Abstract
Purpose
To identify a clinically meaningful threshold for change in retinal thickness measured by optical coherence tomography (OCT) for patients with uveitic macular edema, using correlation with change in visual acuity.
Design
Cross-sectional and longitudinal study.
Methods
128 eyes (101 individuals) with macular edema enrolled in the Multicenter Uveitis Steroid Treatment (MUST) trial. At enrollment and after six months of follow-up, retinal thickness was measured at the central subfield with time domain OCT and visual acuity was measured with logarithmic (ETDRS) visual acuity charts. Participants were classified as having macular edema if the retinal thickness was ≥260μm.
Results
A threshold for change in retinal center subfield thickness of 20% balanced the percentage of false positives and negatives for predicting greater than 10-letter change in visual acuity with sensitivity of 77% and a specificity of 75%. The results were similar for greater than 5 or 15 or greater letter changes. Those with a 20% or greater reduction in retinal thickness had a mean 11.0 letter improvement (95% CI: 7.7 to 14.3) as compared to a -0.4 letter change (95% CI: -4.1 to 3.3) in visual acuity for those without a 20% reduction (p < 0.01).
Conclusions
In addition to being above the level of measurement uncertainty, a 20% change in retinal thickness in patients with macular edema appears to be optimal for clinically important changes in visual acuity and may be considered as an outcome for clinical trials of treatments for uveitic macular edema.
Introduction
Macular edema (ME) is among the most frequent structural complications of uveitis and is a common cause of vision loss.1, 2, 3 Initially, ME was evaluated by measuring the area of macular leakage with fluorescein angiography (FA). Optical coherence tomography (OCT) measures retinal thickness in a quantifiable and reproducible fashion and has largely replaced FA for managing ME due in part to the fact that visual acuity is more strongly associated with retinal thickness than macular leakage.4
For measurements graded as abnormal, there are two possible response targets in clinical research: normalization (or resolution) and “improvement”. Visual acuity changes often are reported in both ways: recovery to a “good” acuity (i.e. 20/40 or better) or a 1-, 2-, or 3-line improvement on a standard acuity chart. Similarly, changes in ocular inflammation are reported as resolution (grade 0 inflammation) or “improvement” (a 2-step change in the standardized semi-quantitative grading scales).5
Similarly, retinal thickness changes in patients with uveitic ME could be quantified as “resolution” (return to a normal retinal thickness) or “improvement” (change by a clinically meaningful amount). Previous data have suggested that the inter-measurement variability of OCT, regardless of machine, is less than 10%.6 Nevertheless, it is unclear whether a change of 10% is clinically meaningful or is the optimal threshold for change in retinal thickness as an outcome measurement for clinical research.
Visual acuity loss in patients with uveitis often is multifactorial, and the causes of visual acuity loss include ME, cataract, disc damage from uveitic glaucoma, and other media opacities from the inflammation. Nevertheless, ME is the most common cause of vision loss in patients with uveitis. Therefore, one potential way to determine a threshold for considering a change in macular edema clinically significant is to correlate change in macular thickness with change in visual acuity. However, currently there are no data to determine what level of change in retinal thickness best correlates with clinically meaningful changes in visual acuity in patients with uveitic macular edema. Even though visual acuity is affected by multiple factors and OCT changes would not be expected to correlate perfectly with changes in VA, if it were necessary to decide whether or not ME had improved, then the change in thickness that best correlated with improvement in visual acuity would seem to be the most reasonable choice. Therefore, we decided to define a clinically meaningful change in retinal thickness for uveitic macular edema as the change in thickness that has optimal sensitivity and specificity for predicting clinically meaningful changes in VA and is above the reproducibility threshold. Furthermore, in order to be machine independent, we based the definition on a percent change rather than a fixed change in the number of microns of thickness.
The Multicenter Uveitis Steroid Treatment (MUST) trial provides an opportunity to identify this threshold since masked visual acuity and objective OCT measurements were obtained at regularly specified time points throughout the trial. In this report, we measure the association between changes in retinal thickness and visual acuity to determine a threshold of retinal thickness change that best predicts clinically meaningful changes in visual acuity.
Methods
This analysis includes data collected as of 1 June 2010 in the Multicenter Uveitis Steroid Treatment (MUST) Trial, a prospective, multi-center, international clinical trial (ClinicalTrials.gov registration number: NCT00132691) comparing the safety and effectiveness of local therapy with a fluocinolone acetonide implant to systemic therapy for patients with severe non-infectious intermediate, posterior, or panuveitis.1
Study population
A detailed version of the enrollment criteria for the MUST trial is published elsewhere.1 Eyes were included in this analysis if the following criteria were met at enrollment: (1) a high quality OCT scan was available and (2) retinal thickness, measured at the central subfield, was greater than or equal to 260 μm.
Data collection
Best corrected visual acuity (BCVA) was measured in a masked fashion using logarithmic (ETDRS) visual acuity charts7 according to a standardized protocol. Optical coherence tomography was performed by certified photographers using Stratus OCT 3 machines (Carl Zeiss Meditec, Jena Germany) to obtain Fast Macular retinal thickness maps and high resolution cross hair scans. The images were graded by a centralized reading center at the University of Wisconsin, Madison.1 The current analysis was restricted to those eyes for which high quality scans (i.e., sufficient quality to derive reliable central subfield data from the automated retinal thickness map) could be attained, providing the best possible data for center point and center subfield measurements.8, 9, 10 The parameters required for high quality scans included proper centration of the scan, proper determination of RPE and ILM boundaries by automated algorithm, signal strength of 5 or greater and standard deviation of the center point and center subfield measurements of less than 10%.11 An eye was defined as having macular edema (ME) if the retinal thickness at the central subfield was 260 μm or greater (normative values 212 ± 20 μm). 8
Main outcome measures
The primary outcomes of interest are the change in visual acuity from enrollment to six-months and the percentage change in retinal thickness over the same time period. The six-month time point was chosen to allow sufficient follow-up in which to observe meaningful changes in both retinal thickness and visual acuity, as the latter may lag behind the former.12 Furthermore, it was necessary to allow sufficient recovery time post-surgery for patients treated with an implant to reduce the likelihood of observing any transient vision loss associated with implant surgery.
Three cut-offs for change in visual acuity were evaluated: 5, 10, and 15 letters. A change of greater than 5 letters is considered reproducible.13, 14 A change of greater than 10 letters is considered clinically meaningful.15 A change of 15 or more letters represents a doubling of the visual angle (e.g. a change from 20/20 to 20/40) and has been a standard outcome measurement in clinical trials.7
The percentage change in retinal thickness was calculated rather than the absolute change. An absolute change assumes that a specific value has the same influence regardless of the thickness at enrollment. In contrast, the percentage change takes into account the initial value. This quantification parallels the logOCT scale proposed by Ferris et al16 in which a one-step change is approximately equivalent to a 20% change. Furthermore, this method has the advantage of being generalizable to all OCT machines, each of which has a different “normal” range.
Statistical analysis
Summary statistics were computed for continuous (medians, inter-quartile ranges, and ranges) and categorical (counts and percentages) variables. Spearman’s rank correlation was used to estimate the association between visual acuity and retinal thickness for specific time points as well as change over time. The linear, logistic, and ordinal relationships between visual acuity and retinal thickness outcomes were modeled using generalized estimating equations (GEEs) in order to adjust for within person, between eye correlations.17
Sensitivity and specificity were used to assess the ability of changes in retinal thickness to accurately predict clinically meaningful changes in visual acuity.18 Receiver operating characteristic (ROC) curves and plots of the sensitivity and specificity versus percentage change in retinal thickness were generated to graphically display the ability of the change in retinal thickness to predict changes in visual acuity for a range of thresholds. Bootstrap confidence intervals were computed for the correlations and prediction characteristics to adjust for within person, between-eye correlations. Multiple imputation techniques were used to assess the effect of missing data.19 Statistical analyses were performed using the SAS (SAS/STAT User’s Guide, Version 9.1, Cary, NC:SAS Institute) and R (The R Project for Statistical Computing, Version 2.11.1, http://www.r-project.ortg/) statistical packages.
Results
A total of 255 patients (479 eyes) with uveitis were enrolled in the MUST trial. Of the 322 eyes with high quality OCT scans at enrollment, 126 eyes (101 individuals) were diagnosed with macular edema and 194 did not have macular edema. The remaining eyes were excluded from the analysis due to the fact that the OCT was missing (N = 26, 6%), the OCT was unreadable (N = 18, 4%), or the OCT did not have sufficient quality to derive reliable central subfield data from the automated retinal thickness map (N = 113, 24%). The large number eyes without a high quality OCT at enrollment was to be expected as there was no requirement for high quality images at enrollment, in order to enroll patients representative of the spectrum of the disease and to encourage recruitment. Indeed, patients with media opacity (primarily cataract) and inability to dilate the pupil (primarily due to posterior synechiae) were eligible for the trial. The proportion of eyes that had a cataract or were aphakic or pseudophakic was significantly higher (p = 0.04) in those eyes for which the OCT was missing or ungradable (92% and 94%, respectively) as compared to those eyes with an OCT that was gradable manually (81%) or using the automated retinal thickness map (74%).
The 128 eyes identified as having macular edema with a high quality OCT constitute the basis of the report. Of these, 75 eyes (53 individuals) had complete enrollment and follow-up data for visual acuity as well as a high quality OCT scan at six months. Incomplete data were due to missing the six month visit (N = 9), missing an OCT (N = 4), ungradable OCT (N = 3), missing visual acuity (N = 1), having an OCT scan that was not of sufficient quality (N = 36) at the six month visit.
Characteristics of the Study Population
The characteristics of the entire MUST cohort are described elsewhere.1 Table 1 compares the demographic and clinical characteristics for eyes with macular edema at enrollment who had complete data for visual acuity and retinal thickness at enrollment and six months with those who did not. In brief, for those eyes with complete follow-up, the median age was 50 (25th to 75th percentile: 40 to 57), the median time from diagnosis with uveitis was 3.8 years (25th to 75th percentile: 1.1 to 7.9), 33% were male, and 69% were white. These eyes were likely to have vitreous haze (N = 53, 73%), vitreous cells (N = 65, 89%) and to have cataracts (N = 33, 44%) or be aphakic or pseudophakic (N = 30, 40%). The median visual acuity was 68 letters (25th to 75th percentile: 57 to 79), which is approximately 20/40 Snellen equivalent (20/70 to 20/25). The majority of the characteristics of uveitis and its complications were similar for those eyes that did not have complete follow-up. However, those eyes that did not have complete follow-up were more likely to be aphakic or pseudophakic (66% vs 40%). There was no significant difference between the change in visual acuity over six months for those who had an OCT of sufficient quality and those that did not (p = 0.2). The remainder of the paper focuses on the characteristics of those 75 eyes for which high quality OCTs and visual acuity measurements were available at enrollment and six months.
Table 1.
Characteristics at enrollment for uveitic eyes diagnosed with macular edema (ME), defined as retinal thickness ≥ 260 μm at the central subfield using optical coherence tomography (OCT) stratified by the availability of complete data for visual acuity and retinal thickness at enrollment and at six-months.
| Characteristic | Eyes with complete Follow-up (N = 75) |
Eyes with incomplete Follow-up (N = 53) |
|---|---|---|
| Age (years)a | 50 (40 to 57) | 58 (48 to 69) |
| Female, n (%)b | 50 (67%) | 42 (79%) |
| White, non-Hispanic, n (%)b | 52 (69%) | 31 (58%) |
| Any systemic disease, n (%)b | 25 (33%) | 11 (21%) |
| Bilateral uveitis, n (%)b | 68 (91%) | 47 (89%) |
| Time since diagnosis (years)a | 3.8 (1.1 to 7.9) | 4.2 (1.2 to 9.9) |
| Missing, n (%)b | 3 (4%) | 3 (5%) |
| Vitreous hazec, n (%)b | 54 (73%) | 41 (77%) |
| Missing, n (%)b | 1 (1%) | |
| Vitreous cellsd, n (%)b | 65 (89%) | 51 (96%) |
| Missing, n (%)b | 2 (3%) | |
| Lens status, n (%)b | ||
| Phakic | 12 (16%) | 3 (6%) |
| Cataract | 33 (44%) | 15 (28%) |
| Aphakic/pseudophakic | 30 (40%) | 35 (66%) |
Continuous variables are summarized using medians and interquartile ranges (25th-75th percentiles).
Percentages are calculated out of the total cohort for the ‘Missing’ category and out of the total observed cohort for all other categories.
Vitreous haze greater than grade 0.
Vitreous cells greater than grade 0.
Change in Retinal Thickness and Visual Acuity
Visual acuity and retinal thicknenss measurements at enrollment and after six months of follow-up, and change during the initial six months of follow-up are summarized in Table 2. Median visual acuity at enrollment was 68 letters (Range: 7 to 94) and the median change in visual acuity was 4 letters (Range: -28 to 35). The median change for the 47 eyes with an improvement in vision was 10 letters (Range: 1 to 35) and for the 28 eyes without improvement in vision was -6 letters (Range: -28 to 0). Thirty-five (47%) eyes had an improvement of greater than 5 letters, 22 (29%) had an improvement of greater than 10 letters and 13 (17%) had a halving of the visual angle (i.e. an improvement of 15 or more letters). The median retinal thickness at the central subfield at enrollment was 327 μm (Range: 260 to 1195) and the median percentage change from enrollment to six months was a 15% reduction (Range: -86% to 152%). The median change for those with improvement was a reduction in retinal thickness of 25% (Range: 1% to 86%) and for those without improvement was an increase of 13% (Range: 0% to 52%).
Table 2.
Visual acuity and retinal thickness measured by optical coherence tomography (OCT) at enrollment and six months for uveitic eyes with macular edema, defined as ≥ 260 μm at the central subfield, at enrollment.
| Characteristic | Eyes with Macular Edemaf At Enrollment (N = 75) |
|---|---|
| Visual Acuity (EDTRS letters)a | |
| Enrollmentb | 68 (7 to 94) |
| Six monthsb | 73 (19 to 93) |
| Change from enrollment to six monthsb | 4 (-28 to 35) |
| > 5 letter improvement, n (%) | 35 (47%) |
| > 10 letter improvement, n (%) | 22 (29%) |
| ≥ 15 letter improvement, n (%) | 13 (17%) |
| Retinal thickness (μm)c | |
| Enrollmentb | 327 (260 to 1195) |
| Six monthsb | 261 (133 to 840) |
| Change from enrollment to six monthsb,d | -43 (-870 to 428) |
| Percentage changeb,c,d,e | -15% (-86% to 152%) |
85 letters = 20/20, 70 letters = 20/40, 50 letters = 20/100.14
Continuous variables are summarized with medians and ranges.
Retinal thickness (RT) is measured by optical coherence tomography (OCT) at the central subfield.
A negative change, representing a decrease in retinal thickness, is considered an improvement.
Percent change = (RT at six months – RT at enrollment)/(RT at enrollment).
Macular edema (ME) is defined as retinal thickness at the center subfield ≥ 260 μm as measured by OCT.
Association between Retinal Thickness and Visual Acuity
For each 100 μm lower retinal thickness, the visual acuity was 6.5 letters higher (95% CI: 5.2 to 7.6, p < 0.01) at enrollment and 5.3 letters higher (95% CI: 1.9 to 8.8, p < 0.01) after six months of follow-up, respectively. The cross-sectional Spearman’s correlation between visual acuity and retinal thickness at enrollment was -0.56 (95% CI: -0.74 to -0.35) and at six months was -0.24 (95% CI: -0.46 to 0.02). As retinal thickness decreased over time, vision improved (Figure 1, Spearman’s correlation: -0.46, 95% CI: -0.62 to -0.27, p < 0.01). Over the six month follow-up period, the average change in visual acuity was 1.5 letters (95% CI: 0.7 to 2.2, p < 0.01) for each 10% decrease in retinal thickness, the minimum reproducible difference.
Figure 1.
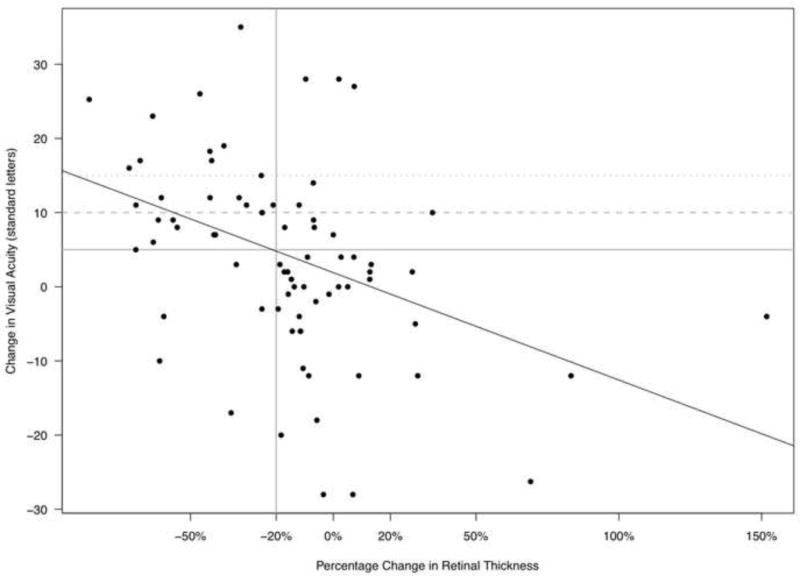
Plot of the percentage change in retinal thickness at the central subfield measured by optical coherence tomography (OCT) versus the change in visual acuity (VA) from enrollment to six months in eyes with uveitic macular edema.
Identifying a Threshold for Retinal Thickness
The performance of a range of potential retinal thickness cut-offs for predicting changes in visual acuity was similar for all three of the vision thresholds as demonstrated by the overlap in the ROC curves, which plot the sensitivity versus 1-specificity (Supplemental Figure 1). The relationship between the percentage change in retinal thickness and the sensitivity (percentage of true positives) and specificity (percentage of true negatives) for identifying a greater than 10 letter change in visual acuity is presented in Figure 2. The point at which the two curves cross represents the percentage change in retinal thickness for which the sensitivity and specificity are equal, i.e. the level of false positives and false negatives are equal. Overall, a 20% reduction in retinal thickness appears to balance the two error types for a change in visual acuity of greater than 10 letters with a sensitivity of 77% (95% CI: 60% to 95%) and a specificity of 75% (95% CI: 62% to 86%). Those with a 20% or greater reduction in retinal thickness had on average a 11.0 letter improvement (95% CI: 7.7 to 14.3) as compared to a -0.4 letter change (95% CI: -4.1 to 3.3) in visual acuity for those without a 20% reduction (Difference: 11.4, 95% CI: 6.9 to 15.9, p < 0.01).
Figure 2.
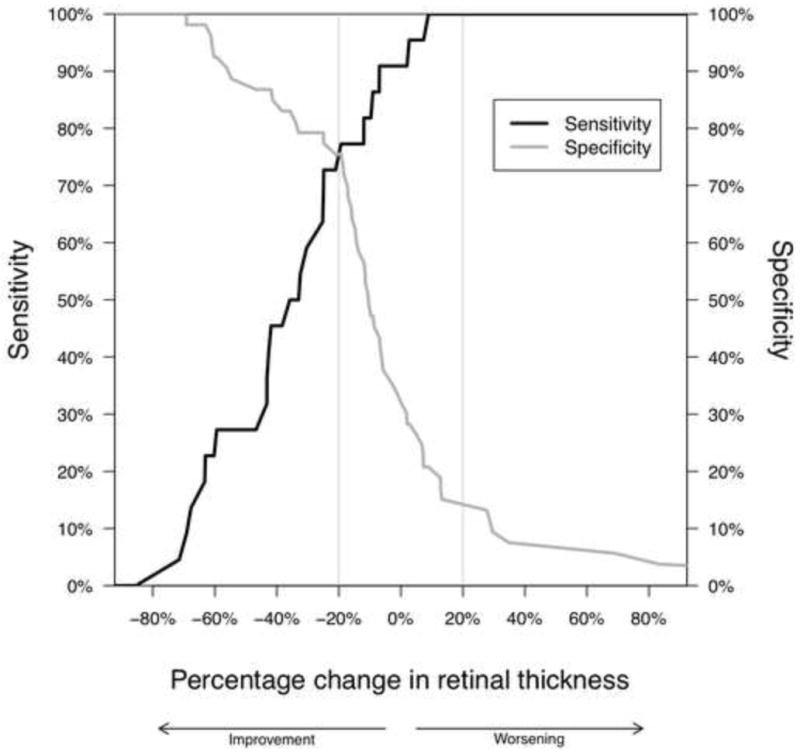
Plot of the sensitivities and specificities associated with different thresholds of percentage change in retinal thickness (measured by optical coherence tomography, OCT, at the central subfield) for identifying a greater than 10 letter increase in visual acuity in eyes with uveitic macular edema.
The association between a 20% change in retinal thickness and clinically meaningful changes in visual acuity (> 5 letters, > 10 letters and ≥ 15 letters) is explored in Table 3. Comparisons were made for changes in either direction (e.g. ≥ 20% reduction, < 20% change or ≥ 20% increase) as well as for improvement only (e.g. ≥ 20% reduction versus < 20% reduction). In both cases, the 20% change was significantly associated with all three visual acuity thresholds.
Table 3.
Association between change in retinal thickness measured by optical coherence tomography (OCT) at the central subfield and change in visual acuity for eyes with uveitic macular edema (defined as retinal thickness ≥ 260 μm). Comparisons are made for scores documenting the overall trends and improvement.
| Change in visual acuity
| ||||
|---|---|---|---|---|
| Overall trenda | ≥ 20% reduction | < 20% change | ≥ 20% increase | P-valueb |
| > 5 letter improvement | 25 (83%) | 9 (24%) | 1 (14%) | < 0.001 |
| ≤ 5 letter change | 3 (10%) | 19 (50%) | 3 (43%) | |
| > 5 letter decline | 2 (7%) | 10 (26%) | 3 (43%) | |
| > 10 letter improvement | 17 (57%) | 5 (13%) | 0 (0%) | < 0.001 |
| ≤ 10 letter change | 11 (36%) | 26 (69%) | 4 (57%) | |
| > 10 letter decline | 2 (7%) | 7 (18%) | 3 (43%) | |
| > 15 letter improvement | 10 (33%) | 3 (8%) | 0 (0%) | < 0.001 |
| ≤ 15 letter change | 19 (63%) | 31 (82%) | 6 (86%) | |
| > 15 letter decline | 1 (3%) | 4 (10%) | 1 (14%) | |
|
| ||||
| Improvementa | ||||
| ≥ 20% reduction | < 20% reduction | P-valuec | ||
| > 5 letter improvement | 25 (83%) | 10 (22%) | < 0.001 | |
| ≤ 5 letter improvement | 5 (17%) | 35 (78%) | ||
| > 10 letter improvement | 17 (57%) | 5 (11%) | < 0.001 | |
| ≤ 10 letter improvement | 13 (43%) | 40 (89%) | ||
| > 15 letter improvement | 10 (33%) | 3 (7%) | 0.006 | |
| ≤ 15 letter improvement | 20 (67%) | 42 (93%) | ||
The counts and column percentages are included for each combination.
P-values are based upon ordinal regression using generalized estimating equations (GEE) to account for within-person, between-eye correlation.
P-values are based upon logistic regression using generalized estimating equations (GEE) to account for within-person, between-eye correlation.
A summary of the sensitivity and specificity associated with a 20% decrease in retinal thickness for each of the visual acuity thresholds is presented in Table 4. For 71% of eyes with an improvement in visual acuity greater than 5 letters the decrease in retinal thickness was 20% or greater. This is the sensitivity of the 20% threshold for predicting a change of greater than 5 letters. Conversely, if the improvement in visual acuity was 5 letters or less, then the percentage of eyes with a decrease in retinal thickness less than 20% was 88%, which represents the specificity.
Table 4.
Estimates of the sensitivity and specificity of a 20% improvement in retinal thickness measured by optical coherence tomography (OCT) at the central subfield for identifying clinically important changes in visual acuity in eyes with uveitic macular edema (defined as retinal thickness ≥ 260 μm). Bootstrap estimates of the 95% confidence intervals are provided in parentheses.
| Change in visual acuity | Sensitivity (95% CIa) | Specificity (95% CIa) |
|---|---|---|
| > 5 letters | 71% (55% to 89%) | 88% (75% to 97%) |
| > 10 letters | 77% (60% to 95%) | 75% (62% to 86%) |
| ≥ 15 letters | 77% (50% to 100%) | 68% (54% to 80%) |
CI = Confidence interval.
A sensitivity analysis, in which missing values for retinal thickness and visual acuity were imputed for the eyes identified as having macular edema at enrollment by high quality OCT, produced similar results. The 20% threshold again proved to be strongly associated with clinically meaningful changes in visual acuity (data not shown). An additional sensitivity analysis was conducted excluding eyes with the following characteristics: VA < 10 letters at enrollment, VA > 70 letters at enrollment, retinal thickness greater than 600, cataract surgery between enrollment and 6 months. A 20% change was still optimal for balancing the two error types however the sensitivity and specificity were reduced (69% and 63% respectively).
Discussion
Optical coherence tomography has revolutionized the approach to uveitic macular edema. With OCT, the response of an eye to treatment for macular edema can be measured directly, as can a return to normal retinal thickness, which typically indicates resolution of the edema. Clinicians now routinely follow macular thickness in managing uveitic macular edema, as visual acuity in patients with uveitis can be influenced by other structural complications such as cataract. In addition, OCT provides an objective method for evaluating the edema that is unlikely to be influenced by the patient or clinician and, when a centralized reading center is used, is easy to mask. Clinical studies evaluating uveitic macular edema can use either improvement in macular edema or resolution of macular edema (i.e. return to normal retinal thickness) as an outcome. Although a 10% change in thickness is considered to be reproducible,9 we have shown that a 20% change in retinal thickness, in addition to being above the level of random variation, is optimally associated with a greater than 10 letter improvement in visual acuity. Similar results were found for other vision thresholds, although the analysis of a ≥ 15 letter change is limited due to the small number of eyes with a change of that magnitude. Hence, we propose that a 20% change in macular thickness be used as an outcome measure for determining whether macular edema has improved. Given the small number of individuals with a 20% or greater increase in retinal thickness, we cannot form highly reliable estimates about the threshold for worsening.
As previously noted,20 retinal thickness is not intended to be a surrogate for vision. Eyes with retinal pigment epithelium (RPE) damage due to chronic edema, chorioretinal lesions or scars at the fovea may experience improvement in their macular edema but not their visual acuity following treatment. Media problems, such as cataract may limit visual acuity even after treatment has resulted in resolution of the macular edema. Improvement of other contributing inflammatory sequela (e.g. vitreous haze, optic nerve swelling, retinitis) might lead to improvement in vision but not in macular edema. Similarly, cataract surgery might have a positive impact on vision while leaving the macular edema unchanged or worse.21 Damage to the retina, such as cystoid degeneration, may affect retinal thickness without impacting visual acuity. However, since BCVA remains the preferred outcome for clinical trials, it remains the most appropriate metric on which to base the selection of a threshold for defining improvement.
The characteristics of the eyes examined in this study were similar to those with macular edema in previous studies of both uveitis and diabetes except for a higher fraction of individuals with bilateral uveitis and a higher fraction of eyes with cataract or aphakic or pseudophakic status.20, 22 The correlation between retinal thickness and visual acuity at enrollment (-0.56) was similar to that observed in previous studies for diabetic macular edema (-0.35 to -0.52),20 and uveitic macular edema (-0.41).22 The cross-sectional correlation after 6 months, while not excluding the enrollment correlation levels, was much lower (-0.24, 95% CI: -0.46 to 0.02), which may be due to cataract progression. It should be noted that 20% of eyes that were not already aphakic or pseudophakic either developed a cataract (9%) or experienced a worsening of an existing cataract that required surgery (11%). Despite this limitation, the correlation between the change in retinal thickness and the change in visual acuity (-0.45) was nearly identical to that observed for diabetic macular edema,20 where a 4.4 letter improvement was observed for every 100 μm decrease in retinal thickness at the center point.
The strengths of the study include the prospective design, use of standardized masked data collection protocols, a centralized masked reading center for the evaluation and grading of OCT images, and recruitment from sites that were diverse both geographically (21 sites across 14 states in the U.S. and 1 each in London, U.K. and Melbourne, Australia) and in terms of access (community- and university-based centers). The primary limitation of this study is the modest number of eyes included in the analysis, which limits the precision of the estimates.
The inability to obtain OCT images of sufficient quality to derive reliable central subfield data from the automated retinal thickness map was the main factor limiting the sample size. Although some of the issues were related to the machine process (e.g. border determination and centering), the majority of problems arose due to disease characteristics affecting the media (e.g. cataracts, poor dilation due to posterior synechiae).
Eyes were excluded for failing to have sufficient quality OCT images both at enrollment and during follow-up. It is important to note that having media clarity sufficient for high quality images was not a requirement for enrollment in the MUST Trial and that individuals with poor visual acuity, severe vitritis, and poor optical media were allowed to enroll. In fact, 77% of the eyes either had cataracts or were aphakic or pseudophakic at enrollment. The individuals included in this analysis represent the population that would be enrolled in trials of treatments for uveitic macular edema, where media clarity and pupillary dilation sufficient to obtain high quality OCT scans would be eligibility criteria.
Only 75 (59%) of the 128 eyes that had a high quality OCT scan indicating macular edema at enrollment also had a high quality OCT scan at 6 months. However, one of the two treatment arms (implant) is known to be associated with a high rate of cataract formation leading to cataract surgery (roughly 33% per year), which would further inhibit the ability to collect OCT images.23 Indeed, as noted above, 20% of the eyes either developed cataracts or had a worsening of an existing cataract that required surgery during the six months of follow-up considered in this analysis. The distribution of eyes between the two treatment arms was similar (Implant: 39 eyes, Systemic: 36 eyes), however there was insufficient power to detect differences between the two groups. It should be noted that a sensitivity analysis imputing missing visual acuity data and follow-up OCT data showed results similar to those obtained when limiting the analysis to eyes with complete data.
The majority of eyes in the cohort had moderate degree of thickening (< 600 μm) with only 8 at or above 600μm (Median: 705 μm, Range: 604-1195). It is possible that eyes with a greater initial retinal thickness might require a higher or lower threshold of change prior to eliciting a change in vision. In our case, all of the eyes with retinal thickness above 600 μm experienced both a 20% decrease in retinal thickness and a 10-letter improvement in visual acuity (5/8 experienced a 15 letter increase in VA). In a sensitivity analysis exploring effect of retinal thickness at enrollment (categorized as < 400, 400-500, 500-600, ≥ 600), no significant difference or trend was observed relating the fraction of individuals with a 5-, 10-, or 15-letter change in visual acuity and retinal thickness at enrollment either for those with a 20% change or those without a 20% change. However, the number of eyes in each category, especially > 600 μm, were small. Therefore, the generalizability of the results is limited primarily to eyes with an initial thickness of 600 μm or less. A further exploration of the potential for such a trend should be considered for cohorts with a greater range of initial retinal thicknesses.
In summary, a 20% change in retinal thickness appears to be both reproducible and to correlate with clinically important changes in visual acuity for eyes with uveitic macular edema. For studies of treatments for uveitic macular edema in which improvement is monitored through changes in retinal thickness, this threshold is optimal in terms of balancing the sensitivity and specificity associated with clinically meaningful changes in visual acuity.
Supplementary Material
Acknowledgments
Funding/Support: Supported by cooperative agreements from the National Eye Institute to Mount Sinai School of Medicine (U10 EY 014655), The Johns Hopkins University Bloomberg School of Public Health (U10 EY 014660), and the University of Wisconsin, Madison, School of Medicine (U10 EY 014656). Bausch & Lomb provided support to the study in the form of donation of a limited number of fluocinolone implants for patients randomized to implant therapy who were uninsured or otherwise unable to pay for the implants. Dr. Thorne is the recipient of the Research to Prevent Blindness Sybill Harrington Special Scholars Award.
Statement about Conformity with Author Information: The study has been registered at ClinicalTrials.gov (identifier NCT00132691) and was approved by the governing institutional review boards of all participating institutions.
Other Acknowledgments: None
Footnotes
Contributions to Authors: Design of the study (EAS, DAJ, MMA, JET); Conduct of the study (EAS, DAJ, MMA, JET); Collection of data (EAS, MMA, SL, NA, ATV); Management of data (EAS); Analysis of data (EAS); Interpretation of data (EAS, DAJ, MMA, SL, NA, ATV, JET); Preparation of the manuscript (EAS); Review of the manuscript (EAS, DAJ, MMA, SL, NA, ATV, JET); Approval of the manuscript (EAS, DAJ, MMA, SL, NA, ATV, JET).
Financial Disclosures: Douglas A. Jabs, M.D., M.B.A: Abbott Laboratories, Alcon Laboratories, Allergan Pharmaceutical Corporation, Applied Genetic Technologies Corporation, Corcep Therapeutics, Genentech Inc., Genzyme Corporation, GlaxoSmithKline, Novartis Pharmaceuticals Corporation, Roche Pharmaceuticals.
Michael M. Altaweel, MD: Pfizer, Regeneron, GlaxoSmithKline.
Sue Lightman, MD, PhD: Allergan Pharmaceutical Corporation, Abbott Laboratories Ltd, Bausch & Lomb, Genactis Ltd, GlaxoSmithKline, Immunocore Ltd, Schering Plough, Virtuoso Healthcare Communications, The Wellcome Trust.
Albert T. Vitale: Bausch & Lomb, ACIONT Inc.
Jennifer E. Thorne, MD, PhD: Allergan Pharmaceutical Corporation.
Elizabeth A Sugar, PhD, and Nisha Acharya, MD, MS: None.
Supplemental Material available at AJO.com
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The Multicenter Uveitis Steroid Treatment Trial Research Group. The Multicenter Uveitis Steroid Treatment Trial: rationale, design, and baseline characteristics. Am J Ophthalmol. 2010;149(4):550–561. doi: 10.1016/j.ajo.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lardenoye CW, van Kooij B, Rothova A. Impact of macular edema on visual acuity in uveitis. Ophthalmology. 2006;113(8):1446–1449. doi: 10.1016/j.ophtha.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 3.Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80(4):332–336. doi: 10.1136/bjo.80.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nussenblatt RB, Kaufman SC, Palestine AG, Davis MD, Ferris FL., 3rd Macular thickening and visual acuity. Measurement in patients with cystoid macular edema. Ophthalmology. 1987;94(9):1134–1139. doi: 10.1016/s0161-6420(87)33314-7. [DOI] [PubMed] [Google Scholar]
- 5.The Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diabetic Retinopathy Clinical Research Network. Reproducibility of macular thickness and volume using Zeiss optical coherence tomography in patients with diabetic macular edema. Ophthalmology. 2007;114(8):1520–1525. doi: 10.1016/j.ophtha.2006.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferris FL, 3rd, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94(1):91–96. [PubMed] [Google Scholar]
- 8.Chan A, Duker JS, Ko TH, Fujimoto JG, Schuman JS. Normal macular thickness measurements in healthy eyes using Stratus optical coherence tomography. Arch Ophthalmol. 2006;124(2):193–198. doi: 10.1001/archopht.124.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SJ, Belair ML, Bressler NM, et al. A method of reporting macular edema after cataract surgery using optical coherence tomography. Retina. 2008;28(6):870–876. doi: 10.1097/IAE.0b013e318169d04e. [DOI] [PubMed] [Google Scholar]
- 10.Diabetic Retinopathy Clinical Research Network. Optical coherence tomography measurements and analysis methods in optical coherence tomography studies of diabetic macular edema. Ophthalmology. 2008;115(8):1366–1371. doi: 10.1016/j.ophtha.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altaweel MM, Johnson DL. Optical coherence tomography. In: Albert DM, Miller JW, Azar DM, Blodi BA, editors. Albert & Jakobiec’s Principles and Practice of Ophthalmology. 3. Philadelphia: Saunders Elsivier; 2008. pp. 1725–1739. [Google Scholar]
- 12.Nagasawa T, Naito T, Matsushita S, Sato H, Katome T, Shiota H. Efficacy of intravitreal bevacizumab (Avastin) for short-term treatment of diabetic macular edema. J Med Invest. 2009;56(3-4):111–115. doi: 10.2152/jmi.56.111. [DOI] [PubMed] [Google Scholar]
- 13.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135(2):194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 14.Blackhurst DW, Maguire MG the Macular Photocoagulation Study Group. Reproducibility of refraction and visual acuity measurement under a standard protocol. Retina. 1989;9(3):163–9. [PubMed] [Google Scholar]
- 15.Beck RW, Maguire MG, Bressler NM, Glassman AR, Lindblad AS, Ferris FL. Visual acuity as an outcome measure in clinical trials of retinal diseases. Ophthalmology. 2007;114(10):1804–1809. doi: 10.1016/j.ophtha.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 16.Ferris FL, 3rd, Miller KM, Glassman AR, Beck RW the Diabetic Retinopathy Clinical Research Network. A proposed method of logarithmic transformation of optical coherence tomography data for use in clinical research. Ophthalmology. 2010;117(8):1512–1516. doi: 10.1016/j.ophtha.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 18.Pepe MS. The statistical evaluation of medical tests for classification and prediction. New York: Oxford University Press Inc; 2003. pp. 22–23. [Google Scholar]
- 19.Dai JY, Ruczinski I, LeBlanc M, Kooperberg C. Imputation methods to improve inference in SNP association studies. Genet Epidemiol. 2006;30(8):690–702. doi: 10.1002/gepi.20180. [DOI] [PubMed] [Google Scholar]
- 20.Diabetic Retinopathy Clinical Research Network. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114(3):525–536. doi: 10.1016/j.ophtha.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SJ, Equi R, Bressler NM. Analysis of macular edema after cataract surgery in patients with diabetes using optical coherence tomography. Ophthalmology. 2007;114(5):881–889. doi: 10.1016/j.ophtha.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 22.Tran TH, de Smet MD, Bodaghi B, Fardeau C, Cassoux N, Lehoang P. Uveitic macular oedema: correlation between optical coherence tomography patterns with visual acuity and fluorescein angiography. Br J Ophthalmol. 2008;92(7):922–927. doi: 10.1136/bjo.2007.136846. [DOI] [PubMed] [Google Scholar]
- 23.Callanan DG, Jaffe GJ, Martin DF, Pearson PA, Comstock TL. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Arch Ophthalmol. 2008;126(9):1191–1201. doi: 10.1001/archopht.126.9.1191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


