ABSTRACT
The adult mouse penis represents the end point of masculine sex differentiation of the embryonic genital tubercle and contains bone, cartilage, the urethra, erectile bodies, several types of epithelium, and many individual cell types arrayed into specific anatomical structures. Using contemporary high-resolution imaging techniques, we sought to provide new insights to the current description of adult mouse penile morphology to enable understanding of penile abnormalities, including hypospadias. Examination of serial transverse and longitudinal sections, scanning electron microscopy, and three-dimensional (3D) reconstruction provided a new appreciation of the individual structures in the adult mouse penis and their 3D interrelationships. In so doing, we discovered novel paired erectile bodies, the male urogenital mating protuberance (MUMP), and more accurately described the urethral meatus. These morphological observations were quantified by morphometric analysis and now provide accurate morphological end points of sex differentiation of mouse penis that will be the foundation of future studies to identify normal and abnormal penile development.
Keywords: developmental biology, differentiation, external genitalia, male reproductive tract, os penis, penile urethra, penis, prepuce, sex differentiation
Complete formation of the adult mouse penis is the end point of masculine sex differentiation of the external genitalia.
INTRODUCTION
The evolution of internal fertilization has resulted in the emergence of anatomic male and female structures required for this complex reproductive process. Accordingly, specific sets of male and female external genitalia have evolved, namely, the penis and scrotum in males and the labia majora, labia minora, clitoris, and vagina in females, along with internal urogenital organs. Sexually dimorphic external genitalia have evolved in insects, fishes, amphibians, birds, reptiles, and mammals [1]. Within these groups there is broad diversity of anatomical forms, particularly in the penis [2, 3]. Indeed, external and internal penile morphology within mammals is different in both species and taxonomic families [2–4].
The primary functions of the penis are urination, copulation, and sperm transfer [2, 5]. Secondary functions of the penis include stimulation of the female and rival sperm displacement, which are achieved via anatomic specializations such as penile shape, length, papillae (spines), and other appendages [2, 3].
Given the importance of the penis in reproduction and the impact of penile malformations on its function, a significant body of literature has described the cellular and molecular development of the external genitalia [1, 3, 6, 7]. Most of these developmental studies have focused on morphogenesis of the ambisexual genital tubercle in the mouse embryo and have used a range of genetically engineered mutants having a variety of informative malformations.
The penis and clitoris emerge from the ambisexual genital tubercle (GT), a common primordium of identical morphology in embryos of both sexes. In mice, the elements of the ambisexual GT are assembled over a 4-day period (Gestation Days 12–16) [6]. Sex differentiation of the ambisexual GT occurs from Embryonic Day 16 onward. At birth, the external genitalia of both sexes are profoundly undifferentiated even though subtle sex differences are discernible. Morphological sex differences between male and female external genitalia emerge postnatally via complex developmental processes affected by hormonal signaling through steroid receptors and culminate in adult morphology (penis and clitoris) [8].
According to current literature, the anatomy of the mouse penis is described as comprising a body proximally and a glans distally that connect at a right-angle bend of the urethra. The body contains the corpus cavernosum penis and the penile urethra. The glans is the segment distal to the right angle bend and contains the penile urethra, the corpus cavernosum glandis, the corpus cavernosum urethrae, and skeletal elements (collectively called the os penis) [9–11]. The os penis is composed of proximal and distal elements. The proximal element contains hyaline growth cartilage proximally and bone distally. The distal element is comprised of fibrocartilage that ossifies after puberty [10, 11]. In conjunction with hydrostatic mechanisms, these skeletal elements presumably provide rigidity required for successful mating [2].
Accurate description of normal adult penile morphology is imperative to understand how and why abnormal development occurs. Much of the literature is decades old and is based on two-dimensional information derived from histological sections. Contemporary imaging and morphological techniques using Three-dimensional (3D) reconstruction have transformed our view of complex structures such as the penis and clitoris. In this study we confirm some of the historic descriptions of the adult mouse penis but also reveal and correct inaccuracies in the literature and discover new anatomic features not previously described.
MATERIALS AND METHODS
Adult wild-type mice CD-1 and C57BL/6 mice (Charles River Breeding Laboratories, Wilmington, MA) were housed in polycarbonate cages (20 × 25 × 47 cm3) with laboratory-grade pellet bedding. The mice were given Purina lab diet and tap water ad libitum and killed at 60 days of age. The Institutional Animal Care and Use Committee at the University of California, San Francisco, approved all animal use protocols. The external genitalia were photographed to identify typical surface characteristics with a digital camera and a dissecting microscope. External genitalia were dissected and fixed in formalin, paraffin embedded, and serially sectioned (transversely and longitudinally) at 7 μm for histologic staining.
Morphometric analysis of key structures was obtained by direct microscopic measurement of longitudinal sections and by counting the number of serial transverse sections containing the object of interest, including the urethra, bone, and cartilage. A total of five C57BL/6 mice and five male CD-1 mice were examined morphometrically. All parameters were not significantly different in C57BL/6 and CD-1 mice, and thus their measurements were combined. The C57BL/6 strain was chosen because many mutant strains utilize this strain background. The CD-1 strain was utilized because it is a frequently used, multipurpose, outbred strain.
Anatomical 3D computer reconstructions were created from the serial transverse sections utilizing SURF driver 3.5 software (SURF driver, University of Hawaii and University of Alberta) or Maya (Autodesk, Inc., San Rafael, CA). Sections were digitized to achieve linear tracings of relevant structures, including bone, cartilage, urethra, preputial glands, and the outer contour of the external genitalia. Digital linear tracings were oriented using Photoshop software (Adobe, Inc. San Rafael) and then exported into either SURF driver or Maya software for 3D reconstruction.
Surface details were elucidated via scanning electron microscopy (SEM). External genitalia were dissected and fixed in 2% glutaraldehyde 0.1 M sodium cacodylate buffer at pH of 7.2 for 6 h. The specimens were then fixed in 2% osmium tetraoxide for 2 h, subsequently dehydrated in serial alcohol solutions, and critical point dried in a Tousimis AutoSamdri 815 Critical Point Dryer (Tousimis, Rockville, MD). The specimens were then mounted on a stub with carbon tape, and images were obtained using a Hitachi TM-1000 SEM (Hitachi High Technologies America, Inc., Pleasanton, CA).
RESULTS
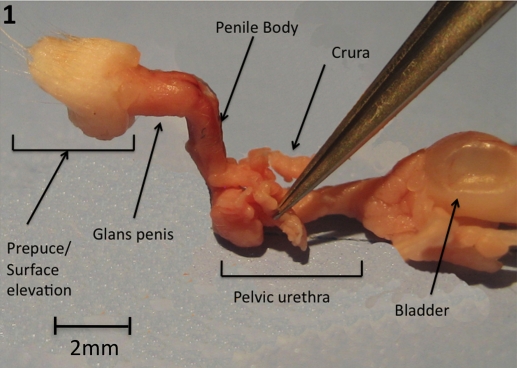
The surface elevation in the perineum of adult male mice is not the glans penis; rather, it is the prepuce. The glans penis lies deep to the surface elevation and can be exposed when the prepuce is retracted. The penis and foreskin develop from genital tubercle of the fetus. The prepuce is bifid distally and is covered with hair on its outer surface. The midline cleft on the prepuce is the opening of the preputial space [8]. The preputial gland ducts open into the preputial space near the tip of the prepuce. The body of the penis begins proximally at the pelvic outlet where the pelvic urethra makes a right-angle bend. At this point the paired corpora cavernosa (crura) emanating from the penile body diverge laterally to attach to the pubic bone (Fig. 1). As described previously, the body of the penis contains the urethra and the corpus cavernosum (once the right and left crura fuse in the midline). The penile body ends distally at a right-angle bend where the penile body joins the glans penis (Fig. 1).
FIG. 1.
Gross image of adult mouse urogenital tract. Bladder and seminal vesicles on right, distal tip of genital tubercle on left. Note that bilateral crura project laterally at level of the bulbar urethra. One crus is held by forceps.
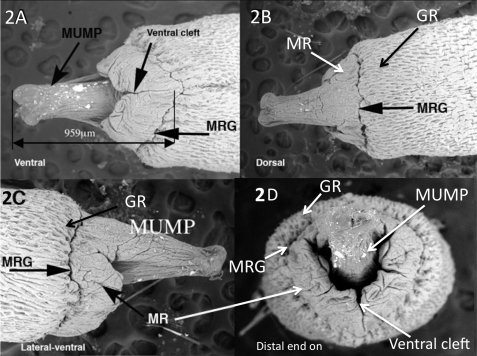
On the distal aspect of the glans penis is the male urogenital mating protuberance (MUMP) that we briefly described (Fig. 2) [8]. The MUMP extends from the dorsal aspect of a ridge encircling the glans, which we call the MUMP ridge (Fig. 2). The MUMP ridge is defined proximally by a circumferential groove (MUMP ridge groove) that invaginates proximally into the glans. Proximal to the MUMP ridge groove (MRG) is another ridge encircling the glans penis herein called the glanular ridge (GR). The MUMP ridge is clefted in the ventral midline. Closure of this ventral cleft occurs about 1 mm from the tip of the MUMP and defines the urethral meatus. The urethral lumen is Y shaped distally (Fig. 2D) and becomes oval shaped proximally (Figs. 2D and 5).
FIG. 2.
SEM images of adult distal glans penis. A) Ventral view showing the ventral cleft ending near the MUMP ridge groove (MRG). B) Dorsal view, showing MUMP ridge (MR) and glanular ridge (GR) separated by MUMP ridge groove. C) Lateral view. D) Distal view, showing Y-shaped urethral meatus and ventral cleft. Modified from Yang et al. 2010 [8].
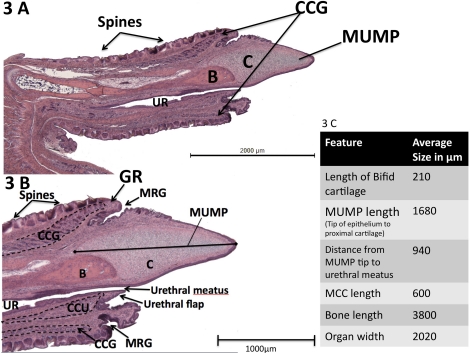
FIG. 5.
Scanning electron micrograph (top), transverse H&E sections (middle) and midsagittal H&E section (bottom) of the glans penis. Arrows indicate exact corresponding location between transverse and longitudinal sections. Modified from Yang et al. 2010 [8].
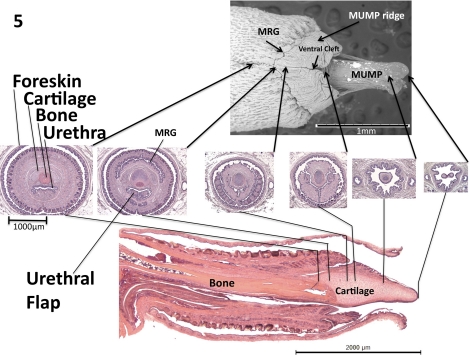
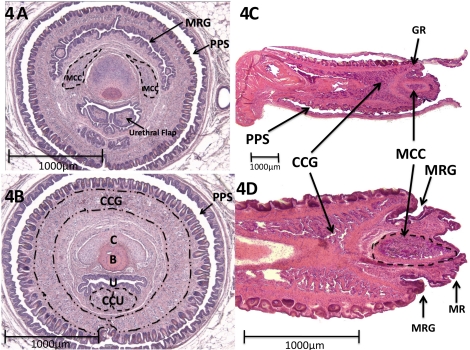
Figure 3 demonstrates midsagittal views of the glans penis, with corresponding morphometric data. In this specimen the foreskin has been removed, but normally the penis is located within the preputial space (Figs. 4 and 5). The surface of the glans penis is covered circumferentially with epithelial spines (Figs. 2, 3, and 4, A and B). These are keratinized projections found circumferentially around the penile skin as described previously [10–12]. The core element of the MUMP is cartilage. The MUMP measures approximately 1.7 mm in length (from its distal tip to the most proximal extent of its cartilaginous core), contains a central body of fibrocartilage, and is covered by epithelium, devoid of spines. The MRG and GR seen on SEM (Fig. 2) are better visualized in longitudinal sections (Figs. 3 and 4). Longitudinal sections clearly indicate that the urethra does not extend to the tip of the penis as previously described [11] but rather begins ∼1 mm proximal to the distal tip of the MUMP (Figs. 2A and 3). Proximal to the urethral meatus, distinct short bilateral intraluminal projections (urethral “flaps”) emanate from the ventral aspect of the urethra. These previously unreported bilateral projections are untethered distally and coalesce proximally into a single structure that attaches ventrally to the urethral wall (Figs. 3–5). These flaps are first identifiable at about 25 days of age. The function of these urethral flaps is unknown. Centrally, bone extends from the right-angle bend of the penis to the level of the MRG and measures approximately 3.8 mm in length. Distally, the bone is overlapped dorsally by the fibrocartilage of the MUMP (Figs. 3–6). Hyaline cartilage, which is the growth plate of the bone, is present on the proximal aspect of the bone as previously described [10, 11]. This cartilage eventually undergoes endochondral ossification and thus gradually disappears by approximately 6 mo of age [13].
FIG. 3.
Mid-sagittal H&E images of adult mouse penis with corresponding morphometric data. A) Midsagittal H&E section of glans penis. B) Close-up midsagittal view of the distal glans penis. C) Morphometric data table highlighting important features and average sizes, lengths, or distances in microns. B, bone; C, cartilage; CCG, corpus cavernosa glandis; CCU, corpus cavernosa urethrae; UR, urethra.
FIG. 4.
Erectile bodies of the adult mouse penis. A) Distal transverse section demonstrating the bilateral distal MUMP corpus cavernosum (MCC) flanking the cartilage. Note at this level that the urethral flap is bifid. Surrounding the penis is the foreskin and preputial space (PPS). B) More proximal transverse section. Note the corpora cavernosa glandis (CCG) is not in continuity ventrally at this level. C) Paramedial sagittal image showing the corpus cavernosa glandis (CCG) extending into glanular ridge (GR). D) Another paramedical section showing ellipsoid MUMP corpus cavernosum (MCC) extending into the MUMP ridge. B, bone; C, cartilage; CCU, corpus cavernosa urethrae; U, urethra.
FIG. 6.
3D reconstructions of the distal glans. The components are not to scale, as they have been elongated to better demonstrate 3D relationships. A–C) Dorsal views showing that the MUMP cartilage is bifid distally and overlaps the bone proximally. D–F) Side and ventral views demonstrate that both cartilage and bone lie dorsal to the penile urethra. Note that the tubular urethra does not extend to the distal tip of the penis. In B, the skin has been rendered semitransparent. In F, the urethra has been rendered semitransparent. Blue, bone; purple, skin; red, cartilage; yellow, urethra.
Figure 4 depicts the erectile bodies in transverse and sagittal sections. Two distinct erectile bodies have been described previously within the glans penis [11] and are outlined in the figures. The corpus cavernosum glandis is circumferential. For the first time we report that the corpus cavernosum glandis extends distally into the GR in close relation to the MRG (Fig. 4, B–D). The corpus cavernosum urethrae is linear, runs ventral to the urethra as described previously [11], and appears to be homologous to the corpus spongiosum of the human. Additionally, our analyses discovered paired bilateral erectile bodies not previously described. These bilaterally paired structures (MUMP corpus cavernosum) flank the cartilaginous core of the MUMP within the glans penis (Fig. 4, A, C, and D). They extend from the level of the proximal MUMP and fuse proximally in the midline dorsal to the cartilage (not illustrated), ending at the level where the MUMP cartilage overlaps the bone. Note that the MUMP corpus cavernosum is distinct from the circumferential corpus cavernosum glandis, which does not extend distally beyond the GR (Fig. 4, C and D).
In Figure 5, key histologic transverse sections are seen in parallel with a midsagittal histologic section and a ventral SEM of the glans penis. By correlating the transverse sections to the corresponding levels of both the SEM and the longitudinal images, we obtain a particularly informative perspective of proportionate sizes, location, and orientation of the penile elements. Further, to better appreciate spatial relationships, 3D reconstructions were prepared from 7-μm serial section sets. A Maya-based 3D reconstruction (artificially elongated for clarity; Fig. 6) reinforces the SEM showing that the MUMP is bifid distally and contains cartilage that extends proximally into the glans to dorsally overlap the os penis. Both the cartilage and the bone lie dorsal to the penile urethra. As stated above, the tubular urethra begins about 1 mm proximal to the distal tip of the MUMP, where the edges of the ventral cleft fuse.
DISCUSSION
Using contemporary morphological techniques including SEM, 3D reconstruction, and morphometric analysis, we re-examined the morphology of the adult mouse glans penis. In doing so, we have advanced understanding of the morphology of the adult mouse penis, better defined the end points of male sex differentiation, clarified past inconsistencies, and added new detailed information. We have precisely defined the MUMP as well as the MUMP corpus cavernosum, a paired erectile body associated with the MUMP. Our study revises the position of the urethral meatus. The tubular urethra opens ventrally ∼1 mm from the tip of the MUMP and not distally near the penile tip as described previously [11]. Another novel finding of the current study is the description of the MUMP ridge, the MRG, the GR, and the fact that the corpus cavernosa glandis extends into the GR. While previously published diagrams of the mouse and rat glans penis show these structures [10, 11, 14], the actual grooves and ridges were not labeled or discussed in relation to function. The MRG and the GR constitute a notable redundancy in the surface of the glans. An erectile body, namely, the corpus cavernosa glandis, extends into the GR and thus provides a mechanism for circumferential expansion of the glans during erection. Finally, urethral “flaps” that project into the urethral lumen were discovered, and their function is unknown.
We have demonstrated that the penis terminates distally in a distinctive bifid, semirigid penile process (the MUMP), as described recently [8]. The function of the MUMP and its cartilaginous core is unknown but may play a role in stimulation of the female. In addition to the previously described corpus cavernosum glandis, corpus cavernosum penis, and corpus cavernosum urethrae [11], we have discovered the MUMP corpus cavernosum, paired erectile bodies associated with the cartilaginous core of the MUMP. These elements (cartilage and the MUMP corpus cavernosum) are presumably involved in stiffening, supporting, or expanding the MUMP during mating. Finally, we present morphometric data on a variety of parameters describing penile morphology.
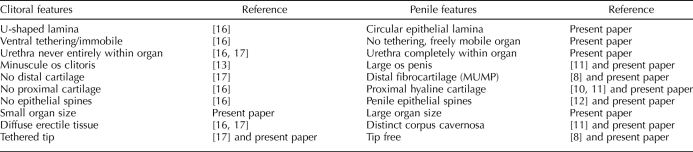
Our precise and comprehensive description of adult mouse penile morphology that encompasses SEM, histological serial sections, morphometrics, and 3D reconstruction provides more accurate and detailed end points of sex differentiation of the penis to which abnormal development can be compared. Information from this article on adult mouse penile morphology in conjunction with the literature on the adult mouse clitoris and previous and ongoing studies of the mouse clitoris allow us to define at least 10 homologous anatomic features that distinguish the adult mouse penis and clitoris (Table 1). It is important to recognize that each of these homologous features arises during sex differentiation (which occurs mostly postnatally) from the common constellation of structures comprising the embryonic ambisexual stage. Understanding the salient features distinguishing the adult penis and clitoris is essential for future studies focusing on specific morphogenetic effects of hormones, gene manipulation, medication, or environmental chemicals on sex differentiation of the external genitalia. In a generic sense, a variety of substances having hormonal (especially estrogenic) activity are known to adversely affect penile development [9, 15]. The more detailed description of adult penile morphology presented herein provides a wider range of specific morphologic end points for assessing adverse hormonal (or genetic) effects on internal or external features of the penis. External genitalia play a critical role in reproduction by facilitating sperm deposition, stimulating the female (an essential event for reflex ovulators), and/or removing sperm of competitors [2]. Thus, understanding adult penile morphology and its development is a crucial aspect of reproductive biology.
TABLE 1.
Comparison of homologous penile and clitoral morphological features.
ACKNOWLEDGMENT
Thanks are in order for the technical assistance of Joshua Migliardi.
Footnotes
Co-first authors; these authors contributed equally to this work.
Supported by NSF grant IOS-0920793, NIH grant RO1 DK0581050, and NH&MRC grant APP1002733.
REFERENCES
- Yamada G, Suzuki K, Haraguchi R, Miyagawa S, Satoh Y, Kamimura M, Nakagata N, Kataoka H, Kuroiwa A, Chen Y. Molecular genetic cascades for external genitalia formation: an emerging organogenesis program. Dev Dyn 2006; 235: 1738 1752 [DOI] [PubMed] [Google Scholar]
- Simmons MN, Jones JS. Male genital morphology and function: an evolutionary perspective. J Urol 2007; 177: 1625 1631 [DOI] [PubMed] [Google Scholar]
- Williams-Ashman HG. Enigmatic features of penile development and functions. Perspect Biol Med 1990; 33: 335 374 [DOI] [PubMed] [Google Scholar]
- Seney ML, Kelly DA, Goldman BD, Sumbera R, Forger NG. Social structure predicts genital morphology in African mole-rats. PLoS One 2009; 4: e7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Bachiller D, Chen YP, Kamikawa M, Ogi H, Haraguchi R, Ogino Y, Minami Y, Mishina Y, Ahn K, Crenshaw EB, III, Yamada G. Regulation of outgrowth and apoptosis for the terminal appendage: external genitalia development by concerted actions of BMP signaling. Development 2003; 130: 6209 6220 [DOI] [PubMed] [Google Scholar]
- Cohn MJ. Developmental genetics of the external genitalia. Adv Exp Med Biol 2004; 545: 149 157 [DOI] [PubMed] [Google Scholar]
- Yamada G, Satoh Y, Baskin LS, Cunha GR. Cellular and molecular mechanisms of development of the external genitalia. Differentiation 2003; 71: 445 460 [DOI] [PubMed] [Google Scholar]
- Yang JH, Menshenina J, Cunha GR, Place N, Baskin LS. Morphology of mouse external genitalia: Implications for a role of estrogen in sexual dimorphism of the mouse genital tubercle. J Urol 2010; 184: 1604 1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal HO, Braden TD, Williams CS, Williams JW. Role of estrogen in induction of penile dysmorphogenesis: a review. Reproduction 2007; 134: 199 208 [DOI] [PubMed] [Google Scholar]
- Murakami R. Histogenesis of the os penis and os clitoridis in rats. Dev Growth Differ 1984; 26: 419 426 [DOI] [PubMed] [Google Scholar]
- Murakami R. A histological study of the development of the penis of wild-type and androgen-insensitive mice. J Anat 1987; 153: 223 231 [PMC free article] [PubMed] [Google Scholar]
- Iguchi T, Irisawa S, Uesugi Y, Kusunoki S, Takasugi N. Abnormal development of the os penis in male mice treated neonatally with tamoxifen. Acta Anat 1990; 139: 201 208 [DOI] [PubMed] [Google Scholar]
- Glucksmann A, Ooka-Souda S, Miura-Yasugi E, Mizuno T. The effect of neonatal treatment of male mice with antiandrogens and of females with androgens on the development of the os penis and os clitoridis. J Anat 1976; 121: 363 370 [PMC free article] [PubMed] [Google Scholar]
- Murakami R. Development of the os penis in genital tubercles cultured beneath the renal capsule of adult rats. J Anat 1986; 149: 11 20 [PMC free article] [PubMed] [Google Scholar]
- Wang MH, Baskin LS. Endocrine disruptors, genital development and hypospadias. J Androl 2008; 29: 499 505 [DOI] [PubMed] [Google Scholar]
- Martin-Alguacil N, Pfaff DW, Shelley DN, Schober JM. Clitoral sexual arousal: an immunocytochemical and innervation study of the clitoris. BJU Int 2008; 101: 1407 1413 [DOI] [PubMed] [Google Scholar]
- Weiss DA, Rodriguez E, Jr, Cunha T, Barcellos D, Chan LY, Risbridger G, Baskin L, Cunha G. Morphology of the external genitalia of the adult male and female mice as an end point of sex differentiation. Mol Cell Endocrinol 2012. (in press). Published online ahead of print 27 August 2011; DOI:10.1016/j.mce.2011.08.009 [DOI] [PMC free article] [PubMed]