of the relative importance of IGP by H. axyridis and C. septempunctata. In 2005, relative IGP rates were more similar among species. The ranking using raw was: C. maculata > H. axyridis > C. septempunctata > P. quatuordecimpunctata (Figure 2), which was almost unaltered when weighted DS50 values were used, except that the relative strengths of IGP for C. septempunctata and P. quatuordecimpunctata were the same (Figure 2).
Table 1. Number (N) of specimens tested and levels of intraguild predation (raw data) among four coccinellid species with molecular gut-content detection of one to three different intraguild prey species in a same predator, in 2004 and 2005.
| N | One intraguild prey species | Two intraguild prey species | Three intraguild prey species | Total IGP | |||||
| n | % | n | % | n | % | n | % | ||
| 2004 | 188 | 72 | 38.30 | 14 | 7.45 | 2 | 1.06 | 88 | 46.81 |
| 2005 | 180 | 74 | 41.11 | 29 | 16.11 | 3 | 1.67 | 106 | 58.89 |
Figure 2. Relative strength of intraguild predation by each of the four coccinellid species measured by molecular gut content analysis in soybean fields in Québec, Canada, in 2004 and 2005.
Results are shown for raw and weighted data*. Ha = Harmonia axyridis, C7 = Coccinella septempunctata, Cmac = Coleomegilla maculata, P14 = Propylea quatuordecimpunctata.
Second, the results indicate that IGP is reciprocal with each of the four coccinellid species feeding on each of the other three species (Figure 1). However, although levels of IGP were high in both years, the relative proportion of intraguild prey species varied between years. In 2004, H. axyridis was strongly represented as an intraguild prey species, whereas in 2005 P. quatuordecimpunctata and C. septempunctata were the dominant intraguild prey species.
Third, we report multiple prey detection (Table 1). When results from both years are combined, 11.8% of the intraguild predators contained the DNA of two other coccinellid species in their gut, and we detected three intraguild prey species simultaneously in the guts of 1.4% of the sampled coccinellids. Consumption of two intraguild prey species was most common in H. axyridis (48.1% of all cases) and C. maculata (35.7%), whereas only H. axyridis was feeding on three intraguild prey species.
Discussion
Our results indicate that IGP is very common among coccinellid species in soybean fields. Levels of IGP were high, with 52.9% of all sampled individuals containing the DNA of one, two and even three other coccinellid species in their gut. The interaction is reciprocal, as each of the four coccinellid species has the capacity to feed on the other three species. To our knowledge, this study represents the most convincing field evidence of the prevalence of IGP among predatory arthropods.
Our demonstration reflects the reality of the field situation. We used a sampling technique that entails no perturbation to the ecosystem or to the members of the community. Coccinellids were sampled in situ, without altering their behavior or distribution, thereby reducing potential artifacts that invariably arise through experimental manipulations conducted under laboratory conditions or within field cages. Molecular analyses allow the detection of minute amounts of prey material by PCR after DNA extraction. Molecular gut-contents analyses led to a demonstration of complex predation events between co-existing species and open the opportunity to better understand the dynamics and structure of communities. However, molecular gut-content analyses have their limits as well [23]. First, it is very difficult or impossible to determine the number of prey items a given predator has consumed, even using quantitative PCR [37], [47]. This is because the size of prey items and the degree of digestion per prey item can vary so widely. For this reason, the ecological significance of intraguild predation can be difficult to determine because we cannot compare the amount of intraguild prey eaten in relation to the extraguild prey. However, using DS50 correction allowed a comparison of intraguild predation rates between predator species that have different digestion times [30], [45], [46]. Second, scavenging or secondary predation (in which a predator eats another predator species containing the prey of interest in its gut) cannot be discriminated from true predation using PCR [48], [49]. And lastly, PCR detection of cannibalism is not achievable because conspecific DNA cannot be discriminated from predator DNA. Thus, we still lack a basic understanding of the relative importance of IGP and cannibalism, a common phenomenon in Coccinellidae [50], [51] for population dynamics. Monoclonal antibody-based ELISA could be useful in detecting cannibalism because it can be used to distinguish different life stages [52], [53].
While IGP models of predator-predator interactions, as well as the effects of omnivory on extraguild prey suppression have recently received considerable attention from both empiricists and theoreticians [2], [6], [7], [19], [54]-[57] very few studies have explicitly measured levels of IGP in arthropods under field conditions. To our knowledge only three other field studies using molecular techniques have directly quantified levels of IGP in arthropods. In the soybean agroecosystem, Harwood et al. [58] examined predation between H. axyridis and the predatory bug Orius insidiosus (Say) (Hemiptera: Anthocoridae) using molecular gut-content analysis. Less than 2.5% of O. insidiosus tested positive for the detection of H. axyridis. Chacon et al. [17] detected aphid parasitoid DNA in two predator species using PCR in a study examining IGP of released parasitoids of the soybean aphid. In this study, percentages of predators testing positive for parasitoid DNA ranged from 8 to 17. Hautier et al. [59] reported that 9 out of 28 H. axyridis collected in potato fields had fed on heterospecific species of coccinellids, based on alkaloid quantification by gas-chromatograph-mass spectroscopy (GC-MS). Although this latter technique is promising, identification of prey species is only possible at the genus level and this method has also been estimated to be more expensive than other analyses of gut contents [60]. More information about IGP levels measured under natural conditions is available for larger predators from different taxa (see Table 2 for selected examples), probably because predation events can be more easily detected through different sampling techniques. The first published study quantifying the incidence of IGP in nature was conducted by Polis and McCormick [4] who observed relatively high proportions of intraguild prey in the diet of desert scorpions, from 8 to 21.9%, and up to 45% for the species Paruroctonus mesaensis. Feeding information was easily collected on scorpions through observation because they digest their prey externally. Nevertheless, available data, both for arthropods and other taxa containing predators, are still too sparse to suggest patterns about the relative strength of IGP.
Table 2. Selected examples of intraguild predation under field conditions among different taxa.
| IG predator | IG prey | Extraguild prey | % IGP | Method of detection | Region | Authors |
| White-tailed sea eagle (Haliaeetus albicilla L.) | Mink (Mustela vison Schreb.) | Fish and birds | <7% (for all mammal species) | Behavioral observation | Finland | [62], [63] |
| Cougar, wolf | Coyote | Small mammals | 43–67% | Radio-tracked animals | Alaska, Idaho | [64] |
| Lion, spotted hyena | African wild dog | 13–50% | South Africa, Tanzania | |||
| Red fox | American marten | 4% | Ontario | |||
| Scorpion Paruroctonus mesaensis | P. luteolus H. arizonensis V. confuses | Insects | 8–22% (in some months higher than 40%) | External digestion (direct observation) | [4] | |
| Eagle owl | Tawny owl | Mammals, birds, fish, invertebrates | 0.6% | Pellets and prey remains found under nests and roost sites | Italy | [65] |
| Dingo | Feral catRed fox | NA | 1.2–6.1% | Dissection of gut-content | Australia | [66] |
| Many intertidal herbivores | Many intertidal herbivores | NA | 0.37–10% | Dissection of intestinal content | Chile | [67] |
Several factors may contribute to the very high levels of IGP we quantified in coccinellids. First, coccinellids respond numerically to high aphid densities [24]-[27] a condition that may favour encounters between predators; although high prey abundance may also lead to predator satiation and thereby a reduction in intraguild interactions. Second, by eating a heterospecific, intraguild predators eliminate a competitor and thereby improve access to the aphid resource. Third, aphids are a relatively low quality prey resource [61], and coccinellids may benefit by complementing their diet by feeding on other coccinellids. A recent study also showed high levels of predation on coccinellid eggs in soybean fields in Michigan, USA [18]. However we still have a poor understanding of ecological factors that influence the strength and direction of intraguild interactions, and there is a need for more empirical studies that examine the effect of factors such as seasonality, vegetation-structured complexity, habitat productivity, extraguild prey density, as well as the behaviors and life histories of protagonists.
Over the last 20 years, several models and experimental studies have examined the nature and role of intraguild interactions in both terrestrial and aquatic communities. Intraguild predation is now considered to be ubiquitous in most species assemblages [12]. However, previous studies conducted in natural or managed ecosystems have largely overlooked the prevalence of IGP among top predators. Our results on coccinellids emphasize the importance of quantifying IGP in the field. This basic information is central for understanding the role of top predators in population dynamics and community structure, and from a more applied perspective, to predict their impact in programs devoted to the biological control of pest species or the management of native endangered or invasive exotic species.
Acknowledgments
We are very grateful to Émilie Lemaire, Véronique Janelle and Julie Mainguy for assistance in the field and laboratory. Thanks to Jay A. Rosenheim and Edward W. Evans who provided valuable comments on an earlier version of the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was funded by the Fonds Québécois de Recherche sur la Nature et les Technologies and the Canada Research Chair program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Paine RT. A note on trophic complexity and community stability. Am Nat. 1969;103:91–93. [Google Scholar]
- 2.Rosenheim JA. Higher-order predators and the regulation of insect herbivore populations. Annu Rev Entomol. 1998;43:421–447. doi: 10.1146/annurev.ento.43.1.421. [DOI] [PubMed] [Google Scholar]
- 3.Polis GA, Myers CA, Holt RD. The ecology and evolution of intraguild predation - potential competitors that eat each other. Annu Rev Ecol Syst. 1989;20:297–330. [Google Scholar]
- 4.Polis GA, McCormick SJ. Intraguild predation and competition among desert scorpions. Ecology. 1987;68:332–343. [Google Scholar]
- 5.Borer ET, Briggs CJ, Murdoch WW, Swarbrick SL. Testing intraguild predation theory in a field system: does numerical dominance shift along a gradient of productivity? Ecol Lett. 2003;6:929–935. [Google Scholar]
- 6.Holt RD, Polis GA. A theoretical framework for intraguild predation. Am Nat. 1997;149:745–764. [Google Scholar]
- 7.Holt RD, Huxel GR. Alternative prey and the dynamics of intraguild predation: Theoretical perspectives. Ecology. 2007;88:2706–2712. doi: 10.1890/06-1525.1. [DOI] [PubMed] [Google Scholar]
- 8.Briggs CJ, Borer ET. Why short-term experiments may not allow long-term predictions about intraguild predation. Ecol Appl. 2005;15:1111–1117. [Google Scholar]
- 9.Rosenheim JA, Kaya HK, Ehler LE, Marois JJ, Jaffee BA. Intraguild predation among biological control agents - theory and evidence. Biol Control. 1995;5:303–335. [Google Scholar]
- 10.Muller CB, Brodeur J. Intraguild predation in biological control and conservation biology. Biol Control. 2002;25:216–223. [Google Scholar]
- 11.Snyder WE, Clevenger GM, Eigenbrode SD. Intraguild predation and successful invasion by introduced ladybird beetles. Oecologia. 2004;140:559–565. doi: 10.1007/s00442-004-1612-5. [DOI] [PubMed] [Google Scholar]
- 12.Arim M, Marquet PA. Intraguild predation: a widespread interaction related to species biology. Ecol Lett. 2004;7:557–564. [Google Scholar]
- 13.Messing R, Roitberg BD, Brodeur J. Measuring and predicting indirect impacts of biological control: competition, displacement, and secondary interactions. In: Bigler F, Babendreier D, Kuhlmann U, editors. Environmental impact of invertebrates for biological control of arthropods: methods and risk assessment. Wallinford, UK: CABI int; 2006. pp. 64–77. [Google Scholar]
- 14.Lucas E, Coderre D, Brodeur J. Intraguild predation among aphid predators: Characterization and influence of extraguild prey density. Ecology. 1998;79:1084–1092. [Google Scholar]
- 15.Rosenheim JA, Wilhoit LR, Armer CA. Influence of intraguild predation among generalist insect predators on the suppression of an herbivore population. Oecologia. 1993;96:439–449. doi: 10.1007/BF00317517. [DOI] [PubMed] [Google Scholar]
- 16.Hoogendoorn M, Heimpel GE. Competitive interactions between an exotic and a native ladybeetle: a field cage study. Entomol Exp Appl. 2004;111:19–28. [Google Scholar]
- 17.Chacon JM, Landis DA, Heimpel GE. Potential for biotic interference of a classical biological control agent of the soybean aphid. Biol Control. 2008;46:216–225. [Google Scholar]
- 18.Gardiner MM, O'Neal ME, Landis DA. Intraguild predation and native lady beetle decline. PLoS ONE. 2011;6(9):e23576. doi: 10.1371/journal.pone.0023576. doi: 10.1371/journal.pone.0023576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vance-Chalcraft HD, Rosenheim JA, Vonesh JR, Osenberg CW, Sih A. The influence of intraguild predation on prey suppression and prey release: A meta-analysis. Ecology. 2007;88:2689–2696. doi: 10.1890/06-1869.1. [DOI] [PubMed] [Google Scholar]
- 20.Kindlmann P, Houdkova K. Intraguild predation: fiction or reality? Popul Ecol. 2006;48:317–322. [Google Scholar]
- 21.Rosenheim JA, Limburg DD, Colfer RG. Impact of generalist predators on a biological control agent, Chrysoperla carnea: Direct observations. Ecol Appl. 1999;9:409–417. [Google Scholar]
- 22.Rosenheim JA, Glik TE, Goeriz RE, Ramert B. Linking a predator's foraging behavior with its effects on herbivore population suppression. Ecology. 2004;85:3362–3372. [Google Scholar]
- 23.Sheppard SK, Harwood JD. Advances in molecular ecology: tracking trophic links through predator-prey food-webs. Funct Ecol. 2005;19:751–762. [Google Scholar]
- 24.Rhainds M, Roy M, Daigle G, Brodeur J. Toward management guidelines for the soybean aphid in Québec. I. Feeding damage in relationship to seasonality of infestation and incidence of native predators. Can Entomol. 2007;139:728–741. [Google Scholar]
- 25.Evans EW, Youssef NN. Numerical responses of aphid predators to varying prey density among Utah alfalfa fields. J Kansas Entomol Soc. 1992;65:30–38. [Google Scholar]
- 26.Donaldson JR, Myers SW, Gratton C. Density-dependent responses of soybean aphid (Aphis glycines Matsumura) populations to generalist predators in mid to late season soybean fields. Biol Control. 2007;43:111–118. [Google Scholar]
- 27.Chacon JM, Heimpel GE. Density-dependent intraguild predation of an aphid parasitoid. Oecologia. 2010;164:213–220. doi: 10.1007/s00442-010-1611-7. [DOI] [PubMed] [Google Scholar]
- 28.Hironori Y, Katsuhiro S. Cannibalism and interspecific predation in two predatory ladybirds in relation to prey abundance in the field. Entomophaga. 1997;42:153–163. [Google Scholar]
- 29.Lucas E, Brodeur J. A fox in sheep's clothing: Furtive predators benefit from the communal defense of their prey. Ecology. 2001;82:3246–3250. [Google Scholar]
- 30.Gagnon A-È, Doyon J, Heimpel GE, Brodeur J. Mol Ecol Resour: In press; 2011. Prey DNA detection success following digestion by intraguild predators: influence of prey and predator species. [DOI] [PubMed] [Google Scholar]
- 31.Symondson WOC. Molecular identification of prey in predator diets. Mol Ecol. 2002;11:627–641. doi: 10.1046/j.1365-294x.2002.01471.x. [DOI] [PubMed] [Google Scholar]
- 32.Harwood JD, Obrycki JJ. Quantifying aphid predation rates of generalist predators in the field. Eur J Entomol. 2005;102:335–350. [Google Scholar]
- 33.Harwood JD, Desneux N, Yoo HJS, Rowley DL, Greenstone MH, et al. Tracking the role of alternative prey in soybean aphid predation by Orius insidiosus: a molecular approach. Mol Ecol. 2007;16:4390–4400. doi: 10.1111/j.1365-294X.2007.03482.x. [DOI] [PubMed] [Google Scholar]
- 34.Traugott M, Symondson WOC. Molecular analysis of predation on parasitized hosts. B Entomol Res. 2008;98:223–231. doi: 10.1017/S0007485308005968. [DOI] [PubMed] [Google Scholar]
- 35.Ragsdale DW, Landis DA, Brodeur J, Heimpel GE, Desneux N. Ecology and management of the soybean aphid in North America. Annu Rev Entomol. 2011;56:375–399. doi: 10.1146/annurev-ento-120709-144755. [DOI] [PubMed] [Google Scholar]
- 36.Mignault MP, Roy M, Brodeur J. Soybean aphid predators in Québec and the suitability of Aphis glycines as prey for three Coccinellidae. BioControl. 2006;51:89–106. [Google Scholar]
- 37.Ives AR, Kareiva P, Perry R. Response of a predator to variation in prey density at 3 hierarchical scales - Lady beetles feeding on aphids. Ecology. 1993;74:1929–1938. [Google Scholar]
- 38.Harwood JD. Are sweep net sampling and pitfall trapping compatible with molecular analysis of predation? Environ Entomol. 2008;37:990–995. doi: 10.1603/0046-225x(2008)37[990:asnsap]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 39.Chapman EG, Romero SA, Harwood JD. Maximizing collection and minimizing risk: does vacuum suction sampling increase the likelihood for misinterpretation of food web connections? Mol Ecol Resour. 2010;10:1023–1033. doi: 10.1111/j.1755-0998.2010.02857.x. [DOI] [PubMed] [Google Scholar]
- 40.Greenstone MH, Weber DC, Coudron TC, Payton ME. Unnecessary roughness? Testing the hypothesis that predators destined for molecular gut-content analysis must be hand-collected to avoid cross-contamination. Mol Ecol Resour. 2011;11:286–293. doi: 10.1111/j.1755-0998.2010.02922.x. [DOI] [PubMed] [Google Scholar]
- 41.Hoogendoorn M, Heimpel GE. PCR-based gut content analysis of insect predators: using ribosomal ITS-1 fragments from prey to estimate predation frequency. Mol Ecol. 2001;10:2059–2067. doi: 10.1046/j.1365-294x.2001.01316.x. [DOI] [PubMed] [Google Scholar]
- 42.Bender W, Spierer P, Hogness DS. Chromosomal walking and jumping to isolate DNA from Ace and Rosy loci and the bithorax complex in Drosophila melanogaster. J Mol Biol. 1983;168:17–33. doi: 10.1016/s0022-2836(83)80320-9. [DOI] [PubMed] [Google Scholar]
- 43.Noda H, Munderloh UG, Kurtti TJ. Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. App Environ Microbiol. 1997;63:3926–3932. doi: 10.1128/aem.63.10.3926-3932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y, Giles KL, Payton ME, Greenstone MH. Identifying key cereal aphid predators by molecular gut analysis. Mol Ecol. 2000;9:1887–1898. doi: 10.1046/j.1365-294x.2000.01100.x. [DOI] [PubMed] [Google Scholar]
- 45.Greenstone MH, Szendrei Z, Payton ME, Rowley DL, Coudron TC, et al. Choosing natural enemies for conservation biological control: use of the prey detectability half-life to rank key predators of Colorado potato beetle. Entomol Entomol Exp Appl. 2010;136:97–107. [Google Scholar]
- 46.Szendrei Z, Greenstone MH, Payton ME, Weber DC. Molecular gut-content analysis of a predator assemblage reveals the effect of habitat manipulation on biological control in the field. Basic Appl Ecol. 2010;11:153–161. [Google Scholar]
- 47.Lundgren JG, Ellsbury ME, Prischmann DA. Analysis of the predator community of a subterranean herbivorous insect based on polymerase chain reaction. Ecol Appl. 2009;19:2157–2166. doi: 10.1890/08-1882.1. [DOI] [PubMed] [Google Scholar]
- 48.Foltan P, Sheppard S, Konvicka M, Symondson WOC. The significance of facultative scavenging in generalist predator nutrition: detecting decayed prey in the guts of predators using PCR. Mol Ecol. 2005;14:4147–4158. doi: 10.1111/j.1365-294X.2005.02732.x. [DOI] [PubMed] [Google Scholar]
- 49.Sheppard SK, Bell J, Sunderland KD, Fenlon J, Skervin D, et al. Detection of secondary predation by PCR analyses of the gut contents of invertebrate generalist predators. Mol Ecol. 2005;14:4461–4468. doi: 10.1111/j.1365-294X.2005.02742.x. [DOI] [PubMed] [Google Scholar]
- 50.Majerus M. London: Harper Collins; 1994. Ladybirds. [Google Scholar]
- 51.Takizawa T, Snyder WE. Cannibalism and Intraguild Predation of Eggs Within a Diverse Predator Assemblage. Environ Entomol. 2011;40:8–14. doi: 10.1603/EN10047. [DOI] [PubMed] [Google Scholar]
- 52.Sigsgaard L, Greenstone MH, Duffield SJ. Egg cannibalism in Helicoverpa armigera on sorghum and pigeonpea. BioControl. 2002;47:151–165. [Google Scholar]
- 53.Fournier V, Hagler J, Daane K, de Leon J, Groves R. Identifying the predator complex of Homalodisca vitripennis (Hemiptera : Cicadellidae): a comparative study of the efficacy of an ELISA and PCR gut content assay. Oecologia. 2008;157:629–640. doi: 10.1007/s00442-008-1095-x. [DOI] [PubMed] [Google Scholar]
- 54.van der Hammen T, de Roos AM, Sabelis MW, Janssen A. Order of invasion affects the spatial distribution of a reciprocal intraguild predator. Oecologia. 2010;163:79–89. doi: 10.1007/s00442-010-1575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hall RJ. Intraguild predation in the presence of a shared natural enemy. Ecology. 2011;92:352–361. doi: 10.1890/09-2314.1. [DOI] [PubMed] [Google Scholar]
- 56.Law YH, Rosenheim JA. Effects of combining an intraguild predator with a cannibalistic intermediate predator on a species-level trophic cascade. Ecology. 2011;92:333–341. doi: 10.1890/10-0156.1. [DOI] [PubMed] [Google Scholar]
- 57.Rosenheim JA, Harmon J. The influence of intraguild predation on the suppression of a shared prey population: an empirical reassessment. In: Brodeur J, Boivin G, editors. Trophic and guild interactions in biological control. New York, USA: Springer; 2006. pp. 1–20. [Google Scholar]
- 58.Harwood JD, Yoo HJS, Greenstone MH, Rowley DL, O'Neil RJ. Differential impact of adults and nymphs of a generalist predator on an exotic invasive pest demonstrated by molecular gut-content analysis. Biol Invasions. 2009;11:895–903. [Google Scholar]
- 59.Hautier L, Gregoire JC, de Schauwers J, Martin GS, Callier P, et al. Intraguild predation by Harmonia axyridis on coccinellids revealed by exogenous alkaloid sequestration. Chemoecology. 2008;18:191–196. [Google Scholar]
- 60.Aebi A, Brown P, De Clercq P, Hautier L, Howe A, et al. Detecting arthropod intraguild predation in the field. BioControl. 2011;56:429–440. [Google Scholar]
- 61.Snyder WE, Joseph SB, Preziosi RF, Moore AJ. Nutritional benefits of cannibalism for the lady beetle Harmonia axyridis (Coleoptera : Coccinellidae) when prey quality is poor. Environ Entomol. 2000;29:1173–1179. [Google Scholar]
- 62.Sulkava S, Tornberg R, Koivusaari J. Diet of the white-tailed eagle Haliaeetus albicilla in Finland. Ornis Fennica. 1997;74:65–78. [Google Scholar]
- 63.Salo P, Nordstrom M, Thomson RL, Korpimaki E. Risk induced by a native top predator reduces alien mink movements. J Anim Ecol. 2008;77:1092–1098. doi: 10.1111/j.1365-2656.2008.01430.x. [DOI] [PubMed] [Google Scholar]
- 64.Palomares F, Caro TM. Interspecific killing among mammalian carnivores. Am Nat. 1999;153:492–508. doi: 10.1086/303189. [DOI] [PubMed] [Google Scholar]
- 65.Sergio F, Marchesi L, Pedrini P, Penteriani V. Coexistence of a generalist owl with its intraguild predator: distance-sensitive or habitat-mediated avoidance? Anim Behav. 2007;74:1607–1616. [Google Scholar]
- 66.Glen AS, Dickman CR. Complex interactions among mammalian carnivores in Australia, and their implications for wildlife management. Biol Rev. 2005;80:387–401. doi: 10.1017/s1464793105006718. [DOI] [PubMed] [Google Scholar]
- 67.Camus PA, Daroch K, Opazo LF. Potential for omnivory and apparent intraguild predation in rocky intertidal herbivore assemblages from northern Chile. Mar Ecol-Prog Ser. 2008;361:35–45. [Google Scholar]