Abstract
Background
Ninety percent of HIV-1-infected children live in sub-Saharan Africa. In the absence of diagnosis and antiretroviral therapy (ART), approximately 50% die before 2 years.
Methods
We evaluated sensitivity and specificity of clinical algorithms for diagnosis of HIV-1 infection and ART initiation among HIV-1-exposed children aged less than 18 months. Children were identified with routine HIV-1 testing and assessed using 3 sets of criteria: 1) Integrated Management of Childhood Illnesses (IMCI), 2) World Health Organization Presumptive Diagnosis (WHO-PD) for HIV-1 infection, and 3) CD4 T-lymphocyte cell subsets. HIV-1 infection status was determined using DNA PCR testing.
Findings
A total of 1,418 children (median age 5.4 months) were screened for HIV-1 antibodies, of whom 144 (10.2%) were seropositive. Of these, 134 (93%) underwent HIV-1 DNA testing and 80 (60%) were found to be HIV-1-infected. Compared to HIV-1 DNA testing, sensitivity and specificity of the IMCI were 19% and 96% and for WHO-PD criteria 43% and 88%, respectively. Inclusion of severe immune deficiency determined by CD4 percent improved sensitivity of IMCI and WHO-PD to 74% and 84% respectively, however, specificity declined to 43% and 41%, respectively.
Interpretation
Diagnosis of HIV-1 infection among exposed children less than 18 months in a high prevalence, resource-limited setting remains a challenge and current recommended algorithms have low sensitivity. This underscores the need for rapid scale-up of viral assays for early infant diagnosis.
Keywords: HIV-1, infant diagnosis, clinical algorithms
Introduction
Of the 2.2 million children currently living with human immunodeficiency virus type 1 (HIV-1) globally, 90% reside in sub-Saharan Africa (UNAIDS 2004). In Kenya alone, over 150,000 children are estimated to be HIV-1 infected. HIV-1 in African children progresses rapidly and is characterized by high mortality. A pooled analysis on the mortality of infants born to HIV-1 infected mothers in Africa demonstrated that without antiretroviral therapy (ART), 30% of HIV-1-infected infants died by the age of 1 year and 50% died by their second birthday (1,2). Early diagnosis and treatment of infected children may decrease this high mortality.
Infants of HIV-1 seropositive women have passively acquired HIV-1 specific antibodies, and therefore diagnosis of HIV-1 infection in infants requires virologic assays that are currently unavailable to the majority of infants in areas of high HIV-1 seroprevalence. In Kenya at the time of the study, HIV-1 Virologic assays for diagnosis were available in only 4 labor oratories, and 2 were purely for research(3). The diagnostic gold standard laboratory test for HIV-1 infection is the HIV-1 qualitative DNA PCR, with sensitivities and specificities in the high 90th percentile in Africa ((4, 5). Screening technology using dried whole blood spots (DBS), has been successfully used for PCR-based detection of human immunodeficiency virus (6, 7, 8, 9, 10) In areas where virological testing is unavailable two algorithms are recommended by the World Health Organization (WHO) for diagnosis of HIV-1 infection and initiation of ART in infants. The Integrated Management of Childhood Illnesses (IMCI) criteria identifies children with suspected HIV-1 infection for HIV-1 testing and referral (11, 12). The adapted IMCI criteria for Kenya, suspicion of HIV in a child is based on a positive antibody test and 3 or more of the following; history of tuberculosis in parent, recccurent pneumonia, lymphadenopathy, oral thrush, diarrhea lasting 7 or more days, growth faltering, parotid enlargement and ear discharge. The WHO Presumptive Diagnosis (WHO-PD) criteria is designed to diagnose HIV-1 infection among symptomatic HIV-1-exposed children aged less than 18 months and uses a scoring system based on clinical factors (2 or more of the following; oral thrush, severe pneumonia, severe wasting or weight <5th centile) to identify those in most need of ART (Revised WHO Paediatric ART guidelines for resource limited settings 2005). The present guidelines are part of WHO’s commitment to achieving universal access for all HIV-1 infected individuals to ART by 2010. We conducted a study to determine sensitivity and specificity of the IMCI and WHO-PD clinical algorithms, with and without incorporation of CD4 T cell results, for diagnosis of HIV-1 infection and ART initiation in HIV-1 exposed Kenyan children aged less than 18 months.
Methods
Study design and subjects
This was a cross-sectional study carried out at Kenyatta National Hospital’s (KNH) general paediatric wards and outpatient clinics in Nairobi, Kenya. KNH is a national public referral hospital which also serves as the teaching facility for the University of Nairobi Medical School. At the initiation of this study, the hospital was in the implementation phase of routine offer of provider initiated testing and counseling (PITC) with HIV-1 immunoglobulin (IgG) antibody testing using ELISA assays to all children accessing services in the general paediatric wards and outpatient clinics as a strategy to increase ART access to children. While this would identify children who had been exposed to HIV-1, it would not definitively identify HIV-1-infected children under 18 months old. Virologic assays that would enable definitive HIV-1 diagnosis were not routinely available at KNH.
Caregivers of HIV-1 ELISA positive children aged 1 month to less than 18 months were invited to participate after post-test counseling. The research objectives, procedures, risks and benefits were explained to caregivers who were then asked to provide written informed consent for study participation. Children without an identifiable adult caregiver and those whose HIV-1 infection status was already known through prior testing were excluded from the study. A caregiver was defined as a parent or guardian who lives with the child and is responsible for the child’s upkeep and heath care.
Study procedures
Clinic procedures
Caregivers were interviewed using a standard structured questionnaire to obtain information about their socio-demographic characteristics, economic status, clinical history and HIV-1 status. In addition we obtained information on use of prevention of mother-to-child HIV-1 transmission (PMTCT) interventions, including use of antiretroviral drugs for the study infant. Clinical history was confirmed using the available medical records (outpatient card, discharge summary, patient file). A physical examination to establish the clinical and nutritional status of the child and to diagnose opportunistic infections was conducted by the Study Pediatrician according to the IMCI and the revised WHO-PD guidelines. In a separate consent process, caregivers were asked to consent to HIV-1 DNA PCR testing for their infants. Subsequently, caregivers underwent post-test counseling with disclosure of infant HIV-1 DNA PCR results and referred to Kenyattta National Hospital HIV care center where they all received cotrimoxazole prophylaxis and assessed further for initiation of antiretroviral drugs per the national guidelines.
Laboratory procedures
Infants whose caregivers consented to HIV-1 DNA PCR testing underwent phlebotomy and a blood specimen (3 ml) was drawn. Using a micropipette, approximately 80 μl of blood was applied to a pre-labeled filter paper. Filter paper HIV-1 DNA PCR testing (Roche Diagnostics, Branchburg, NJ, USA) for infants was done at the CDC laboratory at the Kenya Medical Research Institute, Kisumu. The remaining blood was taken to the Department of Paediatrics Laboratory at the University of Nairobi for assessment of the haemogram and T-lymphocyte cell subsets (CD4 count and CD4 percent). The CD4% was used to determine immunologic status as per the newly recommended WHO criteria 2005. Severe immune suppression was considered as CD4% < 25% for children < 12 months and CD4% < 20% for children 12–18 months.
Data analysis
All clinical and laboratory data were entered using the SPSS (Version 12) data entry program. A model was developed combining medical history and clinical findings constituting the IMCI and WHO-PD criteria for HIV infection. Different combinations of variables were entered into the model, which then was used to calculate how many children met the criteria. This was compared to HIV-1 DNA PCR test results and used to calculate the performance of each algorithm. Sensitivity, specificity, positive and negative predictive values (PPV and NPV) were calculated for the different algorithms. The baseline HIV-1 prevalence among HIV-1 exposed infants was estimated to be 25% in this population based on reported transmission rates in published Nairobi-based PMTCT studies (13).
To determine if combined criteria improved the performance of the data, the IMCI and the WHO-PD criteria were then assessed in combination with the CD4% information. The test was designated to be positive if a child met the IMCI or WHO-PD clinical criteria and/or the CD4% criteria for severe immunosuppression and was negative if neither of the criteria were met. In a separate analysis, we included information on PMTCT interventions to improve the performance of the clinical algorithms. If the caregiver reported both baby and mother did not receive ARV drugs for PMTCT, this was counted as a positive criterion in both the IMCI and the WHO-PD algorithms. For example, a child would be considered HIV-infected if neither mother nor child had received ARVs and 2 additional criteria were met.
Ethical approval
The study was approved by the Kenyatta National Hospital/University of Nairobi Ethics and Research Committee and the University of Washington Human Subjects Division. Written informed consent was obtained for all the caregiver-child pairs participating in the study.
Results
Characteristics of caregiver-infant pairs
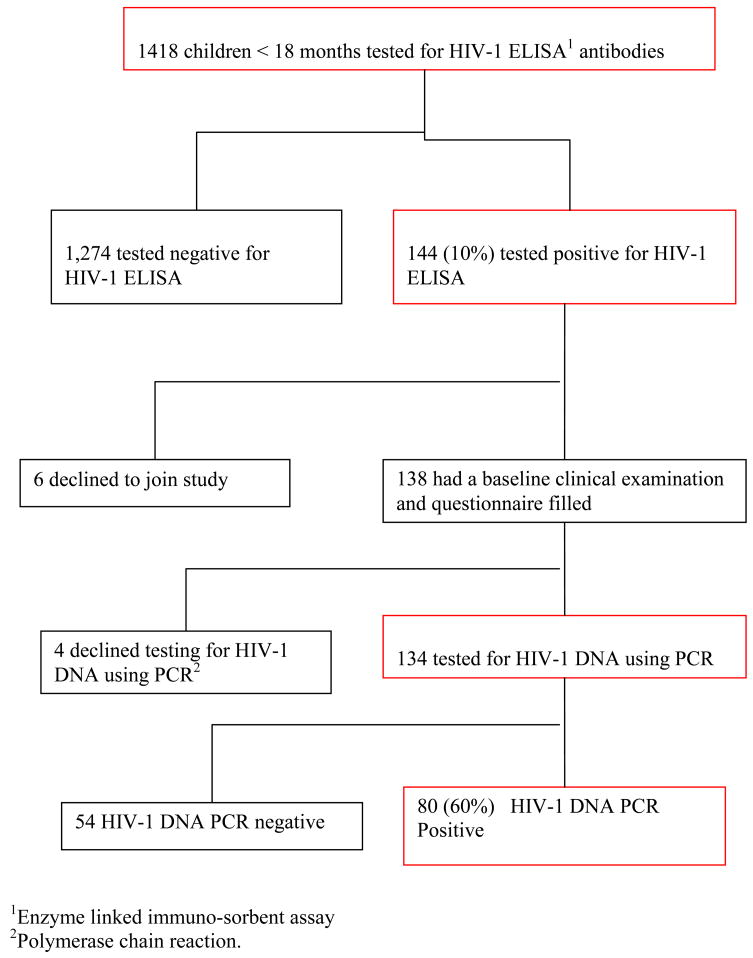
Between September 2005 and June 2006, 1,418 children less than 18 months of age were tested for HIV-1 antibodies using ELISA assays. One hundred forty-four (10%) children were HIV-1 seropositive, and among these, 6 (4%) of the 144 caregivers declined to participate in the study. Of the 138 enrolled caregivers, 4 (3%) declined to have their child tested for HIV-1 DNA. The study population for analyses included the remaining 134 caregiver-infant pairs who had infant HIV-1 DNA testing (Figure 1). Of 134 caregivers, 133 (99%) were female, of whom 119 (89%) were the child’s biological mother. Median age of caregivers was 27 years (Interquartile range [IQR] 24 – 31). The majority of the caregivers were literate, with 127 (95%) having completed at least 8 years of education, and 119 (89%) were unemployed. Ninety-five (81%) of 117 caregivers who responded to the question, reported having had an HIV-1 test, and 62 (72%) of the 86 who received their results reported their status as HIV-1-seropositive and 24 (28%) as seronegative (Table 1a).
Figure 1.
Selection of study subjects
Table 1.
| Table 1a. Baseline characteristics of the 134 enrolled caregivers and reported uptake of prevention of mother-to-child transmission of HIV-1 (PMTCT) interventions | ||
|---|---|---|
| Number or median | Percent or IQR1 | |
|
Caregiver characteristic | ||
| Age (years) | 27 | 24 – 31 |
| Female gender | 133 | 99% |
| Biological mother | 119 | 89% |
| Primary education2 | 127 | 95% |
| Married | 102 | 76% |
| Unemployed | 119 | 89% |
| Rooms in the house | 2 | 1 – 4 |
| Shared toilet3 | 95 | 71% |
| Tested for HIV-1 infection4 | 95 | 81% |
| HIV-1 infected5 | 62 | 72% |
|
| ||
|
Infant feeding first 6 months of life (n=124) | ||
| Breast only | 36 | 29% |
| Formula or cow milk only | 27 | 22% |
| Breast plus other | 61 | 49% |
|
| ||
|
ARVs6 for PMTCT(n = 119) | ||
| Used no ARVs | 53 | 44% |
| Only mother received | 5 | 4% |
| Only infant received | 9 | 8% |
| Both mother and infant received | 52 | 44% |
|
| ||
| Table 1b. Baseline characteristics of the 134 children enrolled and tested for HIV-1 | ||
| Characteristic | Number or median | Percent or IQR1 |
|
| ||
| Age (months) | 5.4 | 3.2 – 9.2 |
| Female gender | 72 | 54% |
| Mother alive | 127 | 95% |
| Father alive | 119 | 89% |
| Number of siblings | 2 | 2 –3 |
| Referred from the paediatric wards | 73 | 55% |
|
| ||
|
Referral diagnosis2 | ||
| Pneumonia | 64 | 48% |
| Diarrhea | 33 | 25% |
| Malnutrition | 26 | 20% |
| Oral Candidiasis | 48 | 36% |
| Tuberculosis | 9 | 7% |
|
| ||
|
Laboratory data | ||
| WBC3 (109/liter) | 10.5 | 7.4 –14.7 |
| TLC4 (109/liter) | 6.1 | 3.9 – 8.5 |
| CD4 count5(cells/mm3) | 1056 | 504 – 1670 |
| CD4%6 | 17 | 9.3 – 23.7 |
| Hemoglobin (g/dl) | 11.1 | 9.3 – 12.5 |
Interquartile ranges
Completed a minimum of eight years of education
Shared toilets with people outside the household
N= 117
N = 86
Antiretroviral medication
Interquartile range
Referral diagnosis in isolation or combination
White blood cell count
Total lymphocyte count
Absolute count of the CD4 subset of the T-lymphocyte cells
CD4 cells as a percentage of the total lymphocyte cell count
Seventy-two children (54%) were female and the median age was 5.4 months (IQR 3.2 –9.2). Eighty (60%) of the 134 HIV-1 ELISA positive infants were found to be HIV-1-infected as determined by HIV-1 DNA testing. Ten (12.5%) of the 80 HIV-1-infected children had insufficient data collected to permit clinical staging. Of the remaining 70 children, 12 (17.2%) were classified as WHO stage 1, 4 (5.7%) stage 2, 22 (31.4%) stage 3 and 32 (45.7%) stage 4. Eighty-seven (78%) of 124 caregivers who responded to the question, reported that their infant had been breastfed, with less than one-third (29%) reporting exclusive breastfeeding for 6 months. Over half (56%) of the 119 caregivers who responded to the question on prevention of mother-to-child transmission of HIV-1 (PMTCT), reported use of antiretroviral drugs for prevention of HIV-1 transmission (Table 1a).
Seventy-three (55%) children were referred from the general paediatric wards of Kenyatta National Hospital, while the remaining 61 (45%) were from the outpatient clinics. The referral diagnoses included pneumonia [64 (48%)], diarrhea [33 (25%)], malnutrition [25 (20%)], oral thrush [48 (36%)], and tuberculosis [9 (7%)]. Median CD4 count was 1,056 (IQR 504-1670) and median CD4% was 17% (IQR 9.3 -23.6). Results of absolute CD4 and CD4% were available for 124 (92.5%) of the 134 HIV-1 exposed children. Eighty-four (68%) had a CD4% which classified them as having severe immune suppression. Medians for white blood cell count, total lymphocyte count, and hemoglobin level were 10.5×109/liter (IQR 7.4 – 14.7), 6.1 (IQR 3.9 – 8.5) ×109/liter, 11.0 (IQR 9.3 – 12.5) grams per deciliter, respectively. Baseline characteristics of the children are shown in Table 1b.
Performance of different algorithms on HIV-1 diagnosis
Sensitivity
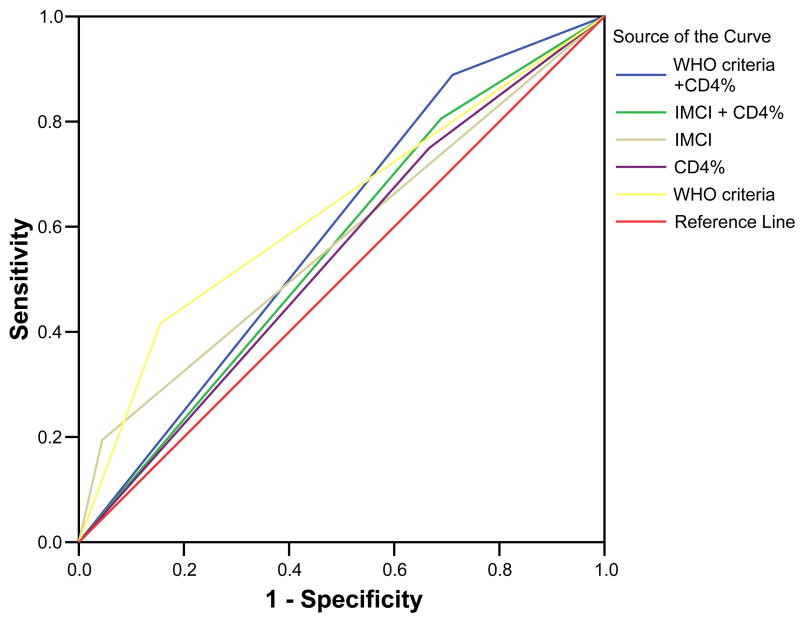
The IMCI criteria had a sensitivity of 19% (95% CI 0.12– 0.29), identifying 15 of 80 HIV-1 infected children while the WHO-PD criteria was found to have a sensitivity of 43% (95% CI 0.32– 0.54), identifying 33 of 77, and the immunological criteria (CD4 percent) had a sensitivity of 72% (95% CI 0.61– 0.81), identifying 54 of 75 HIV-1 infected children assessed. Adding CD4 percent criteria for severe immunosuppression to the IMCI and WHO-PD criteria improved the sensitivity of the IMCI criteria to 74% (95% CI 0.63– 0.82) and the WHO-PD criteria to 84% (95% CI 0.74– 0.90), (Table 2a).
Table 2.
| Table 2a. Sensitivity, specificity and predictive values for HIV-1 infection in infants using different algorithms | ||||
|---|---|---|---|---|
| Test | Sensitivity (95% CI) | Specificity (95% CI) | PPV1 | NPV2 |
| IMCI3 | 0.19 (0.12, 0.29) | 0.96 (0.87, 0.98) | 0.13 | 0.67 |
| WHO-PD criteria4 | 0.43 (0.32, 0.54) | 0.88 (0.77, 0.94) | 0.54 | 0.82 |
| CD4 %5 | 0.72 (0.61, 0.81) | 0.39 (0.26, 0.53) | 0.42 | 0.88 |
| IMCI plus CD4%6 | 0.74 (0.63, 0.82) | 0.43 (0.30, 0.56) | 0.30 | 0.83 |
| WHO-PD plus CD4%7 | 0.84 (0.74, 0.90) | 0.41 (0.29, 0.54) | 0.32 | 0.88 |
| Table 2b. Performance of the integrated management of childhood infections (IMCI) criteria for HIV-1 infection in infants by WHO clinical stage | ||||
| Test | Sensitivity (95% CI) | Specificity (95% CI) | PPV1 | NPV2 |
|
| ||||
| IMCI | ||||
| Stage 1 n = 34 |
0.00 (0.00, 0.24) | 1.00 (0.85, 1.00) | 0.00 | 0.75 |
| Stage 2 n = 8 |
0.00 (0.00, 0.49) | 1.00 (0.51, 1.00) | 0.00 | 0.75 |
| Stage 3 n = 30 |
0.18 (0.07, 0.39) | 1.00 (0.67, 1.00) | 1.00 | 0.79 |
| Stage 4 n = 38 |
0.25 (0.13, 0.42) | 0.67 (0.30, 0.90) | 0.20 | 0.73 |
| Table 2c. Performance of the integrated management of childhood infections (IMCI) and WHO presumptive diagnosis of HIV-1 infection (WHO-PD) criteria in infants by age | ||||
| Test | Sensitivity (95% CI) | Specificity (95% CI) | ||
|
| ||||
| IMCI | ||||
| 0–5.9 months n = 67 |
0.20 (0.11, 0.31) | 0.94 (0.79, 0.99) | ||
| 6–11.9 months n = 32 |
0.26 (0.10, 0.48) | 1.00 (0.66, 1.00) | ||
| 12–18 months n = 21 |
0.14 (0.02, 0.43) | 1.00 (0.59, 1.00) | ||
| WHO-PD | ||||
| 0–5.9 months n = 67 |
0.38 (0.22, 0.56) | 0.88 (0.71, 0.96) | ||
|
| ||||
| 6–11.9 months n = 32 |
0.56 (0.34, 0.77) | 0.89 (0.52, 1.00) | ||
|
| ||||
| 12–18 months n = 21 |
0.36 (0.13, 0.65) | 0.71 (0.29, 0.96) | ||
Positive predictive value
Negative predictive value
Integrated management of childhood illnesses (IMCI) criteria for HIV-1 infection
WHO presumptive diagnosis of HIV-1 infection
WHO criteria for severe immunosuppression using CD4%
IMCI criteria for HIV-1 infection and/or WHO criteria for severe immunosuppression
WHO presumptive diagnosis of HIV-1 infection and/or WHO criteria for severe immunosuppression
Positive predictive value
Negative predictive value
Specificity
The specificity of the IMCI criteria was 96% (95% CI 0.87– 0.98) identifying 52 of the 54 children without the disease, while that for the WHO-PD criteria in this population was 88% (95% CI 0.77 – 0.94) identifying 44 of the 50 children without the disease. Specificity using CD4% was 39% (95% CI 0.26– 0.53) identifying 19 of the 49 children without the disease. Addition of the CD4% to the IMCI criteria and WHO-PD criteria reduced the specificity to 43% (95% CI 0.30– 0.56), identifying 23 of the 54 children without the disease and 41% (95% CI 0.29– 0.54), identifying 22 of the 54 children without the disease, respectively (Table 2a).
Positive predictive value (PPV)
Using IMCI criteria, 13% of the children with a positive test were HIV-1 infected. Thus, the PPV for the IMCI criteria was 13%. The PPV for the WHO-PD and CD4% criteria were 54% and 42%, respectively. The PPV for IMCI and WHO-PD criteria changed after incorporating the CD4% to 30% and 32%, respectively (Table 2a).
Negative predictive value (NPV)
IMCI criteria had a NPV of 67%. Thus, 36 children of the 54 diagnosed as not being infected using these criteria had no HIV-1 DNA detected by PCR. The NPV for WHO-PD and CD4% criteria were 82% and 88% respectively. Incorporating the CD4% criteria to the IMCI and WHO-PD criteria minimally changed the NPV to 83% and 88%, respectively (Table 2a).
Performance of the IMCI criteria for HIV-1 infection in infants by WHO clinical stage
None of the 16 HIV-1-infected children classified as WHO Stage 1 or 2 were diagnosed using the IMCI criteria, giving a sensitivity of 0%, while all the 26 uninfected children would have correctly been identified as uninfected, giving a specificity of 100%. Only 12 (22%) of the 54 HIV-1 infected children classified as severe disease (WHO stage 3 and 4) were identified using the IMCI criteria, while 12 (86%) of the 14 uninfected were correctly identified giving a sensitivity of 22% and a specificity of 86%, respectively (Table 2b).
Performance of the IMCI and WHO-PD criteria by age
Analysis of the performance of the IMCI and WHO-PD criteria in infants by age of the infant showed that sensitivity for IMCI increases from 20% for infants aged less than 6 months to peak at 26% for ages 6-less than 12 months, then declines to 14% for those 12–18 months. A similar trend was observed for the WHO-PD where those less than 6 months, the sensitivity was 38%, for ages 6-less than 12 months 56% and 36% for ages 12–18 months (Table 2c)
Performance of algorithms with inclusion of no peripartum use of ARVs for PMTCT
We evaluated algorithms incorporating information on use of ARVs for PMTCT to determine if this information enhanced performance of the clinical algorithms. Reported failure to use PMTCT interventions was identified as a significant independent predictor of HIV-1 infection in this population. If neither mother nor child received ARVs for PMTCT, the child was 4-fold more likely to be HIV-1 infected (Odds ratio (OR) 4.45; 95% CI 1.75 – 11.63; p value 0.005) compared to a child where both the mother and child received ARVs. In the adjusted analysis controlling for pneumonia, oral thrush, malnutrition, and diarrhea, no peripartum ARVs remained significantly associated with HIV-1 infection (OR 3.03; 95% CI 1.20– 7.66; p-value 0.02).
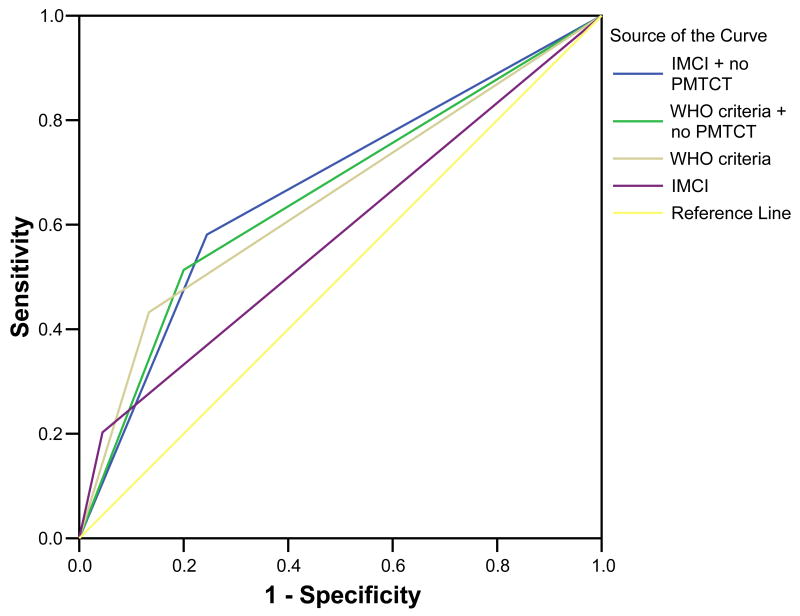
If the criterion ‘no peripartum use of ARVs for PMTCT’ was added to the IMCI and WHO-PD criteria, the sensitivity and specificity of the IMCI criteria were 58% and 75%, respectively, correctly classifying 43 of 74 infected and 34 of 45 uninfected children, while those for the WHO-PD criteria were 51% and 80%, respectively, correctly classifying 38 of the 74 infected and 36 of 45 uninfected children. The positive and negative predictive value for IMCI were 44% and 84%, while those for the WHO-PD criteria were 46% and 83% respectively with the inclusion of the no peripartum use of ARVs for PMTCT as a criterion (Figure 2b).
Figure 2.
Figure 2a. Receiver operating curves for the different clinical algorithms
Figure 2b Receiver operating curves for clinical algorithms with inclusion of no perinatal use of antiretroviral drugs (ARVs) for prevention of mother to child transmission of HIV-1
Stratified analysis for children in different stages of the disease, incorporating no peripartum use of ARVs for PMTCT as a criterion to the IMCI criteria would result in enhanced sensitivity correctly classifying 10 (62.5%) of the 16 HIV-1 infected and 20 (77%) of 26 uninfected children, for children in WHO disease stage 1 and 2, respectively. For children with severe disease (WHO stage 3 and 4) the IMCI criteria with addition of no peripartum use of ARVs for PMTCT as a criterion correctly classified 31 (57%) of the 54 infected and 8 (57%) of the 14 uninfected children. Thus the sensitivity of the IMCI criteria for children in WHO clinical stage 1 and 2 was increased from 0% to 62.5%, while specificity was reduced by 10% from 100% to 90.9%. For children in WHO stage 3 and 4 sensitivity was increased from 22% to 57% (2.6 fold), while the specificity reduced by 30% from 86% to 57%.
Discussion
This study critically assesses the Integrated Management of Childhood Illnesses (IMCI) and the WHO presumptive diagnosis (WHO-PD) criteria for diagnosis of HIV-1 infection in symptomatic, HIV-1-exposed children less than 18 months using HIV-1 DNA PCR testing to definitively diagnose children infected with HIV-1. Infant HIV-1 prevalence in this cross-sectional sample of HIV-1 exposed infants was 60% and both clinical algorithms were found to be relatively insensitive as screening tests in this setting. However we found clinical algorithms have a good specificity and negative predictive value and are useful in identifying children who might not need HIV-1 DNA PCR testing which could improve appropriate referral and effective use of resources available for viral tests.
In high disease prevalence, low resource settings with high infant mortality rates, early identification and access to care of HIV-1 infected infants is critical to prevent rapid HIV-1 disease progression and mortality in perinatally-infected infants. Thus, a highly sensitive algorithm is desirable. However, there are also reasons for wanting a highly specific test. ART drugs are expensive and known to have toxic effects. A highly sensitive algorithm with a low specificity would over-diagnose and expose some children unnecessarily to ART. Furthermore, diagnosis of HIV-1 may cause the family psychological stress, social discrimination and sometimes violence and false positive results should be minimized.
The IMCI criteria in this hospital based population yielded a sensitivity of 19%, specificity of 96%, positive predictive value of 13% and negative predictive value of 67%. The WHO-PD criteria for ART initiation performed better with a sensitivity of 43%, specificity of 88%, positive predictive value of 54% and a negative predictive value of 82%. Thus, WHO-PD had better sensitivity than IMCI without compromising specificity (Table 2a). When IMCI criteria were analyzed for performance in children at different WHO disease stages (Table 2b), we found that the algorithm completely failed to identify HIV-1 infected children in WHO disease stage 1 and 2. Thus, only children who are in stage 3 and 4 would be referred for HIV-1 testing and care. Even in these children the sensitivity was low (22%), which is particularly concerning because children in advanced stages of disease need treatment and should be started on ART.
The findings of this study add to the few studies which have evaluated these criteria in high HIV-1 prevalence regions. In contrast to previous studies, children in this study were not in a PMTCT cohort. They were very young with a median age of 5.4 months and about half (55%) were hospitalized (Table 1a). The young age of our study would be expected to result in lower sensitivity of clinical criteria as was noted in a prospective study from South Africa in which sensitivity for the IMCI criteria was 17% at 6 weeks improving to 50% at 12 months of age (14). In this South African study, as well as a second one also conducted in South Africa, the populations were PMTCT cohorts, in care from birth and were older than the children in this study (14, 15). Furthermore, in a PMTCT cohort, caregivers are likely to have more information on infant feeding and children are more commonly on cotrimoxazole prophylaxis, reducing frequency and severity of common childhood infections (16). As seen in this study, young hospitalized HIV-1 exposed children may not have enough combination of symptoms to be picked by the clinical algorithms, hence exposed children sick enough to be admitted should be considered priority for virological testing and where such facilities are not available for further evaluation for antiretroviral treatment.
The WHO recommends that symptomatic HIV-1 exposed children less than 18 months who are severely immunosupressed by CD4% criterion be started on treatment where virological testing is not available until a definitive diagnosis can be made. CD4% criterion among HIV-1 exposed infants had a sensitivity of 72% and specificity of 39% for diagnosing HIV-1 infection in this population (Table 2a). While many studies show that CD4% is low in HIV-1-infected children (17, 18), we did not find published data on the performance of this criterion in a high HIV prevalence region where other concurrent childhood conditions might cause reduction of T-lymphocyte cell subsets. Hospitalized children are more likely to have conditions which reduce lymphocyte count, and this might explain why the CD4% criterion was non-specific. IMCI criteria’s sensitivity increased to 74% and the WHO-PD criteria with CD4% had an improved sensitivity of 84%, however both had lowered specificity (43% and 41%, respectively) (Table 2a). Adding CD4% to clinical algorithms is expensive and requires laboratory infrastructure, and even though many children eligible for ARV would be identified early, there are many other who would have unnecessary exposure to antiretroviral drugs because of the reduced specificity if empiric ART initiation was based on these criteria.
Prevention of mother-to-child transmission of HIV-1 interventions are being rapidly scaled up in Africa. We found that asking a simple question about peripartum use of ARVs for PMTCT to the caregivers improved the performance of the clinical algorithms in a resource limited, high prevalence setting. Addition of ‘no peripartum use of ARVs for PMTCT’ as a criterion improved the IMCI criteria’s sensitivity to 58% and reduced the specificity to 75%, and it increased WHO-PD criteria’s sensitivity to 51% with a reduction in specificity to 80%. This was a 3-fold improvement in the sensitivity of the IMCI criteria versus 22% decline in the specificity. The gains in the WHO-PD criteria were more modest with 18.6% increase in the sensitivity versus 9% decline in the specificity. The positive and negative predictive value for IMCI was 44% and 84%, while that for the WHO-PD criteria was 46% and 83% respectively. Inclusion of this question in the IMCI criteria would help identify the children in early disease stage as shown by a sensitivity and specificity of 63% and 91%, respectively, for children in WHO disease stages 1 and 2. This question on use of ARVs for PMTCT is clinically relevant as it guides the choice of drugs used for treatment of infected children, is widely applicable, and is practical to include in training packages for healthcare workers caring for children. Including a question of no peripartum use of ARVs for PMTCT improved the performance of the clinical algorithms by increasing the numbers accessing diagnosis and treatment, as well as identifying children early. We conclude from this study that it should be considered when adapting WHO clinical algorithms for use in similar settings. Infants whose mothers used ARVs for PMTCT were unlikely to be HIV-1 infected; hence the need to scale up ARVs for PMTCT in resource limited settings where HIV-1 virological tests are not readily available.
This study is the first to evaluate these widely implemented clinical algorithms in a population not in long term follow-up that included in-patients. However, the fact that children were recruited from an urban tertiary hospital is also a limitation because it may make results less generalizable to all health facilities. In addition, the study was underpowered to evaluate the effects of different disease status on CD4%. The low rates of sensitivity for these algorithms for diagnosing HIV-1 infection in this population underscore challenges in diagnosing many childhood infections using algorithms in regions with high disease prevalence. Nevertheless these sensitivity rates compare with those found for algorithms to diagnose acute lower respiratory infections (40 – 61%) in infants (19), pertussis (40–80%) (20) and malaria (40–65%) (21, 22). It is clear that diagnosis of many illnesses, including HIV-1 infection, remains a challenge even in a high prevalence, resource limited setting, and current recommended algorithms have a low sensitivity, especially in children under the age of 18 months. However, our findings clearly emphasize the need for rapid scale up of diagnostic viral assays to determine HIV-1 infection in infants early.
Acknowledgments
Funding: I. Inwani and D. Wamalwa were scholars in the International AIDS Research and Training Program supported by the National Institutes of Health/Fogarty International Center D43 TW000007. C. Farquhar was supported by NIH grant K23 HD41879.
We thank the study participants for volunteering to take part in the study, staff at the Kenyatta National Hospital for help with data collection, CDC Kenya Medical Research Institute (KEMRI), Kisumu for doing the HIV-1 DNA PCR testing, Network of AIDS Researchers In East and Southern Africa (NARESA) for providing support for data analysis and management, the International AIDS Research and Training Program (IARTP) staff for administrative support. Our appreciation also goes to the PEPFAR program for providing laboratory and ARV support, through the University of Nairobi AIDS Care and Treatment program.
Footnotes
This study was presented as an abstract poster, at the 4th IAS Conference on HIV Pathogenesis, Treatment and Prevention, held in Sydney, Australia 22–25 July 2007Abstract No A-042-0077-01536
References
- 1.Newell ML, Coovadia H, Cortina-Borja M, et al. Mortality of infected and uninfected infants born to HIV infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 2.Obimbo E, Mbori-Ngacha D, Ochieng J, et al. Predictors of early mortality in a cohort of human immunodeficiency virus type 1-infected African children. Paediatr Infect Dis J. 2004;23:536–5. doi: 10.1097/01.inf.0000129692.42964.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cherutich P, Inwani I, Nduati R, Mbori-Ngacha D. Optimizing paediatric HIV care in Kenya: challenges in early infant diagnosis. Bulletin of the World Health Organization. 2008;86:155–160. doi: 10.2471/BLT.07.040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherman GG, Cooper PA, Coovadia AH, Puren AJ, Jones SA, Mokhachane M, Bolton KD. Polymerase chain reaction for diagnosis of human immunodeficiency virus infection in infants in low resource Settings. Paediatr Infect Dis J. 2005;24:993–997. doi: 10.1097/01.inf.0000187036.73539.8d. [DOI] [PubMed] [Google Scholar]
- 5.Zijenah LS, Humphrey J, Nathoo K, Malaba L, Zvandasara P, Mahomva A, Iliff P, Mbizvo MT. Evaluation of the prototype Roche DNA amplification kit incorporating the new SSK145 and SKCC1B Primers in detection of human immunodeficiency virus type 1 DNA in Zimbabwe. J Clin Microbiol. 1999;37:3569–3571. doi: 10.1128/jcm.37.11.3569-3571.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck IA, Drennan KD, Melvin AJ, Mohan KM, Herz AM, Alarcón J, Piscoya J, Velázquez C, Frenkel LM. Simple, sensitive, and specific detection of human immunodeficiency virus type 1 subtype B DNA in dried blood samples for diagnosis in infants in the field. J Clin Microbiol. 2001;39:29–33. doi: 10.1128/JCM.39.1.29-33.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassol SA, Kinard Butcher S, Spadoro J, Sy T, Lapointe N, Read S, Gomez P, Fauvel M, Major C, O’Shaughnessy M. Rapid screening for early detection of mother-to-child transmission of human immunodeficiency Virus type 1. J Clin Microbiol. 1994;32:2641–2645. doi: 10.1128/jcm.32.11.2641-2645.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer AC, Lambert Lejczak C, Servais J, Makombe N, Rusine J, Staub T, Hemmer R, Schneider F, Schmit JC, Arent V. Simple DNA extraction method for dried blood spots and comparison of two PCR assays for diagnosis of vertical human immunodeficiency virus type 1 transmission in Rwanda. J Clin Microbiol. 2004;42:16–20. doi: 10.1128/JCM.42.1.16-20.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nyambi PN, Fransen K, De Beenhouwer H, Chomba EN, Temmerman M, Ndinya-Achola JO, Piot P, van der Groen G. Detection of human immunodeficiency virus type 1 (HIV-1) in heel prick blood on filter paper from children born to HIV-1 seropositive mothers. J Clin Microbiol. 1994;32:2858–2860. doi: 10.1128/jcm.32.11.2858-2860.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherman GG, Stevens G, Jones SA, Horsfield P, Stevens WS. Dried blood spots improve access to HIV diagnosis and care for infants in low-resource settings. J Acquir Immune Defic Syndr. 2005;38:615–617. doi: 10.1097/01.qai.0000143604.71857.5d. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization Division of Diarrhoeal and Acute Respiratory Disease Control. Integrated management of the sick child. Bull World Health Organ. 1995;73:735–40. [PMC free article] [PubMed] [Google Scholar]
- 12.Gove S. Integrated management of childhood illness by outpatient health workers: technical basis and overview. Bull World Health Organ. 1997;75 (Suppl 1):7–2. [PMC free article] [PubMed] [Google Scholar]
- 13.Nduati R, John G, Mbori-Ngacha D, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;282:1167–74. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 14.Jones SA, Sherman GG, Coovadia AH. Can clinical algorithms deliver an accurate diagnosis of HIV infection in infancy? Bull World Health Organ. 2005 Jul;83(7):559–60. [PMC free article] [PubMed] [Google Scholar]
- 15.Horwood C, Liebeschuetz S, Blaauw D, et al. Diagnosis of paediatric HIV infection in a primary health care setting with a clinical algorithm. Bull World Health Organ. 2003;81(12):858–66. [PMC free article] [PubMed] [Google Scholar]
- 16.Chintu C, Bhat GJ, Walker AS, et al. Co-trimoxazole as prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): a double-blind randomized placebo-controlled trial. Lancet. 2004 Nov 20–26;364(9448):1865–71. doi: 10.1016/S0140-6736(04)17442-4. [DOI] [PubMed] [Google Scholar]
- 17.Zijenah LS, Katzenstein DA, Nathoo KJ, et al. T lymphocytes among HIV- infected and uninfected infants: CD4/CD8 ratio as a potential tool in diagnosis of infection in infants under the age of 2 years. J Transl Med. 2005;3(6):3–6. doi: 10.1186/1479-5876-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Embree J, Bwayo J, Nagelkerke N, et al. Lymphocyte subsets in human immunodeficiency virus type 1 infected and uninfected children in Nairobi. Pediatr Infect Dis J. 2001;20:397–403. doi: 10.1097/00006454-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Berman S. Overview of pneumonia in early infancy. In: Gadomski A, editor. Acute respiratory infection and child survival in developing countries, understanding the current status and directions for the 1990s. Baltimore: Institute for International Programs, Johns Hopkins University; 1990. pp. 39–52. [Google Scholar]
- 20.Voorhoeve AM, Muller AS, Schulpen TWJ, et al. Machakos project studies: agents affecting health of mother and child in a rural area of Kenya. IV: the epidemiology of pertussis. Trop Geogr Med. 1978;30:125–39. [PubMed] [Google Scholar]
- 21.Greenwood BM, Bradley AK, Greenwood AM, et al. Mortality and morbidity from malaria among children in a rural area of The Gambia, West Africa. Trans Roy Soc of Trop Med Hyg. 1987;81:478–86. doi: 10.1016/0035-9203(87)90170-2. [DOI] [PubMed] [Google Scholar]
- 22.Trape JF, Zoulani A, Quinet MC. Assessment of the incidence and prevalence of clinical malaria in semi-immune children exposed to intense and perennial transmission. Am J Epidemiol. 1987;126(2):193–201. doi: 10.1093/aje/126.2.193. [DOI] [PubMed] [Google Scholar]