Abstract
Background
Preservation of original organic components in fossils across geological time is controversial, but the potential such molecules have for elucidating evolutionary processes and phylogenetic relationships is invaluable. Chitin is one such molecule. Ancient chitin has been recovered from both terrestrial and marine arthropods, but prior to this study had not been recovered from fossil marine mollusks.
Methodology/Principal Findings
Organics consistent with β-chitin are recovered in cuttlebones of Mississaepia mississippiensis from the Late Eocene (34.36 million years ago) marine clays of Hinds County, Mississippi, USA. These organics were determined and characterized through comparisons with extant taxa using Scanning Electron Microscopy/Energy Dispersive Spectrometry (SEM/EDS), Field Emission Scanning Electron Microscopy (Hyperprobe), Fourier Transmission Infrared Spectroscopy (FTIR) and Immunohistochemistry (IHC).
Conclusions/Significance
Our study presents the first evidence for organics consistent with chitin from an ancient marine mollusk and discusses how these organics have been degraded over time. As mechanisms for their preservation, we propose that the inorganic/organic lamination of the cuttlebone, combined with a suboxic depositional environment with available free Fe2+ ions, inhibited microbial or enzymatic degradation.
Introduction
Chitin is a complex amino-polysaccharide produced by many living organisms, including arthropods, mollusks, nematodes, algae and fungi. It is, after cellulose, the second most abundant biopolymer found in nature [1]. Chitin is a linear polymer consisting mainly of β-(1→4)-linked 2-acetamido-2-deoxy-β-d-glucopyranose units and partially of β-(1→4)-linked 2-amino-2-deoxy-β-d-glucopyranose [2]. In nature, chitin occurs in three polymorphs, either α or β-allomorphs or in γ-form [2], distinguished by differences in the types of chemical linkage employed or by the orientation of the constituent micro-fibrils relative to these linkages [1].
Based primarily on morphological comparisons with extant forms and not on chemical analyses of fossils, chitin is hypothesized to have been a major structural component of invertebrates since the Cambrian, but it may have originated sometime in the Proterozoic [3]. Detection of chitin in fossils is not frequent. There are reports of fossil chitin in pogonophora, and in insect wings from amber [4]. Chitin has also been reported from beetles preserved in an Oligocene lacustrine deposit of Enspel, Germany [5] and chitin-protein signatures have been found in cuticles of Pennsylvanian scorpions and Silurian eurypterids [6].
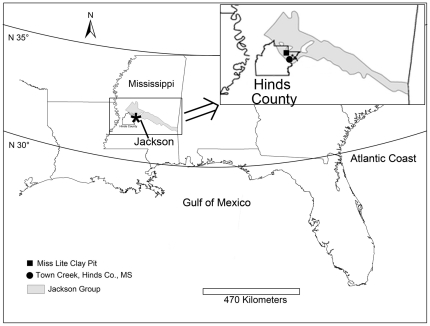
Initial examination of cuttlebones of Mississaepia mississippiensis [7] from the Late Eocene (34.36mya) [8] from the Yazoo Clay, Jackson Group, Hinds County, Mississippi (Figure 1) by light microscopy revealed yellowish-brown sheet-like structures that appeared to have been originally organic. EDS examination of these structures revealed phosphorus. These observations led to questions 1) could any original organics be preserved in these cuttlebones? 2) What mechanisms might have preserved original endogenous organics? The present paper reports evidence of organics consistent with β-chitin from the Late Eocene cephalopod M. mississippiensis and presents evidence of ultrastructural and environmental factors which might have contributed to their preservation.
Figure 1. Generalized locality map.
Generalized locality map showing sites where M. mississipiensis were collected and the geographical extent of the Jackson Group in Mississippi.
Materials and Methods
Nine cuttlebones of M. mississippiensis from collections at The Mississippi Geological Survey were examined. For comparison, five cuttlebones of extant cuttlefish (Sepia sp. NCSM 11399–11403; collections at the North Carolina Museum of Natural Sciences) were examined. Eight fossil specimens (MGS 1943, 1944, 1946–1949, 1956, 1963) and one extant specimen were analyzed using SEM/EDS (Text S1). Specimen MGS 1951 (Figure 2A,B) and extant Sepia specimen NCSM 11399 were cut laterally and longitudinally for ground sections. The remaining portion of fossil specimen MGS 1951 and extant specimens NCSM 11402 and 11403 were de-mineralized for Hyperprobe, FTIR and IHC analyses (Text S1). For comparison, three specimens of extant Loligo sp. pens were de-mineralized for FTIR analysis. Complete methods are given in the supporting data (Text S1).
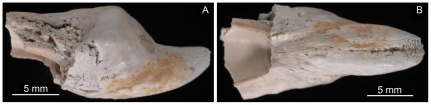
Figure 2. Fragmentary preserved cuttlebone of M. mississippiensis (MGS 1951).
Fragmentary preserved cuttlebone of M. mississippiensis (MGS 1951), late Eocene, Mississippi, USA: A, B – left lateral and ventral view, respectively.
Results
Scanning Electron Microscopy/Energy Dispersive Spectrometry (SEM/EDS)
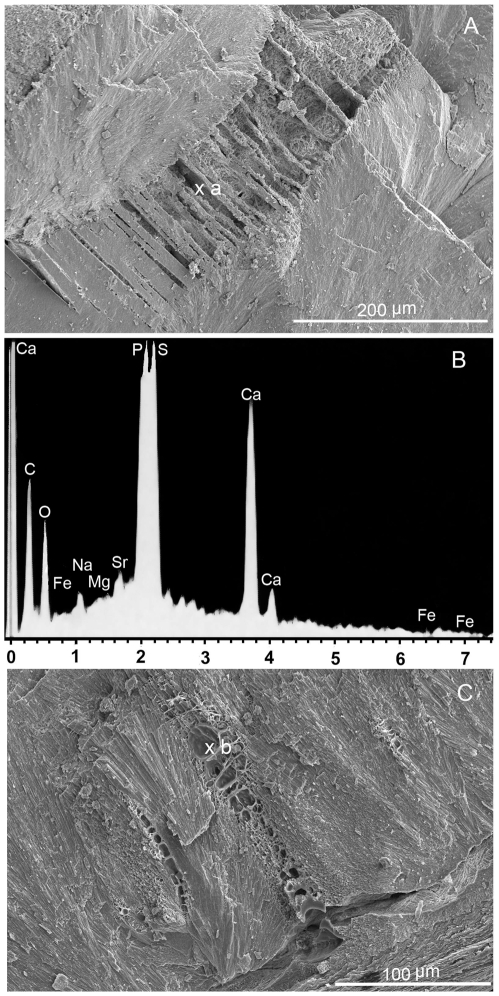
Analysis of M. mississippiensis under SEM (Text S1) revealed ultrastructures (Figure 3Aa), between carbonate spherulites, morphologically similar to the chitin membranes found in extant Sepia (Figure 3Cb). Elemental analyses show these “sheet-like” structures in M. mississippiensis contain high amounts of phosphorous (Figure 3B). These sheet-like structures may have been phosphatized during fossilization as is typical of chitin structures in cephalopods, such as gladii and mandibles [9], [10]. Alternatively these originally organic structures could have served as a template for phosphatization [11] and some of the original organic material may have been preserved.
Figure 3. SEM/EDS images comparing similar structures of M. mississippiensis and Sepia.
Sheet-like structures in between carbonate spherulites in M. mississippiensis (MGS1956, Fig. 2Aa) and Sepia sp. (Fig. 2Cb). 2B, EDS analysis of M. mississipiensis (spot a) showing phosophorus in the sheet-like structures.
Hyperprobe
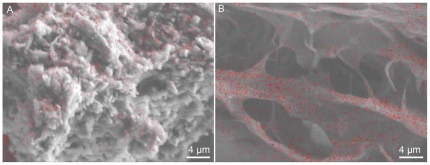
To test for presence of original organics, cuttlebones of M. mississipiensis and Sepia sp. were demineralized in HCL (Text S1) then mapped for nitrogen. Nitrogen, a reliable indicator of organic material [12], was detected in de-mineralized powdered samples of Mississaepia mississippiensis and extant Sepia sp. using a JEOL X-ray Analyzer JXA-85-30F Hyperprobe (Text S1). Though not a specific indicator of chitin, nitrogen signals obtained from powders indicate original organics have been preserved in the fossil. Nitrogen was detected only on places where there was sample and thus is not a byproduct of nitrogen within the epoxy of the carbon tape (Figure 4A,B). Because these samples are three dimensional no quantitative analyses are possible. Overall morphology of de-mineralized material from M. mississippiensis and extant Sepia sp. was different (Figure 4A,B).
Figure 4. Hyperprobe comparison of HCL de-mineralized M. mississippiensis and extant Sepia sp.
A) Hyperprobe analysis of HCL de-mineralized fossil (MGS 1951) mapped for nitrogen (red). B) Hyperprobe analysis of HCL de-mineralized extant cuttlebone mapped for nitrogen (red).
Fourier Transform Infrared (FTIR)
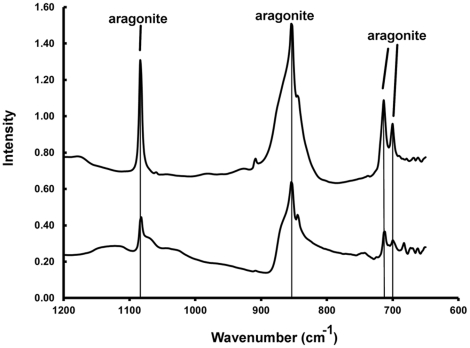
Cuttlebones of M. mississippiensis and Sepia sp. were analyzed using FTIR (Text S1) to determine the mineral phase of the carbonates. The cuttlebone of M. mississippiensis shows two peaks of the carbonate ν4 vibration at 700 and 713 cm−1 (Figure 5) which are diagnostic of aragonite [13]. The peaks at 1083 and 854 cm−1 are also characteristic of aragonite [14]. Similar peaks were detected in cuttlebone of extant Sepia (Figure 5). In contrast calcite would show a peak at 876 cm−1 and no peak at 1083 cm−1 [14]. Preservation of aragonite, a meta-stable form of calcium carbonate, rather than the more stable calcite form, indicate the cuttlebones have undergone minimal diagenesis and thus had a greater potential to preserve original organics.
Figure 5. FTIR spectra of cuttlebones of Sepia sp. and M. mississippiensis.
Spectra of Sepia sp. (top) and M. mississippiensis (bottom). Spectra indicate aragonite. By comparison, calcite has peaks at 848 and 876 cm−1and a singlet at 713 cm−1 [14].
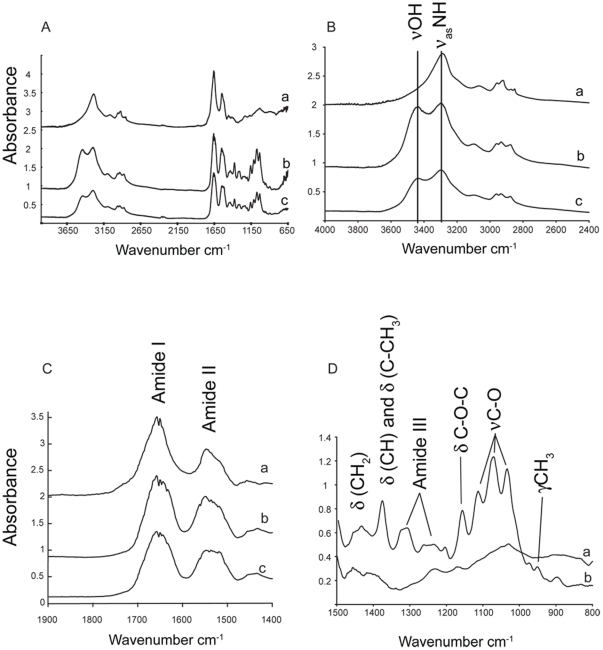
FTIR (Text S1) was also used to examine in detail the organic components preserved in M. mississippiensis. After demineralization of extant Loligo sp. pens (squid pens are commonly used as the standard for β-chitin), Sepia sp. and fossil M. mississippiensis, spectra (Figure 6A) were obtained and band assignments (Table 1) were made according to published works [13], [15]. Spectra of organics obtained for fossil and extant specimens were similar (Table 1, Figure 6A), though some peaks were not as well resolved in the fossil. The OH stretching absorbance (Figure 6B) was examined using spectral decomposition analysis with Peakfit®. The OH stretching mode in the fossil is greatly reduced as compared to the extant cuttlebone, and shifted to slightly lower wavenumbers (Table 2). The N-H asymmetric stretching vibration is also shifted to slightly lower wavenumbers.
Figure 6. FTIR spectra of HCL de-mineralized M. mississippiensis, Sepia sp. and Loligo sp.
FTIR spectra of fossil (a) M. mississippiensis and chitin from extant (b) Sepia sp. and (c) Loligo sp. A) All spectra are consistent with beta chitin. B) FTIR spectra of the hydrogen stretching region of a, b and c showing reduction in OH of M. mississippiensis. C) FTIR spectra of a, b and c emphasizing Amide I and Amide II absorbances which are distinctive for beta chitin. D) FTIR spectra of a and b; portions of the Amide III region are missing in the fossil. Overall broadening and loss of structure of the peaks is interpreted as breakdown of chitin chains into shorter fragments.
Table 1. Band assignments (cm−1) for fossil and extant materials.
| Band assignment [13], [15] | Squid [15] | Loligo sp. (NCSM, Sea of Japan) | Sepia officinalis [13] | Sepia sp. (NCSM 11403 & 11402) | M. mississipiensis MGS 1951 |
| νOH | 3479 (?) & 3426 | 3431 | 3421 | 3431 | – |
| νasNH | 3290 | 3292 | 3280 | 3395 | 3281 |
| νsNH | 3102 | 3085 | 3104 | 3091 | 3065 |
| νasCH3 | 2962 | 2961 | 2966 | 2959 | 2956 |
| νsCH2 | 2929 | 2933 | 2933 | 2931 | 2918 |
| νasCH3 | 2880 | 2876 | 2884 | 2873 | 2849 |
| νC = O,Amide I | 1656 | 1656 | 1652 | 1657 & 1649 | 1656 & 1650 |
| νC-N,C-N-H, Amide II +δN-H Amide II | 1556 | 1533 | 1553 | 1541 | 1545 |
| δCH2 | 1424 | 1433 | 1422 | 1437 | 1456, 1417 |
| δCH and δC-CH3 | 1376 | 1375 | 1377 | 1376 | 1397 |
| νC-N and δNH,Amide III | 1314 | 1324 & 1304 | 1317 | 1306 | – |
| δNH, Amide III | 1262 | 1260 | 1262,1229, 1205 | 1260,1236, 1204 | 1231 |
| νas C-O-C ring | 1155 | 1157 | 1155 | 1159 | 1171 |
| νC-O | 1111 | 1113 | 1112 | 1115 | – |
| νC-O | 1069 | 1071 | 1073 | 1074 | – |
| νC-O | 1032 | 1033 | 1033 | 1032 | 1030 |
| γCH3 | 948 | 949 | 948 | 947 | – |
| γCH (C1axial) (β bond) | 902 | 895 | – | 895 | 905 |
| ρCH2 | – | – | – | 705 | 703 |
| γNH (Amide V) | 692 | 690 | – | 695 | 691 |
Table 2. Peak positions (cm−1) from spectral decomposition using Peakfit®.
| Band Assignment | Loligo sp. | Sepia sp. | M . mississippiensis |
| νasNH | 3296 | 3285 | 3290 |
| νOH | 3444 | 3446 | 3405 |
Peaks at 1649 cm−1 (Amide I, Figure 6C) and 1544 cm−1(Amide II, Figure 6C) in cuttlefish, squid and fossil spectra indicate organics consistent with β-chitin. Closer examination of the region ca. 600–1400 cm−1(Figure 6D) shows the principle change in M. mississippiensis is the loss of peaks in Amide III region. Amide I and II peaks may arise from chitin, but also from proteins, humics or other chemicals. It is parsimonious to assume the presence of chitin-like molecules rather than an admixture of substances displaying a similar spectrum.
Immunohistochemistry (IHC)
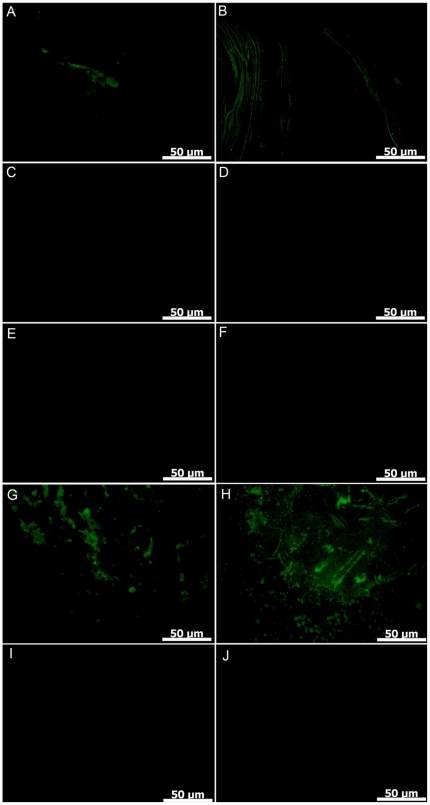
IHC has been used successfully to detect and characterize ancient biomolecules such as collagen in birds [16] and dinosaurs [17]. Because chitin antibodies and IHC have been used to successfully detect chitin in extant organisms [18], [19], IHC (Text S1) was used here to further elucidate the specific nature of biomolecules found in M. mississippiensis. Analysis of extant Sepia sp. and fossil M. mississippiensis showed specific antibody binding to chitin with comparable avidity and specificity (Figure 7A,B) indicating some endogenous chitin is retained in the fossil, though its morphology is slightly different from chitin in extant Sepia sp. (Figure 7A,B). Fluorescent signal from antibody-antigen interaction was greater than that seen in negative controls (secondary antibody only; Figure 7C,D). When sections were subjected to digestion by chitinase, immunological signal was either reduced where tissues were completely digested (Figure 6E,F), or enhanced where tissues were not completely digested (Figure 7G,H). This signal enhancement seems contradictory, but enzymes are often used to help unmask antigens [20]–[22] resulting in an enhanced immunological signal. Degradation by chitinase is indicated by the lack of order (as seen in Figure 7A,B). The pattern of enhanced signal is common to both fossil and extant specimens, indicating commonality in chemical make-up. An irrelevant antibody (human testosterone) was applied at identical concentrations and under identical conditions to some sections to verify specificity of the antibody-antigen interaction. Results showed no antigen/antibody binding (Figure 7I,J). IHC analysis showed fluorescence consistent with and specific to chitin in both fossil and extant samples which is consistent with the interpretation that original chitin was preserved in these specimens.
Figure 7. IHC comparison of HCL de-mineralized M. mississippiensis and Sepia sp.
A,C,E,G,I, fossil M. mississipiensis (MGS 1951); B,D,F,H,J, extant Sepia sp. A, B) Fossil/extant incubated in chitin antibody and secondary antibody, show antibody/antigen binding. C,D) Fossil/extant incubated in secondary antibody only, no antibody/antigen binding. E,F) Fossil/extant incubated in chitinase, chitin anti-body and secondary antibody. Tissues have been digested by the chitinase giving a much reduced immunological signal. G,H) Fossil/extant incubated in chitinase, chitin antibody and secondary antibody. Tissues in these samples were not completely digested by the chitinase, eptiopes were unmasked, and show enhanced antibody/antigen retrieval. I,J) Fossil/extant incubated in human testosterone antibody and secondary antibody, shows no antibody/antigen binding.
Discussion
The initial analysis of fossil M. mississippiensis by multiple methods yielded results that, taken alone, were insufficient to support the presence of a specific organic material. High percentage of phosphorus detected in EDS analysis shows possible preservation of organic material between aragonitic spherulites. Hyperprobe reveals nitrogen clearly unassociated with carbonate minerals, though the overall morphology of de-mineralized M. mississippiensis looks different from extant Sepia sp. FTIR analysis shows presence of amides, and spectra that resembled extant beta chitin. Finally, IHC reveals presence of molecules with specific reactivity to chitin anti-bodies indicating preserved organic material in M. mississippiensis is still recognizable as chitin.
Results of IHC support our interpretation of FTIR data as representative of chitin. Because spectra obtained in FTIR indicate chitin, the lack of a doublet in the Amide I region identifies the fossil material as beta chitin, which is also found in extant mollusks. Differences between spectra of fossil and extant chitin can be used to further illuminate changes in the β- chitin structure of the fossil.
β-chitin is characterized by parallel chains of chitin molecules held together with inter-chain hydrogen bonding [2]. The OH stretching absorbance (Figure 5B), at about 3445 cm−1 in extant chitin, is diminished in the fossil and shifted to lower wavenumbers (Table 2), showing that the specimen is losing OH by an as yet undetermined mechanism. The N-H asymmetric stretching vibration is shifted to slightly lower wavenumbers, showing that it is no longer hydrogen bonding exactly as in extant specimens. Changes in the region 2800–3600 cm−1 indicate that biomolecules have been degraded via disruption of interchain hydrogen bonds.
Examination of the region 800–1500 cm−1 shows that the spectrum of fossil material roughly follows the spectrum of the extant material (Figure 5D). This region has been referred to as the “crystallinity region” for oligosaccharides [23]. Loss of well-defined peaks in the fossil material is ascribed here as a loss of long-range ordering in strands of chitin due to shortening of the chitin strands. Similar changes in FTIR spectra have been observed in the breakdown of chitosan [24] and the organic components of nacre [25].
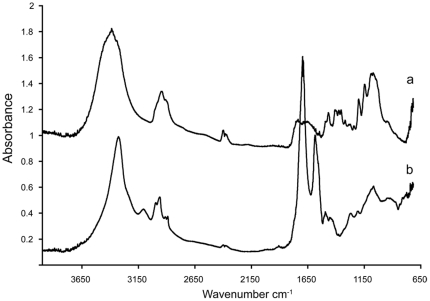
Shortening of strands and loss of hydrogen bonds could explain morphological differences between de-mineralized M. mississipiensis and extant Sepia sp. seen in Hyperprobe (Figure 3A,B) and IHC (Figure 6A,B). In addition, not all pieces of de-mineralized fossil showed amide or lipid peaks (Figure 8). Differences observed in fossil chitin, as compared to extant, could be a result of mechanical destruction, preservational biases or partial chemical degradation within microenvironments of the specimen.
Figure 8. Comparison of two FTIR spectra of M. mississippiensis (MGS 1951).
a) spectrum lacks Amide I and Amide II peaks, b) for comparison, spectrum shows the Amide I peak at 1656 cm−1 and Amide II peak at 1545 cm−1.
There is a remote possibility that the organics detected in the fossil are a result of contamination. This is highly unlikely. Chitin is not a common lab contaminant. Fossil specimens used in this study were surface collected, washed in water and stored in climate controlled metal cabinets until they were sent to us in individual sealed plastic bags. None of the samples used for Hyperprobe, FTIR or IHC were preserved using epoxies or other glues. To reduce possible contaminants from extant samples chemical preparations were done in separate labs and FTIR was conducted in a mineralogy lab that has never housed invertebrate specimens. Still, there is a slight possibility that the chitin detected in our samples could a result of extant fungus. This also is unlikely, as we saw no evidence of fungi in SEM. FTIR results showed β-chitin in our samples not γ-chitin found in fungi [2]. Results of IHC were specific for chitin (Figure 7), thus it is unlikely that the amide peaks seen in FTIR (Figure 6, Table 1) are result of contaminating proteins from human or other sources.
Mechanisms for preservation
In vivo inorganic-organic structure of the cuttlebone [13], in combination with physical and geochemical conditions within the depositional environment [26], [27] and favorable taphonomic factors likely contributed to preservation of organics in M. mississippiensis. Available clays within the Yazoo Clay in conjunction with suboxic depositional environment [28]–[30] may have facilitated preservation of original organics by forming a physical and geochemical barrier to degradation [31]–[33]. One key to the preservation of organic tissues, in particular chitin and chitosan, is cessation of bacterial degradation within environments of deposition. Bacterial breakdown of polymeric molecules is accomplished through activities of both free extracellular enzymes (those in the water column) and ektoenzymes (those on the surface of the microbial cell) such as chitinases [31], [32]. Chitinases function either by cleaving glycosidic bonds that bind repeating N-acetyl-D-glucosamine units within chitin molecules or by cleaving terminal N-acetyl-D-glucosamine groups [33]–[35]. These enzymes adsorb to the surface of clay particles, which inactivates them [33]–[35]. Strong ions in solution like iron may act in the same manner [33]. Once bound to functional groups within these polymeric molecules, Fe2+ ions prevent specific bond configuration on the active-site cleft of specific bacterial chitinases and prevents hydrolysis [31], [33], thus contributing to preservation.
Organic layers within cuttlebones are protected by mineralized layers, similar to collagen in bones, and this mineral-organic interaction may also have played a role in their preservation [36]–[38]. Specimens of M. mississipiensis show preserved original aragonite as well as apparent original organics. These organics appear to be endogenous and not a function of exogenous fungal or microbial activity. Fungi contain the γ form of chitin not the β allomorph found in our samples. Also, SEM analyses shows there is no evidence of tunneling, microbes, or wide-spread recrystallization of the aragonite. Therefore the chitin-like molecules detected in fossil sample are most likely endogenous. Similar to collagen in bone, perhaps, organics could not be attacked by enzymes or other molecules until some inorganic matrix had been removed [39].
Conclusions
Analysis by Hyperprobe, FTIR and IHC of cuttlebones of the Eocene coleoid cephalopod M. mississipiensis show preservation of degraded original organics consistent with β-chitin. These are oldest known organics consistent with β-chitin (34.36 mya) [8] and are the first from a fossil marine mollusk. Preservation of organics consistent with β-chitin was possible due to the inorganic/organic construction of cuttlebone and available free Fe2+ ions in suboxic depositional environment, which inhibited microbial or enzymatic degradation. Future work will focus on comparisons with other Eocene cuttlefish and the phylogenetic implications of chitinous structures with regards to the origin cuttlefish.
Supporting Information
Supplementary text.
(DOC)
Acknowledgments
The authors are indebted to David Dockery III (Mississippi Geological Survey) for the loan of M. mississippiensis, James Bain (NC Fossil Club) for donation of Sepia sp., Mary Schweitzer, Timothy Cleland and Elizabeth Johnson (NC State University) for guidance with chemical analyses, Rainer Martin (University of Ulm) for his donation of chitin antibodies, Stefan Bengtson (Swedish Museum of Natural History) for discussion on EDS data, Steven Singletary (Fayetteville State University) for assistance with Hyperprobe and Janet Edgerton (NCSM) for bibliographic assistance.
Footnotes
Competing Interests: The authors have the following competing interest: JC is an employee of FAI Materials Testing Laboratory. There are no patents, products in development or marketed products to declare. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: FTIR analysis in this study was supported by NSF grant EAR-0929898 to RT. LD received travel and subsisitence support from the Swedish Academy of Sciences and PW received travel support from the NC Museum of Natural Sciences and the NC Fossil Club. FAI Materials Testing Lab Inc. supported the study through the employment of JC, who contributed to all aspects of the study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Majtán J, Bíliková, K, MarkovǏc, O, Gróf, J, Kogan, G, et al. Isolation and characterization of chitin from bumblebee (Bombus terrestris). International Journal of Biological Macromolecules. 2007;40:237–241. doi: 10.1016/j.ijbiomac.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Kumirska J, Czerwicka, M, Kaczyński, Z, Bychowska, A, Brzozowski, K, et al. Application of spectroscopic methods for structural analysis of chitin and chitosan. Marine Drugs. 2010;8:1567–1636. doi: 10.3390/md8051567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller RF. Chitin paleoecology. Biochemical Systematics and Ecology. 1991;19:401–411.3. [Google Scholar]
- 4.Muzzarelli RAA. Chitin paleoecology. In: Jollès P, Muzarelli, RAA, editors. Chitin and Chitinases. Basel, Switzerland: Birkhäuser Verlag; 1999. pp. 1–6. [Google Scholar]
- 5.Stankiewicz BA, Briggs DEG, Evershed RP, Flannery MB, Wuttke M. Preservation of chitin in 25-million-year-old fossils. Science. 1997;276:1541–1543. [Google Scholar]
- 6.Cody GD, Gupta, NS, Briggs, DEG, Kilcoyne, ALD, Summons, RE, et al. Geology; 2011. Molecular signature of chitin-protein complex in Paleozoic arthropods. doi: 10.1130/G31648.1. [Google Scholar]
- 7.Weaver PG, Dockery DT, Ciampaglio CN. A new genus of coleoid cephalopod from the Jackson Group (Late Eocene), Hinds County, Mississippi. Palaeontographica Abteilung A. 2010;292:53–63. [Google Scholar]
- 8.Fluegeman RH, Grigsby, JD, Hurley, JV Koeberl C, Montanari, A, editors. Eocene-Oligocene greenhouse to icehouse transition on a subtropical clastic shelf: The Jackson-Vicksburg Groups of the eastern Gulf Coastal Plain of the United States. The Late Eocene Earth-Hothouse, Icehouse, and Impacts: Geological Society of America Special Papers. 2009. pp. 261–277.
- 9.Doguzhaeva LA, Mutvei, H Ultrastructural and chemical comparison between gladii in living coleoids and Aprian coeloids from Central Russia. Acta Universitatis Carolinae Geologica. 2006;49:83–93. [Google Scholar]
- 10.Mapes RH. Upper Paleozoic cephalopod mandibles: frequency of occurrence, modes of preservation, and paleoecological implications. Journal of Paleontology. 1987;61:521–538. [Google Scholar]
- 11.Martill DM, Wilby, PR Lithified prokaryotes associated with fossil soft-tissues from the Santana Formation (Cretaceous) of Brazil. Kaupia. 1994;2:71–77. [Google Scholar]
- 12.Oehler DZ, Robert, F, Mostefaoui, S, Meibom, A, Selo, M, et al. Seckbach J, Walsh, M, editors. Nanoisms opens a new window for deciphering organic matter in terrestrial and extraterrestrial samples. From Fossils to Astrobiology: Records of Life on Earth and Search for Extraterrestrial Biosignatures: Springer Science + Business Media B.V. 2009. pp. 7–23.
- 13.Florek M, Fornal, E, Gómez-Romero, P, Zieba, E, Paszkowicz, W, et al. Complementary microstructural and chemical analyses of Sepia officinalis endoskeleton. Materials Science and Engineering C. 2009;C29:1220–1226. [Google Scholar]
- 14.Adler HH, Kerr, PF Infrared study of aragonite and calcite. American Mineralogist. 1962;47:700–717. [Google Scholar]
- 15.Cardenas G, Cabrera, G, Taboada, E, Miranda, SP Chitin characterization by SEM, FTIR, XRD, and 13C Cross Polarization/Mass Angle Spinning NMR. Journal of Applied Polymer Science. 2004;93:1876–1885. [Google Scholar]
- 16.Avci R, Schweitzer, MH, Boyd, RD, Witmeyer, JL, Arce, FT, et al. Preservation of bone collagen from the late Cretaceous Period studied by immunological techniques and atomic force microscopy. Langmuir. 2005;21:3584–3590. doi: 10.1021/la047682e. [DOI] [PubMed] [Google Scholar]
- 17.Schweitzer MH, Zheng, W, Organ, CL, Avci, R, Suo, Z, et al. Biomolecular characterization and protein sequences of the Campanian hadrosaur B. canadensis. Science. 2009;324:626–631. doi: 10.1126/science.1165069. [DOI] [PubMed] [Google Scholar]
- 18.Martin R, Hild, S, Walther, P, Ploss, K, Boland, W, et al. Granular chitin in the epidermis of nudibranch molluscs. Biological Bulletin. 2007;213:307–315. doi: 10.2307/25066648. [DOI] [PubMed] [Google Scholar]
- 19.Nudelman F, Gotliv, BA, Addadi, L, Weiner, S Mollusk shell formation: Mapping the distribution of organic matrix components underlying a single aragonitic tablet in nacre. Journal of Structural Biology. 2007;153(2):176–187. doi: 10.1016/j.jsb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Werner M, von Wasielewski, R, Komminoth, P Antigen retrieval, signal amplification and intensification in immunohistochemistry. Histochemistry and Cell Biology. 1996;105:253–260. doi: 10.1007/BF01463928. [DOI] [PubMed] [Google Scholar]
- 21.McNichol AM, Richmond, JA Optimizing immunohistochemistry: antigen retrieval and signal amplification. Histopathology. 1998;32:97–103. doi: 10.1046/j.1365-2559.1998.00342.x. [DOI] [PubMed] [Google Scholar]
- 22.Ramos-Vara JA. Technical aspects of Immunohistochemistry. Vet Pathology. 2005;42:405–426. doi: 10.1354/vp.42-4-405. [DOI] [PubMed] [Google Scholar]
- 23.Tul'chinsky V, Zurabyan, SE, Asankozhoev, KA, Koga, GA, Khorlin, AT Study of the infrared spectra of oligosaccharides in the region 1000-40 cm-1. Carbohydrate Research. 1976;51:1–8. doi: 10.1016/s0008-6215(00)84031-8. [DOI] [PubMed] [Google Scholar]
- 24.Ratajska M, Boryniec, S Physical and chemical aspects of biodegradation of natural polymers. Reactive & Functional Polymers. 1998;38:35–49. [Google Scholar]
- 25.Balmain J, Hannoyer, B, Lopez, E Fourier Transform Infrared Spectroscopy (FTIR) and x-ray diffraction anaylses of mineral and organic matrix during heating of mother of pearl (nacre) from the shell of the mollusc Pinctada maxima. Journal of Biomedical Materials Research. 1999;48:749–754. doi: 10.1002/(sici)1097-4636(1999)48:5<749::aid-jbm22>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 26.Tew BH. Sequence stratigraphy, lithofacies relationships and paleogeography of Oligocene strata in southeastern Mississippi and southwestern Alabama. Alabama Geological Survey Bulletin. 1992;146:1–73. [Google Scholar]
- 27.Tew BH, Mancini, EA An integrated stratigraphic model for paleogeographic reconstruction: Examples from the Jackson and Vicksburg Groups of the eastern Gulf Coastal Plain. Palaios. 1995;10:133–153. [Google Scholar]
- 28.Moore WH, Bicker, Jr, AR, McCutchen, TE, Parks, WS . Mississippi Geological, Economic and Topographical Survey, Bulletin 105; 1965. Hinds County geology and mineral resources.244 [Google Scholar]
- 29.May JH, Baughman, WT . Mississippi Geological Survey Bulletin 117; 1974. Wayne County geology and mineral resources.199 [Google Scholar]
- 30.Gilliland WA, Harrelson, DW . Mississippi Department of Natural Resources Bureau of Geology Bulletin 121; 1980. Clarke County geology and mineral resources.199 [Google Scholar]
- 31.Muzzarelli RAA, Tubertini, O Chitin and chitosan as chromatographic supports and adsorbents for collection of metal ions from organic and aqueoussolutions and sea-water. Talanta. 1969;16:1571–1577. doi: 10.1016/0039-9140(69)80218-3. [DOI] [PubMed] [Google Scholar]
- 32.Butterfield NJ. Organic preservation of non-mineralizing organisms and the taphonomy of the Burgess Shale. Paleobiology. 1990;16:272–286. [Google Scholar]
- 33.Petrovich R. Mechanisms of fossilization of the soft-bodied and lightly amored faunas of the Burgess Shale and of some other classic localities. American Journal of Science. 2001;301:683–726. [Google Scholar]
- 34.Chróst RJ. Environmental control of the synthesis and activity of aquatic microbial ectoenzymes. In: Chróst RJ, editor. Microbial Enzymes in Aquatic Environments. Berlin: Springer-Verlag; 1991. pp. 29–59. [Google Scholar]
- 35.Gooday GW. Physiology of microbial degradation of chitin and chitosan. Biodegradation. 1990;1:177–190. [Google Scholar]
- 36.Englington G, Logan, GA Molecular preservation. Philosophical Transactions of the Royal Society of London B. 1991;333:315–323. doi: 10.1098/rstb.1991.0081. [DOI] [PubMed] [Google Scholar]
- 37.Collins MJ, Nielsen-Marsh, CM, Hiller, J, Smith, CI, Roberts, JP, et al. The survival of organic matter in bone: a review. Archaeometry. 2002;44:383–394. [Google Scholar]
- 38.Schmidt-Schultz TH, Schultz, M Bone protects proteins over thousands of years: extraction, analysis, and interpretation of extracellular matrix proteins in archeological skeletal remains. American Journal of Physical Anthropology. 2004;123:30–39. doi: 10.1002/ajpa.10308. [DOI] [PubMed] [Google Scholar]
- 39.Hedges REM. Bone diagenesis: an overview of the processes. Archaeometry. 2002;44:319–328. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary text.
(DOC)