Abstract
Objective
To prospectively estimate the risk for earlier ovarian failure among women undergoing hysterectomy with ovarian preservation, as compared to women of similar age without hysterectomy.
Methods
A prospective cohort study was conducted among women aged 30 to 47 years undergoing hysterectomy without bilateral oophorectomy (n=406) and women with intact uteri (n=465). Blood samples and questionnaire data were obtained at baseline and annually for up to 5 years. Hazard ratios (HR) for ovarian failure, defined as follicle-stimulating hormone (FSH) levels 40 IU/L or higher, were calculated using Cox proportional hazards models.
Results
Ovarian failure occurred among 60 of the women with hysterectomy and 46 of the control women. Women undergoing hysterectomy were at nearly a twofold increased risk for ovarian failure as compared to women with intact uteri (HR=1.92, 95% confidence interval (CI) 1.29 – 2.86). The proportional hazards model further estimated that 14.8% of women with hysterectomy experienced ovarian failure after four years of follow-up compared to 8.0% of the control women. Risk for ovarian failure was greater for women who had a unilateral oophorectomy along with their hysterectomy (HR=2.93, 95% CI 1.57 – 5.49), but also was significantly increased for women who retained both ovaries (HR=1.74, 95% CI 1.14 – 2.65).
Conclusions
Increased risk of earlier ovarian failure is a possible consequence of premenopausal hysterectomy. While it is unresolved whether it is the surgery itself or the underlying condition leading to hysterectomy that is the cause of earlier ovarian failure, physicians and patients should take into account this possible sequela when considering options for treatment of benign conditions of the uterus.
INTRODUCTION
Hysterectomy is the most common non-obstetrical surgical procedure among women in the United States. Although the increasing number of procedures performed in ambulatory settings makes estimation from hospital-based registries difficult, sources report from 460,000 to 600,000 procedures annually in the United States.(1, 2) Data from the Behavioral Risk Factor Surveillance System show that more than one-quarter of American women aged 18 to 75 have had a hysterectomy, and it is estimated that up to 40% of women will have a hysterectomy during their lifetimes. (3, 4) Although uterine artery embolization, endometrial ablation or progestin-releasing intrauterine devices are increasingly used for treating common indications for hysterectomy such as fibroids and dysfunctional uterine bleeding, hysterectomy rates remain high, (1) and the long-term outcomes after surgery are an important clinical consideration.
An unresolved concern with hysterectomy is whether it increases risk for early menopause. More than half of all hysterectomies are performed on women younger than age 44, with the highest rates among women aged 40 to 44 years.(5) The majority of women having pre-menopausal hysterectomies retain at least one ovary (5) because of evidence that the physical and psychological benefits derived from keeping the ovaries outweigh the possibility of ovarian pathology, including cancer.(6, 7) Although it is clear that most women do not lose ovarian function in the short-term after hysterectomy without bilateral oophorectomy, there has long been suspicion that these women are at increased risk for early ovarian failure.(8) Earlier menopause in turn has serious health implications including increased risk for osteoporosis, cardiovascular disease and all-cause mortality.(9-13)
The association between hysterectomy and early menopause has been examined in a number of studies dating back several decades, but most of them had important limitations including cross-sectional or retrospective study designs, lack of a control group, menopause classification based only on symptoms, small sample sizes or insufficient follow-up time. (8, 14-22) In the one prospective study with long-term follow-up and serial hormone measurements that evaluated risk for menopause among women having a hysterectomy (n=257) as compared to women of similar age with intact uteri (n=258), Farquhar and colleagues estimated that menopause occurred nearly four years earlier in women undergoing hysterectomy as compared to women who did not have the surgery.(20)
In this report we estimate the risk of earlier ovarian failure (defined as a serum follicle stimulating hormone (FSH) ≥ 40 IU/L) after pre-menopausal hysterectomy using data from a prospective study of ovarian function after hysterectomy conducted in North Carolina.
METHODS
Study subjects were pre-menopausal women in a prospective cohort known as the PROOF (Prospective Research on Ovarian Function) Study. Methods for the study have been described previously.(23) Between 2004 and 2007, we identified women scheduled to undergo hysterectomy from operating room schedules of the two hospitals in Durham, NC, both of which are part of the Duke University Health System. Potentially eligible women received a letter from their physician describing the study and informing them that an interviewer would be contacting them to ask them to take part. The interviewer verified that the women met the eligibility criteria of age 30 to 47 years, were pre-menopausal as evidenced by at least one menstrual period in the previous three months, had no personal history of cancer (except non-melanoma skin cancer), were able to complete an interview in English and at least one ovary was expected to be left intact after the hysterectomy. Eligible women who agreed to participate were scheduled for an interview visit during which they signed an informed consent form, completed an interviewer-administered questionnaire, had a blood specimen drawn and had body measurements taken (height, weight, waist circumference and hip circumference). All baseline visits occurred before the women’s hysterectomies, and most were done in conjunction with their pre-operative visits. The study subjects were re-contacted annually for follow-up visits to obtain updated questionnaire information and blood samples.
Control women were recruited using study brochures and ads in publications that were placed in clinics and offices of gynecology and family medicine practices within Duke University Health System. The eligibility criteria were age 30 to 47 years, pre-menopausal, no personal history of cancer, not currently pregnant and able to complete an interview in English. Recruitment was targeted such that the age distribution was similar to that of the cases and the race distribution was similar to that of Durham County. Baseline and follow-up interview visit procedures for the control women were the same as for the women undergoing hysterectomy. The study protocol was approved by the Duke University Medical Center Institutional Review Board.
Information obtained from the questionnaires included demographic characteristics, menstrual cycle characteristics, pregnancy history, history of contraceptive and hormone use, menopause symptoms, current use of medications and supplements and lifestyle characteristics such as smoking history and alcohol consumption. Clinical information for the women undergoing hysterectomy including type of surgery, pre- and post-operative diagnoses and pathologic information was abstracted from the medical records.
Blood samples obtained from the study subjects were spun down, the serum was divided into two or more aliquots depending on the volume obtained and the tubes were stored in a −80°C freezer until the time of analysis. All samples were analyzed for FSH at the Duke Clinical and Research Laboratories, which serves as the core laboratory for all general testing requirements for Duke Hospital and Clinics. The samples were analyzed in batches with samples from the women with hysterectomies and control women intermixed in each batch. Laboratory personnel were blinded to hysterectomy status.
A total of 902 women scheduled for hysterectomy were identified for the study. We were unable to contact or schedule an interview with 58 women before their surgery and 145 women were found to be ineligible (73 were planning to have a bilateral oophorectomy, 27 had a prior history of cancer, 14 decided against hysterectomy, and 31 did not meet other the eligibility criteria including age, ability to complete an interview in English, or menstrual periods in prior three months). Of the 699 women who were contacted and eligible, baseline surveys and blood samples were obtained from 504 (72.1%). Of these 504 women, we excluded from analysis those for whom medical record review after their surgery showed that they were ineligible due to bilateral oophorectomy or a cancer diagnosis (n=45), those with a baseline FSH value 40 IU/L or higher (n=6), and those who did not complete any follow-up visits (n=47), for a total of 406 women with hysterectomy included in the analysis.
Baseline interviews and blood samples were obtained from 518 control women. We excluded from our analysis women with baseline FSH values ≥ 40 IU/L (n=15), women who did not complete any follow-up visits (n=31), and women with a cancer diagnosis (n=6) or bilateral oophorectomy (n=1) during the first year of follow-up. The final number of control women included in the analysis was 465. When comparing characteristics of the women we excluded to those included in the study, we found no statistically significant differences except that the excluded controls had a lower educational level and were more likely to be current smokers.
Follow-up of the cohort continued until November 2009. The median number of blood draws (baseline and follow-up) was four and the maximum was six. Women enrolled as controls who subsequently had a hysterectomy were censored at the time of their surgery. Likewise, women were censored if they had a bilateral oophorectomy or a diagnosis of cancer. The primary outcome of ovarian failure was defined as an FSH value ≥ 40 IU/L.
The racial composition of the cohort was 48.9% White, 47.5% African American and 3.6% other races. For race-specific results, we conducted analyses comparing African American women to White women and African American women to all other races. Because the results were substantively the same in these analyses, the results presented in this paper compare African American women to all other races.
Statistical Analyses
Comparisons between women with hysterectomy and controls and between racial groups were made with t-tests for continuous variables and chi-square or Fisher’s exact test for categorical variables. Kaplan-Meier plots were used to show the time to ovarian failure comparing women with and without hysterectomy. Cox proportional hazard modeling was performed to calculate the hazard ratios (HR) and 95% confidence intervals (CI) for ovarian failure. Variables that are established predictors of menopause or were associated with risk for ovarian failure in bivariate analyses were included in multivariable models. The proportional hazards assumption was tested by evaluating interactions between the variables of concern and log (time). None of the time-dependent variables was significant, supporting the assumption of proportional hazards. Analyses were performed using SAS statistical software, version 9.2 (Cary, NC).
RESULTS
Descriptive characteristics of women undergoing hysterectomy and controls for the total study population and stratified by race are presented in Table 1. Women undergoing hysterectomy were more likely than controls to be parous and have a history of tubal ligation. No statistically significant differences were observed for age at menarche, history of cesarean delivery, history of infertility or duration of oral contraceptive use. In the overall group, body mass index was higher among the women undergoing hysterectomy than controls, with significant differences among non-African Americans but not among African American women. As would be expected, a greater proportion of women undergoing hysterectomy reported a history of uterine fibroids, endometriosis, ovarian cysts and myomectomy, with some differences by race. Uterine fibroids and a prior history of myomectomy were more commonly reported by African American women, whereas a history of endometriosis or ovarian cysts was more common among non-African Americans. Baseline FSH values were not significantly different between women undergoing hysterectomy and controls, or between African Americans and non-African Americans.
Table 1.
Baseline Characteristics of Women Undergoing Hysterectomy and Controls, for Total Study Population and Stratified by Race
| All Women | Non-African American Women | African American Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Hysterectom | Control | Hysterectom | Control | Hysterectomy | Control | ||||
| y n=406 (%) |
n=465 (%) |
P | y n=199 (%) |
n=264 (%) |
P |
n=207 (%) |
n=201 (%) |
P | |
| Age category (years) | |||||||||
| 30 – 34 | 5.9 | 9.0 | 0.2 | 8.0 | 9.8 | 0.8 | 3.9 | 8.0 | 0.04 |
| 35 – 39 | 24.4 | 26.9 | 27.1 | 24.2 | 21.7 | 30.3 | |||
| 40 – 44 | 43.1 | 37.9 | 38.2 | 37.5 | 47.8 | 38.3 | |||
| 44 - 47 | 26.6 | 26.2 | 26.6 | 28.4 | 26.6 | 23.4 | |||
| Full-term pregnancies | |||||||||
| None | 16.5 | 31.7 | <0.001 | 17.1 | 36.0 | <0.001 | 15.9 | 25.9 | 0.04 |
| 1 – 2 | 57.7 | 50.0 | 56.8 | 50.4 | 58.9 | 49.3 | |||
| ≥3 | 25.6 | 18.5 | 26.1 | 13.6 | 25.1 | 24.9 | |||
| Cesarean deliveries* | |||||||||
| None | 69.9 | 68.2 | 0.7 | 67.3 | 73.4 | 0.3 | 72.4 | 62.4 | 0.14 |
| 1 | 16.5 | 19.2 | 16.4 | 15.4 | 16.7 | 23.5 | |||
| ≥2 | 13.6 | 12.6 | 16.4 | 11.2 | 10.9 | 14.1 | |||
| Oral contraceptive duration |
|||||||||
| Never | 8.5 | 8.8 | 0.7 | 9.7 | 11.4 | 0.7 | 7.3 | 5.6 | 0.4 |
| <1 year | 11.0 | 11.3 | 13.3 | 9.8 | 8.8 | 13.1 | |||
| 1 year to <5 years | 21.7 | 24.1 | 24.0 | 24.3 | 19.5 | 23.7 | |||
| 5 years to <10 years ≥10 years |
35.2 | 30.9 | 32.1 | 30.2 | 38.0 | 31.8 | |||
| Reported history of: | |||||||||
| Tubal ligation | 45.0 | 25.9 | <0.001 | 38.2 | 16.3 | <0.001 | 51.7 | 38.3 | 0.007 |
| Infertility | 17.7 | 19.2 | 0.6 | 12.6 | 16.7 | 0.2 | 22.7 | 22.4 | 0.9 |
| Leiomyomas | 74.4 | 21.6 | <0.001 | 55.8 | 12.9 | <0.001 | 92.8 | 33.2 | <0.001 |
| Endometriosis | 15.3 | 8.7 | 0.002 | 21.1 | 8.0 | <0.001 | 9.7 | 9.6 | 0.9 |
| Myomectomy | 9.9 | 3.7 | <0.001 | 4.5 | 2.3 | 0.2 | 15.0 | 5.5 | 0.002 |
| Polycystic ovary syndrome |
2.5 | 2.8 | 0.9 | 3.5 | 3.4 | 0.9 | 1.5 | 2.0 | 0.7 |
| Ovarian cysts | 32.2 | 18.2 | <0.001 | 42.7 | 20.9 | <0.001 | 22.2 | 14.5 | 0.04 |
|
| |||||||||
| Body mass index (kg/m2) |
22.7 | 32.5 | 0.001 | 34.9 | 45.6 | 0.04 | 11.2 | 15.4 | 0.3 |
| <25 | 25.9 | 26.9 | 28.2 | 27.0 | 23.8 | 26.9 | |||
| 25 to <30 | 51.4 | 40.5 | 36.9 | 27.4 | 65.0 | 57.7 | |||
| ≥30 | 51.4 | 40.5 | 36.9 | 27.4 | 65.0 | 57.7 | |||
| Smoking status | |||||||||
| Never | 60.0 | 67.1 | 0.09 | 57.8 | 67.0 | 0.1 | 63.3 | 67.2 | 0.5 |
| Former smoker | 19.5 | 18.1 | 24.6 | 20.1 | 14.5 | 15.4 | |||
| Current smoker | 20.0 | 14.8 | 17.6 | 12.9 | 22.2 | 17.4 | |||
| Age at menarche (years), mean (SD) |
20.0 | 14.8 | 17.6 | 12.6 | 22.2 | 17.4 | |||
| BMI (kg/m2), mean (SD) |
31.3 (7.8) | 29.7 (8.2) | 0.01 | 28.6 (6.6) | 27.4 (6.7) | 0.05 | 33.7 (8.0) | 33.0 | 0.3 |
| FSH IU/L (at baseline), mean (SD) |
7.2 (5.9) | 7.5 (5.8) | 0.4 | 7.0(6.0) | 7.2(5.7) | 0.7 | 7.3(5.8) | 8.0 (6.0) | 0.3 |
SD, standard deviation; BMI, body mass index; FSH, follicle-stimulating hormone.
Among women with full-term pregnancies.
Clinical characteristics of the women with hysterectomies stratified by race are shown in Table 2. The mean uterine weight among African American women was more than double that of non-African American women (459 versus 211 grams, p<0.0001). African American women were more likely to undergo abdominal hysterectomy. No statistically significant differences were observed in the organs removed at surgery, although a slightly higher proportion of the non-African American women had supracervical hysterectomies or hysterectomies with unilateral oophorectomy. Fibroids and menorrhagia/dysfunctional uterine bleeding were the most common operative diagnoses reported for both African American and non-African American women. However, racial differences in the frequency of diagnoses were observed, with African American women more likely to have a diagnosis of fibroids whereas non-African American women were more likely to have endometriosis or pelvic organ prolapse.
Table 2.
Clinical Characteristics of Women Undergoing Hysterectomy, Stratified by Race
| Non-African American Women (n=199) |
African American Women (n=207) |
||||
|---|---|---|---|---|---|
| n | (%) | n | (%) | P | |
| Type of hysterectomy | |||||
| Abdominal | 85 | (42.9) | 141 | (68.1) | <0.001 |
| Vaginal | 94 | (47.5) | 58 | (28.0) | |
| Laparoscopic | 19 | (9.6) | 8 | (3.9) | |
| Organs removed | |||||
| Uterus only | 20 | (10.1) | 11 | (5.3) | 0.1 |
| Uterus, cervix | 146 | (73.4) | 171 | (82.6) | |
| Uterus, one ovary | 3 | (1.5) | 2 | (1.0) | |
| Uterus, cervix, one ovary | 30 | (15.1) | 23 | (11.1) | |
| Postoperative diagnosis* | |||||
| Leiomyomas | 90 | (45.2) | 178 | (86.0) | <0.001 |
| Dysmenorrhea | 37 | (18.6) | 36 | (17.4) | 0.8 |
| Menorrhagia/dysfunctional uterine bleeding |
127 | (63.8) | 140 | (67.6) | 0.4 |
| Pelvic pain | 20 | (10.1) | 8 | (3.9) | 0.01 |
| Endometriosis | 23 | (11.6) | 3 | (1.4) | <0.00 |
| Pelvic organ prolapse | 2 | (1.0) | 0 | (0.0) | 0.1 |
| Endometrial hyperplasia | 7 | (3.5) | 3 | (1.4) | 0.2 |
| Cervical dysplasia/carcinoma in situ Adenomyosis |
3 | (1.5) | 5 | (2.4) | 0.5 |
|
|
|||||
| Uterine weight (g), mean (SD) | 210.8 | (272.4) | 458.5 | (431.7) | <0.001 |
| Hemoglobin, mean (SD) | 12.9 | (1.5) | 11.8 | (1.7) | <0.001 |
| Baseline FSH (IU/L), mean (SD) | 7.0 | (6.0) | 7.3 | (5.8) | 0.7 |
SD, standard deviation; FSH, follicle-stimulating hormone.
More than one diagnosis may have been reported.
In Figure 1, the Kaplan-Meier curves depict the risk for ovarian failure (defined on the basis of an FSH value ≥ 40 IU/L) comparing women with hysterectomy to controls. During 2,410 person-years of follow-up, 106 women experienced ovarian failure, 60 of the women with hysterectomy and 46 of the control women.
Figure 1.
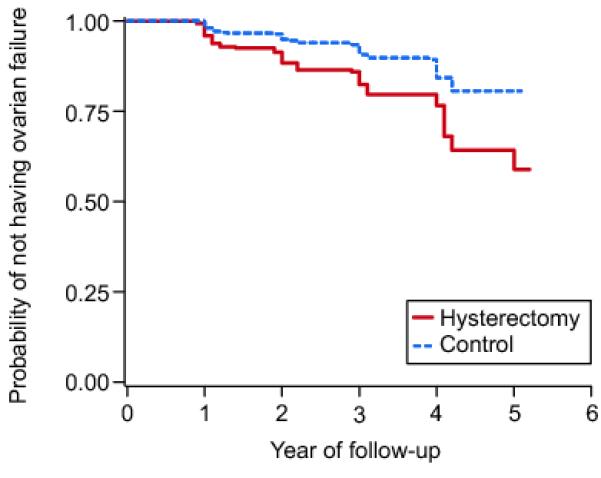
Kaplan-Meier plot showing the probability of not experiencing ovarian failure over years of follow-up for women with hysterectomy (solid line) and control women (dashed line). P-value for difference between hysterectomy and control group is <.001 by log-rank test.
Table 3 presents hazard ratios derived from proportional hazards models for ovarian failure by hysterectomy status. The hazard ratio for ovarian failure among the women with hysterectomy as compared to controls was 1.92, 95% CI 1.29 – 2.86, adjusting for age, race, BMI, smoking status and number of pregnancies. Further, the model estimated that after four years of follow-up, ovarian failure occurred among 14.8% (95% CI 8.6 – 20.7%) of the women with hysterectomy and 8.0% (95% CI 4.4 – 11.6%) of the control women. Other statistically significant predictors of menopause in the multivariable model were age (HR per year 1.36, 95% CI 1.27 – 1.46) and race (HR for non-AA vs. AA 0.59, 95% CI 0.39 – 0.90). As compared to women who had a hysterectomy with both ovaries left intact, the risk was higher for women who had a unilateral oophorectomy along with their hysterectomy.
Table 3.
Hazard Ratios and 95% Confidence Intervals for Ovarian Failure, Defined as Follicle-Stimulating Hormone Greater Than 40 IU/L, by Hysterectomy Status, Overall and Within Subgroups
| Hazard Ratio* |
95% Confidence Interval |
P | |
|---|---|---|---|
| All women | |||
| Controls | 1 | Reference | |
| Hysterectomy (all) | 1.92 | 1.29 – 2.86 | 0.001 |
| Hysterectomy only | 1.74 | 1.14 – 2.65 | 0.01 |
| Hysterectomy with unilateral oophorectomy |
2.93 | 1.57 – 5.49 | 0.001 |
| African American women | |||
| Controls | 1 | Reference | |
| Hysterectomy (all) | 1.58 | 0.92 – 2.72 | 0.1 |
| Hysterectomy only | 1.43 | 0.81 – 2.53 | 0.2 |
| Hysterectomy with unilateral oophorectomy |
2.74 | 1.10 – 6.87 | 0.03 |
| Non-African American women | |||
| Controls | 1 | Reference | |
| Hysterectomy (all) | 2.66 | 1.47 – 4.81 | 0.001 |
| Hysterectomy only | 2.48 | 1.30 – 4.74 | 0.01 |
| Hysterectomy with unilateral oophorectomy |
3.19 | 1.34 – 7.60 | 0.01 |
| Women aged 40 years and older | |||
| Controls | 1 | Reference | |
| Hysterectomy (all) | 1.79 | 1.18 – 2.71 | 0.006 |
| Hysterectomy only | 1.65 | 1.06 – 2.57 | 0.03 |
| Hysterectomy with unilateral oophorectomy |
2.49 | 1.27 – 4.87 | 0.01 |
| Women aged younger than 40 years | |||
| Controls | 1 | Reference | |
| Hysterectomy (all) | 4.29 | 0.83 – 22.26 | 0.08 |
| Hysterectomy only | 3.11 | 0.55 – 17.60 | 0.2 |
| Hysterectomy with unilateral oophorectomy |
19.17 | 2.15 – 171.2 | 0.01 |
Adjusted for age at baseline, body mass index, smoking status, number of full-term pregnancies and race (except in race-specific models).
We also used the model to calculate the difference in time when a given proportion of women in each group experienced ovarian failure as an estimate of how much earlier ovarian failure occurred among women with hysterectomy. The time difference between when 15% of the women in the control group and the hysterectomy group experienced ovarian failure was 1.88 years (95% CI 1.39 – 2.37). Although the study was not powered for sub-group analyses, we did exploratory analyses to examine risk for ovarian failure by hysterectomy status within race and age categories. In race-specific analyses, the multivariable-adjusted hazard ratio associated with hysterectomy was 2.66, 95% CI 1.47 – 4.81 for non-African American women and 1.58, 95% CI 0.92 – 2.72 for African American women. A test for interaction of race by hysterectomy status was not statistically significant. We also performed analyses within age strata (< 40 years and ≥ 40 years at baseline) to compare risk among groups of women who were earlier or later in their reproductive life. The hazard ratio for the younger women was markedly higher, albeit with a wide confidence interval, than for the older women (HR=4.29, 95% CI 0.83 – 22.3 and HR=1.79, 95% CI 1.18 – 2.71, respectively). The wide confidence interval for the younger women reflects the small number of events in this age range (7 instances of ovarian failure among women with hysterectomy versus 2 among controls).
DISCUSSION
This study found a nearly twofold increased risk for ovarian failure among women undergoing hysterectomy without bilateral oophorectomy as compared to women of similar age with intact uteri. In race-specific analyses, we found hazard ratios for non-African American women were higher than for African American women but tests for interaction between race and hysterectomy status were not statistically significant. Comparisons of Kaplan-Meier curves suggest that the increased risk was not due to an abrupt disruption of ovarian function after surgery, but rather a proportional excess risk throughout the period of follow-up.
When our study was designed, we used age-specific estimates of the risk for menopause (24) and the age distribution of women in our study to project that approximately 11% of the control women would experience ovarian failure during approximately four years of follow-up. Based on the proportional hazards model, we estimated that ovarian failure occurred in approximately 8% of the control women by four years of follow-up. The HR of 1.92 for women with hysterectomy corresponds to approximately 15% of women experiencing ovarian failure within four years of hysterectomy.
We also used the proportional hazards model to estimate that the difference in time to ovarian failure between women with and without hysterectomy was approximately 1.88 years, based on the time in which approximately 15% of the women in each group experienced ovarian failure. While a nearly two-year difference in age at ovarian failure is clinically important, these data should be interpreted cautiously because of the limited duration of follow-up of our study population. We do not have the data to conclude that this difference in time to ovarian failure would remain constant between the groups with further follow-up.
Our findings are generally consistent with the only other long-term, prospective study of ovarian function after hysterectomy in which serial hormone measurements were performed and there was a control group of women from the general population.(20) Farquhar and colleagues also reported an increased risk for ovarian failure among women with hysterectomy that was more pronounced for women having unilateral oophorectomy; however their estimate of the difference in time when 15% of women in each group experienced ovarian failure was 3.7 years, compared to our estimate of 1.88 years.(20)
Other recent studies that have examined risk of ovarian failure after hysterectomy have been inconsistent in their conclusions, with two studies reporting no effect (19, 21) while another reported an adverse effect of hysterectomy on ovarian function (22). However, because these studies either had no control group (19) or compared women with hysterectomy to women with uterine artery embolization (21, 22), the results are not strictly comparable to our study.
The strengths of our study include its large sample size, prospective design, serial measurements of hormone levels and the inclusion of large numbers of African American women. African American women have the highest rates of hysterectomy of any racial group within the U.S., with the most striking differences in rates during the mid to late pre-menopausal years (35 to 44 years) when their rates are one and half times those of White women.(5)
A limitation of our analysis is the use of FSH as a marker of ovarian failure. Although FSH is the biomarker most commonly used to categorize menopausal status, it is well-recognized that there is no cut-point that absolutely distinguishes pre-menopausal from post-menopausal women.(25) A value of 40 IU/L, which is commonly used in the menopause literature, is a very specific but less sensitive indicator of ovarian failure. Few pre-menopausal women will have an FSH value >40 IU/L, but some post-menopausal women will have lower FSH values. Using a cut-point of 20 or 30 IU/L would likely have increased sensitivity, but more pre-menopausal women would have been classified incorrectly as having ovarian failure. The misclassification of pre-menopausal women using a lower cut-point of FSH is a particular problem in a study of women with hysterectomy where it is not possible to time the blood draw around the menstrual cycle to ensure collection during the follicular phase. Consequently, it would have been difficult to differentiate if moderately high FSH values were indicative of menopause or the mid-cycle spike indicative of ovulation. Although using a FSH value of 40 IU/L undoubtedly misclassified some post-menopausal women as pre-menopausal, the stringent definition of menopause probably resulted in a conservative estimate of the likelihood of ovarian failure in the study population.
Another potential limitation of our study was the use of volunteers from the same health system as controls. We do not believe that the results were significantly influenced by our choice of control subjects since the study participants were unaware of the study hypothesis and the outcome was based on a biological measure. Observed differences in baseline characteristics between women with hysterectomies and controls are consistent with risk factors for hysterectomy reported in other studies including higher BMI, more pregnancies, and a history of tubal ligation.(26-28) The effects on risk of ovarian failure of these and other potential confounders were evaluated and those that were significantly associated with ovarian failure were controlled for in the proportional hazards models. Most importantly, the baseline FSH values were not statistically significantly different between the women having hysterectomies and the control women (7.2 and 7.5 IU/L, respectively, p=0.4), indicating the comparability of our groups at baseline.
Our findings are consistent with the long-standing hypothesis that women with hysterectomy experience ovarian failure at a younger age.(8) While there is now compelling evidence from two large prospective studies comparing FSH levels in women with hysterectomies and controls to support this impression, the causal pathways remain unknown. One of the most prominent hypotheses is that the surgery to remove the uterus compromises the blood flow to the ovaries, which could result in reduced production of hormones leading to earlier ovarian failure.(8, 29) The evidence for this mechanism is mixed, with most but not all studies finding a reduction in ovarian blood flow after hysterectomy.(30-33) Another hypothesis is that the uterus has an inhibitory influence of pituitary FSH secretions and consequently has an effect on follicular atresia.(29) It is posited that removal of the uterus allows FSH levels to rise and accelerates follicular depletion, leading to earlier menopause.
An alternative explanation for the earlier menopause observed among women undergoing hysterectomy is that it is not the surgery itself but the condition that led to the surgery that places women at increased risk for early ovarian failure. There are scant data on the risk of ovarian failure associated with common indications for hysterectomy such as dysfunctional uterine bleeding, fibroids or endometriosis. It is possible that certain cases of dysfunctional uterine bleeding that lead to hysterectomy are a more extreme manifestation of the menstrual changes that many women experience in the months or years preceding natural menopause. Whether other common indications for hysterectomy such as fibroids or endometriosis also could be associated with increased risk for early menopause is unknown. Indirect evidence based on decreased risk of fracture or higher bone mineral density among women with a history of fibroids suggests that estrogen levels and by extension, ovarian function, may be higher among women with fibroids,(34, 35) which would argue against women with fibroids being at higher risk for early ovarian failure.
The possibility that the increased risk for ovarian failure is due to the underlying condition leading to hysterectomy cannot be adequately addressed with the data currently available from this study. An important area for future research is the evaluation of ovarian reserve comparing women undergoing hysterectomy to control women. Anti-Müllerian hormone (AMH) has been proposed as a useful marker of ovarian reserve and possible predictor of age at menopause. (36) AMH levels correlate with antral follicle counts, exhibit near-linear declines after approximately age 30 and show little variability throughout the menstrual cycle. Repeated measurements of AMH before and after hysterectomy may be a useful tool for sorting out whether it is the indication for hysterectomy or the surgery itself that places women at higher risk for early menopause. A comparison of baseline AMH levels in women undergoing hysterectomy and control women would give insight into whether women undergoing hysterectomy (or women with specific indications for hysterectomy) have lower ovarian reserve as compared to women of similar age. Serial measurements that compare the rate of change in AMH levels over time between women with hysterectomy and controls could give an indication of whether removal of the uterus accelerates the rate of decline in ovarian reserve.
The major finding from our study is that women undergoing hysterectomy are at significantly increased risk for earlier ovarian failure as measured by serum FSH levels. While it is unresolved whether it is the surgery itself or the underlying condition leading to hysterectomy that is the cause of earlier ovarian failure, it is important that physicians consider this possible sequela when discussing with patients options for treatment of benign conditions of the uterus. In addition, because not all women will experience overt symptoms of menopause, women who have undergone premenopausal hysterectomy may warrant closer monitoring of bone density or cardiovascular risk factors because of their possible risk of early ovarian failure.
Acknowledgments
Supported by grants from the National Institutes of Health, National Institute on Aging (R01 AG020162) and National Center for Research Resources (UL1 RR024128-01).
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Whiteman MK, Hillis SD, Jamieson DJ, Morrow B, Podgornik MN, Brett KM, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008 Jan;198(1):34 e1–7. doi: 10.1016/j.ajog.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 2.Sample NI. Estimates for all discharges with any procedure code for abdominal and vaginal hysterectomy, Nationwide Inpatient Sample, 2009. 2009.
- 3.Erekson EA, Weitzen S, Sung VW, Raker CA, Myers DL. Socioeconomic indicators and hysterectomy status in the United States, 2004. J Reprod Med. 2009 Sep;54(9):553–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Merrill RM, Layman AB, Oderda G, Asche C. Risk estimates of hysterectomy and selected conditions commonly treated with hysterectomy. Ann Epidemiol. 2008 Mar;18(3):253–60. doi: 10.1016/j.annepidem.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Keshavarz HHS, Burney A, Marchbanks P. Hysterectomy surveillance - United States, 1994-1999. Surveillance Summaries MMWR. 2002:1–7. [Google Scholar]
- 6.Hickey M, Ambekar M, Hammond I. Should the ovaries be removed or retained at the time of hysterectomy for benign disease? Hum Reprod Update. 2010 Mar-Apr;16(2):131–41. doi: 10.1093/humupd/dmp037. [DOI] [PubMed] [Google Scholar]
- 7.Parker WH, Broder MS, Chang E, Feskanich D, Farquhar C, Liu Z, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses’ health study. Obstet Gynecol. 2009 May;113(5):1027–37. doi: 10.1097/AOG.0b013e3181a11c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddle N, Sarrel P, Whitehead M. The effect of hysterectomy on the age at ovarian failure: identification of a subgroup of women with premature loss of ovarian function and literature review. Fertil Steril. 1987 Jan;47(1):94–100. doi: 10.1016/s0015-0282(16)49942-5. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher JC. Effect of early menopause on bone mineral density and fractures. Menopause. 2007 May-Jun;14(3 Pt 2):567–71. doi: 10.1097/gme.0b013e31804c793d. [DOI] [PubMed] [Google Scholar]
- 10.Jacobsen BK, Heuch I, Kvale G. Age at natural menopause and all-cause mortality: a 37-year follow-up of 19,731 Norwegian women. Am J Epidemiol. 2003 May 15;157(10):923–9. doi: 10.1093/aje/kwg066. [DOI] [PubMed] [Google Scholar]
- 11.Mondul AM, Rodriguez C, Jacobs EJ, Calle EE. Age at natural menopause and cause-specific mortality. Am J Epidemiol. 2005 Dec 1;162(11):1089–97. doi: 10.1093/aje/kwi324. [DOI] [PubMed] [Google Scholar]
- 12.Jacobsen BK, Knutsen SF, Fraser GE. Age at natural menopause and total mortality and mortality from ischemic heart disease: the Adventist Health Study. J Clin Epidemiol. 1999 Apr;52(4):303–7. doi: 10.1016/s0895-4356(98)00170-x. [DOI] [PubMed] [Google Scholar]
- 13.Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006 Mar-Apr;13(2):265–79. doi: 10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]
- 14.Riedel HH, Lehmann-Willenbrock E, Semm K. Ovarian failure phenomena after hysterectomy. J Reprod Med. 1986 Jul;31(7):597–600. [PubMed] [Google Scholar]
- 15.Kaiser R, Kusche M, Wurz H. Hormone levels in women after hysterectomy. Arch Gynecol Obstet. 1989;244(3):169–73. doi: 10.1007/BF00931295. [DOI] [PubMed] [Google Scholar]
- 16.Derksen JG, Brolmann HA, Wiegerinck MA, Vader HL, Heintz AP. The effect of hysterectomy and endometrial ablation on follicle stimulating hormone (FSH) levels up to 1 year after surgery. Maturitas. 1998 Jun 3;29(2):133–8. doi: 10.1016/s0378-5122(98)00018-8. [DOI] [PubMed] [Google Scholar]
- 17.Metcalf MG, Braiden V, Livesey JH. Retention of normal ovarian function after hysterectomy. J Endocrinol. 1992 Dec;135(3):597–602. doi: 10.1677/joe.0.1350597. [DOI] [PubMed] [Google Scholar]
- 18.Cooper GS, Thorp JM., Jr FSH levels in relation to hysterectomy and to unilateral oophorectomy. Obstet Gynecol. 1999 Dec;94(6):969–72. doi: 10.1016/s0029-7844(99)00429-9. [DOI] [PubMed] [Google Scholar]
- 19.Read MD, Edey KA, Hapeshi J, Foy C. The age of ovarian failure following premenopausal hysterectomy with ovarian conservation. Menopause Int. 2010 Jun;16(2):56–9. doi: 10.1258/mi.2010.010022. [DOI] [PubMed] [Google Scholar]
- 20.Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005 Jul;112(7):956–62. doi: 10.1111/j.1471-0528.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- 21.Rashid S, Khaund A, Murray LS, Moss JG, Cooper K, Lyons D, et al. The effects of uterine artery embolisation and surgical treatment on ovarian function in women with uterine fibroids. BJOG. 2010 Jul;117(8):985–9. doi: 10.1111/j.1471-0528.2010.02579.x. [DOI] [PubMed] [Google Scholar]
- 22.Hehenkamp WJ, Volkers NA, Broekmans FJ, de Jong FH, Themmen AP, Birnie E, et al. Loss of ovarian reserve after uterine artery embolization: a randomized comparison with hysterectomy. Hum Reprod. 2007 Jul;22(7):1996–2005. doi: 10.1093/humrep/dem105. [DOI] [PubMed] [Google Scholar]
- 23.Moorman PG, Schildkraut JM, Myers ER, Wang F. Reported symptoms before and one year after hysterectomy in African American and white women. J Womens Health (Larchmt) 2011 Jul;20(7):1035–42. doi: 10.1089/jwh.2010.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato I, Toniolo P, Akhmedkhanov A, Koenig KL, Shore R, Zeleniuch-Jacquotte A. Prospective study of factors influencing the onset of natural menopause. J Clin Epidemiol. 1998 Dec;51(12):1271–6. doi: 10.1016/s0895-4356(98)00119-x. [DOI] [PubMed] [Google Scholar]
- 25.Backer LC, Rubin CS, Marcus M, Kieszak SM, Schober SE. Serum follicle-stimulating hormone and luteinizing hormone levels in women aged 35-60 in the U.S. population: the Third National Health and Nutrition Examination Survey (NHANES III, 1988-1994) Menopause. 1999 Spring;6(1):29–35. [PubMed] [Google Scholar]
- 26.Powell LH, Meyer P, Weiss G, Matthews KA, Santoro N, Randolph JF, Jr., et al. Ethnic differences in past hysterectomy for benign conditions. Womens Health Issues. 2005 Jul-Aug;15(4):179–86. doi: 10.1016/j.whi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Bower JK, Schreiner PJ, Sternfeld B, Lewis CE. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009 Feb;99(2):300–7. doi: 10.2105/AJPH.2008.133702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hillis SD, Marchbanks PA, Tylor LR, Peterson HB. Higher hysterectomy risk for sterilized than nonsterilized women: findings from the US. Collaborative Review of Sterilization. The U.S. Collaborative Review of Sterilization Working Group. Obstet Gynecol. 1998 Feb;91(2):241–6. doi: 10.1016/s0029-7844(97)00648-0. [DOI] [PubMed] [Google Scholar]
- 29.Chalmers C. Does hysterectomy in a premenopausal woman affect ovarian function? Med Hypotheses. 1996 Jun;46(6):573–5. doi: 10.1016/s0306-9877(96)90134-6. [DOI] [PubMed] [Google Scholar]
- 30.Lee DY, Park HJ, Kim BG, Bae DS, Yoon BK, Choi D. Change in the ovarian environment after hysterectomy as assessed by ovarian arterial blood flow indices and serum anti-Mullerian hormone levels. Eur J Obstet Gynecol Reprod Biol. Jul;151(1):82–5. doi: 10.1016/j.ejogrb.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 31.Petri Nahas EA, Pontes A, Nahas-Neto J, Borges VT, Dias R, Traiman P. Effect of total abdominal hysterectomy on ovarian blood supply in women of reproductive age. J Ultrasound Med. 2005 Feb;24(2):169–74. doi: 10.7863/jum.2005.24.2.169. [DOI] [PubMed] [Google Scholar]
- 32.Xiangying H, Lili H, Yifu S. The effect of hysterectomy on ovarian blood supply and endocrine function. Climacteric. 2006 Aug;9(4):283–9. doi: 10.1080/13697130600865774. [DOI] [PubMed] [Google Scholar]
- 33.Chan CC, Ng EH, Ho PC. Ovarian changes after abdominal hysterectomy for benign conditions. J Soc Gynecol Investig. 2005 Jan;12(1):54–7. doi: 10.1016/j.jsgi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Rozenberg S, Ham H, Peretz A, Robyn C, Degueldre M. Bone mineral content of women with uterine fibromyomas. Int J Fertil Menopausal Stud. 1994 Mar-Apr;39(2):77–80. [PubMed] [Google Scholar]
- 35.Randell KM, Honkanen RJ, Tuppurainen MT, Kroger H, Jurvelin JS, Saarikoski S. Fracture risk and bone density of peri- and early postmenopausal women with uterine leiomyomas. Maturitas. 2006 Feb 20;53(3):333–42. doi: 10.1016/j.maturitas.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 36.La Marca A, Volpe A. Anti-Mullerian hormone (AMH) in female reproduction: is measurement of circulating AMH a useful tool? Clin Endocrinol (Oxf) 2006 Jun;64(6):603–10. doi: 10.1111/j.1365-2265.2006.02533.x. [DOI] [PubMed] [Google Scholar]


