ABSTRACT
A hypothesis to explain the maternal age-dependent increase in formation of aneuploid eggs is deterioration of chromosome cohesion. Although several lines of evidence are consistent with this hypothesis, whether cohesion is actually reduced in naturally aged oocytes has not been directly tested by any experimental perturbation. To directly target cohesion, we increased the activity of separase, the protease that cleaves the meiotic cohesin REC8, in oocytes. We show that cohesion is more susceptible to premature separase activation in old oocytes than in young oocytes, demonstrating that cohesion is significantly reduced. Furthermore, cohesion is protected by two independent mechanisms that inhibit separase, securin and an inhibitory phosphorylation of separase by CDK1; both mechanisms must be disrupted to prematurely activate separase. With the continual loss of cohesins from chromosomes that occurs throughout the natural reproductive lifespan, tight regulation of separase in oocytes may be particularly important to maintain cohesion and prevent aneuploidy.
Keywords: aging, aneuploidy, meiosis, oocyte, separase
Age increases susceptibility of chromosome cohesion to premature separase activation, and cohesion is protected independently by securin and an inhibitory phosphorylation of separase.
INTRODUCTION
It is well established that errors in meiotic chromosome segregation increase with maternal age [1]. A leading hypothesis to explain age-related aneuploidy is deterioration of chromosome cohesion with increasing maternal age. Cohesion is established in the premeiotic S phase during fetal development, and it must remain functional until meiosis resumes before ovulation in the adult, which can be up to 50 yrs later in humans. Several lines of evidence in mouse are consistent with the cohesion hypothesis. Cohesion can only be established when the meiotic cohesin protein REC8 is expressed before birth [2], and cohesion can be maintained for at least 8 mo without replenishment if expression of another meiotic cohesin, SMC1B, is restricted to before birth [3]. These results suggest that cohesins load onto chromosomes and establish functional cohesion only during fetal development and that the cohesin complexes initially laid down must last for the long period of time before meiosis resumes. Furthermore, in SMC1B-deficient mice and flies, the loss of cohesion worsens with age (e.g., from 17 days to 2 mo in mice), suggesting that cohesion function is susceptible to age [4, 5].
In natural aging mouse models, REC8 protein is continually lost from chromosomes with age, and the observed segregation errors in oocytes from old mice (old oocytes) are consistent with reduced cohesion [6–8]. These studies provide correlative support for the cohesion hypothesis; however, a crucial element—reduced cohesion in naturally aged oocytes—has not been directly tested by any experimental perturbation. To test the cohesion hypothesis, we increased the activity of separase, the protease that cleaves REC8, to directly reduce cohesion, with the prediction that chromosomes in old oocytes should be more susceptible to increased separase activity. Because most maternal errors originate from meiosis I (MI) [9, 10], we focused on MI cohesion.
MATERIALS AND METHODS
Oocyte Collection and Culture
B6D2F1/J female mice were used for all experiments. B6D2F1/J mice were obtained from the Jackson Laboratory and National Institutes of Aging and were aged to 6–14 wk (young), 11–12 mo (12m), or 17–20 mo (old). Oocytes from young mice were used unless otherwise noted. Oocytes from gonadotropin-treated females were collected in modified Hepes-buffered Whitten medium and cultured in Chatot-Ziomek-Bavister (CZB) medium as previously described [11]. All animal experiments were approved by the Institutional Animal Use and Care Committee and were consistent with National Institutes of Health guidelines.
Securin Morpholino and AA-Separase Mutant Design
Morpholino (MO) against securin (GATAAGAGTAGCCATTCTGGATTAC) and a 5-bp mismatch MO control (GATAACACTACCGATTCTCGATTAC; sequences obtained from Nabti et al. [12]) were ordered through Gene Tools LLC. The AA-separase mutant (AA-separase) was constructed by mutating both CDK1 phosphorylation sites, S1121A and T1342A [13], in a full-length mouse separase (Espl1) cDNA clone (Source BioScience) using the Quikchange Multi Site-Directed Mutagenesis kit (Agilent Technologies). The resulting cDNA was cloned into the pIVT vector, and cRNA was synthesized using the TranscriptAid T7 High Yield Transcription Kit (Thermo Fisher Scientific). Approximately 5–7 pl of cRNA were microinjected into the cytoplasm of germinal vesicle-intact oocytes as previously described [11].
Immunocytochemistry
Oocytes were fixed in 2% paraformaldehyde with 0.1% Triton-X-100 for 30 min, then permeabilized and blocked as previously described [11]. REC8, securin, kinetochores, and DNA were detected using REC8 antiserum (1:1000 dilution) [6], a securin antibody (ab3305; 1:200 dilution; Abcam), CREST (calcinosis, Raynaud syndrome, esophageal dysmobility, sclerodactyly, telangiesctasia) autoimmune serum (1:40 dilution; ImmunoVision), and SYTOX Green nucleic acid stain (1:5000 dilution; Invitrogen), respectively. The primary antibodies were detected by Alexa Fluor secondary antibodies (1:100 dilution; Invitrogen). Images were collected on a spinning disk confocal microscope (DMI4000 B; Leica Microsystems, Inc.) with a 100× 1.4 NA objective, an XY piezo-z stage (Applied Scientific Instrumentation), a spinning disk (Yokogawa), an electron multiplier charge-coupled device camera (ImagEM; Hamamatsu Photonics), and an LMM5 laser merge module equipped with 440-, 488-, and 593-nm lasers (Spectral Applied Research) controlled by Metamorph software (MDS Analytical Technologies).
Live Imaging and Kinetochore Distance Measurements
An H2B-targeted sensor for proteolytic activity of separase, based on a mitotic separase sensor (developed by Toru Hirota, Cancer Institute, Japanese Foundation for Cancer Research) was constructed to contain REC8 cleavage sites in between CFP and YFP fluorescent proteins. However, results using the sensor were difficult to interpret in our system, so we did not make separase activity measurements. Instead, these constructs were used to mark chromosomes in our live imaging experiments and, in the text, are referred to as fluorescently labeled H2B. Complementary RNA was synthesized using the T7 mMESSAGE mMACHINE kit (Ambion). Oocytes were microinjected with AA-separase cRNA, securin MO, and/or a cRNA-encoding fluorescently labeled H2B, then arrested in 2.5 μM milrinone for 18–19 h and transferred to individual 3-μl drops of CZB medium on a coverslip 5 h after milrinone washout. Differential interference contrast and CFP images were acquired on a Leica DM6000 microscope with a 63× 1.4 NA objective and a charge-coupled device camera (Orca-AG; Hamamatsu Photonics) controlled by Metamorph Software. A z-series of three images was collected at 3-μm intervals for oocytes in MI (5 or 8 h after milrinone washout). Images were processed using ImageJ software (NIH). Some oocytes from the live imaging experiments were then fixed and stained for kinetochores (CREST) and DNA (SYTOX Green). Images of fixed cells were collected with the spinning disk confocal microscope described above. Outer kinetochore distances were measured from the outer edges of all kinetochore staining as previously described [6].
RESULTS
Cohesion Is Maintained by Two Independent Mechanisms that Inhibit Separase
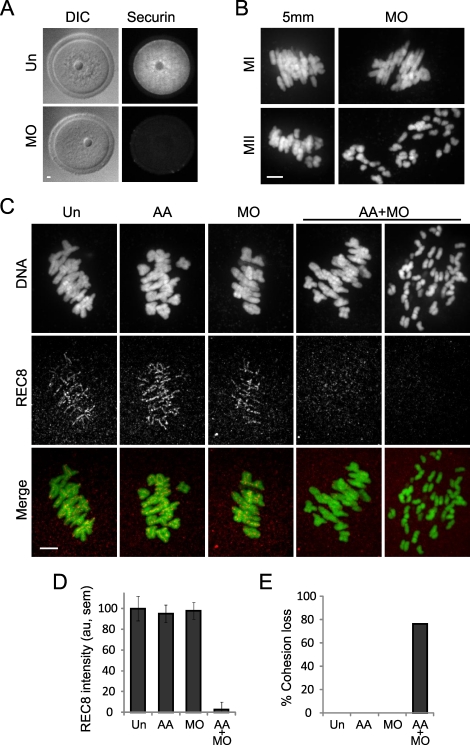
Two independent mechanisms contribute to keep separase inactive until anaphase: securin (PTTG1 in mouse) binding and inhibitory phosphorylation of separase by CDK1 [13–16]. The relative importance of these two mechanisms is unclear in MI [12, 17, 18], where separase inhibition may be particularly important due to the extended duration of both the long prophase arrest (months to years) and metaphase (∼8 h). To increase separase activity, we independently disrupted each of the two inhibitory mechanisms. First, we used an established MO to knock down securin protein expression [12] (Fig. 1A). Consistent with previous results, securin MO alone did not lead to premature chromosome separation in MI but resulted in complete chromatid separation by meiosis II (MII) (Fig. 1B). Second, we microinjected cRNA encoding mouse separase with two known CDK1 phosphorylation sites mutated to alanines: S1121A and T1342A (AA-separase), mutations previously shown to prevent inhibition by CDK1 [13].
FIG. 1.
Cohesion is maintained by two independent mechanisms that inhibit separase. A) Uninjected oocytes (Un; n = 10) or oocytes microinjected with securin MO (n = 17) were fixed and stained for securin protein in germinal vesicle-intact oocytes. B) Oocytes microinjected with securin MO or a 5-bp mismatch MO (5 mm) were matured in vitro to MI (MO, n = 19; 5 mm, n = 11) or MII (MO, n = 10; 5 mm, n = 8), fixed, and stained for DNA. C and D) Un oocytes (n = 23) or oocytes microinjected with AA-separase cRNA (AA; n = 18), securin MO (n = 14), or both (AA+MO; n = 21) were fixed 8 h after meiotic resumption, and REC8 protein was detected by immunocytochemistry. Oocytes were collected from a total of five mice. Representative images (C) are maximal intensity projections of confocal z-series showing DNA (green) and REC8 (red). Note that in AA+MO oocytes, REC8 levels are low even when bivalents are intact. REC8 fluorescence intensity (mean ± SEM) was normalized to the mean intensity of Un oocytes and quantified for each group (D). E) Percentage of oocytes with cohesion loss, as indicated by prematurely separated bivalents and chromatids, was determined for each group. Bar = 5 μm.
To determine if separase is active, we tested for loss of cohesin protein and loss of functional cohesion by measuring chromosome-associated REC8 levels and the incidence of premature chromosome separation in MI, respectively. Oocytes were microinjected with AA-separase cRNA, securin MO, or AA-separase cRNA and securin MO together (AA+MO), then were matured to MI in vitro, fixed 8 h after meiotic resumption, and stained for DNA and REC8 (Fig. 1C). We observed similar levels of chromosome-associated REC8 in uninjected, AA-separase, and securin MO oocytes. However, in AA+MO oocytes, REC8 levels were reduced at least 90% (P < 0.05) (Fig. 1, C and D). Furthermore, all chromosomes were intact in uninjected, AA-separase, and securin MO oocytes, whereas the majority of AA+MO oocytes showed premature chromosome separation of both bivalents and sister chromatids (Fig. 1, C and E). REC8 levels were indistinguishable in AA+MO oocytes where chromosomes separated compared to those where bivalents remained intact and aligned at the metaphase plate. These results indicate that either securin expression or separase phosphorylation is sufficient to suppress separase activity. Only when both inhibitory mechanisms are abolished is REC8 lost and premature chromosome separation found.
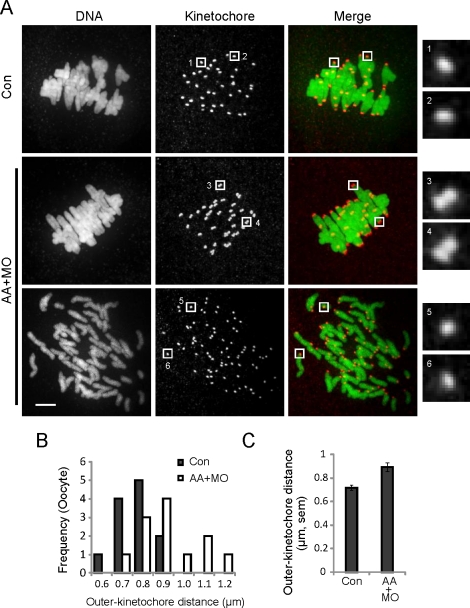
Premature chromosome separation in MI reflects loss of cohesion from chromosome arms, but centromere cohesion has another important function in MI: holding kinetochores of sister chromatids together so they attach to the same pole, or mono-orient. Weakened centromere cohesion leads to increased separation of sister kinetochores, which promotes erroneous biorientation [19–21]. To test whether any loss of functional cohesion occurs in cells where REC8 is reduced but chromosome arm cohesion remains intact, we examined centromere cohesion in MI. Control or AA+MO oocytes were matured in vitro to MI, then fixed and stained for DNA and kinetochores (Fig. 2A). In all control oocytes (n = 12), bivalents were intact and aligned at the metaphase plate. Approximately half of the AA+MO oocytes (n = 11 of 23) displayed premature bivalent and chromatid separation, whereas the rest (n = 12 of 23) contained intact bivalents. To assess centromere cohesion, kinetochore pairs in the latter group were compared to those in control oocytes. Based on kinetochore staining, sister kinetochores were overlapping or indistinguishable in the controls, whereas most sister kinetochores were distinct in AA+MO oocytes (Fig. 2A). Distances between sister kinetochores, as measured from the outer edges of the pairs, were significantly greater in AA+MO oocytes (0.89 ± 0.03 μm; values are mean ± SEM throughout) than in control oocytes (0.72 ± 0.02 μm; P < 0.05) (Fig. 2, B and C). These results show that centromere cohesion is reduced even when bivalents are intact, as in oocytes from old mice [6, 7], and suggest that centromeres are more susceptible than chromosome arms to cohesion loss.
FIG. 2.
Centromeres are more susceptible than chromosome arms to cohesin loss. A) Control oocytes (Con; n = 12) or oocytes microinjected with both AA-separase cRNA and securin MO (AA+MO; n = 23) were fixed 8 h after meiotic resumption and stained for DNA (green) and kinetochores (CREST; red). The AA+MO group includes oocytes with intact chromosomes (middle; n = 12 of 23) and prematurely separated chromosomes (bottom; n = 11 of 23). Images are maximal intensity projections of confocal z-series, with insets of single optical sections to show kinetochores. Insets 1–4 include the two kinetochores of a sister pair; insets 5 and 6 show single kinetochores in AA+MO oocytes where chromosomes prematurely separated. Oocytes were collected from a total of four mice. Bar = 5 μm. B and C) Outer kinetochore distances were measured from the outer edges of sister kinetochore pairs for control and AA+MO oocytes with intact chromosomes (n = 240 kinetochore pairs from 12 oocytes in each group). The populations are represented by histograms (B) and by the mean ± SEM for each group (C).
Cohesion Is Reduced in Old Oocytes
To determine if natural aging leads to reduced cohesion, we tested whether oocytes from older mice are more susceptible to separase activity. We compared oocytes from mice at three age groups: young, 12m, and old. In the 12m group, chromosome-associated REC8 is already below the level that we can accurately quantify. However, cohesion remains functional, and the incidence of aneuploidy in MII eggs is similar to that in young mice (∼5%). By 19 mo (old group), the incidence of aneuploidy is substantially increased, from approximately 5% to approximately 20% [6].
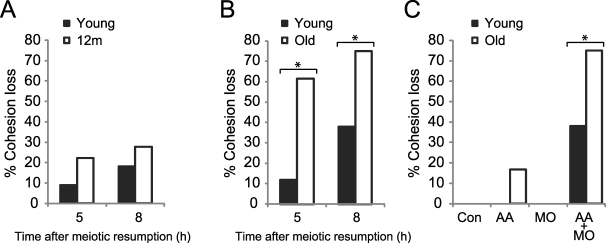
To test separase susceptibility, oocytes were microinjected with both AA-separase cRNA and securin MO, together with a cRNA-encoding fluorescently labeled H2B. In these experiments, we used lower concentrations of both AA-separase cRNA and securin MO compared to our previous experiments to increase our sensitivity to differences between the age groups. The oocytes were then matured in vitro and imaged live by fluorescence to monitor chromosome configuration at 5 and 8 h after meiotic resumption. At both time points, most bivalents were intact in both young (n = 22) and 12m (n = 18) oocytes (Fig. 3A), indicating a comparable susceptibility to cohesion loss. Using the same assay, however, we found significantly more old oocytes with prematurely separated bivalents and chromatids compared to young oocytes (Fig. 3B) (P < 0.05), indicating a reduction of functional cohesion in old oocytes. We also tested effects of single separase perturbations in old oocytes and found cohesion to be largely functional: Some AA-separase oocytes did contain prematurely separated chromosomes (n = 2 of 12), but the increase was not statistically significant (Fig. 3C).
FIG. 3.
Cohesion is reduced in old oocytes. A) Young (n = 22 from four mice) and 12m oocytes (n = 18 from eight mice) microinjected with a cRNA-encoding fluorescently labeled H2B, AA-separase cRNA, and securin MO to increase separase activity were matured in vitro and imaged live by fluorescence at 5 and 8 h after meiotic resumption. Percentage of oocytes with cohesion loss, as indicated by prematurely separated bivalents and chromatids, was determined for each group. At both time points, bivalents in young and 12m oocytes are similarly intact. B) Young and old oocytes were microinjected as in A and imaged at 5 h (n = 17 young and 13 old) and 8 h (n = 29 young and 20 old) after meiotic resumption. Percentage of oocytes with cohesion loss is significantly higher in old oocytes compared to young (asterisks) at both 5 h (Fisher exact test, P = 0.007) and 8 h (P = 0.019). Oocytes were collected from 11 young and 10 old mice. C) Young and old oocytes were microinjected with a cRNA-encoding fluorescently labeled H2B (Control; n = 94 young and 14 old), AA-separase cRNA (AA; n = 6 young and 12 old), securin MO (MO; n = 13 young and 9 old), or both AA and MO (AA+MO; n = 29 young and 20 old) and imaged at 8 h after meiotic resumption. The increased cohesion loss in old AA oocytes is not statistically significant. Oocytes were collected from 34 young and 34 old mice.
DISCUSSION
We introduced an experimental perturbation of cohesion in naturally aged oocytes to directly test the cohesion hypothesis of age-related aneuploidy. When separase activity is increased in MI, old oocytes are more prone to premature separation of bivalents and sister chromatids compared to young counterparts. This result demonstrates that cohesion is reduced in old oocytes, at the age when the risk of chromosome segregation errors and aneuploidy is elevated. Furthermore, increased separase activity in young oocytes mimics the loss of cohesion during natural aging [6, 7]. In both cases, REC8 levels are severely reduced, and sister kinetochores are farther apart even when bivalents remain intact, suggesting that centromere cohesion is more vulnerable than arm cohesion. Our results also show that separase is regulated by two independent mechanisms in MI: binding to securin and inhibitory phosphorylation by CDK1. Either is sufficient to protect cohesion, and both must be disrupted to have any effect on either chromosome-associated REC8 levels or premature chromosome separation. These complementary mechanisms may be particularly important in oocytes, because current evidence suggests that cohesion can only be established in premeiotic S phase and must subsequently be maintained as cohesins are continually lost from chromosomes during aging [2, 3, 6, 7]. Tight regulation of separase likely is essential for the fidelity of meiotic chromosome segregation throughout the normal female reproductive lifespan.
ACKNOWLEDGMENTS
T.C. thanks Karen Schindler and Olga Davydenko for useful discussions.
Footnotes
Supported by a grant from the National Institutes of Health (HD 058730) to R.M.S. and M.A.L. and a Searle Scholar award to M.A.L.
REFERENCES
- Hassold T, Hall H, Hunt P. The origin of human aneuploidy: where we have been, where we are going. Hum Mol Genet 2007; 16(spec no. 2): R203 R208 [DOI] [PubMed] [Google Scholar]
- Tachibana-Konwalski K, Godwin J, van der Weyden L, Champion L, Kudo NR, Adams DJ, Nasmyth K. Rec8-containing cohesin maintains bivalents without turnover during the growing phase of mouse oocytes. Genes Dev 2010; 24: 2505 2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revenkova E, Herrmann K, Adelfalk C, Jessberger R. Oocyte cohesin expression restricted to predictyate stages provides full fertility and prevents aneuploidy. Curr Biol 2010; 20: 1529 1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges CA, Revenkova E, Jessberger R, Hassold TJ, Hunt PA. SMC1beta-deficient female mice provide evidence that cohesins are a missing link in age-related nondisjunction. Nat Genet 2005; 37: 1351 1355 [DOI] [PubMed] [Google Scholar]
- Subramanian VV, Bickel SE. Aging predisposes oocytes to meiotic nondisjunction when the cohesin subunit SMC1 is reduced. PLoS Genet 2008; 4: e1000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang T, Duncan FE, Schindler K, Schultz RM, Lampson MA. Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr Biol 2010; 20: 1522 1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister LM, Kouznetsova A, Hyslop LA, Kalleas D, Pace SL, Barel JC, Nathan A, Floros V, Adelfalk C, Watanabe Y, Jessberger R, Kirkwood TB. et al Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol 2010; 20: 1511 1521 [DOI] [PubMed] [Google Scholar]
- Liu L, Keefe DL. Defective cohesin is associated with age-dependent misaligned chromosomes in oocytes. Reprod Biomed Online 2008; 16: 103 112 [DOI] [PubMed] [Google Scholar]
- Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet 2001; 2: 280 291 [DOI] [PubMed] [Google Scholar]
- Warren WD, Gorringe KL. A molecular model for sporadic human aneuploidy. Trends Genet 2006; 22: 218 224 [DOI] [PubMed] [Google Scholar]
- Duncan FE, Chiang T, Schultz RM, Lampson MA. Evidence that a defective spindle assembly checkpoint is not the primary cause of maternal age-associated aneuploidy in mouse eggs. Biol Reprod 2009; 81: 768 776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabti I, Reis A, Levasseur M, Stemmann O, Jones KT. Securin and not CDK1/cyclin B1 regulates sister chromatid disjunction during meiosis II in mouse eggs. Dev Biol 2008; 321: 379 386 [DOI] [PubMed] [Google Scholar]
- Stemmann O, Zou H, Gerber SA, Gygi SP, Kirschner MW. Dual inhibition of sister chromatid separation at metaphase. Cell 2001; 107: 715 726 [DOI] [PubMed] [Google Scholar]
- Ciosk R, Zachariae W, Michaelis C, Shevchenko A, Mann M, Nasmyth K. An ESP1/PDS1 complex regulates loss of sister chromatid cohesion at the metaphase to anaphase transition in yeast. Cell 1998; 93: 1067 1076 [DOI] [PubMed] [Google Scholar]
- Huang X, Hatcher R, York JP, Securin Zhang P. and separase phosphorylation act redundantly to maintain sister chromatid cohesion in mammalian cells. Mol Biol Cell 2005; 16: 4725 4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorr IH, Boos D, Stemmann O. Mutual inhibition of separase and Cdk1 by two-step complex formation. Mol Cell 2005; 19: 135 141 [DOI] [PubMed] [Google Scholar]
- Herbert M, Levasseur M, Homer H, Yallop K, Murdoch A, McDougall A. Homologue disjunction in mouse oocytes requires proteolysis of securin and cyclin B1. Nat Cell Biol 2003; 5: 1023 1025 [DOI] [PubMed] [Google Scholar]
- Gorr IH, Reis A, Boos D, Wuhr M, Madgwick S, Jones KT, Stemmann O. Essential CDK1-inhibitory role for separase during meiosis I in vertebrate oocytes. Nat Cell Biol 2006; 8: 1035 1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuno T, Tada K, Watanabe Y. Kinetochore geometry defined by cohesion within the centromere. Nature 2009; 458: 852 858 [DOI] [PubMed] [Google Scholar]
- Li X, Dawe RK. Fused sister kinetochores initiate the reductional division in meiosis I. Nat Cell Biol 2009; 11: 1103 1108 [DOI] [PubMed] [Google Scholar]
- Chelysheva L, Diallo S, Vezon D, Gendrot G, Vrielynck N, Belcram K, Rocques N, Marquez-Lema A, Bhatt AM, Horlow C, Mercier R, Mezard C. et al AtREC8 and AtSCC3 are essential to the monopolar orientation of the kinetochores during meiosis. J Cell Sci 2005; 118: 4621 4632 [DOI] [PubMed] [Google Scholar]