Abstract
The centrosome is essential for the formation of the cilia and has been implicated in cell polarization and signaling during early embryonic development. A number of Wnt pathway components were found to localize at the centrosome, but how this localization relates to their signaling functions is unclear. In this study, we assessed a role for Diversin, a putative Wnt pathway mediator, in developmental processes that involve cilia. We find that Diversin is specifically localized to the basal body compartment near the base of the cilium in Xenopus multi-ciliated skin cells. Overexpression of Diversin RNA disrupted basal body polarization in these cells, suggesting that tightly regulated control of Diversin levels is crucial for this process. In cells depleted of endogenous Diversin, basal body structure appeared abnormal and this was accompanied by disrupted polarity, shortened or absent cilia and defective ciliary flow. These results are consistent with the involvement of Diversin in processes that are related to the acquisition of cell polarity and require ciliary functions.
Keywords: Diversin, cilia, basal body, Xenopus, polarity, PCP, Wnt
1. Introduction
The centrosome serves as a microtubule organizing center and forms a template for basal body and cilia development (Dawe et al., 2007; Ou and Rattner, 2004). The cilium is a microtubule-based hair-like structure that extends from the apical surface of the cell. Virtually all cells in vertebrate embryos contain either a single (primary) cilium or hundreds of cilia (multi-ciliated cells)(Dawe et al., 2007; Satir and Christensen, 2007). In multi-ciliated cells, basal bodies and cilia are uniformly oriented, which is necessary to achieve directional fluid flow (Billett and Gould, 1971; Konig and Hausen, 1993; Mitchell et al., 2007; Ou and Rattner, 2004). Primary cilia-based motility is thought to underlie left-right axis specification in vertebrate embryos (Aw and Levin, 2009; Blum et al., 2009; Hirokawa et al., 2006; Yost, 2003). Moreover, the centrosome and its derivatives have recently attracted much attention as regulators of cell polarity and coordinators of major embryonic signaling pathways, including those triggered by Hedgehog, PDGF, FGF and Wnt proteins (Eggenschwiler and Anderson, 2007; Gerdes et al., 2009; Neugebauer et al., 2009; Singla and Reiter, 2006; Wessely and Obara, 2008). These critical roles of the centrosome and cilia in cell physiology and the existence of multiple developmental disorders connected to these cellular structures (Badano et al., 2005; Sharma et al., 2008) warrant detailed molecular analysis of underlying mechanisms.
Wnt signaling is one of the major pathways regulating cell polarity, cell movements, cell proliferation and fate determination (Chien et al., 2009; MacDonald et al., 2009; Nakaya et al., 2005; Nascone and Mercola, 1997). Wnt ligands activate gene transcription largely through the canonical, β-catenin/TCF-dependent pathway (Behrens et al., 1996; Clevers, 2006; Moon et al., 2002; Nusse, 2005) and are commonly thought to regulate cell polarity and morphogenesis in the β-catenin/TCF-independent manner (Adler, 2002; Mlodzik, 2000; Sokol, 2000; Wallingford et al., 2000; Zallen, 2007). The Wnt pathways have been reported to modulate ciliary and centrosomal functions, including basal body polarization and left-right asymmetry (Mitchell et al., 2009; Nakaya et al., 2005; Park et al., 2006; Park et al., 2008), and, conversely, the centrosome and its derivatives have been implicated in the regulation of Wnt signaling (Alexandrova and Sokol, 2009; Corbit et al., 2008; Gerdes et al., 2007; Itoh et al., 2009). Although several Wnt pathway components were reported to associate with the centrosomes or cilia (Bahmanyar et al., 2008; Fumoto et al., 2009; Hadjihannas et al., 2006; Kaplan et al., 2004; Kim et al., 2009; Louie et al., 2004), the specific mechanisms underlying the control of ciliary and centrosomal functions by Wnt signaling remain to be elucidated.
Diversin is an ankyrin repeat-containing protein, which is structurally related to vertebrate Inversin and Drosophila Diego (Moeller et al., 2006; Schwarz-Romond et al., 2002; Simons et al., 2005). In zebrafish embryos Diversin was reported to be required for aspects of axial patterning and morphogenetic movements, consistent with the hypothesis that Diversin is a regulator of Wnt signaling (Moeller et al., 2006; Schwarz-Romond et al., 2002). Since Diversin physically associates with the centrosome (Itoh et al., 2009), it is a good candidate for regulating ciliary functions. Our study explores this possibility in Xenopus embryos using multi-ciliated skin cells (Billett and Gould, 1971; Konig and Hausen, 1993) and primary cilia-containing cells of the gastrocoel roof plate (GRP) (Neugebauer et al., 2009; Schweickert et al., 2007). We find that Diversin is localized to a specific compartment of the basal body and functions in ciliogenesis to establish basal body polarity of multi-ciliated cells and is responsible for ciliary functions of the gastrocoel roof cells in Xenopus early embryos.
2. Results
2.1. Diversin localizes at the basal bodies of multi-ciliated skin cells
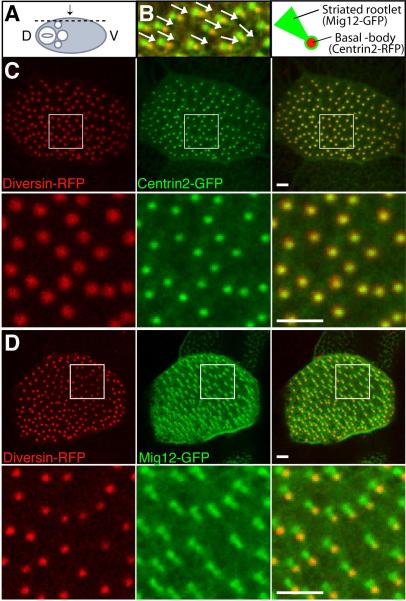
Since Diversin was reported to localize at the centrosome (Itoh, 2009), we studied its distribution in Xenopus embryo epidermis, which contain multi-ciliated cells (Fig. 1A). At stage 34, multi-ciliated cells are well differentiated and basal body polarity is visible upon coexpression of Centrin2-RFP, a basal body marker, and Mig12-GFP that marks both the basal body and the striated rootlet (Fig. 1B, (Park et al., 2008). The striated rootlet is an accessory structure attached to the basal side of the basal body (Dawe et al., 2007; Park et al., 2008). The striated rootlet can be also labeled by CLAMP (Dawe et al., 2007; Park et al., 2008), which colocalizes with Mig12-GFP (Hayes et al., 2007). Four-cell embryos were co-injected with mRNAs, encoding Diversin-RFP (Div-RFP) and Centrin2-GFP or Mig12-GFP, and double fluorescence was analyzed in the embryonic skin at stage 34 (Fig. 1C, D). At low doses of Div-RFP RNA (0.1–0.2 ng), basal bodies were doubly labeled with Centrin2-GFP and Div-RFP (Fig. 1C). On the other hand, upon coexpression of Mig12-GFP and Div-RFP, Div-RFP was not detected in the striated rootlet marked by Mig12-GFP (Fig. 1D). These results indicate that Diversin is specifically localized to the basal body compartment near the base of the cilium, segregating from the striated rootlet. Considering this subcellular distribution, we wanted to investigate a role for Diversin in regulating cilia development and functions.
Fig. 1. Diversin localizes to the basal body in multi-ciliated cells.
Both ventral blastomeres of four-cell embryos were injected with the following mRNAs as indicated: Div-RFP (0.1–0.2 ng), Centrin2-RFP (0.4 ng), Centrin2-GFP (0.1 ng) or Mig12-GFP (0.1 ng). Injected embryos were fixed at stage 34 and cryosectioned for confocal imaging. (A) Experimental scheme. Plane of sectioning is indicated by a dashed line; arrow shows direction of viewing. D, Dorsal; V, Ventral. (B) Mig12-GFP and Centrin2-RFP label the striated rootlet and the basal body, respectively, as indicated in the cartoon on the right. Arrows represent basal body polarity. (C, D) Div-RFP localizes to the basal bodies labeled with Centrin2-GFP (C), but not to the striated rootlets marked by Mig12-GFP (D). Lower panels show boxed images at higher magnification, merged files are on the right (B–D). Representative confocal images of embryonic epidermis are shown. Scale bar, 2 μm.
2.2. Diversin is necessary for basal body polarity and striated rootlet formation
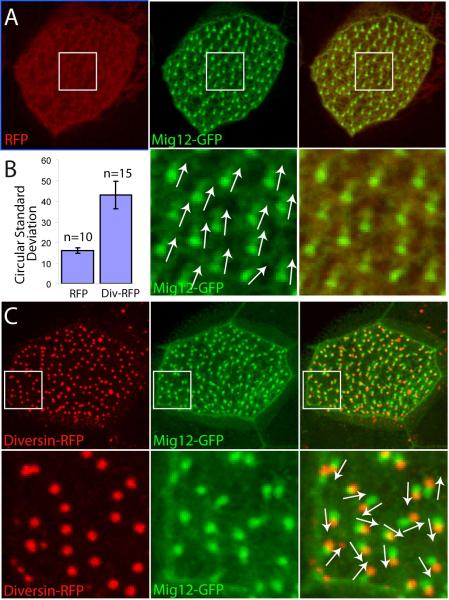
To assess a role for Diversin in basal body structure and function, we first analyzed the effect of Diversin on Xenopus multi-ciliated cells in gain-of-function experiments. Div-RFP, but not RFP RNA, interfered with basal body polarity (Fig. 2), similar to the effects of Disheveled MOs and dominant interfering Dishevelled constructs (Park et al., 2008). This effect was dose-dependent, with the majority of multi-ciliated cells affected at high doses of Div-RFP RNA (0.3–0.5 ng, Fig. 2, see Fig. 1D, for comparison) or Flag-Diversin RNA (2 ng, Suppl. Fig. 1), indicating that Diversin expression levels need to be tightly regulated during this process. These observations suggest that Diversin is involved in the establishment of basal body polarity.
Fig. 2. Overexpressed Diversin disrupts basal body polarity.
(A) Basal body polarity in the control RFP-expressing cell. (B) Basal body polarity was quantified by calculating circular standard deviations for individual multi-ciliated cells (see Materials and Methods). Results of a representative experiment are shown as means +/− SEM (n = 10 and 15 for RFP and Div-RFP, respectively). (C) Disrupted basal body polarity in Div-RFP-expressing cell. Div-RFP (0.3–0.5 ng) and control RFP (1 ng) RNAs were injected as described in Fig. 1. Merged files are on the right, lower panels are at higher magnification. Basal body polarity is represented by arrows.
We next investigated the function of Diversin in a loss-of-function approach by injecting a translation-blocking antisense morpholino oligonucleotide (MO)(Heasman et al., 2000). As the sequence of Xenopus laevis Diversin has not been available in DNA databases, we generated a partial cDNA clone by polymerase chain reaction with primers complementary to highly conserved Diversin sequences. We then used the 5'-RACE approach (see Materials and Methods for details) to obtain 5'- region sequence information and synthesized a specific MO based on its sequence (DivMO). DivMO completely blocked the translation of a co-injected RNA encoding Xenopus tropicalis Diversin-GFP and containing MO target sequence (Fig. 3A). A control MO (CoMO) with a different sequence did not have this effect. In contrast, DivMO did not inhibit the translation of an unrelated RNA lacking target sequence (Myc-γ-tubulin, Fig. 3A). These findings demonstrate efficiency and specificity of DivMO. Further confirming specificity, Div-RFP RNA partially rescued embryonic defects caused by DivMO injection (data not shown).
Fig. 3. Diversin is required for basal body polarity and ciliogenesis.
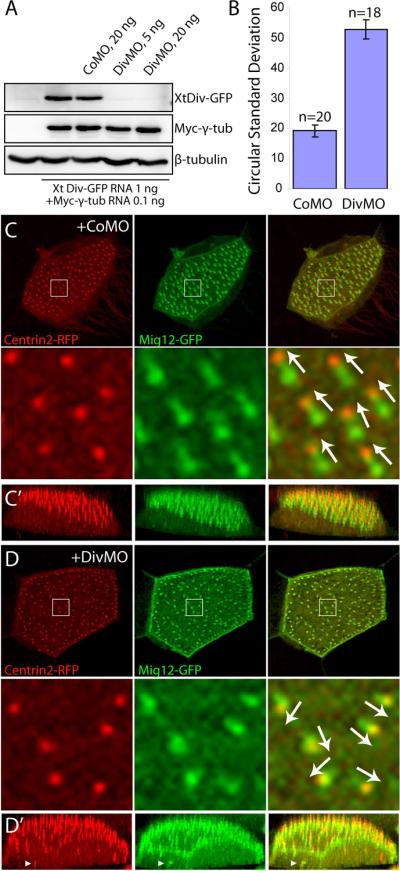
(A) Specificity of DivMO. Embryos were injected with CoMO or DivMO, as indicated, together with XtDiv-GFP RNA (1 ng) and myc-γ tubulin RNA (0.1 ng). Western analysis of st. 11.5 embryo lysates shows specific inhibition of XtDiv-GFP by DivMO, but not CoMO. XtDiv-GFP was detected with anti-GFP, whereas myc-γ tubulin was detected with anti-Myc antibody. Anti-β-tubulin antibody was used to control loading. (B–D) MOs (20 ng) and RNAs encoding Centrin2-RFP (0.4 ng) and Mig12-GFP (0.1 ng) were injected into the animal-ventral region of four-cell embryos. (B) Basal body polarity was quantified in a representative experiment by calculating circular standard deviations (see Materials and Methods). Results are shown as means +/− SEM (n = 20 and 18 for CoMO and DivMO respectively). (C, D) The apical regions of multi-ciliated cells scored in (B) are shown as x–y (C, D) or x–z plane (C', D') projections of serial optical sections (see also Fig. 1 legend). Arrows indicate basal body polarity. Top is apical in C' and D'. (C, C') CoMO does not affect basal body apical localization and polarity. (D, D') DivMO disrupted basal body polarity and striated rootlet structure. Arrowheads point to defects in basal body apical docking.
Embryos were injected with MOs and Centrin2-RFP and Mig12-GFP RNAs to indicate basal body polarity marked by the relative positions of the basal body and the striated rootlet (Fig. 1B). Basal body polarity was evident in CoMO-injected embryos (n=20; Fig. 3B, C). By contrast, knockdown of Diversin severely impaired basal body polarity and increased the circular standard deviation compared with that of controls (n=18; Fig. 3B, D). Apical localization of basal bodies, a prerequisite for basal body polarization, was also weakly affected (Fig. 3D'; arrowheads). In addition to these defects, we observed that basal bodies from DivMO-containing multi-ciliated cells had abnormal striated rootlet morphology. In control embryos, the rootlet is a wedge-shaped structure marked by Mig12-GFP (Fig. 1B and Fig. 3C). In DivMO-injected embryos, the rootlet appeared shorter, since Mig12-GFP essentially colocalized with Centrin2-RFP marking the basal body (Fig. 3D). Alternatively, the rootlet may have been less tilted relative to the apical surface. This defect was confirmed using CLAMP-RFP as another marker for the striated rootlet (data not shown).
2.3. A requirement for Diversin in ciliogenesis and ciliary function
Since the striated rootlet has been implicated in ciliogenesis (Hayes et al., 2007), we assessed cilia formation in Diversin-depleted embryos. Labeling with anti-acetylated tubulin revealed ciliary defects in Diversin-depleted cells, (Fig. 4A and B). We observed that both basal body number and cilia length were decreased (Fig. 4C, D). These effects of DivMO on cilia are consistent with the hypothesis that Diversin regulates the morphology of the basal body.
Fig. 4. Diversin is required for ciliary function in multi-ciliated cells.
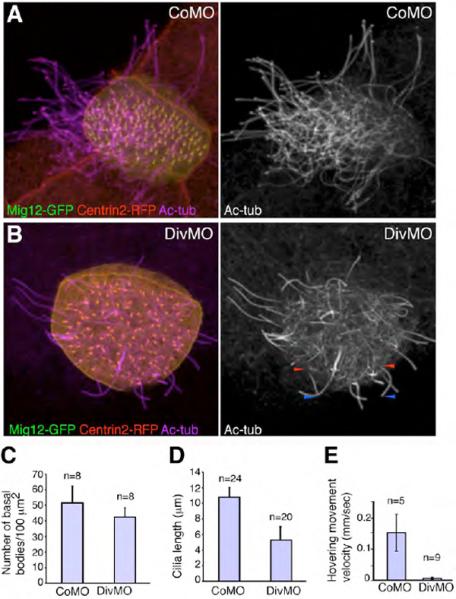
(A, B) Effects of Diversin MO (DivMO) on ciliogenesis. Morpholinos (20 ng each) and RNAs for Centrin2-RFP (0.4 ng) and Mig12-GFP (0.1 ng) were injected as described in Fig. 1, embryos were cultured until stage 34, fixed and stained for cilia with anti-acetylated tubulin antibody. (A) CoMO-injected cell. (B) DivMO-injected cell. Short cilia are marked by arrowheads at the base (red) and the tip (blue). (C) Basal body density is slightly reduced in multi-ciliated cells from embryos injected with DivMO. Number of basal bodies was counted in eight randomly picked multi-ciliated cells of CoMO or DivMO injected embryos. (D) DivMO-injected embryos contain shorter cilia. Cilia length was measured in multi-ciliated cells of CoMO- or DivMO-injected embryos shown in A, B. (E) Effects of DivMO on hovering movements. Morpholinos (20 ng) were injected into four ventral blastomeres of eight-cell embryos. Compared to CoMO, DivMO significantly decreased hovering movement velocity that was measured when control siblings reached stage 36 (see Materials and Methods). Means +/− standard deviations are shown, *p < 0.005.
We next wanted to evaluate if the existing cilia in Diversin-depleted embryos retain their function. Since beating of cilia in Xenopus multi-ciliated cells cause embryos to hover in a Petri dish, this phenomenon was used to assess the effect of DivMO on coordinated cilia motility. DivMO-injected embryos showed significantly lower velocity (0.007 ± 0.004 mm/sec, n=9), compared with the CoMO-injected embryos (0.16 ± 0.06 mm/sec, n=5; Fig. 4E). A slight delay in development that was observed for DivMO-injected embryos is not likely to be the cause for this effect, because the injected embryos did not recover even at the later stages. This defect in cilia function is consistent with abnormal basal body polarity and impaired ciliogenesis in DivMO-injected embryos. Together with the localization data, our observations indicate that Diversin is an essential basal body constituent, which regulates the function of cilia in multi-ciliated cells.
2.4. Lack of cilia in the gastrocoel roof plate in DivMO-injected embryos
The Xenopus gastrocoel roof plate (GRP), similarly to the mouse node or zebrafish Kupffer's vesicle, contains cilia that have been implicated in the control of left-right asymmetry (Hashimoto et al., 2010; Qiu et al., 2005; Schweickert et al., 2007; Song et al., 2010; Vick et al., 2009; Wallingford, 2010)(Hyatt and Yost, 1998; Okada et al., 2005). To determine whether ciliogenesis in the GRP is perturbed in embryos depleted of Diversin, we prepared GRP explants from embryos injected with DivMO or control MO and stained for cilia using an antibody against anti-acetylated tubulin. Injection of DivMO, but not CoMO, led to the significant decrease in the staining of cilia in GRP cells (Fig. 5A, B). In GRP cells, in which Diversin has been knocked down, both the number of cilia has decreased and the remaining cilia appeared shorter (Fig 5C and data not shown). These observations are consistent with Diversin playing a general role in ciliogenesis in vertebrate embryos.
Fig. 5. Defective ciliogenesis in the gastrocoel roof plate of Diversin-depleted embryos.
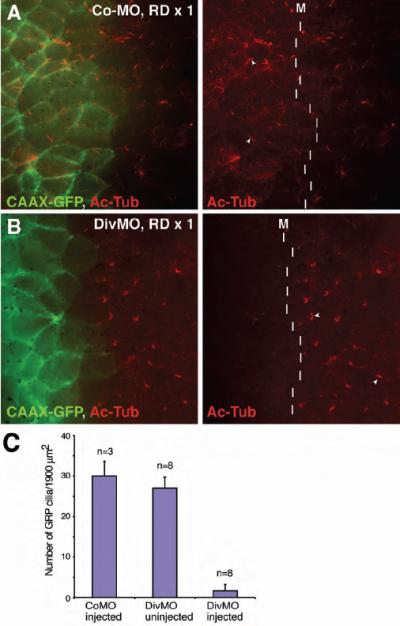
Morpholino oligonucleotides and GFP-CAAX RNA (150 pg) were coinjected into one dorsal blastomere of four-cell embryos. Gastrocoel roof plate (GRP) was dissected at stage 17 and stained with anti-GFP and anti-acetylated tubulin antibodies. Arrowheads (red channel only) indicate GRP cilia. The midline (M) is shown by a dashed line. Merged images are shown on the left. Div-MO, but not Co-MO, inhibited cilia formation in the GRP. (A) Control MO, 40 ng. (B) Diversin MO, DivMO, 40 ng. (C) Effects of DiVMO on GRP cilia. The number of GRP cilia was scored in the area of 1900 μm2 (adjacent to the midline), which was randomly selected in morpholino-injected embryos. The area on the contra-lateral side was used as an additional control. DivMO drastically reduced cilia number.
3. Discussion
In this study, we have investigated the localization of Diversin, a putative Wnt signaling component, in multi-ciliated cells of Xenopus epidermis and examined its roles in cilia-dependent processes. We discovered that Diversin is specifically localized to the basal body compartment near the base of the cilium, segregating from the striated rootlet, an accessory structure to the basal body. Depletion of Diversin resulted in inhibited ciliogenesis, misoriented basal bodies and disrupted ciliary functions.
Whereas our data reveal a role for Diversin in ciliogenesis in multi-ciliated cells and the GRP cells, the underlying mechanisms remain unclear. Diversin may be involved in ciliogenesis and basal body polarity, because of its interaction with the canonical Wnt/β-catenin pathway that has also been linked to ciliogenesis. For example, Chibby, a β-catenin inhibitor (Takemaru et al., 2003), localizes at the base of cilia in airway epithelial cells and is required for proper basal body localization (Voronina et al., 2009). Both Dishevelled and Axin/Conductin, another inhibitor of the canonical pathway, are found at the basal bodies (Alexandrova and Sokol, 2009; Fumoto et al., 2009; Park et al., 2008), and Diversin was reported to associate with both proteins (Schwarz-Romond et al., 2002). Arguing against the requirement for Diversin in canonical Wnt signaling, Diversin depletion did not lead to significant β-catenin stabilization in ectodermal cells (data not shown).
The role of Diversin in ciliary functions could also be related to its involvement in noncanonical Wnt signaling and planar cell polarity (PCP) (Schwarz-Romond et al., 2002). Supporting this possibility, Diego, another ankyrin-domain protein that is similar to Diversin, has been shown to be required for the PCP pathway in Drosophila embryos (Feiguin et al., 2001; Simons and Mlodzik, 2008). Consistent with the above hypothesis, the components of the PCP pathway Dvl2/Dsh, Frizzled3 and Vangl2 have been reported to modulate basal body polarity (Antic et al., 2010; Borovina et al., 2010; Ganner et al., 2009; Hashimoto et al., 2010; Mitchell et al., 2009; Park et al., 2008). On the other hand, Diversin colocalizes with Centrin 2 in the basal body (Fig. 1C), whereas Dishevelled localizes adjacent to Centrin 2 (Park et al., 2008), implying distinct functions for the two PCP proteins during basal body formation and cilia assembly. Whether or not the requirement of Diversin for ciliogenesis reflects its function in the PCP pathway, the abnormal striated rootlet morphology and basal body docking/polarity in DivMO-injected embryos are likely to cause the observed ciliary defects in multi-ciliated cells.
The effects of DivMO on GRP cilia may also be related to PCP signaling. In vertebrates, the leftward fluid flow created by motile primary cilia of the mammalian node, the Kupffer's vesicle in zebrafish, and the GRP in Xenopus, is pivotal for the establishment of left-right polarity (Aw and Levin, 2009; Blum et al., 2009; Essner et al., 2005; Hirokawa et al., 2006; Okada et al., 2005). Polarization of cilia to the posterior region of each cell in GRP (or equivalent structures in other species) is regulated by the PCP pathway (Antic et al., 2010; Hashimoto et al., 2010; Song et al., 2010) and appears critical for determining the rotational axis of cilia and hence for the generation of leftward flow (Maisonneuve et al., 2009; Okada et al., 2005). Consistent with the effect of Diversin MO on GRP cilia, we have observed frequent gut coiling reversals in DivMO-injected embryos (data not shown). Of interest, the inversin mutation also causes left-right polarity defects as a result of defective nodal ciliary flow (Mochizuki et al., 1998; Okada et al., 1999; Serluca et al., 2009). Inversin, which is distantly related to Diversin, has been reported to modulate Wnt signaling (Simons et al., 2005) and promote ubiquitin-mediated degradation of Dvl2 (Ganner et al., 2009). Since Inversin-depleted zebrafish embryos are partially rescued by Diversin (Simons et al., 2005), the two proteins might function in a similar manner. On one hand, lack of cilia in the GRP of Diversin-depleted embryos revealed in our study is consistent with the role of Diversin in ciliogenesis, rather than GRP cilia motility as demonstrated for Dynein heavy chains (Vick et al., 2009) or sensorial function of cilia as shown for mouse polycystin 2 (Pennekamp et al., 2002). On the other hand, the absence of cilia in the chicken node and the specification of the left-right axis before node appearance (Zhang and Levin, 2009) indicate that PCP components may be crucial for asymmetry generation in the chick embryo independently of ciliogenesis. Thus, additional studies are required to elucidate how Diversin and other PCP proteins regulate ciliary function and left-right patterning, and whether this regulation involves Wnt/PCP or Wnt/β-catenin signaling.
4. Materials and methods
4.1. DNA constructs and morpholinos
RFP-tagged mouse Diversin has been previously described (Itoh et al., 2009). Centrin2-RFP in pCS105 was generated by fusing in frame the coding regions of Xenopus Centrin2 (GenBank accession number: BC108522) and mRFP (at the N-terminus). Mig12-GFP and Centrin2-GFP in pCS2 were generated by fusing in frame the coding regions of Xenopus Mig12 (GenBank accession number: BC108773) or Xenopus Centrin2 to GFP (at the C-terminus). Myc-γ-tubulin in pXT7 was generated by in-frame subcloning of PCR-amplified coding sequence of mouse γ-tubulin (GenBank accession number: BC006581) into pXT7-Myc. XtDiv-GFP in pCS2 was generated by fusing the partial 5'UTR plus the coding region of Xenopus tropicalis Diversin (cDNA clone MGC172370, obtained from Open Biosystems) to GFP (at the C-terminus). Sequences of primers used for PCR are listed in Table 1. All constructs were verified by sequencing. Further details of cloning are available on request.
Partial Xenopus laevis Diversin cDNA was isolated by PCR from Xenopus gastrula stage cDNA using primers complementary to the nucleotide sequence that is highly conserved in zebrafish, mouse and X. tropicalis Diversin homologues. The following primers have been used: forward, 5'-ATG AGC CAG CAG GAT GT-3'; reverse, 5'-CCA TCC TTA TCT TGT CT-3'. Based on this partial Diversin clone, the sequence corresponding to the 5' UTR and the N-terminus of the protein was obtained by 5'RACE (Invitrogen), following the manufacturer's protocol. Morpholinos (MOs) were from GeneTools: Diversin MO, 5'-GGC CAC ATC CTG CTG GCT CAT GAA T-3'; Control MO, 5'-CCT CTT ACC TCA GTT ACA ATT TAT A-3'.
4.2. Embryo culture, microinjections and manipulation
Capped synthetic mRNAs were generated by in vitro transcription with SP6 or T7 RNA polymerase using the mMessage mMachine kit (Ambion). Xenopus laevis eggs and embryos were obtained by in vitro fertilization, treated with 3 % cysteine (pH 7.8) to remove jelly coat and have been cultured in 0.1 x Marc's modified Ringer's solution (MMR)(Newport and Kirschner, 1982). For microinjection, 4–8-cell embryos were transferred to 3 % Ficoll in 0.5 × MMR and injected with 10 nl of solution containing RNAs and/or MOs.
4.3. Immunostaining
For multi-ciliated cell analysis, MOs (20 ng) and RNAs encoding fluorescent markers for the basal body or striated rootlet were coinjected into the animal region of ventral blastomeres at the four- to eight-cell stage. The injected embryos at stage 34 were fixed for one hour with MEMFA (0.1 M MOPS, pH 7.4, 2 mM EGTA, 1 mM MgSO4 and 3.7% formaldehyde)(Brivanlou and Harland, 1989), washed with PBS, and embedded in 15 % fish gelatin/15 % sucrose solution. The embedded embryos were quickly frozen on dry ice and skin cryosections were obtained on Leica Cryostat (see also Fig. 1A). Cryosections were immunostained essentially as described (Itoh et al., 2009), using anti-acetylated-tubulin hybridoma supernatant (6-11B-1, 1:5), followed by Cy5-conjugated anti-mouse IgG antibodies (Jackson ImmunoResearch, 1:200).
For gastrocoel roof plate (GRP) analysis, CAAX-GFP RNA (0.15 ng) was coinjected with control or Diversin MO (40 ng) into one right or left blastomere in the dorsal marginal zone at 4-cell stage. Dorsal explants including GRP were isolated when the injected embryos reached stage 17. The explants were dissected in Danilchik's buffer with 0.1% BSA, fixed in 4% paraformaldehyde in PBS and processed for immunostaining as described by Antic et al. (2010). Immunostaining was carried out with rabbit anti-GFP antibodies (Invitrogen, 1:500) and with anti-acetylated-tubulin hybridoma supernatant (1:10), followed by Alexa488-conjugated anti-rabbit IgG (1:200) and Cy3-conjugated anti-mouse IgG (1:200).
4.4. Imaging of multi-ciliated cells and cilia-directed hovering movements
Fluorescent 3D projection images were obtained with a Leica SP5 confocal microscope. To quantify basal body polarity, angular measurements were obtained for the orientation of each basal body using the Image J software (http://rsbweb.nih.gov/ij/) and analyzed with Oriana 2.0 statistical program (Kovach computing Service). Circular standard deviations (CSD) were calculated for individual multi-ciliated cells and compared as described (Park et al., 2008). Cells were randomly picked from three different embryos. Experiments were repeated two to three times with an independent batch of embryos.
For measuring hovering movements, MOs (20 ng per injection) and GFP mRNA as a tracer (200 pg per injection) were injected into the ventral side of eight-cell embryos. The embryos with fluorescent skin were selected at the neurula stage and further cultured until the sibling embryos reached stage 36. The embryos were placed in a Petri dish and those with the relatively straight movement were selected for videorecording (45 sec). Movement distance was measured by tracing the tip of the tail using Openlab 3.1.4 (Improvision) and the velocity was calculated.
4.5. Western blot analysis
Western analysis was carried out using standard techniques as previously described (Itoh et al., 2000). Four animal blastomeres were injected with indicated RNAs/MOs at the four-cell stage. Cell lysates from the injected embryos were prepared at stage 11.5. Antibodies used were mouse anti-Myc (hybridoma supernatant 9E10, 1:200), mouse anti-β-tubulin (BioGenex, 1:1000) and mouse anti-GFP (B-2, Santa Cruz, 1:1000).
Supplementary Material
Highlights
In multi-ciliated cells, the ankyrin-domain protein Diversin is localized to the basal body.
In gain-of-function assays, Diversin disrupts basal body polarization.
The knockdown of Diversin leads to shortened or absent cilia and defective ciliary functions in multi-ciliated cells and gastrocoel roof plate cells.
Acknowledgements
We thank C. Iomini for anti-acetylated tubulin antibody and members of the Sokol laboratory for technical advice and helpful discussions. Confocal laser microscopy was performed at the MSSM Microscopy shared research facility, supported by a NIH/NCI shared instrumentation grant. This study was supported by NIH grants to S. S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler PN. Planar signaling and morphogenesis in Drosophila. Dev Cell. 2002;2:525–35. doi: 10.1016/s1534-5807(02)00176-4. [DOI] [PubMed] [Google Scholar]
- Alexandrova EM, Sokol SY. Xenopus axin-related protein: A link between its centrosomal localization and function in the Wnt/beta-catenin pathway. Dev Dyn. 2010;239:261–70. doi: 10.1002/dvdy.22125. [DOI] [PubMed] [Google Scholar]
- Antic D, Stubbs JL, Suyama K, Kintner C, Scott MP, Axelrod JD. Planar cell polarity enables posterior localization of nodal cilia and left-right axis determination during mouse and Xenopus embryogenesis. PLoS One. 2010;5:e8999. doi: 10.1371/journal.pone.0008999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw S, Levin M. Is left-right asymmetry a form of planar cell polarity? Development. 2009;136:355–66. doi: 10.1242/dev.015974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badano JL, Teslovich TM, Katsanis N. The centrosome in human genetic disease. Nat Rev Genet. 2005;6:194–205. doi: 10.1038/nrg1557. [DOI] [PubMed] [Google Scholar]
- Bahmanyar S, Kaplan DD, Deluca JG, Giddings TH, Jr., O'Toole ET, Winey M, Salmon ED, Casey PJ, Nelson WJ, Barth AI. beta-Catenin is a Nek2 substrate involved in centrosome separation. Genes Dev. 2008;22:91–105. doi: 10.1101/gad.1596308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–42. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Billett FS, Gould RP. Fine structural changes in the differentiating epidermis of Xenopus laevis embryos. J Anat. 1971;108:465–80. [PMC free article] [PubMed] [Google Scholar]
- Blum M, Beyer T, Weber T, Vick P, Andre P, Bitzer E, Schweickert A. Xenopus, an ideal model system to study vertebrate left-right asymmetry. Dev Dyn. 2009;238:1215–25. doi: 10.1002/dvdy.21855. [DOI] [PubMed] [Google Scholar]
- Borovina A, Superina S, Voskas D, Ciruna B. Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nat Cell Biol. 2010;12:407–12. doi: 10.1038/ncb2042. [DOI] [PubMed] [Google Scholar]
- Brivanlou AH, Harland RM. Expression of an engrailed-related protein is induced in the anterior neural ectoderm of early Xenopus embryos. Development. 1989;106:611–7. doi: 10.1242/dev.106.3.611. [DOI] [PubMed] [Google Scholar]
- Chien AJ, Conrad WH, Moon RT. A Wnt survival guide: from flies to human disease. J Invest Dermatol. 2009;129:1614–27. doi: 10.1038/jid.2008.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter JF. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10:70–6. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- Dawe HR, Farr H, Gull K. Centriole/basal body morphogenesis and migration during ciliogenesis in animal cells. J Cell Sci. 2007;120:7–15. doi: 10.1242/jcs.03305. [DOI] [PubMed] [Google Scholar]
- Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol. 2007;23:345–73. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essner JJ, Amack JD, Nyholm MK, Harris EB, Yost HJ. Kupffer's vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development. 2005;132:1247–60. doi: 10.1242/dev.01663. [DOI] [PubMed] [Google Scholar]
- Feiguin F, Hannus M, Mlodzik M, Eaton S. The ankyrin repeat protein Diego mediates Frizzled-dependent planar polarization. Dev Cell. 2001;1:93–101. doi: 10.1016/s1534-5807(01)00010-7. [DOI] [PubMed] [Google Scholar]
- Fumoto K, Kadono M, Izumi N, Kikuchi A. Axin localizes to the centrosome and is involved in microtubule nucleation. EMBO Rep. 2009;10:606–13. doi: 10.1038/embor.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganner A, Lienkamp S, Schafer T, Romaker D, Wegierski T, Park TJ, Spreitzer S, Simons M, Gloy J, Kim E, Wallingford JB, Walz G. Regulation of ciliary polarity by the APC/C. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0909465106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009;137:32–45. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes JM, Liu Y, Zaghloul NA, Leitch CC, Lawson SS, Kato M, Beachy PA, Beales PL, DeMartino GN, Fisher S, Badano JL, Katsanis N. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet. 2007;39:1350–60. doi: 10.1038/ng.2007.12. [DOI] [PubMed] [Google Scholar]
- Hadjihannas MV, Bruckner M, Jerchow B, Birchmeier W, Dietmaier W, Behrens J. Aberrant Wnt/beta-catenin signaling can induce chromosomal instability in colon cancer. Proc Natl Acad Sci U S A. 2006;103:10747–52. doi: 10.1073/pnas.0604206103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Shinohara K, Wang J, Ikeuchi S, Yoshiba S, Meno C, Nonaka S, Takada S, Hatta K, Wynshaw-Boris A, Hamada H. Planar polarization of node cells determines the rotational axis of node cilia. Nat Cell Biol. 2010;12:170–6. doi: 10.1038/ncb2020. [DOI] [PubMed] [Google Scholar]
- Hayes JM, Kim SK, Abitua PB, Park TJ, Herrington ER, Kitayama A, Grow MW, Ueno N, Wallingford JB. Identification of novel ciliogenesis factors using a new in vivo model for mucociliary epithelial development. Dev Biol. 2007;312:115–30. doi: 10.1016/j.ydbio.2007.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman J, Kofron M, Wylie C. Beta-catenin signaling activity dissected in the early Xenopus embryo: a novel antisense approach. Dev Biol. 2000;222:124–34. doi: 10.1006/dbio.2000.9720. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Tanaka Y, Okada Y, Takeda S. Nodal flow and the generation of left-right asymmetry. Cell. 2006;125:33–45. doi: 10.1016/j.cell.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Hyatt BA, Yost HJ. The left-right coordinator: the role of Vg1 in organizing left-right axis formation. Cell. 1998;93:37–46. doi: 10.1016/s0092-8674(00)81144-7. [DOI] [PubMed] [Google Scholar]
- Itoh K, Antipova A, Ratcliffe MJ, Sokol S. Interaction of dishevelled and Xenopus axin-related protein is required for wnt signal transduction. Mol Cell Biol. 2000;20:2228–38. doi: 10.1128/mcb.20.6.2228-2238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Jenny A, Mlodzik M, Sokol SY. Centrosomal localization of Diversin and its relevance to Wnt signaling. J Cell Sci. 2009;122:3791–8. doi: 10.1242/jcs.057067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DD, Meigs TE, Kelly P, Casey PJ. Identification of a role for beta-catenin in the establishment of a bipolar mitotic spindle. J Biol Chem. 2004;279:10829–32. doi: 10.1074/jbc.C400035200. [DOI] [PubMed] [Google Scholar]
- Kim SM, Choi EJ, Song KJ, Kim S, Seo E, Jho EH, Kee SH. Axin localizes to mitotic spindles and centrosomes in mitotic cells. Exp Cell Res. 2009;315:943–54. doi: 10.1016/j.yexcr.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Konig G, Hausen P. Planar polarity in the ciliated epidermis of Xenopus embryos. Dev Biol. 1993;160:355–68. doi: 10.1006/dbio.1993.1312. [DOI] [PubMed] [Google Scholar]
- Louie RK, Bahmanyar S, Siemers KA, Votin V, Chang P, Stearns T, Nelson WJ, Barth AI. Adenomatous polyposis coli and EB1 localize in close proximity of the mother centriole and EB1 is a functional component of centrosomes. J Cell Sci. 2004;117:1117–28. doi: 10.1242/jcs.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonneuve C, Guilleret I, Vick P, Weber T, Andre P, Beyer T, Blum M, Constam DB. Bicaudal C, a novel regulator of Dvl signaling abutting RNA-processing bodies, controls cilia orientation and leftward flow. Development. 2009;136:3019–30. doi: 10.1242/dev.038174. [DOI] [PubMed] [Google Scholar]
- Mitchell B, Jacobs R, Li J, Chien S, Kintner C. A positive feedback mechanism governs the polarity and motion of motile cilia. Nature. 2007;447:97–101. doi: 10.1038/nature05771. [DOI] [PubMed] [Google Scholar]
- Mitchell B, Stubbs JL, Huisman F, Taborek P, Yu C, Kintner C. The PCP pathway instructs the planar orientation of ciliated cells in the Xenopus larval skin. Curr Biol. 2009;19:924–9. doi: 10.1016/j.cub.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlodzik M. Spiny legs and prickled bodies: new insights and complexities in planar polarity establishment. Bioessays. 2000;22:311–5. doi: 10.1002/(SICI)1521-1878(200004)22:4<311::AID-BIES1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Saijoh Y, Tsuchiya K, Shirayoshi Y, Takai S, Taya C, Yonekawa H, Yamada K, Nihei H, Nakatsuji N, Overbeek PA, Hamada H, Yokoyama T. Cloning of inv, a gene that controls left/right asymmetry and kidney development. Nature. 1998;395:177–81. doi: 10.1038/26006. [DOI] [PubMed] [Google Scholar]
- Moeller H, Jenny A, Schaeffer HJ, Schwarz-Romond T, Mlodzik M, Hammerschmidt M, Birchmeier W. Diversin regulates heart formation and gastrulation movements in development. Proc Natl Acad Sci U S A. 2006;103:15900–5. doi: 10.1073/pnas.0603808103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644–6. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- Nakaya MA, Biris K, Tsukiyama T, Jaime S, Rawls JA, Yamaguchi TP. Wnt3a links left-right determination with segmentation and anteroposterior axis elongation. Development. 2005;132:5425–36. doi: 10.1242/dev.02149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascone N, Mercola M. Organizer induction determines left-right asymmetry in Xenopus. Dev Biol. 1997;189:68–78. doi: 10.1006/dbio.1997.8635. [DOI] [PubMed] [Google Scholar]
- Neugebauer JM, Amack JD, Peterson AG, Bisgrove BW, Yost HJ. FGF signalling during embryo development regulates cilia length in diverse epithelia. Nature. 2009;458:651–4. doi: 10.1038/nature07753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport J, Kirschner M. A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell. 1982;30:675–86. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- Okada Y, Nonaka S, Tanaka Y, Saijoh Y, Hamada H, Hirokawa N. Abnormal nodal flow precedes situs inversus in iv and inv mice. Mol Cell. 1999;4:459–68. doi: 10.1016/s1097-2765(00)80197-5. [DOI] [PubMed] [Google Scholar]
- Okada Y, Takeda S, Tanaka Y, Belmonte JC, Hirokawa N. Mechanism of nodal flow: a conserved symmetry breaking event in left-right axis determination. Cell. 2005;121:633–44. doi: 10.1016/j.cell.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Ou Y, Rattner JB. The centrosome in higher organisms: structure, composition, and duplication. Int Rev Cytol. 2004;238:119–82. doi: 10.1016/S0074-7696(04)38003-4. [DOI] [PubMed] [Google Scholar]
- Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet. 2006;38:303–11. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet. 2008;40:871–9. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennekamp P, Karcher C, Fischer A, Schweickert A, Skryabin B, Horst J, Blum M, Dworniczak B. The ion channel polycystin-2 is required for left-right axis determination in mice. Curr Biol. 2002;12:938–43. doi: 10.1016/s0960-9822(02)00869-2. [DOI] [PubMed] [Google Scholar]
- Qiu D, Cheng SM, Wozniak L, McSweeney M, Perrone E, Levin M. Localization and loss-of-function implicates ciliary proteins in early, cytoplasmic roles in left-right asymmetry. Dev Dyn. 2005;234:176–89. doi: 10.1002/dvdy.20509. [DOI] [PubMed] [Google Scholar]
- Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T, Asbrand C, Bakkers J, Kuhl M, Schaeffer HJ, Huelsken J, Behrens J, Hammerschmidt M, Birchmeier W. The ankyrin repeat protein Diversin recruits Casein kinase Iepsilon to the beta-catenin degradation complex and acts in both canonical Wnt and Wnt/JNK signaling. Genes Dev. 2002;16:2073–84. doi: 10.1101/gad.230402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweickert A, Weber T, Beyer T, Vick P, Bogusch S, Feistel K, Blum M. Cilia-driven leftward flow determines laterality in Xenopus. Curr Biol. 2007;17:60–6. doi: 10.1016/j.cub.2006.10.067. [DOI] [PubMed] [Google Scholar]
- Serluca FC, Xu B, Okabe N, Baker K, Lin SY, Sullivan-Brown J, Konieczkowski DJ, Jaffe KM, Bradner JM, Fishman MC, Burdine RD. Mutations in zebrafish leucine-rich repeat-containing six-like affect cilia motility and result in pronephric cysts, but have variable effects on left-right patterning. Development. 2009;136:1621–31. doi: 10.1242/dev.020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Berbari NF, Yoder BK. Ciliary dysfunction in developmental abnormalities and diseases. Curr Top Dev Biol. 2008;85:371–427. doi: 10.1016/S0070-2153(08)00813-2. [DOI] [PubMed] [Google Scholar]
- Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–43. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Annu Rev Genet. 2008;42:517–40. doi: 10.1146/annurev.genet.42.110807.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla V, Reiter JF. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science. 2006;313:629–33. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- Sokol S. A role for Wnts in morpho-genesis and tissue polarity. Nat Cell Biol. 2000;2:E124–5. doi: 10.1038/35017136. [DOI] [PubMed] [Google Scholar]
- Song H, Hu J, Chen W, Elliott G, Andre P, Gao B, Yang Y. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature. 2010;466:378–82. doi: 10.1038/nature09129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemaru K, Yamaguchi S, Lee YS, Zhang Y, Carthew RW, Moon RT. Chibby, a nuclear beta-catenin-associated antagonist of the Wnt/Wingless pathway. Nature. 2003;422:905–9. doi: 10.1038/nature01570. [DOI] [PubMed] [Google Scholar]
- Vick P, Schweickert A, Weber T, Eberhardt M, Mencl S, Shcherbakov D, Beyer T, Blum M. Flow on the right side of the gastrocoel roof plate is dispensable for symmetry breakage in the frog Xenopus laevis. Dev Biol. 2009;331:281–91. doi: 10.1016/j.ydbio.2009.05.547. [DOI] [PubMed] [Google Scholar]
- Voronina VA, Takemaru K, Treuting P, Love D, Grubb BR, Hajjar AM, Adams A, Li FQ, Moon RT. Inactivation of Chibby affects function of motile airway cilia. J Cell Biol. 2009;185:225–33. doi: 10.1083/jcb.200809144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB. Planar cell polarity signaling, cilia and polarized ciliary beating. Curr Opin Cell Biol. 2010 doi: 10.1016/j.ceb.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–5. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- Wessely O, Obara T. Fish and frogs: models for vertebrate cilia signaling. Front Biosci. 2008;13:1866–80. doi: 10.2741/2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost HJ. Left-right asymmetry: nodal cilia make and catch a wave. Curr Biol. 2003;13:R808–9. doi: 10.1016/j.cub.2003.09.051. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Levin M. “Left-right asymmetry in the chick embryo requires core planar cell polarity protein Vangl2, Genesis. 2009;47:719–728. doi: 10.1002/dvg.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129:1051–63. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.