ABSTRACT
The transforming growth factor beta (TGFB) protein family is renowned for its diverse roles in developmental biology including reproduction. Gremlin is a member of the differential screening-selected gene aberrative in neuroblastoma (DAN)/cerberus family of bone morphogenetic protein (BMP) antagonists. Recent studies on gremlin focus on its involvement in embryonic skeletal, lung, and kidney development. To define the role of gremlin (Grem1) in female reproduction, we analyzed postnatal folliculogenesis using global and conditional knockout (cKO) mice for gremlin. Grem1−/− mice die within 48 h after birth, and ovaries collected from neonatal Grem1−/− mice demonstrated reduced oocyte numbers and delayed primordial follicle development. Transplanting Grem1−/− neonatal ovaries showed that folliculogenesis proceeded to large antral follicle stage, but Grem1−/− ovaries contained corpora lutea-like structures not found in control-transplanted ovaries. However, Grem1 cKO mice had comparable fertility to control mice. These data suggest that gremlin plays a previously uncharacterized role in the regulation of oocyte numbers and the timing of primordial follicle development, but either it is not required for later folliculogenesis or its loss is possibly compensated by other BMP antagonists.
Keywords: fertility, follicular development, folliculogenesis, ovary, primordial follicle, reproduction, transgenic, transgenic/knockout model
The Grem1 knockout has novel defects in early postnatal follicle development, but the conditional knockout in the ovary has normal fertility, due to compensation by other BMP antagonists expressed in the ovary.
INTRODUCTION
Originally identified as a dorsalizing agent with bone morphogenetic protein (BMP) antagonistic activities during Xenopus embryonic development [1, 2], gremlin has emerged as a major regulator of growth, differentiation, and development. Gremlin is found as secreted or cell-associated forms and is a member of the differential screening-selected gene aberrative in neuroblastoma (DAN)/cerberus family of BMP antagonists. Gremlin is recognized mainly for its BMP antagonistic activities, and the majority of our understanding comes from its functions during developmental processes in the embryonic renal, lung, and bone systems. Much of these data were generated from mouse models either completely or cell-specifically devoid of gremlin as well as in mice with cell-specific overexpression [3–5].
BMPs have multiple described roles in embryonic and adult ovaries [6–8]. Knockout mice for the ligands and their downstream signaling proteins and receptors have established the BMP system as essential for primordial germ cell (PGC) specification, migration, and maintenance [9]. However, few mouse models are available to study the role of the BMP family in postnatal ovarian development because of embryonic lethality or genetic redundancy of the ligands or their signaling components. Exceptions to this include Bmp15 or Bmp6 knockout mice, which display subfertility on some genetic backgrounds [10–12], and Bmpr1b (Alk6) knockout mice, which are infertile with defects in cumulus expansion and uterine function [13]. More recently, mice with ovarian somatic cell deletion of BMP receptor-regulated SMADs [14] and type I receptors, Bmpr1a/Alk3 and Bmpr1b/Alk5 [15], develop infertility and metastatic granulosa cell tumors.
Gremlin (Grem1) is expressed in granulosa cells of the mouse ovary [16] along with Grem2, a closely related gene that shares 62% amino acid sequence homology in the mature region [17]. Gremlin regulation of BMP activity in granulosa cells has been reported from our laboratory [16] and others [18, 19]. Given the important developmental roles of gremlin in other systems [3–5], we hypothesized that gremlin plays an integral role in ovarian follicular development. We therefore generated knockout mice (Grem1−/−) and ovary conditional knockout (Grem1 cKO). Ovaries analyzed from neonatal Grem1−/− mice showed defects in germ cell numbers and primordial follicle development. However, transplanted Grem1−/− ovaries and Grem1 cKO ovaries contained all stages of growing follicles, and Grem1 cKO mice had similar fertility to controls. These data suggest that Grem1 has important roles in ovary prior to the primordial follicle stage, but once primordial follicles are established, follicle development proceeds normally in the absence of gremlin.
MATERIALS AND METHODS
Experimental Mice
All mouse lines used in the present study were maintained on a mixed C57BL/6/129S7/SvEv genetic background and manipulated according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved protocols at Baylor College of Medicine. Grem1flox/flox mice (generated as previously described) [4] were rederived in the Transgenic Mouse Core facility at Baylor College of Medicine. Ella-cre (The Jackson Laboratory, http://jaxmice.jax.org/strain/003724.html) transgenic mice [20] were used to generate Grem1+/− mice that were then intercrossed to produce Grem1−/− homozygote mutants. To generate ovary conditional knockouts (cKO), Grem1flox/flox female mice were crossed to Grem1+/− Amhr2cre/+male mice [14, 21–24] to generate Grem1flox/−Amhr2cre/+ experimental mice (denoted Grem1 conditional knockout [cKO]). Control mice (Grem1flox/−) were siblings of experimental mice but negative for cre. Mice were genotyped by PCR analysis of genomic tail DNA as reported [4, 24].
Tissue Collection
All mice were anesthetized by isoflurane inhalation (Abbott Laboratories, Abbott Park, IL) and euthanized by either decapitation (neonatal mice) or by cervical dislocation (adult mice). Ovaries for histology and immunohistology were collected in either Bouin's solution (Sigma, St. Louis, MO) or 10% neutral buffered formalin (Electron Microscopy Sciences, Hatfield, PA). Ovaries for gene expression studies were stored in RNAlater (Ambion, Austin, TX) at −80°C for subsequent use. Neonatal ovaries for transplantation studies (described below) were collected on the day of birth (D0) and immediately placed in 1× PBS for transplantation under the kidney capsule.
Fertility Analysis
Four to six individually housed female mice of each genotype (Grem1flox/−Amhr2cre/+ or Grem1flox/−) were bred at 6 wk of age continuously to wild-type males with known fertility. The number of litters and the number of pups were recorded over a 9-mo period. For the analysis of fertility over time, four control and four experimental female mice at 6 wk of age were crossed to adult wild-type males, the total number of pups generated that month was recorded, and the data were plotted over time.
Morphological, Histological, and Immunohistochemical Analysis
Tissue processing and embedding were performed by the Department of Pathology Core Facility (Baylor College of Medicine) using standard techniques. For counting, newborn ovaries (D0) were serially sectioned at 5 μm and all sections retained. Four mice per genotype were used for quantification. Sections were immunostained with a goat polyclonal antibody against NOBOX (1:500) using previously published protocols [25] to visualize the oocyte nucleus (Supplemental Fig. S1, available online at www.biolreprod.org). Images of ovarian sections were captured with a digital camera (AxioCam MRc5) at a low magnification (100×), and all NOBOX- labeled oocytes in every fifth section were counted manually. Every fifth section was counted to avoid double counting oocytes. From these values, the total sum of NOBOX labeled oocytes (as naked oocytes and primordial follicles) per ovary was calculated. Statistical differences in the total number of oocytes were assessed using Student's t-test. Statistical differences in the numbers of NOBOX-labeled cells between naked oocytes and primordial follicles were assessed by performing a chi-square test using Graph Pad Prism 5 (GraphPad Software, La Jolla, CA).
Immunohistochemistry
Immunohistochemistry was performed using the Vectastain ABC method (Vector Laboratories, Burlingame, CA) as previously described [24, 26] using the following antibodies: goat polyclonal anti-AMH (1:1500 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), rat polyclonal anti-GCNA (kindly provided by G.C. Enders), and goat polyclonal NOBOX (1:500; kindly provided by A. Rajkovic). Immunoreactivity was visualized by diaminobenzidine (Vector Laboratories), and ovaries were counterstained with hematoxylin. Fluorescent immunohistochemistry was utilized to colocalize GCNA and rabbit polyclonal anti-YBX2 (MSY2; kindly provided by R.M. Schultz). Briefly, sections were subjected to antigen retrieval (0.01 M citric acid) and 0.1% Triton X (Sigma), followed by block incubation in normal horse serum (Vector). Rat anti-GCNA was incubated overnight (1:200 dilution) and detected using Alexa Fluor donkey anti-rat 488 (Invitrogen) diluted 1:500 in TBS for 1 h. Sections were reblocked with horse serum and incubated overnight with rabbit anti-YBX2 (MSY2) diluted 1:4000 and detected using Alexa Fluor donkey anti-rabbit 594 (Invitrogen) at 1:500 in TBS for 1 h. Nucleic acids were labeled with TOTO3 (Invitrogen) and washed and mounted with Prolong Gold mounting media (Invitrogen). Fluorescent images were captured using an LSM 510 Axiovert 100M confocal microscope (Carl Zeiss, Jena, Germany), and all images were compiled using Photoshop CS3 (Adobe Systems Inc., San Jose, CA).
Transplantation of Neonatal Ovaries
To assess the role of gremlin in growing follicles, neonatal (i.e., D0) control (Grem1+/+ and Grem1+/−) and mutant (Grem1−/−) ovaries were surgically transplanted beneath the kidney capsule of bilaterally ovariectomized virgin adult female mice. Both ovaries were recovered from the neonatal donor mouse, dissected of surrounding bursal tissue, and placed in PBS. Immunocompetent recipient mice of the same genetic background (C57BL/6J; 129S7/SvEvBrd) at 6–8 wk of age were anesthetized with i.p. injections of 2.5% tribromoethanol (in t-amyl alcohol solution at 0.4–0.75 mg/g; Sigma) and ovariectomized via a dorso-horizontal incision. The recipient's left kidney was exteriorized, and, if possible, both neonatal ovaries (from the same donor) were inserted under the recipient kidney capsule using fine watchmakers' forceps, and the kidney was returned to its normal anatomical position. Incisions were closed with wound clips (BD, Franklin Lakes, NJ). Three weeks posttransplantation, recipient ovaries were collected for histological/immunohistochemical assessment. Transplantation was performed on three separate occasions. Eight recipient mice were used for each genotype. A total of 13 ovaries for Grem1−/− were transplanted; of these, eight Grem1−/− ovaries grafted successfully, and similar numbers were found for the controls.
RT-PCR and Quantitative PCR
RNA was isolated using the Qiagen (Valencia, CA) RNeasy Microkit. RNA concentration was quantified using a NanoDrop Spectrophotometer ND-1000 (NanoDrop Technologies, Wilmington, DE). Complementary DNA was reverse transcribed from 200 ng of total RNA in a 20-μl reaction using High Capacity RNA-to-cDNA Master Mix (Applied Biosystems, Foster City, CA). Five-microliter templates were amplified using 10× ThermoPol reaction buffer and polymerase (New England Biolabs, Ipswich, MA). PCR conditions were as follows: incubation at 95°C for 2 min, 35 cycles of 94°C for 30 sec, annealing temperature for 30 sec, and 72°C for 30 sec, followed by 72°C for 7 min. Quantitative PCR was carried out as previously described [24, 26] using SYBR Green PCR Master Mix (Applied Biosystems) for Grem1 transcript quantification (primers sequences availableon request) or Taqman Master Mix and Gene Expression Assays for Grem1 (Mm00488615_s1) and Grem2 (Mm0051909_m1; Applied Biosystems). All quantitative PCR data were analyzed by the ΔΔ cycle threshold method using the ABI 7500 System Software (version 1.2.3) and normalized to the endogenous reference (Gapdh). The mean of the control samples was used as the calibrator sample, and the mean ± SEM was calculated. Results were plotted using the relative expression of each target gene with each sample compared to the control.
Statistical Analyses
Statistical analysis was carried out using GraphPad Prism 5 (GraphPad Software, La Jolla, CA). Two-tailed unpaired Student t-tests were used for single comparisons. One-way analysis of variance followed by the Fisher least significant difference test was used for multiple comparisons. A minimum of at least three independent experiments were carried out at all times, and P < 0.05 was considered statistically significant.
RESULTS
Loss of Grem1 Results in Fewer Germ Cells at Birth and Delays Primordial Follicle Assembly
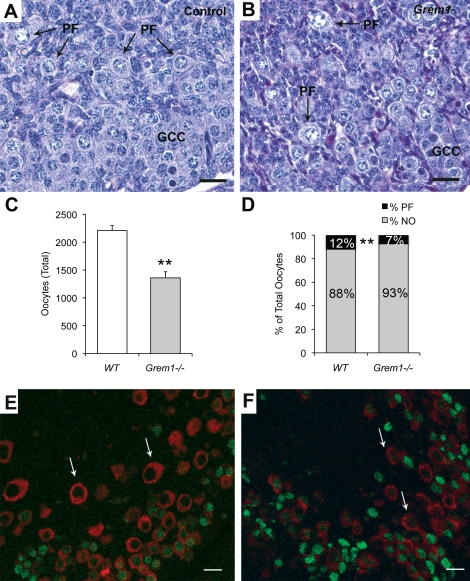
In mouse and human, the number of oocytes at birth is fixed [27, 28]. Because BMPs are essential for primordial germ cell specification and maintenance during embryogenesis [29–31], we analyzed whether loss of gremlin influenced oocyte numbers and primordial follicle formation. Histological analysis of Grem1−/− neonatal ovaries revealed germ cell nests and developing primordial follicles grossly similar to control mice (Fig. 1, A and B). However, the total number of oocytes was significantly decreased in Grem1−/− neonatal ovaries (Fig. 1C and Supplemental Fig. S1). In addition, when categorized into subpopulations (oocytes without somatic cell counterparts [naked oocytes] and oocytes with somatic cell counterparts [primordial follicles]), significantly fewer oocytes had transitioned into primordial follicles in the Grem1−/− ovaries (Fig. 1D; 7% versus 12%). Because there is a known interdependent relationship between meiotic markers during the onset of follicular assembly [32], we further characterized neonatal ovaries utilizing GCNA (a prediplotene marker) and YBX2 (MSY2; a diplotene marker). Oocytes in primordial follicles of control ovaries are GCNA negative and YBX2 positive [32] (Fig. 1E). Grem1−/− ovaries show abundance of GCNA staining (green in Fig. 1F) and less for YBX2 (red; Fig. 1F compared to Fig. 1E). Collectively, these results indicate that loss of gremlin results in developmentally delayed oocytes in the neonate; fewer oocytes are arrested in the diplotene stage of meiosis and found as primordial follicles.
FIG. 1.
Grem1−/− ovaries exhibit a delay in the formation of primordial follicles. A, B) Germ cell cysts (GCC) and primordial follicle (PF) formation are evident in control (A) and Grem1−/− (B) neonatal ovaries (Postnatal Day 0). C) Analysis of total oocyte numbers reveals that germ cells are decreased in Grem1−/− ovaries. Error bars are the mean ± SEM of n = 4 ovaries per genotype. D) The percentage of oocytes found as primordial follicles (PF) versus those found as naked oocytes (NO) is significantly reduced in Grem1−/− ovaries (P < 0.01 by chi-square test). E) Stage-specific molecular markers show that premeiotic-arrested oocytes positive for GCNA (green) are more prominent in Grem1−/− ovaries (F) compared to control ovaries (E), which have more oocytes in the diplotene stage of Meiosis I (arrows in E, F; red, positive for YBX2). **Indicates statistical significance at P < 0.01. Bars = 20 μm.
Transplanted Grem1−/− Ovaries Display Follicles of All Growing Stages but Exhibit Luteal Tissue Not Observed in Control Ovaries
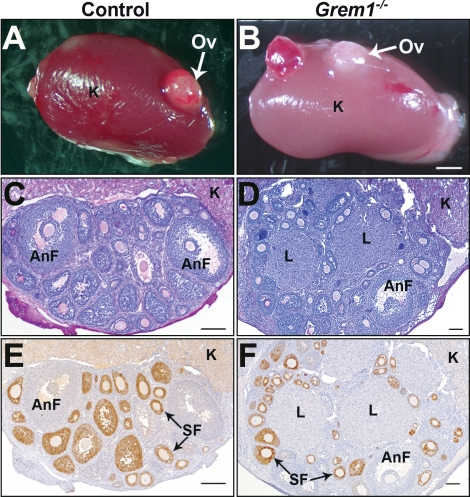
Because of the absence of kidney formation, Grem1−/− mice die within 48 h of birth [5]. Therefore, to investigate whether folliculogenesis proceeds in the absence of gremlin, we transplanted neonatal (i.e., D0) Grem1−/− ovaries under the kidney capsule of ovariectomized wild-type recipient mice for 3 wk. Approximately 60% of control and 60% of Grem1−/− transplanted ovaries survived and exhibited healthy follicles (Fig. 2, A and B). Three weeks posttransplantation, all surviving transplanted ovaries had grown in size and measured between 1.5 and 2.5 mm across their longest axis for both genotypes. Histological assessment of the transplants (n = 8 ovaries for each genotype) revealed that follicles at all stages of development developed in both genotypes (Fig. 2, C and D). Interestingly, in three of the eight Grem1−/− ovarian transplantations, large structures that histologically resemble corpora lutea were observed (Fig. 2D), suggesting that postantral stage follicle development may be altered in the absence of Grem1.
FIG. 2.
Transplanted Grem1−/− ovaries display mature follicles and contain luteinized structures not found in control ovaries. A, B) Gross morphology of neonatal control (A) and Grem1−/− (B) ovaries (Ov) 3 wk posttransplantation under the kidney (K) capsule of ovariectomized adult recipient mice. C, D) Histological assessment of control (C) and Grem1−/− (D) transplanted ovaries reveal follicles at all stages of development. Mature antral follicles (AnF) are present in both genotypes. Interestingly, only Grem1−/− (D) ovaries display luteinized structures (L), suggesting that they are more advanced than control (C) ovaries. E, F) Immunoreactivity for anti-Müllerian hormone (AMH) confirms normal positive staining patterns in secondary follicles (SF) and loss of the protein in the more advanced antral follicles (AnF) and luteinized structures in both wild-type (E) and Grem1−/− (F). Bars in A and B = 1 mm; bars in C–H = 100 μm.
In the postnatal ovary, anti-Müllerian hormone (AMH) is detected in nonatretic preantral and small antral follicles [33, 34] and decreases when follicles become responsive to follicle-stimulating hormone (FSH) [35]. Immunohistochemistry of control-transplanted ovaries confirmed positive AMH staining patterns in secondary follicles, with loss of the protein in the more advanced larger antral follicles (Fig. 2E). Similar patterns were also observed in preantral and antral follicles in transplanted Grem1−/− ovaries (Fig. 2F). Rarely, Grem1−/− mice survive to adulthood, and in the 24 mo of breeding for these studies, three mice (one female and two males) were found at weaning that exhibited the skeletal deformities characteristic of Grem1−/− mice [5]. Genotyping confirmed that all were Grem1−/− and that all had developed one kidney, consequently allowing for longevity after the neonatal period. Gonads from the one 3-wk-old female (Supplemental Fig. S2) and two males (data not shown) were histologically normal, further confirming that prepubertal folliculogenesis to 3 wk of age did not appear to be compromised in the absence of gremlin. No luteinized follicles were visible in these ovaries (such as in the transplants), but none would be expected, as these surviving mice were collected prior to puberty (i.e., at 3 wk of age).
Conditional Deletion of Gremlin in Granulosa Cells Does Not Affect the Overall Fertility in Adult Mice
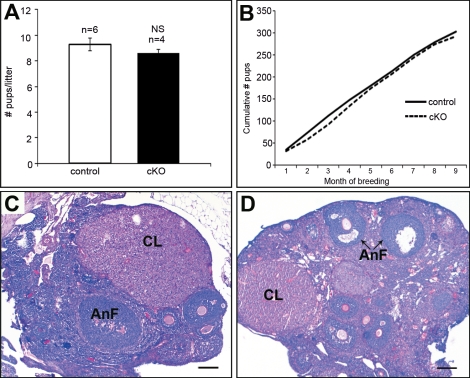
Previous studies from our laboratory [16] and others [19] have proposed a role for gremlin as an important inhibitor of BMPs during ovulation. To assess the in vivo interactions of gremlin in the later stages of folliculogenesis and ovulation, we generated a conditional knockout (Grem1 cKO) using Amhr2cre/+ mice. Confirmation of deletion was tested by quantitative PCR by measuring Grem1 transcript levels in preovulatory granulosa cells isolated from 3-wk-old female mice. Relative levels of Grem1 transcript were approximately 75% lower than controls (Supplemental Fig. 3). Grem1 cKO mice had comparable litter sizes (8–10 pups; Fig. 3A) and a similar reproductive profile (Fig. 3B) as control mice, revealing that conditional loss of gremlin did not affect the overall fertility. Histologic analysis of ovaries from adult Grem1 cKO mice showed normal folliculogenesis with secondary follicles, antral follicles, and postovulatory corpora lutea structures (Fig. 3, C and D).
FIG. 3.
Conditional deletion of gremlin in granulosa cells does not affect overall fertility in adult female mice. A) Adult female mice at 6 wk of age were bred to wild-type males for a 9-mo period (control, n = 6; cKO, n = 4). Gremlin conditional knockout mice (cKO) had comparable litter sizes to control mice. The error bars are mean ± SEM. NS, not significant. B) The cumulative number of pups generated from four breeding females of each genotype is similar. C, D) Histological analysis from adult cycling control (C) and Grem1 cKO (D) mice display normal folliculogenesis with secondary follicles, antral follicles (AnF), and postovulatory corpora lutea (CL). Bars = 200 μm.
Grem1 and Grem2 Are Coexpressed in the Postnatal Mouse Ovary
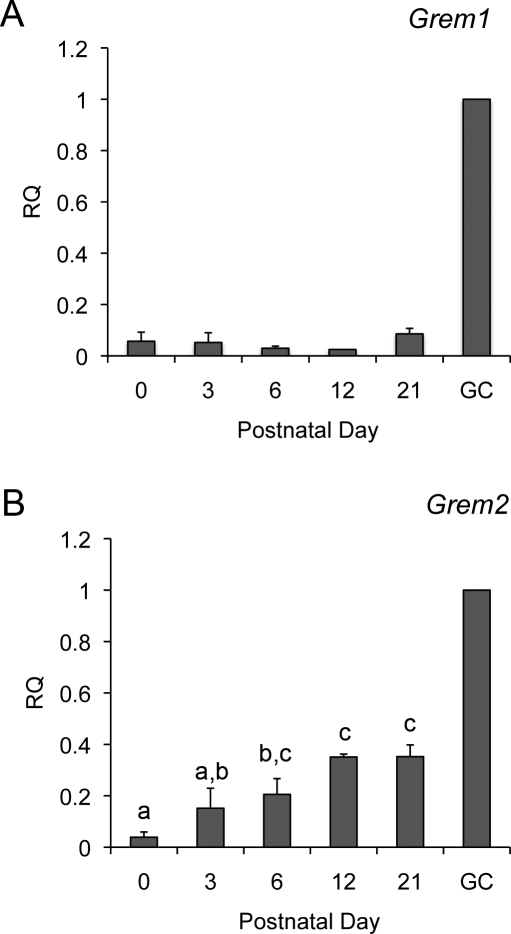
Loss of gremlin in our mouse models could potentially be compensated by expression of other BMP antagonists, including gremlin 2 (Grem2), which has been shown to be expressed in rodent granulosa cells [17]. We analyzed the expression profiles of Grem1 and Grem2 in the postnatal ovary. Both Grem1 and Grem2 are detected in postnatal ovaries from Day 0 to Day 21 (Fig. 4). The mean value of Grem1 expression did not change in ovaries from Day 0 to Day 21. Grem2 showed statistically significant changes in expression during this time frame (P < 0.05; Fig. 4B).
FIG. 4.
Grem1 and Grem2 are coexpressed in the postnatal ovary. Relative quantity (RQ) of Grem1 (A) and Grem2 (B) in the postnatal ovary determined by quantitative PCR. Ovaries were collected at birth (Postnatal Day 0) and Days 3, 6, 12, and 21 (n = 3 ovaries each age). Data are shown relative to the transcript levels in a sample of preovulatory granulosa cells (GC) collected following superovulation at Day 21. Statistical analysis by one-way analysis of variance followed by the Fisher least significant difference test was performed on all samples, excluding the calibrator (GC) sample. Bars with different letters have statistically different means (e.g., “a” is different from “c” but not “a,b”).
DISCUSSION
In the present study, we investigated the role of gremlin in ovarian development and function. Among many of the factors reported to be key for germ cell development, the BMPs and their associated signaling pathway are necessary for primordial germ cell specification, maintenance, and function [29–31, 36–44]. We hypothesized that in the absence of gremlin, BMP signaling would be disrupted and that primordial germ cell specification or development may be affected. Our findings revealed that while newborn Grem1−/− ovaries had similar architecture to control ovaries, they contained fewer oocytes. Decreases in germ cell numbers prior to birth may be due to defects in PGC specification, migration, proliferation, or apoptosis. In vitro organ culture systems used to study PGC numbers and motility have been reported for the human [45] and the mouse [46], though there have been contrasting findings between species. Although BMP4 was reported to increase PGC numbers in the mouse [46], it had the opposite effect in the human and negatively regulated PGC numbers via apoptotic mechanisms [45]. Studies are ongoing to determine the mechanism(s) behind the germ cell loss in the Grem1−/− mouse model.
In addition, we found that Grem1−/− newborn ovaries had proportionally more oocytes found as germ cell cysts and fewer as primordial follicles than would be expected. In the mouse ovary, germ cell cyst breakdown to form primordial follicles occurs within the first few days of birth [47, 48]. While BMPs, in particular BMP4 and BMP7, have been reported to be important paracrine factors in the primordial to primary follicle transition [49–51], defined roles from germ cell cyst to primordial follicle are not known. Our results suggest that gremlin may also have a role in limiting BMP function during primordial follicle formation in the neonatal mouse ovary. An alternative possibility is that loss of Grem1 indirectly results in alterations in the activity or expression of other factors known or suspected to be involved in the formation of primordial follicles, such as estrogen, activin, or notch [52–54]. Thus, additional studies will be necessary to determine which of these pathways are disrupted.
To determine if the delay in primordial follicle development affects later follicular development, we transplanted neonatal Grem1−/− ovaries under the kidney capsule of ovariectomized adult mice. Ovarian transplants from control and Grem1−/− mice exhibited follicles at all stages of development. An intriguing observation from our ovarian transplantation experiments was the appearance of large luteinized structures in 40% of the mutant ovaries that were morphologically similar to corpora lutea. These structures were not observed in any of the control ovaries, suggesting that later aspects of antral follicle development were altered in the absence of Grem1. BMPs are known regulators of granulosa cell differentiation [6]; however, the precise roles of BMPs remain poorly understood. Whether the luteinized structures in Grem1−/− transplanted ovaries represent pre- or postovulatory structures remains to be determined. Interestingly, in a rare 3-wk-old female Grem1−/− mouse that survived (Supplemental Fig. S2), we observed follicles with declining AMH expression, which is associated with the onset of FSH sensitivity and a hallmark of granulosa cell maturation [35] but not as prominent in aged-matched controls. These data suggest that in the absence of Grem1, granulosa cell maturity is possibly accelerated, perhaps leading to premature granulosa cell differentiation. Roles for TGFB family members have been implicated in the timing of luteinization [8, 55] and observed in mouse models with targeted deletions of TGFB family transcription factors Smad4 or Smad2/3 [23, 56].
To study the role of gremlin in adult fertility, we produced granulosa cell-specific knockout mice using cre recombinase under the control of the Amhr2 promoter. Amhr2cre is useful for recombination in somatic cells of growing follicles [22, 23, 57] and thus will represent a different developmental time frame to that observed Grem1−/− neonates and neonatal transplants. Over a 9-mo period, Grem1 cKO mice delivered healthy pups in litter sizes comparable to control mice. No adverse phenotypes were observed in Grem1 cKO mice, and ovarian architecture was normal. The phenotypic differences observed between our two Grem1−/− models and the Grem1 cKO model may reflect the different developmental stages at which gremlin was ablated or that ablation in the Grem1 cKO did not reduce gremlin levels to a point that uncovered the phenotype. Alternatively, the lack of a notable phenotype in the conditional ablation of gremlin may reflect a modest or nonessential role for this BMP antagonist during the later stages of follicular development.
A feature of BMP signaling is the various regulatory mechanisms that exist to control BMP-mediated actions. Most of these signals are inhibitory [58] and include spatial and temporal control of extracellular antagonists, receptor specificity, and downstream effectors and inhibitors of SMAD proteins [59]. Some of the most studied BMP antagonists include noggin, chordin, sclerostin, gremlin, BAMBI (BMP and activin membrane bound inhibitor), SMAD6, SMAD7, DAN (differential screening-selected gene aberrant in neuroblastoma), Cerberus, and gremlin 2 (also known as PRDC [protein related to DAN and cerberus]) [3]. These proteins play the important role of modulating BMP activities within a variety of systems, some of which include early embryo development [60], neural development [61], prostate development [62], inflammation of cardiac tissues [63], and bone development [64]. Noggin has been recently shown to interact with Grem1 during skeletal development [65]. The lack of full loss of oocytes at birth, or effects on postnatal folliculogenesis in our gremlin mouse models, could suggest compensatory mechanisms by other antagonists in the ovary. In particular, the closely related Grem2 is coexpressed in the ovary with Grem1 [16, 17]. We hypothesize that functional redundancies for gremlin also occur in the ovary.
The precise roles of the BMPs during folliculogenesis are not entirely clear, although they have been proposed for multiple developmental stages (Supplemental Fig. S4). To investigate the role of the BMP2, BMP4, and BMP7 antagonist gremlin in the neonatal and adult ovary, we employed three models representing different developmental stage of folliculogenesis (Supplemental Fig. S4). Thus, variations in the phenotype between global and conditional knockouts may partly reflect differences in timing of the deletion. Grem1−/− ovaries contain decreased oocyte numbers and delayed primordial follicle development. This may result from defective embryonic development of the reproductive tract, indirect disruption of other signaling pathways, or a direct consequence of additional BMP signaling in the embryonic ovary. This phenotype would not be evident in the Grem1 cKO because of the timing of recombination of floxed alleles in the conditional knockout. However, progression of folliculogenesis beyond the primordial stage appears normal in both Grem1−/− and Grem1 cKO mice, indicating that Grem1 is not required for preantral and antral stage follicle development or that expression of other BMP antagonists, such as Grem2, may compensate for its loss. BMP antagonists are known to act cooperatively to limit BMP activity; therefore, combinatorial knockouts of BMP antagonists will be required to address these questions and fully uncover their function(s) during folliculogenesis.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Rebecca James (Baylor College of Medicine) for technical assistance with data collection. We would like to thank Robert Cook for helpful discussions. We also thank Aleksandar Rajkovic (Magee-Women's Research Institute) for the NOBOX antibody, George C. Enders (University of Kansas) for the GCNA antibody, and Richard M. Schultz (University of Pennsylvania) for the MSY2 antibody.
Footnotes
Supported by Burroughs Wellcome Career Award in Biomedical Sciences and National Institutes of Health (NIH) grant CA138628 (to S.A.P.) and grant AR21707 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), NIH (to E.C.).
REFERENCES
- Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev 2003; 24: 218 235 [DOI] [PubMed] [Google Scholar]
- Hsu DR, Economides AN, Wang X, Eimon PM, Harland RM. The Xenopus dorsalizing factor Gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol Cell 1998; 1: 673 683 [DOI] [PubMed] [Google Scholar]
- Gazzerro E, Canalis E. Bone morphogenetic proteins and their antagonists. Rev Endocr Metab Disord 2006; 7: 51 65 [DOI] [PubMed] [Google Scholar]
- Gazzerro E, Smerdel-Ramoya A, Zanotti S, Stadmeyer L, Durant D, Economides AN, Canalis E. Conditional deletion of gremlin causes a transient increase in bone formation and bone mass. J Biol Chem 2007; 282: 31549 31557 [DOI] [PubMed] [Google Scholar]
- Khokha MK, Hsu D, Brunet LJ, Dionne MS, Harland RM. Gremlin is the BMP antagonist required for maintenance of Shh and Fgf signals during limb patterning. Nat Genet 2003; 34: 303 307 [DOI] [PubMed] [Google Scholar]
- Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction 2006; 132: 191 206 [DOI] [PubMed] [Google Scholar]
- Pangas SA, Matzuk MM. The TGF-β family in the reproductive tract. : Derynck R, Miyazono K (eds.) The TGF-β Family. New York: Cold Spring Harbor Laboratory Press; 2008: 861 889 [Google Scholar]
- Shimasaki S, Moore RK, Erickson GF, Otsuka F. The role of bone morphogenetic proteins in ovarian function. Reprod Suppl 2003; 61: 323 337 [PubMed] [Google Scholar]
- Dudley B, Palumbo C, Nalepka J, Molyneaux K. BMP signaling controls formation of a primordial germ cell niche within the early genital ridges. Dev Biol 2010; 343: 84 93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvin JA, Yan C, Matzuk MM. Oocyte-expressed TGF-beta superfamily members in female fertility. Mol Cell Endocrinol 2000; 159: 1 5 [DOI] [PubMed] [Google Scholar]
- Solloway MJ, Dudley AT, Bikoff EK, Lyons KM, Hogan BL, Robertson EJ. Mice lacking Bmp6 function. Dev Genet 1998; 22: 321 339 [DOI] [PubMed] [Google Scholar]
- Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL, Celeste AJ, Matzuk MM. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol 2001; 15: 854 866 [DOI] [PubMed] [Google Scholar]
- Yi SE, LaPolt PS, Yoon BS, Chen JY, Lu JK, Lyons KM. The type I BMP receptor BmprIB is essential for female reproductive function. Proc Natl Acad Sci U S A 2001; 98: 7994 7999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangas SA, Li X, Umans L, Zwijsen A, Huylebroeck D, Gutierrez C, Wang D, Martin JF, Jamin SP, Behringer RR, Robertson EJ, Matzuk MM. Conditional deletion of Smad1 and Smad5 in somatic cells of male and female gonads leads to metastatic tumor development in mice. Mol Cell Biol 2008; 28: 248 257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson MA, Nalam RL, Clementi C, Franco HL, Demayo FJ, Lyons KM, Pangas SA, Matzuk MM. Granulosa cell-expressed BMPR1A and BMPR1B have unique functions in regulating fertility but act redundantly to suppress ovarian tumor development. Mol Endocrinol 2010; 24: 1251 1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangas SA, Jorgez CJ, Matzuk MM. Growth differentiation factor 9 regulates expression of the bone morphogenetic protein antagonist gremlin. J Biol Chem 2004; 279: 32281 32286 [DOI] [PubMed] [Google Scholar]
- Sudo S, Avsian-Kretchmer O, Wang LS, Hsueh AJ. Protein related to DAN and cerberus is a bone morphogenetic protein antagonist that participates in ovarian paracrine regulation. J Biol Chem 2004; 279: 23134 23141 [DOI] [PubMed] [Google Scholar]
- Gilchrist RB, Ritter LJ, Myllymaa S, Kaivo-Oja N, Dragovic RA, Hickey TE, Ritvos O, Mottershead DG. Molecular basis of oocyte-paracrine signalling that promotes granulosa cell proliferation. J Cell Sci 2006; 119: 3811 3821 [DOI] [PubMed] [Google Scholar]
- Hussein TS, Froiland DA, Amato F, Thompson JG, Gilchrist RB. Oocytes prevent cumulus cell apoptosis by maintaining a morphogenic paracrine gradient of bone morphogenetic proteins. J Cell Sci 2005; 118: 5257 5268 [DOI] [PubMed] [Google Scholar]
- Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci U S A 1996; 93: 5860 5865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat Genet 2002; 32: 408 410 [DOI] [PubMed] [Google Scholar]
- Jorgez CJ, Klysik M, Jamin SP, Behringer RR, Matzuk MM. Granulosa cell-specific inactivation of follistatin causes female fertility defects. Mol Endocrinol 2004; 18: 953 967 [DOI] [PubMed] [Google Scholar]
- Pangas SA, Li X, Robertson EJ, Matzuk MM. Premature luteinization and cumulus cell defects in ovarian-specific Smad4 knockout mice. Mol Endocrinol 2006; 20: 1406 1422 [DOI] [PubMed] [Google Scholar]
- Pangas SA, Jorgez CJ, Tran M, Agno J, Li X, Brown CW, Kumar TR, Matzuk MM. Intraovarian activins are required for female fertility. Mol Endocrinol 2007; 21: 2458 2471 [DOI] [PubMed] [Google Scholar]
- Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science 2004; 305: 1157 1159 [DOI] [PubMed] [Google Scholar]
- Myers M, Middlebrook BS, Matzuk MM, Pangas SA. Loss of inhibin alpha uncouples oocyte-granulosa cell dynamics and disrupts postnatal folliculogenesis. Dev Biol 2009; 334: 458 467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kezele P, Nilsson E, Skinner MK. Cell-cell interactions in primordial follicle assembly and development. Front Biosci 2002; 7: d1990 d1996 [DOI] [PubMed] [Google Scholar]
- Pepling ME. From primordial germ cell to primordial follicle: mammalian female germ cell development. Genesis 2006; 44: 622 632 [DOI] [PubMed] [Google Scholar]
- Dunn NR, Winnier GE, Hargett LK, Schrick JJ, Fogo AB, Hogan BL. Haploinsufficient phenotypes in Bmp4 heterozygous null mice and modification by mutations in Gli3 and Alx4. Dev Biol 1997; 188: 235 247 [DOI] [PubMed] [Google Scholar]
- Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, Korving JP, Hogan BL. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev 1999; 13: 424 436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev 1995; 9: 2105 2116 [DOI] [PubMed] [Google Scholar]
- Paredes A, Garcia-Rudaz C, Kerr B, Tapia V, Dissen GA, Costa ME, Cornea A, Ojeda SR. Loss of synaptonemal complex protein-1, a synaptonemal complex protein, contributes to the initiation of follicular assembly in the developing rat ovary. Endocrinology 2005; 146: 5267 5277 [DOI] [PubMed] [Google Scholar]
- Baarends WM, Uilenbroek JT, Kramer P, Hoogerbrugge JW, van Leeuwen EC, Themmen AP, Grootegoed JA. Anti-mullerian hormone and anti-mullerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology 1995; 136: 4951 4962 [DOI] [PubMed] [Google Scholar]
- Hirobe S, He WW, Lee MM, Donahoe PK. Mullerian inhibiting substance messenger ribonucleic acid expression in granulosa and Sertoli cells coincides with their mitotic activity. Endocrinology 1992; 131: 854 862 [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction 2002; 124: 601 609 [DOI] [PubMed] [Google Scholar]
- Chang H, Lau AL, Matzuk MM. Studying TGF-beta superfamily signaling by knockouts and knockins. Mol Cell Endocrinol 2001; 180: 39 46 [DOI] [PubMed] [Google Scholar]
- Chang H, Zwijsen A, Vogel H, Huylebroeck D, Matzuk MM. Smad5 is essential for left-right asymmetry in mice. Dev Biol 2000; 219: 71 78 [DOI] [PubMed] [Google Scholar]
- de Sousa Lopes SM, Roelen BA, Monteiro RM, Emmens R, Lin HY, Li E, Lawson KA, Mummery CL. BMP signaling mediated by ALK2 in the visceral endoderm is necessary for the generation of primordial germ cells in the mouse embryo. Genes Dev 2004; 18: 1838 1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechleider RJ, Ryan JL, Garrett L, Eng C, Deng C, Wynshaw-Boris A, Roberts AB. Targeted mutagenesis of Smad1 reveals an essential role in chorioallantoic fusion. Dev Biol 2001; 240: 157 167 [DOI] [PubMed] [Google Scholar]
- Tremblay KD, Dunn NR, Robertson EJ. Mouse embryos lacking Smad1 signals display defects in extra-embryonic tissues and germ cell formation. Development 2001; 128: 3609 3621 [DOI] [PubMed] [Google Scholar]
- Yang X, Castilla LH, Xu X, Li C, Gotay J, Weinstein M, Liu PP, Deng CX. Angiogenesis defects and mesenchymal apoptosis in mice lacking SMAD5. Development 1999; 126: 1571 1580 [DOI] [PubMed] [Google Scholar]
- Ying Y, Liu XM, Marble A, Lawson KA, Zhao GQ. Requirement of Bmp8b for the generation of primordial germ cells in the mouse. Mol Endocrinol 2000; 14: 1053 1063 [DOI] [PubMed] [Google Scholar]
- Ying Y, Zhao GQ. Cooperation of endoderm-derived BMP2 and extraembryonic ectoderm-derived BMP4 in primordial germ cell generation in the mouse. Dev Biol 2001; 232: 484 492 [DOI] [PubMed] [Google Scholar]
- Zhao C, Hu Y. [The effect of implantation of reconstituted bone xenograft on the production of IL-2]. Zhonghua Wai Ke Za Zhi 1996; 34: 589 591 [PubMed] [Google Scholar]
- Childs AJ, Kinnell HL, Collins CS, Hogg K, Bayne RA, Green SJ, McNeilly AS, Anderson RA. BMP signaling in the human fetal ovary is developmentally regulated and promotes primordial germ cell apoptosis. Stem Cells 2010; 28: 1368 1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley BM, Runyan C, Takeuchi Y, Schaible K, Molyneaux K. BMP signaling regulates PGC numbers and motility in organ culture. Mech Dev 2007; 124: 68 77 [DOI] [PubMed] [Google Scholar]
- Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev 2009; 30: 438 464 [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol 1991; 124: 43 101 [DOI] [PubMed] [Google Scholar]
- Lee WS, Yoon SJ, Yoon TK, Cha KY, Lee SH, Shimasaki S, Lee S, Lee KA. Effects of bone morphogenetic protein-7 (BMP-7) on primordial follicular growth in the mouse ovary. Mol Reprod Dev 2004; 69: 159 163 [DOI] [PubMed] [Google Scholar]
- Nilsson EE, Skinner MK. Kit ligand and basic fibroblast growth factor interactions in the induction of ovarian primordial to primary follicle transition. Mol Cell Endocrinol 2004; 214: 19 25 [DOI] [PubMed] [Google Scholar]
- Tanwar PS, O'Shea T, McFarlane JR. In vivo evidence of role of bone morphogenetic protein-4 in the mouse ovary. Anim Reprod Sci 2008; 106: 232 240 [DOI] [PubMed] [Google Scholar]
- Trombly DJ, Woodruff TK, Mayo KE. Suppression of Notch signaling in the neonatal mouse ovary decreases primordial follicle formation. Endocrinology 2009; 150: 1014 1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Cook RW, Kipp JL, Shea LD, Mayo KE, Woodruff TK. Postnatal regulation of germ cells by activin: the establishment of the initial follicle pool. Dev Biol 2006; 298: 132 148 [DOI] [PubMed] [Google Scholar]
- Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology 2007; 148: 3580 3590 [DOI] [PubMed] [Google Scholar]
- Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr Rev 2004; 25: 72 101 [DOI] [PubMed] [Google Scholar]
- Li Q, Pangas SA, Jorgez CJ, Graff JM, Weinstein M, Matzuk MM. Redundant roles of SMAD2 and SMAD3 in ovarian granulosa cells in vivo. Mol Cell Biol 2008; 28: 7001 7011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerboom D, Paquet M, Hsieh M, Liu J, Jamin SP, Behringer RR, Sirois J, Taketo MM, Richards JS. Misregulated Wnt/beta-catenin signaling leads to ovarian granulosa cell tumor development. Cancer Res 2005; 65: 9206 9215 [DOI] [PubMed] [Google Scholar]
- Wordinger RJ, Clark AF. Bone morphogenetic proteins and their receptors in the eye. Exp Biol Med (Maywood) 2007; 232: 979 992 [DOI] [PubMed] [Google Scholar]
- Massague J, Chen YG. Controlling TGF-beta signaling. Genes Dev 2000; 14: 627 644 [PubMed] [Google Scholar]
- Mine N, Anderson RM, Klingensmith J. BMP antagonism is required in both the node and lateral plate mesoderm for mammalian left-right axis establishment. Development 2008; 135: 2425 2434 [DOI] [PubMed] [Google Scholar]
- McMahon JA, Takada S, Zimmerman LB, Fan CM, Harland RM, McMahon AP. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev 1998; 12: 1438 1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C, Vezina CM, Allgeier SH, Shaw A, Yu M, Peterson RE, Bushman W. Noggin is required for normal lobe patterning and ductal budding in the mouse prostate. Dev Biol 2007; 312: 217 230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa S, Fukuda K. Recent advances in cardiovascular regenerative medicine: the induced pluripotent stem cell era. Expert Rev Cardiovasc Ther 2008; 6: 803 810 [DOI] [PubMed] [Google Scholar]
- Gazzerro E, Minetti C. Potential drug targets within bone morphogenetic protein signaling pathways. Curr Opin Pharmacol 2007; 7: 325 333 [DOI] [PubMed] [Google Scholar]
- Stafford DA, Brunet LJ, Khokha MK, Economides AN, Harland RM. Cooperative activity of noggin and gremlin 1 in axial skeleton development. Development 2011; 138: 1005 1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.