INTRODUCTION
There has been conflicting evidence in the literature as to whether an association exists between sleep apnea and glaucomatous or other forms of optic neuropathy. Several studies have demonstrated a link between sleep apnea and open-angle glaucoma (OAG)1-6, normal-tension glaucoma (NTG)1-3,7-9, nonarteritic ischemic optic neuropathy (NAION)10-12, papilledema13,14, and idiopathic intracranial hypertension (IIH)15-17. However, other studies show no relationship between glaucoma and sleep apnea.18-22 Use of continuous positive airway pressure as a treatment for sleep apnea has also been implicated as a cause of elevated intraocular pressure23 and questioned as an effective means of preventing optic neuropathy24while others studies recommend using continuous positive airway pressure to prevent glaucomatous or ischemic optic neuropathy.21,25
Should such an association exist between sleep apnea and glaucoma or other optic neuropathies, it may provide clues to the pathophysiologic mechanisms by which the optic nerve can become damaged from hypoxia associated with episodes of apnea. Furthermore, if sleep apnea (or its treatment with continuous positive airway pressure) is associated with any of these sight-threatening conditions, this knowledge could help to inform guidelines for monitoring the health of the optic nerves among patients with sleep apnea, or screening for sleep apnea among patients with disease of the optic nerves.
This study uses a large, national cohort to compare the incidence of glaucomatous and other forms of optic neuropathy among individuals with and without sleep apnea, and to assess whether the hazard for these conditions is associated with sleep apnea or its treatment, continuous positive airway pressure.
METHODS
Data Source
The i3 InVision Data Mart database (Ingenix, Eden Prairie, MN) contains detailed fully de-identified records of all beneficiaries in a large managed care network in the United States. We had access to data for beneficiaries in the Data Mart database who had any form of eye care from January 1, 2001 through December 31, 2007. This subset consisted of beneficiaries who had one or more International Classification of Diseases26 (ICD-9CM) code for any eye-related diagnosis (360-379.9), or Current Procedural Terminology27 (CPT-4) code for any eye-related visits, diagnostic or therapeutic procedures (65091-68899 or 92002-92499), or any other ICD-9 or CPT codes adjudicated by an ophthalmologist or optometrist during their time in the medical plan. We had access to all beneficiaries’ medical claims (inpatient, outpatient, skilled nursing facility) for ocular and nonocular medical conditions. The database also contains detailed records of demographic (age, sex, race, race/ethnicity) and socioeconomic information (education, household net worth) for each beneficiary.
Patients
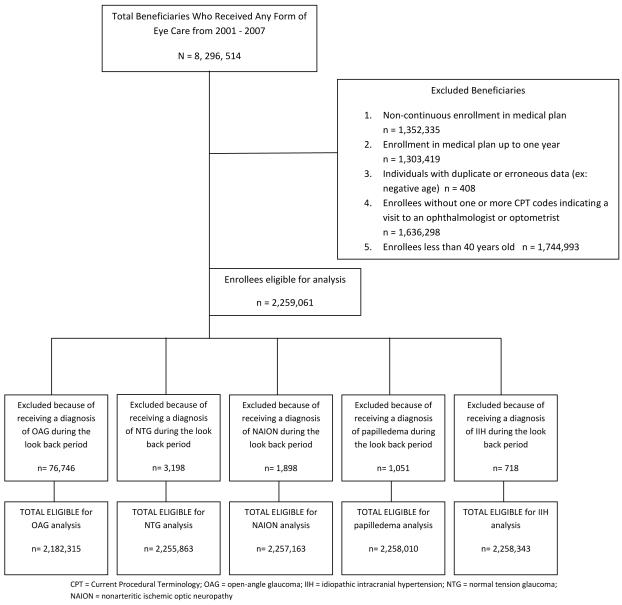
We identified all persons aged 40 or older in the i3 InVision Data Mart database for more than one consecutive year and with at least one visit to an eye-care provider during their time in the medical plan (Figure 1).
Figure 1.
Sample Selection of Enrollees Eligible for Study Inclusion to Determine the Relationship Between Sleep Apnea and Glaucomatous and Other Forms of Optic Neuropathy
Patients were identified as having sleep apnea if they received ≥1 of these International Classification of Diseases 9 Clinical Modification (ICD-9CM) diagnostic codes: 327.2, 327.20, 327.21, 327.23, 327.27, 327.29, 780.53, 780.57, 780.51 at any point during their time in the medical plan. These codes reflect forms of obstructive and central sleep apnea, distinguished further by etiology (e.g., organic, primary, or secondary) or accompanying symptoms, such as hypersomnia or insomnia. Obstructive sleep apnea due to repeated closure or near closure of the upper airway during sleep, affects at least 3% of adult Americans and is much more common than central sleep apnea, in which decreased drive to breathe causes pauses in breathing.28,29 Treatment with continuous positive airway pressure was identified by one or more records of CPT code 94660. (Supplemental Table #1 at AJO.com) shows the ICD-9CM codes used to identify persons with each of these ocular conditions: OAG, NTG, NAION, papilledema, IIH. A patient who received a diagnosis of suspected glaucoma but did not progress to OAG or NTG was not counted as experiencing either of these glaucoma types. The database does not contain information to determine whether persons diagnosed with two different ocular conuditions listed above had them in the same eye. Therefore, individuals could be diagnosed with more than one of these ocular conditions. Sensitivity analyses were performed to assess whether the findings differed significantly by requiring a second, confirmatory diagnosis of the ocular condition of interest to address concerns about misclassification of enrollees.
Incidence rates of ocular conditions were calculated by dividing the number of newly diagnosed beneficiaries with the ocular condition of interest by their time in the plan at risk. Diagnoses were considered incident cases if the enrollee did not have any record of the ocular condition of interest during their first year in the medical plan. A test of rate ratios was performed to compare the incidence rates of each condition among beneficiaries who did and did not have sleep apnea, and among those with sleep apnea who were receiving and did not receive continuous positive airway pressure.
Analyses
All analyses were performed by using SAS 9.2 (Cary, NC). Participant characteristics were summarized for the entire sample by using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. Incidence estimates, stratified according to sleep apnea status, were generated for the following ocular conditions: OAG, NTG, NAION, papilledema, and IIH.
Next, Cox regression models were developed to determine the hazard of developing each ocular condition of interest.30 For the model, we used the first year each beneficiary was enrolled in the medical plan as a look back period. The purpose of the look back period is to identify and exclude non-incident cases of each ocular condition of interest from the models. The models are capturing incident cases since individuals diagnosed with the ocular condition during the look back period were excluded from the analysis. To avoid selection bias, follow-up of all enrollees started at one year after enrollment in the medical plan. Persons were followed until they developed the event (OAG, NTG, NAION, papilledema, IIH) or were censored (either when they left the medical plan or the last day for which we had data, December 31, 2007). For each beneficiary the age to event or the age to censoring was determined. The model is structured in a manner so that individuals must have received their sleep apnea diagnosis prior to experiencing the event (ex: an OAG diagnosis), since they are censored from the model at the time they experience the event. Using age as the time axis and sleep apnea status as the key predictor of interest, the Cox model was left-truncated at the age of index (one year after entry into the medical plan). Adjustments were made for age (the time axis), sex, race, region of residence within the US, education level, household net worth, and the following medical and ocular conditions: diabetes mellitus, systemic arterial hypertension, hyperlipidemia, obesity, systemic hypotension, migraine headache, cataract, pseudophakia or aphakia, diabetic retinopathy, macular degeneration, or Charlson Comorbidity Index, an overall measure of health.(Supplemental Table #1 at AJO.com) A p-value of < 0.05 was considered statistically significant. The University of Michigan Institutional Review Board determined this study was exempt from requiring IRB approval.
RESULTS
Of the 2,259,061 individuals in the medical plan who met the inclusion criteria, 156,336 individuals (6.9%) had received at least one diagnosis of sleep apnea during the study period. The mean age at entry into the plan for those without sleep apnea was 54.8 (±10.5) years; among those with sleep apnea, the mean age at plan entry was 54.2 (±9.2) years. (p<0.0001) There were more males with sleep apnea and fewer Asian Americans with sleep apnea relative to other races. (Table 1). (p<0.0001 for both comparisons).
Table 1.
Demographic Characteristics of Beneficiaries With and Without Sleep Apnea in the Sample
| Enrollees without SA | Enrollees with SA | ||||
|---|---|---|---|---|---|
| N | % | N | % | Total N | |
| Female | 1216582 | 57.8 | 62639 | 40.1 | 1279221 |
| Male | 885934 | 42.1 | 93678 | 59.9 | 979612 |
| White | 1421023 | 86.5 | 113985 | 88.2 | 1535008 |
| Black | 72552 | 4.4 | 5763 | 4.4 | 78315 |
| Latino | 92696 | 5.6 | 6822 | 5.3 | 99518 |
| Asian | 42424 | 2.6 | 1679 | 1.3 | 44103 |
| Other race | 14995 | 0.9 | 1023 | 0.8 | 16018 |
| < HS | 23176 | 1.3 | 1599 | 1.2 | 24775 |
| HS Diploma | 582087 | 33.3 | 47796 | 35.1 | 629883 |
| Some college | 669154 | 38.3 | 54292 | 39.9 | 723446 |
| College diploma | 467159 | 26.7 | 32227 | 23.7 | 499386 |
| Advanced degree | 4938 | 0.3 | 274 | 0.2 | 5212 |
SA = sleep apnea; HS = high school
There are 228 persons with missing sex, 486,099 persons with missing race, and 376,359 persons with missing education levels.
To help validate whether the enrollees who had been diagnosed with sleep apnea indeed had this condition, we reviewed the records to determine the types of medical providers who provided care to these enrollees. Medical providers who frequently care for patients with sleep apnea include pulmonologists, neurologists, sleep specialists, and otolaryngologists. During their time in the plan, 82% of the enrollees who had been diagnosed with sleep apnea had at least one visit to one of these medical providers and among those with sleep apnea who received treatment with continuous positive airway pressure, 92% had seen one of these medical providers. Furthermore, 60% had at least one CPT code for a polysomnogram or some other formal sleep study (CPT codes 95800, 95801, 95806-10). Likewise, among those enrollees who were diagnosed with one of the five ocular conditions of interest for this analysis, greater than 99% of these individuals had records indicating they were under the care of an ophthalmologist or optometrist. Among those who were diagnosed with OAG, 81% had undergone at least one visual field test during their time in the plan and 57% had at least one record of receiving ocular imaging (optical coherence tomography, confocal scanning laser ophthalmoscopy, or scanning laser polarimetry). Of note, not all of the providers caring for these patients may have had access to equipment to be able to perform some of these tests and some patients may have been unable to take these tests because of ocular or systemic comorbidities.
Differences in Incidence of Optic Neuropathies in Persons with and without Sleep Apnea
A total of 55,090 individuals developed OAG over the course of 5,935,107 person-years of follow-up (while at risk) for an OAG incidence rate of 0.93%. There were no significant differences in the incidence of OAG among those with sleep apnea not receiving continuous positive airway pressure (0.94%), those with sleep apnea on continuous positive airway pressure (0.83%), and those without sleep apnea (0.93%). The overall incidence of NTG among those in the plan was 0.08%. Those without sleep apnea had an incidence rate of developing NTG of 0.08% which was higher than those with sleep apnea who were not receiving continuous positive airway pressure (0.07%). Individuals with sleep apnea not treated with continuous positive airway pressure had higher incidence of NAION (0.07% vs 0.05%), papilledema (0.05% vs 0.03%), and IIH (0.04% vs 0.01%) relative to those without sleep apnea. Similarly, persons with sleep apnea receiving treatment with continuous positive airway pressure had higher incidence rates of NAION (0.09% vs 0.05%), and papilledema (0.07% vs 0.03%), compared to those without sleep apnea. The incidence of each ocular condition for sleep apnea patients with and without continuous positive airway pressure treatment did not differ significantly (Table 2). It is important to note that these incidence rates are not adjusted for potential confounding factors.
Table 2.
Incidence of Glaucoma and Other Optic Neuropathies Among Individuals With and Without Sleep Apnea*
| OAG | ||||||
|---|---|---|---|---|---|---|
| N | # OAG cases | Cumulative person years | Incidence rate | Test of rate ratios* | Test of rate ratios# | |
| Without SAS | 2030682 | 50533 | 5449318.76 | 0.93% | ||
| With SAS, untreated | 146172 | 4397 | 466764.19 | 0.94% | 1.02 (0.99-1.05) | |
| With SAS, treated | 5461 | 160 | 19023.79 | 0.84% | 0.91 (0.78-1.06) | 0.89 (0.76-1.05) |
| Overall | 2182315 | 55090 | 5935106.74 | 0.93% | ||
| NTG | ||||||
|---|---|---|---|---|---|---|
| N | # NTG cases | Cumulative person years | Incidence rate | Test of rate ratios* | Test of rate ratios# | |
| Without SAS | 2099555 | 4330 | 5687460.96 | 0.08% | ||
| With SAS, untreated | 150690 | 331 | 487302.66 | 0.07% | 0.89 (0.80-1.00) | |
| With SAS, treated | 5618 | 11 | 19765.2 | 0.06% | 0.73 (0.40-1.32) | 0.82 (0.45-1.49) |
| Overall | 2255863 | 4672 | 6194528.82 | 0.08% | ||
| NAION | ||||||
|---|---|---|---|---|---|---|
| N | # NAION cases | Cumulative person years | Incidence rate | Test of rate ratios* | Test of rate ratios# | |
| Without SAS | 2100853 | 2788 | 5696583.57 | 0.05% | ||
| With SAS, untreated | 150691 | 319 | 487588.69 | 0.07% | 1.34 (1.19-1.50) | |
| With SAS, treated | 5619 | 16 | 19780.97 | 0.08% | 1.65 (1.01-2.70) | 1.24 (0.75-2.04) |
| Overall | 2257163 | 3123 | 6203953.23 | 0.05% | ||
| Papilledema | ||||||
|---|---|---|---|---|---|---|
| N | # Papilledema cases | Cumulative person years | Incidence rate | Test of rate ratios* | Test of rate ratios# | |
| Without SAS | 2101664 | 1799 | 5700011.52 | 0.03% | ||
| With SAS, untreated | 150721 | 256 | 487798.49 | 0.05% | 1.66 (1.46-1.90) | |
| With SAS, treated | 5625 | 14 | 19794.48 | 0.07% | 2.24 (1.32-3.79) | 1.35 (0.79-2.31) |
| Overall | 2258010 | 2069 | 6207604.49 | 0.03% | ||
| IIH | ||||||
|---|---|---|---|---|---|---|
| N | # IIH cases | Cumulative person years | Incidence rate | Test of rate ratios* | Test of rate ratios# | |
| Without SAS | 2102011 | 617 | 5702818.29 | 0.01% | ||
| With SAS, untreated | 150706 | 171 | 487957.95 | 0.04% | 3.24 (2.73-3.84) | |
| With SAS, treated | 5626 | 4 | 19812.25 | 0.02% | 1.87 (0.70-4.99) | 0.58 (0.21-1.55) |
| Overall | 2258343 | 792 | 6210588.49 | 0.01% | ||
comparison of persons with SAS versus those with no SAS
comparison of persons with SAS treated with CPAP to persons with untreated SAS
OAG = open-angle glaucoma; NAION = nonarteritic ischemic optic neuropathy; IIH = idiopathic intracranial hypertension; NTG = normal tension glaucoma; CPAP = continuous positive airway pressure; SA = sleep apnea syndrome
Notes: These incidence rates are unadjusted for confounding factors. The overall n’s differ for each condition listed in the table because there were different numbers of individuals who had each condition of interest during the 1 year look back period and thus were deemed ineligible since they were non-incident cases.
Univariable and Multivariable Analyses
Open Angle Glaucoma
Before adjustment for confounding factors, persons diagnosed with sleep apnea who were not receiving treatment with continuous positive airway pressure had a 7% increased hazard of developing OAG (unadjusted HR=1.07 [95% CI 1.03-1.10]). By comparison, in the unadjusted model, those with sleep apnea treated with continuous positive airway pressure (unadjusted HR=1.01 [CI=0.86-1.18]) did not differ in their hazard of developing OAG relative to individuals without sleep apnea. After adjustment for confounding factors, neither sleep apnea patients with continuous positive airway pressure therapy (adjusted HR=0.99 [CI=0.82-1.18]) nor those without it (adjusted HR=1.01 [CI=0.98-1.05]) had increased hazards of developing OAG relative to persons without sleep apnea. (Table 3)
Table 3.
Univariate and Mulltivariable Analysis of the Hazard of Developing Glaucoma and Other Optic Neuropathies in Persons with Sleep Apnea
| OAG | NTG | NAION | Papilledema | IIH | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate HR |
Multivariable HR |
Univariate HR |
Multivariable HR |
Univariate HR |
Multivariable HR |
Univariate HR |
Multivariable HR |
Univariate HR |
Multivariable HR |
|
| Untreated SA |
1.07 (1.03- 1.10) |
1.01 (0.98- 1.05) |
0.95 (0.85- 1.07) |
0.98 (0.86- 1.12) |
1.41 (1.26- 1.58) |
1.16 (1.01- 1.33) |
1.70 (1.49- 1.93) |
1.29 (1.10- 1.50) |
3.25 (2.74- 3.86) |
2.03 (1.65- 2.49) |
| CPAP- treated SA |
1.01 (0.86- 1.18) |
0.99 (0.82- 1.18) |
0.85 (0.47- 1.53) |
0.79 (0.38- 1.67) |
1.96 (1.20- 3.21) |
1.38 (0.76- 2.50) |
2.32 (1.37- 3.92) |
2.05 (1.19- 3.56) |
1.95 (0.73- 5.22) |
1.50 (0.56- 4.03) |
Reference group in multivariable models: Persons with no sleep apnea
Multivariable models adjusted for age, race, sex, household net worth, education, region of residence, obesity, cataracts, pseudophakia or aphakia, age related macular degeneration, diabetic retinopathy, systemic hypotension, migraines, diabetes mellitus, hypertension, and hyperlipidemia.
OAG = open-angle glaucoma; NTG = normal-tension glaucoma; NAION = non-arteritic ischemic optic neuropathy; IIH = idiopathic intracranial hypertension; HR = hazard ratio; SA = sleep apnea; CPAP = continuous positive airway pressure
Normal-Tension Glaucoma
Unadjusted and adjusted models showed no differences in hazard ratios of developing NTG for sleep apnea patients untreated with continuous positive airway pressure (adjusted HR=0.98 [CI=0.86-1.12]) relative to persons without sleep apnea. Furthermore, the unadjusted and adjusted models demonstrated no differences in the hazard of developing NTG between continuous positive airway pressure-treated sleep apnea patients (adjusted HR=0.79 [CI=0.38-1.67]) and persons without sleep apnea. (Table 3)
Nonarteritic Ischemic Optic Neuropathy
In the unadjusted models, persons with sleep apnea not receiving treatment with continuous positive airway pressure had a 41% increased hazard of experiencing NAION (unadjusted HR=1.41 [CI=1.26-1.58]) relative to individuals without sleep apnea. Those with sleep apnea on continuous positive airway pressure had a 96% increased hazard of developing NAION (unadjusted HR=1.96 [CI=1.20-3.21]) relative to persons without sleep apnea. After adjustment for confounding influences, individuals with sleep apnea not treated with continuous positive airway pressure had a 16% increased hazard of experiencing NAION (adjusted HR=1.16 [CI=1.01-1.33]) as compared with individuals without sleep apnea. By comparison, the adjusted hazard of experiencing NAION was no different between individuals with sleep apnea on continuous positive airway pressure and those without sleep apnea (adjusted HR=1.38 [CI=0.76-2.50]).(Table 3)
Papilledema
In the unadjusted models, individuals with sleep apnea not treated with continuous positive airway pressure had a 70% increased hazard of developing papilledema (unadjusted HR=1.70 [CI=1.49-1.93) relative to individuals without sleep apnea. Those with sleep apnea who were treated with continuous positive airway pressure had a 132% increased unadjusted hazard of developing papilledema (unadjusted HR=2.32 [CI=1.37-3.92]). After adjustment for confounding factors, persons with sleep apnea not receiving continuous positive airway pressure had a 29% increased hazard of papilledema as compared to individuals without sleep apnea (adjusted HR=1.29 [CI=1.10-1.50]). Those with sleep apnea on continuous positive airway pressure therapy had a 105% increased hazard of experiencing papilledema (adjusted HR=2.05 [CI=1.19-3.56]) relative to individuals without sleep apnea. (Table 3)
Idiopathic Intracranial Hypertension
In the unadjusted models, sleep apnea patients not on continuous positive airway pressure had a 225% increased hazard of developing IIH (unadjusted HR=3.25 [CI=2.74-3.86]) relative to persons without sleep apnea. For sleep apnea patients on continuous positive airway pressure, the unadjusted hazard of experiencing IIH was similar to that of persons without sleep apnea (unadjusted HR=1.95 [CI=0.73-5.22). After adjustment for confounders, sleep apnea patients not receiving continuous positive airway pressure had a 103% increased hazard of IIH (adjusted HR=2.03 [CI=1.65-2.49]) as compared to individuals without sleep apnea. In the adjusted model, no differences were noted in the hazard of developing IIH for persons with sleep apnea on continuous positive airway pressure and individuals without sleep apnea (adjusted HR=1.50 [CI=0.56-4.03]). (Table 3)
In sensitivity analysis, the findings from each of the Cox regression models did not change significantly when requiring a confirmatory diagnosis of each ocular condition. (results not shown)
DISCUSSION
There has long been a debate in the literature as to whether a relationship exists between sleep apnea and glaucomatous and other forms of optic neuropathy. (Table 4) In the present analysis, we followed a large cohort of beneficiaries enrolled in a national managed care network longitudinally over time to better understand the relationship between these conditions. After adjustment for a number of important confounding factors, we found no significant relationship between sleep apnea (either treated or untreated with continuous positive airway pressure) and the development of OAG or NTG. We did find a significantly increased hazard of experiencing NAION, papilledema and IIH among individuals with sleep apnea who were not receiving continuous positive airway pressure therapy relative to persons without sleep apnea. By comparison, only the hazard of developing papilledema was increased among sleep apnea patients with continuous positive airway pressure as compared to persons without sleep apnea.
Table 4.
Studies in the Literature Assessing the Relationship Between Sleep Apnea Syndrome and Optic Neuropathies
| First author | Reference # | Year | Ocular condition | Sample size (n) | Main findings | Additional information |
|---|---|---|---|---|---|---|
| Bendel | 1 | 2008 | OAG, NTG | 100 | Prevalence of glaucoma in SAS patients = 27% | No correlation between glaucoma and AHI |
| Boonyaleephan | 18 | 2008 | OAG, NTG | 44 OSA cases, 42 controls | No significant difference between glaucoma prevalence in OSA cases (13.6%) vs. controls (7.1%) | All subjects Thai patients |
| Geyer | 19 | 2003 | OAG, NTG | 228 | Prevalence of glaucoma in SAS patients = 2% | All subjects age > 40 |
| Girkin | 20 | 2006 | OAG, NTG | 667 glaucoma cases, 6667 controls | No significant association between sleep apnea and the development of glaucoma (OR =1.80, 95% CI 0.76 - 4.23) |
All subjects male, age > 50 |
| Kadyan | 21 | 2010 | OAG, NTG | 89 SAS cases, 26 controls | No significant difference between glaucoma prevalence in SAS cases (3.4%) vs. controls (3.8%) | |
| Karakucuk | 2 | 2008 | OAG, NTG | 31 SAS cases, 25 controls | Prevalence of glaucoma in SAS patients = 12.9% | |
| Mojon | 3 | 1999 | OAG, NTG | 69 SAS cases, 45 controls | Significant difference in glaucoma prevalence in SAS cases (7.2%) vs. expected prevalence in normal population (2%) |
No glaucoma cases in SAS controls |
| Roberts | 22 | 2009 | OAG, NTG | 52 glaucoma cases, 60 controls | No significant difference in prevalence of moderate to severe respiratory dysfunction in glaucoma cases (17%) vs. controls (12%) |
All subjects age between 45-80 |
| Mojon | 4 | 2000 | OAG | 30 OAG cases | Significant difference in prevalence of abnormal oximetry in glaucoma cases (20%) vs. historic controls (11%) |
|
| Onen | 5 | 2000 | OAG | 212 OAG cases, 218 controls | Significant association between snoring and OAG (RR=1.44, 95% CI 1.34-1.54) | All subjects age > 40 |
| Mojon | 7 | 2002 | NTG | 16 NTG cases | Prevalence of SAS in NTG = 44% | |
| Sergi | 8 | 2007 | NTG | 51 SAS cases, 40 controls | Significant difference in prevalence of NTG in SAS cases (5.9%) vs. expected prevalence of NTG in control population (0.5%) |
No NTG cases in SAS controls |
| Tsang | 9 | 2006 | Glaucoma (nonspecific) | 41 OSA cases, 35 controls | Significant difference in prevalance of suspicious optic disc change in OSA cases (26.4%) vs. controls (6.8%) |
All subjects Chinese patients |
| Walsh | 6 | 1982 | Glaucoma (nonspecific) | N/A | N/A | Study of three generations of one family demonstrate possible genetic/familial link between sleep apnea and glaucoma |
| Behbehani | 24 | 2005 | NAION | 3 | CPAP did not prevent development of NAION in patients with SAS | |
| Li | 10 | 2007 | NAION | 73 NAION cases, 73 controls | NAION cases significantly more likely to report symptoms/characteristics of SAS (OR 2.62, 95% CI 1.03-6.60) |
Case-control study matched on age and gender |
| Mojon | 11 | 2002 | NAION | 17 NAION cases, 17 controls | Signifcant difference in SAS prevalence between NAION cases (71%) vs. controls (18%) | |
| Palombi | 12 | 2006 | NAION | 27 NAION cases | 89% of NAION cases had SAS | |
| Peter | 13 | 2007 | Papilledema | 35 OSA cases, 35 controls | Significant difference in visual symptoms between OSA cases (40%) and controls (11%) | None demonstrated papilledema on examination Intermittent ICP elevation is sufficient to cause permanent optic disc edema |
| Purvin | 14 | 2000 | Papilledema | 4 | ||
| Bruce | 15 | 2009 | IIH | 721 | Significant difference in prevalence of SAS in men with IIH (24%) vs. prevalence of SAS in women with IIH (4%) |
Men with IIH had significantly worse visual acuity and visual fields than women |
| Lee | 25 | 2002 | IIH | 6 | Treatment with CPAP associated with reduced disc edema | |
| Marcus | 16 | 2001 | IIH | 14 | Sleep related breathing problems in 13 of 14 IIH patients | |
| Kiekens | 23 | 2005 | IOP | 8 OSA cases | Moderate, non-statistically significant nocturnal IOP elevation in OSA patients compared with historical controls |
OAG = open-angle glaucoma; OSA = obstructive sleep apnea; NAION = nonarteritic ischemic optic neuropathy; IIH = idiopathic intracranial hypertension; NTG = normal tension glaucoma; CPAP = continuous positive airway pressure; SAS = sleep apnea syndrome; OR = odds ratio; RR = relative risk; IOP = intraocular pressure; ICP = intracranial pressure; CI = confidence interval; AHI = apnea-hypopnea index
Comparison with Other Studies
Glaucoma
A number of studies in the literature have looked at whether an association exists between sleep apnea and OAG or NTG.1-9,18-20 The largest published study, to date, was a case-control study by Girkin and colleagues of 667 glaucoma patients and 6,667 controls.20 After adjustment for confounding influences, they found no association between glaucoma and sleep apnea. Studies by Geyer and coworkers and Roberts and colleagues also demonstrated no association between sleep apnea and glaucoma, findings similar to those demonstrated in the present analysis.19,22 Among the several studies which report an association between glaucoma and sleep apnea, many of them had small sample sizes (ranging from 16 to 430 participants). Furthermore, since a number of these studies relied on patient symptoms or trend oximetry results to identify patients with sleep apnea, some of the patients may have been misclassified with this condition. Selection bias (recruitment from referral centers) and observer bias may have also affected the findings of some of these studies. Most importantly, many of the existing studies that assessed a relationship between sleep apnea status and glaucoma did not adjust for covariates. Given that we note a positive association between OAG and sleep apnea in our univariable analysis but no association between these conditions in the multivariable analysis, these findings suggest that without adjusting for confounding factors, one may conclude erroneously that a positive association between sleep apnea and glaucoma exists.
Some investigators have suggested that sleep apnea patients might benefit from continuous positive airway pressure because during periods of apnea, blood flow to the optic nerve may diminish and thereby promote glaucomatous or ischemic optic neuropathy.31 Others have expressed concern that use of continuous positive airway pressure can actually cause a rise in intraocular pressure and increase the risk of glaucoma.23, 32 Based on our study findings, we think that the decision to initiate therapy with continuous positive airway pressure in patients with sleep apnea can be based on diagnosis of clinically significant sleep apnea, without concern about increased risk due to continuous positive airway pressure for developing glaucomatous optic neuropathy.
Papilledema / Idiopathic intracranial hypertension
Several small case series and observational studies have reported an association between IIH and sleep apnea.15-17 Marcus and coworkers reviewed the medical records of 53 persons with IIH and found that 70% had evidence of a sleep disturbance.16 Of the 14 patients who underwent polysomnography, 13 were found to have sleep apnea or upper airway resistance syndrome. Bruce and colleagues reviewed the records of 721 patients who had been diagnosed with IIH.15 In their series, they found that 25 of 655 women with IIH (4%) had sleep apnea whereas 16 of 66 men with IIH (24%) did. Given that the males in their study were considerably more likely to experience severe visual loss from IIH, these authors concluded that more research should be undertaken to ascertain whether the worse visual prognosis in the males in this study may be attributable to sleep apnea. In the present analysis, we found no significant differences in the hazard of IIH among females and males with untreated or treated sleep apnea. (results not shown).
The possible mechanism by which sleep apnea is related to IIH is not clearly known. It has been proposed that patients with sleep apnea experience nocturnal episodes of hypercapnea that can lead to increased intracranial pressure and secondary papilledema. The termination of each individual obstructive apnea during sleep is generally associated with a marked though transient spike in intracranial pressure, possibly in association with increased systemic blood pressure, heart rate, and sympathetic tone.17 Episodes that are repeated hundreds of times each night, as often happens in sleep apnea, could conceivably contribute to, or exacerbate IIH. As untreated papilledema or IIH can lead to irreversible vision loss, and remain otherwise asymptomatic, a complete ophthalmologic examination that includes careful assessment of the optic nerve should be considered in patients diagnosed with sleep apnea.
Study Strengths and Weaknesses
There are several strengths of using large administrative databases to study whether an association exists between sleep apnea and ocular conditions which affect the optic nerve. Given the very large sample size of the database, the number of individuals with sleep apnea and each ocular condition of interest are orders of magnitude larger than the sample sizes reported in each of the other studies in the literature. Furthermore, our sample includes ample numbers of enrollees with less common conditions such as NTG or IIH, enabling the performance of multivariable analyses to adjust for important confounding influences. Second, the i3 Data Mart database contains a geographically diverse group of individuals. Unlike studies that recruit patients from a specific city or region of the country which may be limited due to an over- or under-representation of individuals with certain socio-demographic characteristics in that geographic locale, this dataset captures data on a wide array of individuals of different socio-demographic profiles. Third, such an analysis using claims data is less affected by selection bias (recruitment of subjects from referral centers) or observer bias (the status of whether a patient has or does not have sleep apnea is known and may affect classification of ocular conditions). Finally, identifying sleep apnea, use of continuous positive airway pressure, and each of the ocular conditions of interest by using billing codes may be more accurate than studies that rely on patient self-report to determine whether individuals have these conditions.
Several limitations need to be recognized. First, since the data for this analysis were generated from billing records and not from actual medical records, for an enrollee to receive any of the diagnoses included in this analysis, the provider had to accurately diagnose the enrollee with this condition and properly complete the billing records. Some patients may have been misdiagnosed or misclassified with the condition of interest. Second, a number of variables are not included in administrative databases. Our dataset did not capture information on visual acuity, visual field loss, refractive error, axial length, corneal thickness, gonioscopy findings, findings from lumbar punctures, and other important clinical and laboratory measures that ideally would be considered when classifying the presence or absence of the conditions of interest. Third, without access to medical records, we are unable to quantify the severity of sleep apnea, whether differences exist in sleep apnea severity for those using continuous positive airway pressure and those not using continuous positive airway pressure, or the level of patient adherence with continuous positive airway pressure. Fourth, we did not consider other treatments of sleep apnea besides continuous positive airway pressure. Finally, all of the beneficiaries in this dataset had some form of health insurance. Thus, the findings from this analysis may not apply to uninsured individuals or those with other forms of insurance.
Implications
Based on the findings of this analysis, no significant association appears to exist between glaucomatous optic neuropathy and sleep apnea. However, other conditions that can result in damage to the optic nerve -- including NAION, IIH, and papilledema -- appear to be associated with sleep apnea. If additional studies confirm these findings, it may be worthwhile to recommend that newly diagnosed sleep apnea patients undergo an ophthalmologic examination with careful evaluation of the optic nerve to assess for these vision threatening disorders.
Supplementary Material
Acknowledgments
A. Funding: Grant support: National Eye Institute K23 Mentored Clinician Scientist Award (1K23EY019511-01), American Glaucoma Society Clinician Scientist Grant, Blue Cross Blue Shield of Michigan Foundation, an unrestricted grant from Research to Prevent Blindness.
Biography
Joshua D. Stein is an Assistant Professor of Ophthalmology and Visual Sciences at the University of Michigan. He is a health services researcher whose primary research interest involves using large health care claims databases to study utilization patterns and outcomes of eye care throughout the United States.

Nidhi Talwar is a Senior Statistician at the Center for Statistical Consultation and Research at the University of Michigan and the University of Michigan Department of Ophthalmology and Visual Sciences.

Footnotes
Disclosures
B. The authors have no proprietary interest in any material discussed in this manuscript.
Financial Disclosures: Musch: Pfizer, Inc, Glaukos Corp. Stein: Pfizer, Inc. All other authors: None
C. Author Contributions:
Preparation of manuscript: KM, DCM, DSK, JDS Design and conduct of study: DCM, JDS, NT, RDC Collection and management of study data: JDS, NT Analysis of data: BN, DCM, JDS, RDC, NT
D. Statement of Conformity: The University of Michigan Institutional Review Board determined this study was exempt from requiring IRB approval since the data are completely de-identified.
E. Other acknowledgements: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Material available at AJO.com
References
- 1.Bendel RE, Kaplan J, Heckman M, Fredrickson PA, Lin S. Prevalence of glaucoma in patients with obstructive sleep apnoea--a cross-sectional case-series. Eye (Lond) 2008;22(9):1105–9. doi: 10.1038/sj.eye.6702846. [DOI] [PubMed] [Google Scholar]
- 2.Karakucuk S, Goktas S, Aksu M, et al. Ocular blood flow in patients with obstructive sleep apnea syndrome (OSAS) Graefes Arch Clin Exp Ophthalmol. 2008;246(1):129–34. doi: 10.1007/s00417-007-0656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mojon DS, Hess CW, Goldblum D, et al. High prevalence of glaucoma in patients with sleep apnea syndrome. Ophthalmology. 1999;106(5):1009–12. doi: 10.1016/S0161-6420(99)00525-4. [DOI] [PubMed] [Google Scholar]
- 4.Mojon DS, Hess CW, Goldblum D, et al. Primary open-angle glaucoma is associated with sleep apnea syndrome. Ophthalmologica. 2000;214(2):115–8. doi: 10.1159/000027478. [DOI] [PubMed] [Google Scholar]
- 5.Onen SH, Mouriaux F, Berramdane L, et al. High prevalence of sleep-disordered breathing in patients with primary open-angle glaucoma. Acta Ophthalmol Scand. 2000;78(6):638–41. doi: 10.1034/j.1600-0420.2000.078006638.x. [DOI] [PubMed] [Google Scholar]
- 6.Walsh JT, Montplaisir J. Familial glaucoma with sleep apnoea: a new syndrome? Thorax. 1982;37(11):845–9. doi: 10.1136/thx.37.11.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mojon DS, Hess CW, Goldblum D, et al. Normal-tension glaucoma is associated with sleep apnea syndrome. Ophthalmologica. 2002;216(3):180–4. doi: 10.1159/000059625. [DOI] [PubMed] [Google Scholar]
- 8.Sergi M, Salerno DE, Rizzi M, et al. Prevalence of normal tension glaucoma in obstructive sleep apnea syndrome patients. J Glaucoma. 2007;16(1):42–6. doi: 10.1097/01.ijg.0000243472.51461.24. [DOI] [PubMed] [Google Scholar]
- 9.Tsang CS, Chong SL, Ho CK, Li MF. Moderate to severe obstructive sleep apnoea patients is associated with a higher incidence of visual field defect. Eye (Lond) 2006;20(1):38–42. doi: 10.1038/sj.eye.6701785. [DOI] [PubMed] [Google Scholar]
- 10.Li J, McGwin G, Jr, Vaphiades MS, Owsley C. Non-arteritic anterior ischaemic optic neuropathy and presumed sleep apnoea syndrome screened by the Sleep Apnea scale of the Sleep Disorders Questionnaire (SA-SDQ) Br J Ophthalmol. 2007;91(11):1524–7. doi: 10.1136/bjo.2006.113803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mojon DS, Hedges TR, 3rd, Ehrenberg B, et al. Association between sleep apnea syndrome and nonarteritic anterior ischemic optic neuropathy. Arch Ophthalmol. 2002;120(5):601–5. doi: 10.1001/archopht.120.5.601. [DOI] [PubMed] [Google Scholar]
- 12.Palombi K, Renard E, Levy P, et al. Non-arteritic anterior ischaemic optic neuropathy is nearly systematically associated with obstructive sleep apnoea. Br J Ophthalmol. 2006;90(7):879–82. doi: 10.1136/bjo.2005.087452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peter L, Jacob M, Krolak-Salmon P, et al. Prevalence of papilloedema in patients with sleep apnoea syndrome: a prospective study. J Sleep Res. 2007;16(3):313–8. doi: 10.1111/j.1365-2869.2007.00598.x. [DOI] [PubMed] [Google Scholar]
- 14.Purvin VA, Kawasaki A, Yee RD. Papilledema and obstructive sleep apnea syndrome. Arch Ophthalmol. 2000;118(12):1626–30. doi: 10.1001/archopht.118.12.1626. [DOI] [PubMed] [Google Scholar]
- 15.Bruce BB, Kedar S, Van Stavern GP, et al. Idiopathic intracranial hypertension in men. Neurology. 2009;72(4):304–9. doi: 10.1212/01.wnl.0000333254.84120.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcus DM, Lynn J, Miller JJ, Chaudhary O, Thomas D, Chaudhary B. Sleep disorders: a risk factor for pseudotumor cerebri? J Neuroophthalmol. 2001;21(2):121–3. doi: 10.1097/00041327-200106000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Wall M, Purvin V. Idiopathic intracranial hypertension in men and the relationship to sleep apnea. Neurology. 2009;72(4):300–1. doi: 10.1212/01.wnl.0000336338.97703.fb. [DOI] [PubMed] [Google Scholar]
- 18.Boonyaleephan S, Neruntarat C. The association of primary open-angle glaucoma / normal tension glaucoma and obstructive sleep apnea in Thai patients. J Med Health Sci. 2008;15(3):86–93. [Google Scholar]
- 19.Geyer O, Cohen N, Segev E, et al. The prevalence of glaucoma in patients with sleep apnea syndrome: same as in the general population. Am J Ophthalmol. 2003;136(6):1093–6. doi: 10.1016/s0002-9394(03)00709-8. [DOI] [PubMed] [Google Scholar]
- 20.Girkin CA, McGwin G, Jr., McNeal SF, Owsley C. Is there an association between pre-existing sleep apnoea and the development of glaucoma? Br J Ophthalmol. 2006;90(6):679–81. doi: 10.1136/bjo.2005.086082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kadyan A, Asghar J, Dowson L, Sandramouli S. Ocular findings in sleep apnoea patients using continuous positive airway pressure. Eye (Lond) 2010;24(5):843–50. doi: 10.1038/eye.2009.212. [DOI] [PubMed] [Google Scholar]
- 22.Roberts TV, Hodge C, Graham SL, Burlutsky G, Mitchell P. Prevalence of nocturnal oxygen desaturation and self-reported sleep-disordered breathing in glaucoma. J Glaucoma. 2009;18(2):114–8. doi: 10.1097/IJG.0b013e318179f80c. [DOI] [PubMed] [Google Scholar]
- 23.Kiekens S, De Groot Veva, Coeckelbergh T, Tassignon MJ, et al. Continuous positive airway pressure therapy is associated with an increase in intraocular pressure in obstructive sleep apnea. Invest Ophthalmol Vis Sci. 2008;49(3):934–40. doi: 10.1167/iovs.06-1418. [DOI] [PubMed] [Google Scholar]
- 24.Behbehani R, Mathews MK, Sergott RC, Savino PJ. Nonarteritic anterior ischemic optic neuropathy in patients with sleep apnea while being treated with continuous positive airway pressure. Am J Ophthalmol. 2005;139(3):518–21. doi: 10.1016/j.ajo.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Lee AG, Golnik K, Kardon R, Eggenberger E, et al. Sleep apnea and intracranial hypertension in men. Ophthalmology. 2002;109(3):482–5. doi: 10.1016/s0161-6420(01)00987-3. [DOI] [PubMed] [Google Scholar]
- 26.Clinical Modification. 9th Revision Vol 1 and 2. American Medical Association Press; Chicago, IL: 2006. Physician International Classification of Diseases (ICD-9CM) [Google Scholar]
- 27.Current Procedural Terminology 2006. Professional Edition American Medical Association Press; Chicago, IL: 2006. [Google Scholar]
- 28.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 29.American Academy of Sleep Medicine . Diagnostic and Coding Manual. 2nd ed American Academy of Sleep Medicine; Westchester, Illinois: 2005. International Classification of Sleep Disorders. [Google Scholar]
- 30.Cnaan A, Ryan L. Survival analysis in natural history studies of disease. Stat Med. 1989;8(10):1255–68. doi: 10.1002/sim.4780081009. [DOI] [PubMed] [Google Scholar]
- 31.Kremmer S, Niederdraing N, Ayertey HD, Steuhl KP, Selbach JM. Obstructive sleep apnea syndrome, normal tension glaucoma, and nCPAP therapy–a short note. Sleep. 2003;26(2):161–2. doi: 10.1093/sleep/26.2.161. [DOI] [PubMed] [Google Scholar]
- 32.Alvarez-Sala R, García IT, García F, Moriche J, et al. Nasal CPAP during wakefulness increases intraocular pressure in glaucoma. Monaldi Arch Chest Dis. 1994;49(5):394–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.