Abstract
Background
Hepatitis C (HCV) recurrence following orthotopic liver transplantation (OLT) is universal, often with accelerated allograft fibrosis. Donor liver steatosis is frequently encountered and often associated with poor early post-operative outcome. The study’s aim was to test the hypothesis that allograft steatosis alters immune responses to HCV and self-antigens promoting allograft fibrosis.
Methods
Forty-eight HCV OLT recipients (OLTr) were enrolled and classified based on amount of allograft macrovesicular steatosis at time of OLT. Group 1-No Steatosis (0–5% steatosis, n=21), Group 2 – Mild (5–35% - n=16), Group 3 – moderate (>35%, n=11). Cells secreting IL-17, IL-10, IFN-γ in response to HCV antigens were enumerated by ELISpot. Serum cytokines were measured by Luminex, antibodies (Abs) to Collagen (Col) I, II, III, IV, V by ELISA.
Results
OLTr of moderate steatotic grafts had the highest incidence of advanced fibrosis in protocol one-year post-OLT biopsy (10.8% vs. 15.8% vs. 36.6%, r = 0.157, p<0.05). OLTr from Groups 2 and 3 had increased HCV specific IL-17 (p<0.05) and IL-10 (p<0.05) with reduced IFN-γ (p<0.05) secreting cells when compared to group 1. This was associated with increase in serum IL-17, IL-10, IL-1β, IL-6, IL-5 and decreased IFN-γ. In addition, there was development of Abs to Col I, II, III and V in OLTr with increased steatosis (p<0.05).
Conclusion
The results demonstrate that allograft steatosis influences post-OLT HCV specific immune responses leading to a IL-17 T-helper response and activation of humoral immune responses to liver associated self antigens which may contribute to allograft fibrosis and poor outcome.
Keywords: Allograft Steatosis, Hepatitis C, Recurrence, Fibrosis, Liver Transplantation
INTRODUCTION
Hepatitis C virus (HCV) liver disease is the leading indication for orthotopic liver transplantation (OLT) in United States (1, 2). In 2010, among 16,904 UNOS registrants only 5763 OLT were performed (3). To meet the demand, ‘extended criteria’ donors after cardiac death and “steatotic” livers are often used for OLT. Steatotic allografts are cautiously used due to early post-operative complications (4–6). Due to high prevalence (25–50%) of potential donors with significant liver steatosis (7, 8), its effect on outcome in HCV recipients requires further investigation.
HCV recurrence in the allograft is near universal often leading to accelerated fibrosis compared to native liver (9–11). Immunological factors including T-cell responses to HCV (12–15), immunity to extracellular matrix (ECM) antigens (Collagens [Col]) (16) have been implicated in progression of allograft fibrosis. Donor factors including graft quality can influence HCV recurrence (17). Briceňo et al demonstrated that allografts with greater than 30% steatosis were associated with increased fibrosis (18). However, Burra et al found no impact of steatosis on fibrosis and outcome (19). Steatotic allografts have an increased susceptibility to ischemia-reperfusion injury (20, 21) and have poorer functional recovery (5, 22). In this context it is interesting to note the influence of duration and degree of ischemia-reperfusion injury on HCV recurrence (23, 24).
This study’s aim was to evaluate the effect of allograft steatosis on post-OLT HCV immunity. We hypothesized that steatotic allografts increase susceptibility to HCV mediated injury, the development of immunity against ECM antigens (Col), thus promoting fibrosis. The results presented demonstrate that OLTr of steatotic allografts have increased Th17 and Th2 responses to HCV and suppression of Th1. This was also associated with the development of antibodies (Abs) to self-antigens (Col).
RESULTS
Patient Demographics
Eighty-five subjects were included - 48 HCV OLTr, 27 non-HCV OLTr and 10 healthy subjects. OLTr were classified by allograft macrovesicular steatosis at the time of OLT: Group 1–3 HCV OLTr; Group 4–6 Non-HCV OLTr : Group 1 (n=21) and Group 4 (n=11) – No steatosis; Group 2 (n=16) and Group 5 (n=10) - Mild Steatosis; Group 3 (n=11) and Group 6 (n=6) - Moderate/severe steatosis. Among the HCV OLTr, time from OLT for blood and biopsy was similar in all groups (312 ± 10 vs. 340 ± 24 vs. 306 ± 22 days). No differences were noted in clinical demographics (Table 1a) including pre-transplant MELD and donor characteristics. Peak transaminase levels after OLT were significantly higher in Group 3 OLTr compared to groups 1 and 2 (AST – 1905 vs. 2809 vs. 3883 IU/mL, p=0.026, ALT – 1236 vs. 1359 vs. 1776 IU/mL, p=0.039).
Table 1.
| Table 1a. Clinical demographics of HCV orthotopic liver transplant (OLT) recipients divided into three groups by extent of macrovesicular steatosis in the donor liver at the time of OLT. | ||||
|---|---|---|---|---|
| Demographic | Group 1 No Steatosis (upto 5% Steatosis) n=21 |
Group 2 Mild Steatosis (5–35% Steatosis) n=16 |
Group 3 Moderate/Severe Steatosis (>35% Steatosis) n= 11 |
p value |
| Age (years)* | 55.1 ± 2.4 | 54.9 ± 1.03 | 49.6 ± 3.32 | 0.34 |
| BMI (kg/m2)* | 26.4 ± 4.4 | 28.3 ± 3.2 | 27.9 ± 4.5 | 0.39 |
| Days post OLT at analysis* | 312 ± 10 | 340 ± 24 | 306 ± 22 | 0.402 |
| Female:Male % | 19:81% | 31:69% | 16.7:83.3% | 0.248 |
| Race (%) | 0.47 | |||
| Caucasian | 74% | 72% | 89% | |
| African American | 22% | 28% | 11% | |
| Other | 4% | |||
| Viral Genotype (%) | 1 – 33% | 1 – 18.2% | 0.34 | |
| 1a - 14.3% | 1a – 31.25% | 1a – 63.6% | ||
| 1b – 14.3% | 1b – 43.75% | 1b – 9.1% | ||
| 3 – 4.7% | 2a – 6.25% | |||
| 3a – 9.5% | ||||
| N/A - 24.2% | N/A – 18.75% | N/A – 9.1% | ||
| HCV Viral Load (in 106 copies/mL)* | 2.5 ± 0.79 | 2.8 ± 0.88 | 2.71 ± 1.08 | 0.965 |
| Total Bilirubin (mg/dL)* | 0.943 ± 0.13 | 1.01 ± 0.44 | 1.25 ± 0.36 | 0.212 |
| AST (IU/mL)* | 95.8 ± 16.4 | 72.5 ± 11.5 | 115.2 ± 33.6 | 0.337 |
| ALT (IU/mL)* | 168.1 ± 30.9 | 127.3 ± 23.4 | 152.6 ± 47.6 | 0.661 |
| INR* | 1.16 ± 0.05 | 1.10 ± 0.12 | 1.08 ± 0.03 | 0.104 |
| Pretransplant MELD* | 20 ± 1 | 15 ± 4 | 19 ± 1 | 0.093 |
| Pretransplant Viral Load (in 106 copies/mL)* | 0.42 ± 0.33 | 0.44 ± 0.14 | 0.28 ± 0.13 | 0.25 |
| Donor Age (years)* | 41.7 ± 3.9 | 45 ± 2.52 | 39 ± 4.53 | 0.39 |
| Donor Race | 0.256 | |||
| Caucasian | 66.67% | 87.5% | 100% | |
| African American | 14.28% | |||
| Others | 4.77 | |||
| Unknown | 14.28% | 12.5% | ||
| Donor Female : Male % | 38:62 % | 37.5:62.5% | 11:89% | 0.338 |
| Cold Ischemia Time (Hours: Minutes)* | 4:51 ± 0:24 | 5:23 ± 0:21 | 5:10 ± 0:30 | 0.6 |
| Warm Ischemia Time (Hours: Minutes)* | 0:40 ± 0:02 | 0:38 ± 0.02 | 0:39 ± 0:02 | 0.812 |
| Post OLT Peak+ Bilirubin (mg/dL)* | 4.5 ± 0.41 | 4.29 ± 0.44 | 5.68 ± 0.83 | 0.222 |
| Post OLT Peak+ AST (IU/mL)* | 1905 ± 416 | 2809 ± 487 | 3883 ± 598 | 0.026 |
| Post OLT Peak+ ALT (IU/mL)* | 1236 ± 250 | 1359 ± 150 | 1776 ± 164 | 0.039 |
| Post OLT Peak+ ALP (IU/mL)* | 87.5 ± 8.7 | 93.1 ± 12.2 | 78.7 ± 8.1 | 0.66 |
| Immunosuppression - n(%) | ||||
| MMF + Tacrolimus | 16 (76%) | 12 (75%) | 10 (91%) | 0.756 |
| MMF + Sirolimus | 1 (5%) | 2 (12.5%) | ||
| Cyclosporine | 2 (9.5%) | 1 (6.25%) | 1 (9%) | |
| Tacrolimus | 2 (9.5%) | 1 (6.25%) | ||
| Acute Rejection episode – n (%) | 5 (25%) | 3 (27%) | 2 (22%) | 0.47 |
| Episodes of acute rejection** | 1 | 1 | 1 | 1 |
| Severity of Acute Rejection – n(%)*** | 0.69 | |||
| Mild | 3(60%) | 2(67%) | 1(50%) | |
| Moderate | 2 (40%) | 1(23%) | 1(50%) | |
| Severe | 0% | 0% | 0% | |
| Table 1b. Clinical demographics of non-HCV orthotopic liver transplant (OLT) recipients divided into three groups by extent of macrovesicular steatosis in the donor liver at the time of OLT. | ||||
|---|---|---|---|---|
| Demographic | Group 4 No Steatosis (upto 5% Steatosis) n=11 |
Group 5 Mild Steatosis (5–35% Steatosis) n=10 |
Group 6 Moderate/Severe Steatosis (>35% Steatosis) n=6 |
p value |
| Age (years)* | 54.7 ± 0.99 | 55.5 ± 1.78 | 47.8 ± 4.03 | 0.061 |
| BMI (kg/m2) | 28.4 ± 5.3 | 29.9 ± 4.2 | 29.2 ± 5.3 | 0.56 |
| Days post OLT at analysis* | 327 ± 14 | 338 ± 20 | 319 ± 18 | 0.304 |
| Female:Male % | 23:77 % | 20:80 % | 25:75 % | 0.76 |
| Race (%) | 0.62 | |||
| Caucasian | 84% | 80% | 85% | |
| African American | 15% | 20% | 15% | |
| Other | 1% | |||
| Indication for Transplant - n(%) | 0.38 | |||
| Cryptogenic | 2 (18%) | 3 (30%) | 1 (16.7%) | |
| Hepatitis B | 2 (18%) | 2 (20%) | 1 (16.7%) | |
| Alcoholism | 3 (27%) | 2 (20%) | 1 (16.7%) | |
| Autoimmune Hepatitis | 1 (9%) | 1 (10%) | 1 (16.7%) | |
| Non-Alcoholic Steato-hepatitis | 1 (9%) | 0 (0%) | 1 (16.7%) | |
| Primary Biliary Cirrhosis | 1 (9%) | 1 (10%) | 1 (16.7%) | |
| Primary Sclerosing Cholangitis | 1 (9%) | 1 (10%) | 0 (0%) | |
| Total Bilirubin (mg/dL)* | 1.1 ± 0.24 | 0.99 ± 0.61 | 1.2 ± 0.42 | 0.44 |
| AST (IU/mL)* | 99.8 ± 20.4 | 84.5 ± 13.5 | 100.1 ± 29.6 | 0.58 |
| ALT (IU/mL)* | 157.1 ± 27.6 | 130.4 ± 30.8 | 149.6 ± 46.6 | 0.51 |
| INR* | 1.06± 0.09 | 1.22 ± 0.21 | 1.1 ± 0.14 | 0.29 |
| Pretransplant MELD* | 20 ± 1 | 18 ± 2 | 21 ± 1 | 0.11 |
| Donor Age (years)* | 40.3 ± 4.1 | 44.2 ± 3.2 | 42.5 ± 2.8 | 0.31 |
| Donor Race | 0.52 | |||
| Caucasian | 72.8% | 80% | 100% | |
| African American | 18.2% | 20% | ||
| Donor Female : Male % | 45:55% | 40:60% | 50:50% | 0.81 |
| Cold Ischemia Time (Hours: Minutes)* | 5:12 ± 0:32 | 6:02 ± 0:42 | 5:20 ± 0:29 | 0.49 |
| Warm Ischemia Time (Hours: Minutes)* | 0:38 ± 0:01 | 0:36 ± 0.04 | 0:37 ± 0:03 | 0.77 |
| Post OLT Peak+ Bilirubin (mg/dL)* | 4.8 ± 0.31 | 4.5 ± 0.38 | 5.93 ± 0.73 | 0.09 |
| Post OLT Peak+ AST (IU/mL)* | 743 ± 265 | 1309 ± 597 | 2708 ± 696 | 0.03 |
| Post OLT Peak+ ALT (IU/mL)* | 934 ± 243 | 1405 ± 178 | 2017 ± 294 | 0.027 |
| Post OLT Peak+ ALP (IU/mL)* | 84.6 ± 7.8 | 92.9.1 ± 9.9 | 80.4 ± 6.4 | 0.46 |
| Immunosuppression n(%) | ||||
| MMF + Tacrolimus | 9 (82%) | 8 (80%) | 5 (83.3%) | 0.82 |
| Cyclosporine | 1(9%) | 1 (10%) | 0 (0%) | |
| Tacrolimus | 1 (9%) | 1 (10%) | 1 (16.7%) | |
| Acute Rejection episode – n (%) | 4 (36%) | 3 (30%) | 1 (16.7%) | 0.32 |
| Episodes of acute rejection** | 1 | 1 | 1 | 1 |
| Severity of Acute Rejection*** | 0.9 | |||
| Mild | 2 (50%) | 2 (67%) | 1 (100%) | |
| Moderate | 2 (50%) | 1 (23%) | 0% | |
| Severe | 0% | 0% | 0% | |
MELD – Model for Endstage liver disease score, AST – Aspartate aminotransferase, ALT – Alanine aminotransferase, INR – International normalized ratio (for pro-thrombin time), MMF – Mycophenolate mofetil, N/A - not available,
Post OLT peak refers to highest value in the first week of transplant,
Values represented as mean ± SEM.
Refers to Median number of Acute rejection episodes in patients that developed acute rejection.
Highest degree of severity of rejection episodes in patients who developed Acute Rejection graded by the 1997 Banff Schema.
Statistical Comparisons made by Kruskal Wallis Test
In 15 subjects (Group 1 – 6, Group 2 – 5, Group 3 – 4) biopsy due to clinical suspicion (rejection/obstruction) was performed during first year post-OLT. Biopsy confirmed acute rejection (Banff schema (25)) was similar (25%, 27% and 22%) including severity (mild or moderate, no severe rejection). One patient (Group 1) developed biliary obstruction and underwent stent placement. Acute rejection was treated with bolus steroids as first-line therapy and in two non-responders (group 1) with thymoglobulin/OKT3. None of the subjects received anti-viral therapy post-OLT.
Clinical demographics of the non-HCV OLTr were similar (Table 1b). In them also, peak transaminase levels were significantly more in moderate/severe steatotic grafts (AST – 743 vs. 1309 vs. 2708 IU/mL, p=0.03, ALT – 934 vs. 1405 vs. 2017 IU/mL, p=0.027).
Ten healthy subjects (no pre-existing liver or autoimmune disease; age 35 ± 8 years, male:female ratio 6:4) were included to standardize ELISA for detection of Abs to Col. Control subjects were HIV, HBV and HCV negative.
Higher prevalence of advanced fibrosis post-OLT in patients with higher donor graft steatosis at OLT
Allograft fibrosis was determined in biopsy by modified Batts Ludwig score (26). Group 3 HCV OLTr whose grafts had moderate/severe steatosis at OLT had the highest incidence of fibrosis (Stage 3–4) when compared to Group 2 and 1 – 36.6% vs. 15.8% vs. 10.8%, p<0.05. Fibrosis post-OLT had a significant positive correlation with extent of steatosis at time of OLT with a spearman rho of 0.157, p<0.05.
In the biopsy of 15 subjects that underwent biopsy within 6 months post-OLT for clinical indications, there was no significant fibrosis. There was no correlation of other factors including rejection (r=0.024, p=0.45) and donor age etc. with post-OLT fibrosis. Non-HCV OLTr did not demonstrate significant fibrosis post OLT
Increased IL-17, IL-10 and decreased IFN-γ secreting cells to HCV antigens in OLTr of higher grades of graft steatosis
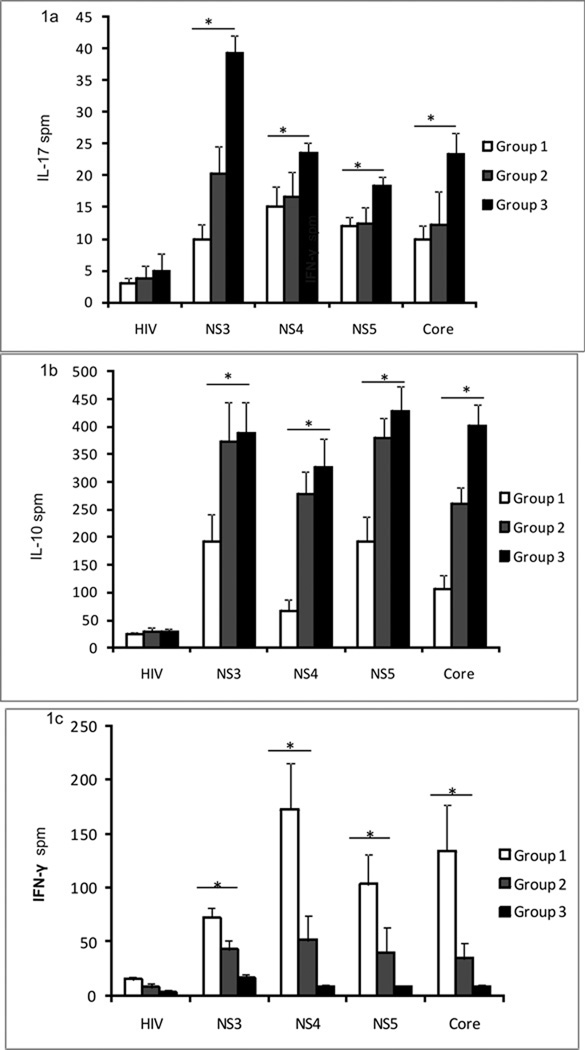
Immune responses to HCV antigens (NS3, NS4, NS5, core) were determined by ELISpot. Group 3 HCV OLTr had significantly higher frequency of HCV specific IL-17 cells when compared to groups 2 and 1 (in mean spots per million cells (spm) ± SE; groups 3 and 2, 1: Core: 23.3 ± 3.4 vs. 12.2 ± 5.2 vs. 10 ± 2.1, p=0.043; NS3 - p=0.03; NS4 p=0.051; NS5 p=0.049;) (Figure 1a). There was no difference in the cells secreting IL-17 in response to HIV and PHA among the groups.
Figure 1. Increased HCV specific IL17 and IL10 and decreased INF-γ secreting cells in HCV OLTr with donor liver grafts of mild to severe steatosis.
ELISpot comparing IL17(1a), IL-10(1b) and IFN-γ(1c) response to HCV antigens (NS3, NS4, NS5, CORE) and non specific peptide (HIV Gp120 – negative control) among three groups – Group 1 (no steatosis – 0–5% macrovesicular steatosis; white bars), Group 2 (mild steatosis – 5-5% macrovescicular steatosis; grey bars), Group 3 (moderate/severe steatosis - >35% steatosis; black bars). 3×105 PBMCs were cultured per well and each antigen was cultured in triplicate. Figure 1a IL-17 between groups 3 and 2, 1: NS3: 39.3 ± 2.7 vs. 20.3 ± 4.2 vs. 10 ± 2.3, p=0.03; NS4: 23.6 ± 1.6 vs. 16.6 ± 3.8 vs. 15 ± 3.2 spm, p=0.051; NS5 18.3 ± 1.4 vs. 12.4 ± 2.6 vs. 12 ± 1.4, p=0.049; Core: 23.3 ± 3.4 vs. 12.2 ± 5.2 vs. 10 ± 2.1, p=0.043. Figure 1b IL-10 between groups 3,2, 1 - NS3: 387.6 ± 56.3 vs. 373.8 ± 69.8 vs. 192 ± 50.3 spm, p<0.01; NS4: 325 ± 52.6 vs. 277.8 ± 40.3 vs. 66.1 ± 20.4 spm, p=0.02; NS5: 427.6 ± 45.5 vs. 378.6 ± 36.9 vs. 193 ± 45.2 spm, p<0.01; Core: 402.3 ± 37.2 vs. 259.6 ± 30.5 vs. 107.2 ± 23.5 spm, p=0.01) Figure 1c IFN-γ – between groups 3, 2, 1 : NS3: vs. 17 ± 2.6 vs. 42.6 ± 8.7 vs. 72.4 ± 8.7 spm, p=0.032; NS4: 8.2 ± 2.3 vs. 51.3 ± 2.1 vs. 172.4 ± 43.6 spm, p=0.02; NS5: 8.5 ± 1.1 vs. 40.1 ± 22.9 vs. 103 ± 28.2; Core: 9.1 ± 2.4 vs. 34.3 ± 14.1 vs. 133.6 ± 42.7, p=0.01 Responses compared by the Mann Whitney U test and values represented as mean ± standard error in spots per million cells (spm), *Denotes p<0.05
Group 2 and 3 HCV OLTr demonstrated significantly increased frequency of HCV specific IL-10 cells when compared to Group 1 (groups 2, 3 and 1; Core: 259.6 ± 30.5 vs. 402.3 ± 37.2 vs. 107.2 ± 23.5 spm, p=0.01; NS3 - p<0.01; NS4 - p=0.02; NS5 - p<0.01) (Figure 1b). When compared to group 1, both groups 2 and 3 HCV OLTr had significantly lower frequency of cells secreting IFN-γ in response to HCV (groups 2, 3 and 1; Core: 34.3 ± 14.1 vs. 9.1 ± 2.4 vs. 133.6 ± 42.7, p=0.01; NS3 - p=0.032; NS4 - p=0.02; NS5 – p=0.045) (Figure 1c). These results indicate that with increasing steatosis, there was an increase in IL-17 and IL-10 in response to HCV antigens with a decrease in IFN-γ.
Increased serum cytokines (IL-1β, IL-4, IL-17, IL-10, IL5, IL-6 and IL-8) and chemokines (IP10, MIP, Eotaxin, MCP1) in HCV OLTr of grafts with moderate to severe steatosis
Serum IL-17, IL-6, IL-1β, IL-4, IL-5, IL-10 and chemokines IL-8, IP10, MIP, Eotaxin and MCP-1 were increased in group 2 and 3 (Table 2). Ten subjects’ were analyzed 6 months post-OLT - Group 1 – 4; Group 2 & 3 – 3 each. Recipients in Group 3, demonstrated a significant increase in these cytokines and chemokines when compared to Groups 1 and 2. In addition IFN-γ in Group 3 OLTr sera were significantly lower than groups 1 and 2 (at 1 year: 23.9 ± 1.1 vs. 27.9 ± 2.3 vs. 25.8 ± 1.8 pg/mL; and at 6 months - 22.74 ± 4.57 vs. 63.29 ± 3.69 vs. 34.69 ± 6.53 pg/mL, p =0.046, Table 2). There were no differences in the levels of other cytokines and chemokines (data not shown). Thus there was an increase in IL-17, pro Th-17 (IL-6, IL-1β), Th2 (IL-10, IL-4) cytokines and pro-inflammatory chemokines in OLTr of higher steatotic grafts with a suppression of Th1 (IFN-γ) cytokines and which was significant in the early post-OLT period.
Table 2.
Cytokine concentration among the three HCV OLTr groups with varying donor liver steatosis measured in serum obtained at the time of biopsy approximately 1 year post OLT – 2a) and 6 months post OLT (2b)
| 2a. | ||||
|---|---|---|---|---|
| Cytokine | Group 1 No Steatosis (0–5% Steatosis) n=21 |
Group 2 Mild Steatosis (5–35% Steatosis) n=16 |
Group 3 Moderate/Severe Steatosis >35% Steatosis n= 11 |
p value |
| IL-1β (pg/mL) | 7.79 ± 1.25 | 8.7 ± 1.1 | 12.3 ± 3.7 | 0.22 |
| IL-4 (pg/mL) | 77.3 ± 6.1 | 80.5 ± 6.9 | 85.5 ± 7.2 | 0.79 |
| IL-5 (pg/mL) | 18.5 ± 5.4 | 26.1 ± 6.1 | 19.5 ± 6.1 | 0.654 |
| IL-6 (pg/mL) | 13.2 ± 1.2 | 15.6 ± 3.2 | 18.3 ± 1.5 | 0.102 |
| IL-8 (pg/mL) | 17.1 ± 1.3 | 18.1 ± 1.6 | 19.7 ± 3.4 | 0.324 |
| IL-10 (pg/mL) | 14.1 ± 1.3 | 17.9 ± 3.7 | 19.4 ± 1.2 | 0.132 |
| IL-17 (pg/mL) | 36.5 ± 4.4 | 37.4 ± 3.6 | 40.1 ± 3.9 | 0.97 |
| IFN-γ (pg/mL) | 27.9 ± 2.3 | 25.8 ± 1.8 | 23.9 ± 1.1 | 0.605 |
| IP-10 (pg/mL) | 3.45 ± 1.47 | 3.01 ± 1.07 | 4.01 ± 2.18 | 0.244 |
| MIP (pg/mL) | 4.7 ± 2.3 | 4.55 ± 2.69 | 8.1 ± 3.11 | 0.156 |
| MCP1 (pg/mL) | 18.7 ± 1.48 | 25.4 ± 7.88 | 15.9 ± 4.9 | 0.554 |
| 2b. | ||||
|---|---|---|---|---|
| Cytokine | Group 1 No Steatosis (0–5% Steatosis) n=4 |
Group 2 Mild Steatosis (5–35% Steatosis) n=3 |
Group 3 Moderate/Severe Steatosis >35% Steatosis n= 3 |
p value |
| IL-1β (pg/mL) | 7.47± 2.01 | 8.06 ± 3.04 | 16.49 ± 2.46 | 0.032 |
| IL-4 (pg/mL) | 74.7± 10.55 | 109.1 ± 16.25 | 159.9 ± 12.35 | 0.04 |
| IL-5 (pg/mL) | 22.22 ± 9.44 | 46.2 ± 13.46 | 81.23 ± 22.15 | 0.03 |
| IL-6 (pg/mL) | 22.44 ± 9.51 | 21.29 ± 10.35 | 50.17 ± 9.85 | 0.028 |
| IL-8 (pg/mL) | 7.71 ± 1.58 | 11.67 ± 2.45 | 27.88 ± 4.46 | 0.044 |
| IL-10 (pg/mL) | 15.45 ± 2.24 | 19.6 ± 3.6 | 33.9 ± 4.35 | 0.038 |
| IL-17 (pg/mL) | 39.1 ± 7.64 | 41.36 ± 10.45 | 128.36 ± 9.56 | 0.002 |
| IFN-γ (pg/mL) | 63.29 ± 3.69 | 34.69 ± 6.53 | 22.74 ± 4.57 | 0.046 |
| IP-10 (pg/mL) | 26.93 ± 4.45 | 138.17 ± 15.63 | 148.3 ± 10.46 | 0.021 |
| MIP (pg/mL) | 25.5 ± 5.85 | 35.2 ± 6.45 | 58.1 ± 5.6 | 0.033 |
| MCP1 (pg/mL) | 154.25 ± 29.5 | 228.34 ± 34.5 | 303.5 ± 23.46 | 0.001 |
Values represented as mean ± SEM.
HCV OLTr of mild to severe steatotic grafts develop increased antibodies to self antigens (Col)
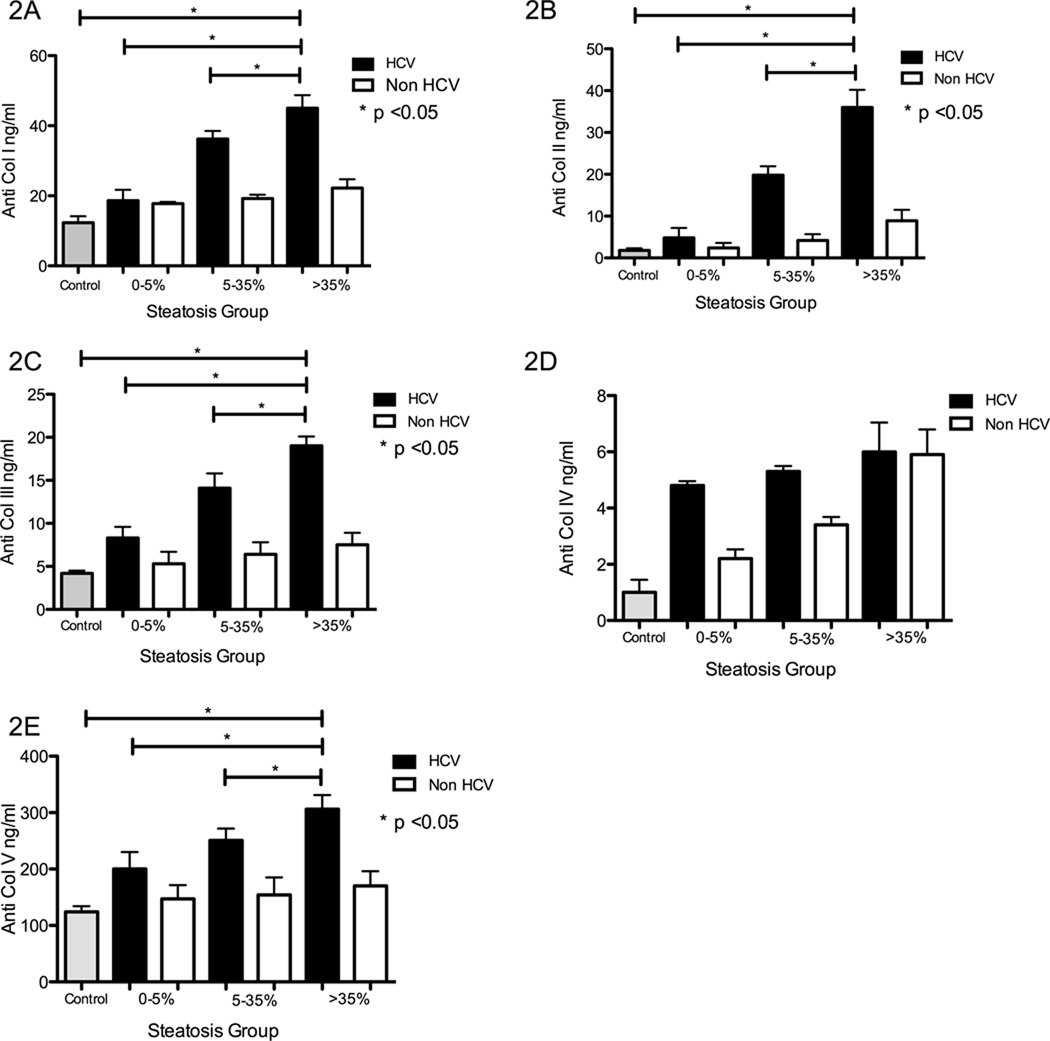
All HCV OLTr developed Abs to Col I, II, III, and V when compared to controls. Additionally, both groups 2 and 3 had significantly higher levels of Abs to Col I (p=0.031), Col II (p=0.029), Col III (p=0.047), Col V (p=0.048) (Figure 2A, B, C, E). There was no significant difference in the Abs to Col IV among the groups (p=0.23) (Figure 2D). The Abs titers in non-HCV OLTr in the various steatotic groups did not significantly differ from healthy controls (Figure 2 A–E).
Figure 2. Development of antibodies to self antigens (Collagen (Col)) in HCV OLTr and increased titer of abs to Col I, II, III and V in recipients of with mild to severe steatotic grafts.
ELISA was performed to detect serum antibodies to Col I (Fig 2A), II (Fig 2B), III (Fig 2C), IV (Fig 2D) and V (Fig 2E) in healthy controls (Grey bars), non-HCV OLTr (white bars), and HCV OLTr (black bars): Groups 1 and 4 (no steatosis – 0–5% macrovesicular steatosis), Group 2 and 5 (mild steatosis – 5–35% macrovescicular steatosis) and Group 3 and 6 (moderate/severe steatosis - >35% steatosis). The titer of abs to Col I, II III and V was significantly higher in OLTr with mild and moderate/severe steatotic grafts (Groups 2 and 3) [Anti Col I - 18.6 ± 3.1 vs. 36.2 ± 2.3 vs. 45.9 ± 3.7ng/mL, p=0.031; Anti Col II - 4.8 ± 2.4 vs. 19.8 ± 2.1 vs. 36.6 ± 4.2 ng/mL, p=0.029; Anti Col III -8.3 ± 1.3 vs. 14.1 ± 1.7 vs. 19.3 ± 0.9 ng/mL, p=0.047; Anti Col V - 200.1 ± 30.1 vs. 250.7 ± 21.2 vs. 306 ± 25.3 ng/mL, p=0.048). Abs to Col IV were similar in all groups (4.88 ± 0.77 vs. 5.3 ± 0.79 vs. 6.06 ± 3.45,, p=0.23). In non-HCV OLTr Abs titers were – Anti Col I -17.8 ± 0.5 vs. 19.2 ± 1.1 vs. 22.2 ± 2.5 ng/mL, Anti Col II – 2.4 ± 1.2 vs. 4.2 ± 1.5 vs. 8.9 ± 2.6 ng/mL; Anti Col III – 5.3 ± 1.4 vs. 6.4 ± 1.4 vs. 7.5 ± 1.7 ng/mL; Anti Col IV – 2.2 ± 1.1 vs. 3.4 ± 0.9 vs. 5.9 ± 2.2 ng/mL; Anti Col V – 247 ± 24.5 vs. 154 ± 31 vs. 170 ± 26 ng/mL. Ab titer in non HCV OLTr did not differ significantly from healthy controls. Values represented as mean ± standard error bars in ng/mL. *denotes p < 0.05
Patient and graft survival in HCV OLTr
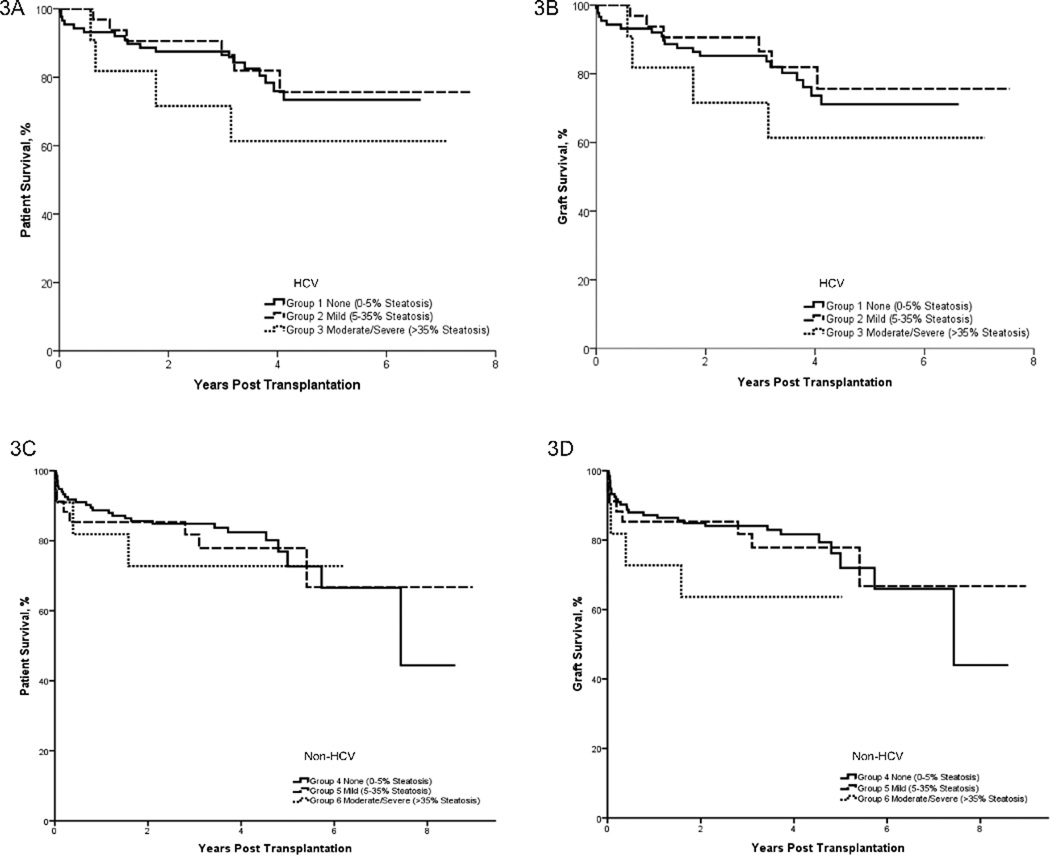
Patient and graft outcome in all OLTr (HCV =131; non-HCV=178) between January 2002 and December 2008 was analyzed (Figure 3A–3D). Mean patient survival was similar in the groups (HCV OLTr – 5.4±0.2, 6.3±0.4, 4.9±0.8 years; non-HCV OLTr – 6.4±0.3, 6.8±0.6, 4.7±0.7 years). Mean graft survival among HCV OLTr was 5.3±0.25, 6.3±0.4, 4.9±0.8 years and among non-HCV OLTr was 6.3±0.4, 6.7±0.6, 4.6±0.7 years. Patient survival at 3 months in HCV OLTr was 97%, 100%, 91% and 1 year - 93% vs. 94% vs. 82% (p=0.24). In non-HCV OLTr 3 month survival was 95%, 88% and 86% and 1 year survival was 90% vs. 85% vs. 71%(p=0.47). There was no difference in survival between HCV and non-HCV OLTr (Figure 3A, C).
Figure 3. HCV OLT recipients with moderate/severe steatotic allografts have poorer early survival.
Survival plots comparing patient graft survival in 131 HCV OLTr (3A, 3B) and 178 non-HCV OLTr (3C, 3D) divided into groups of varying degree of allograft steatosis. IN the HCV OLTr, mean overall patient and graft survival was survival was 5.4±0.2, 6.3±0.4, 4.9±0.8 years and 5.3±0.25, 6.3±0.4, 4.9±0.8 years respectively. In the non-HCV OLTr mean patient and graft survival among groups was – 6.4±0.3, 6.8±0.6, 4.7±0.7 years and 6.3±0.4, 6.7±0.6, 4.6±0.7 years respectively. In HCV OLTr - one-year patient and two year patient survival among the three groups was 93%, 94%, 82% and 88% vs. 92 % vs. 72% respectively. As determined by Log Rank tests there was no significant difference in the survival among the groups in HCV and Non-HCV OLTr as well as between HCV and Non-HCV recipients.
DISCUSSION
Many factors both in the donor and recipient including immune responses affect prognosis in HCV OLTr, (14, 17, 23, 27–32). This study evaluated the impact of donor graft steatosis on changes in post-OLT HCV immunity. In HCV OLTr, increasing grade of allograft steatosis positively correlated with fibrosis one-year post-OLT. These OLTr had increased HCV specific IL-17 and IL-10 with decreased IFN-γ secreting cells (Figure 1). This was associated with an increase in serum IL-17, pro Th-17 (IL-6, IL-1β) and Th2 (IL-4, IL-10) cytokines (Table 2). Additionally, HCV OLTr also developed abs to self-antigens (Col I, II, III, V) whose titer was more in OLTr of moderate/severe steatotic grafts. These results support the contention of donor steatosis being an important factor in viral recurrence and fibrosis (18) and further suggest a role for allograft steatosis in influencing post-OLT HCV immune responses development of immunity to self-antigens.
Earlier studies have associated graft steatosis with poor function (28, 29), but these grafts when appropriately selected can have functional recovery (5). The degree of steatosis decreases soon after OLT (33) and in this study also there was lack of any significant steatosis in the grafts one-year post-OLT(data not shown) with no difference in overall survival. Similar to previous findings (5–7, 20, 22), this report demonstrates that OLTr of moderate/ severe steatotic grafts had higher transaminases (AST and ALT, p<0.05) in the first week following OLT (Table 1) signifying increased reperfusion injury. OLTr of moderate to severe steatotic grafts have increased coagulopathy and a stormier post-operative period (5, 6). Several studies including by Verran and Chui et al (6, 34) report increased early poor function and survival in moderate/severe steatotic grafts. These could serve as a possible explanation for the poor early survival of steatotic allografts, however this was not statistically significant (Figure 3).
HCV recurrence is universal (35, 36) and in peri-OLT period early preservation and reperfusion injuries have been associated with HCV recurrence and poor outcome (23, 24). Therefore, it is likely that post-operative stress sets up an inflammatory process that can alter the host immunological response to HCV. This in turn can promote HCV pathologic injury and recurrence resulting in development of allograft fibrosis. Although events immediately post-OLT couldn’t be analyzed due to non-availability of serial samples, the significant differences in cytokine levels 6 months post-OLT even with a smaller number of samples (Table 2b) support the hypothesis that early inflammatory events may determine the course and progression of HCV infection and fibrosis.
The immune response to HCV is a critical determinant of allograft fibrosis and a predominant Th2 (13, 14, 37) and Th17 (15) immunity is associated with increased fibrosis and poor outcome. Cytokines including IL-1β, IL-6 and IL-8 have been shown to promote fibrosis (38–41). These cytokines also favor Th17 responses and suppress IFN-γ (15). In this study we demonstrate that such HCV immune responses are more prominent in OLTr of grafts with higher grade of steatosis (Figure 1, Table 2). We propose that this prevents viral clearance and enhances liver damage, increased ECM turnover and the development of fibrosis.
The inflammatory process that follows ischemia reperfusion injury and HCV mediated liver damage may lead to exposure of cryptic self-antigens such as Col thus precipitating an immune response. A recent study from our laboratory found that Abs to Col I, II, V are associated with the development of fibrosis both in non-OLT and post-OLT HCV patients (16). In this study as well, the degree of allograft steatosis demonstrated positive correlations with Abs titer and fibrosis (Figure 2). The mechanistic role of these Abs in the development of fibrosis can be postulated from reports in lung and heart transplantation wherein humoral and cellular (especially Th17) responses to self-antigens are implicated in the immunopathogenesis of fibrosis and chronic allograft rejection (42, 43).
This is further explained by an increase in IL-17 that has been shown to play a role in the development of autoimmune B-cells and liver fibrosis (15, 44). An increase in IL-17 in steatotic allografts along with a pro-Th17 cytokine milieu (IL-6, IL-1β) may facilitate Th-17 immune responses to self-antigens (Col) leading to the production of Abs. These immune responses characterized by increased IL-6 and IL-17 can also lead to increase in other pro-fibrotic growth factors (transforming growth factor beta and connective tissue growth factor), which will result in ECM turnover and allograft fibrosis (45).
In non-HCV OLTr however there is no significant immune response to self-antigens (Figure 2) and there is no significant fibrosis post OLT (data not shown). In these non-HCV OLTr, only ischemia reperfusion injury plays a role and the additional HCV mediated responses doesn’t occur. Thus both an early post operative stress and the continued HCV mediated liver damage may be critical for exposure of cryptic self-antigens or determinants for development of immune responses to self-antigens and subsequent allograft fibrosis.
A limitation of this study is that due to lack of serial samples post-OLT, early changes and effects of steatosis on HCV replication and immune responses as well as the temporal and mechanistic correlation of development of Abs to self-antigens and allograft fibrosis could not be determined.
In conclusion, this study demonstrates that extent of graft steatosis significantly influences post-OLT HCV specific immune responses and development of allograft fibrosis. Increasing grades of steatosis favors the development of predominant Th17 type HCV specific responses with a concomitant suppression of Th1 (IFN-γ). In addition, early inflammatory changes in the allograft due to steatosis and inflammatory cytokine milieu can perpetuate the development of Abs to self-antigens (Col). We propose that HCV specific as well as immune responses to self-antigens collectively promote allograft fibrosis and lead to a poor outcome especially in HCV OLTr of grafts with moderate to severe grades of steatosis.
MATERIALS AND METHODS
Patient Population
HCV OLTr at Barnes Jewish Hospital, Washington University, St. Louis were consecutively enrolled (January 2002 to December 2008). Among 131 HCV OLTr, a cross-sectional analysis was conducted on 48 HCV OLTr by obtaining blood and post-OLT biopsy on same day of one-year follow up. Twenty-seven non-HCV OLTr were also enrolled and blood collected at similar time point. HCV infection was confirmed by HCV+ RNA PCR (Roche Diagnostics), anti-HCV Abs (Abbot Laboratories, Chicago) and liver pathology. Patients with Hepatitis B (HBV) and/or Human Immunodeficiency virus (HIV) co-infection were excluded. In 10 HCV OLTr, additional six months post-OLT samples were analyzed. Clinico-demographic data was obtained retrospectively. Laboratory parameters were at time of biopsy and peak transaminase level was their highest concentration immediately following OLT. Control healthy subjects were enrolled when donating blood at HLA laboratory. The study was approved by the Institutional Review Board and informed consent obtained from all subjects.
Allograft Histology
Pathologists estimated steatosis in graft biopsies (wedge or core from left lateral segment) taken either at the time of procurement or immediately post-reperfusion (5). HCV (Group 1–3) and non-HCV (Group 4–6) OLTr were stratified by macrovesicular steatosis estimated by extent of large droplet fat occupying the parenchymal area: Group 1&4 – Up to 5% steatosis, Group 2&5 – 5 to 35% steatosis and Group 3&6 – greater than 35% steatosis.
Protocol one-year post-OLT and biopsies obtained for clinical indications were graded by pathologists for fibrosis by modified Batts Ludwig score (26). Patients were dichotomized into those with advanced fibrosis (Stage 3–4) and without fibrosis (Stage 0–2). Acute rejection was scored by Banff Schema (25).
Isolation of Mononuclear Cells and serum samples
Serum was stored at −70°C. Peripheral blood mononuclear cells (PBMC) were isolated by density gradient using Ficoll-Hypaque and used either immediately or frozen in 10% dimethyl-sulfoxide.
HCV and Peptide Antigens
Recombinant HCV core, and non-structural (NS3, NS4, NS5) (Fitzgerald Industries, Acton MA), HIV (Gp120 peptide, Biosynthesis, TX), PHA (Sigma, St. Louis, MO) antigens were tested endotoxin-free (15). PBMCs were stimulated with antigens (5 µg/mL) in 24-well plates at 37°C in 5% CO2 overnight before use.
Enzyme Linked Immunospot assay (ELISpot)
ELISpot was performed as described previously (14, 15). Stimulated PBMCs were cultured in triplicate (3×105 cells /200µL) in immunospot plates in presence of antigens (5µg/mL) for 72 hours. IFN-γ, IL-10 and IL-17 (BD Bioscience, CA) ELISpot were performed as per manufacturer’s instructions and spots analyzed in ImmunoSpot Analyzer (CTL, Cleveland, OH). Cells cultured in medium (CTL) and irrelevant peptide (HIV) were used as negative and PHA as positive control. Spots in the experimental wells +2 standard deviations (SD) of negative control were considered significantly positive and expressed as spots per million cells (spm).
Luminex Assay for serum cytokines
Serum cytokines and chemokines were measured using human 25-plex immunoassays (Invitrogen, Carlsbad, CA) (15). Plates were read on Luminex xMAP™ (Fischer, Pittsburgh, PA). Concentrations obtained by the standard curve were expressed in pg/mL.
Enzyme Linked Immunosorbent Assay (ELISA) for antibodies to Collagens
Abs to various Col were determined by ELISA (16, 42). ELISA plates were coated with recombinant human - Col I (Cell Sciences, Canton, MA), Col II, III and V (Sigma), and Col IV (Biodesign International, Saco, ME). Serum was tested for binding to Col. Detection done by peroxidase-conjugated goat-anti-human (Jackson Immunoresearch, West grove, PA), developed using tetramethylbenzidine and read at 450nm. Concentration of Abs was calculated using standard curve of known concentration of anti-Col (Santa Cruz Biotechnology, CA). Positive cut off was set as +2SD of mean in normal subjects. This was 14ng/mL for anti Col I, 2ng/mL - anti Col II, 5ng/mL - anti Col III, 1ng/mL - anti Col IV, 140ng/mL - anti Col V.
Statistical Analysis
Analysis performed using SPSS v17 (SPSS Inc., Chicago). Schapiro-Wilks test was used to check for normality and non-normal data log transformed. Kruskal Wallis and Mann Whitney U-test were used to compare clinical demographics, cellular responses and cytokine and Abs concentrations between groups. Correlation analysis was performed by Spearman rank test. Patient and allograft survival were compared by Kaplan Meier and Log-rank tests. Two-sided level of significance set at p<0.05.
ACKNOWLEDGMENTS
The authors acknowledge Dr. Elizabeth M. Brunt in Pathology and Immunology for assistance in discussing histopathology and reviewing the manuscript, and Ms. Billie Glasscock for assistance in manuscript preparation.
Funding Source: This publication was made possible by an award from the NIH DK065982 (TM), and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. AS was supported by 5-T32-DK07301-35 NIH Training Grant. TM is supported by the BJC Foundation. CDA is supported in part by the ASTS-Astellas Faculty Development Award, and NIH Grants: P30 DK056341 and L30 DK082350
Abbreviations
- Abs
antibodies
- Col
Collagens
- ECM
Extracellular matrix
- ELISpot
Enzyme Liked Immunospot
- ELISA
Enzyme linked immunosorbent assay
- HBV
Hepatitis B Virus
- HCV
Hepatitis C Virus
- HIV
Human immunodeficiency virus
- IL
Interleukin
- IFN-γ
Interferon gamma
- MELD
Model for end stage liver disease
- NS
non-structural
- OLT
Orthotopic Liver Transplantation
- OLTr
Orthotopic liver transplantation recipient
- PBMC
peripheral blood mononuclear cells
- PHA
Phytohemagglutinin
- PCR
polymerase chain reaction
- SPM
spots per million cells
- SE
standard error
- Th cell
T- helper cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interests: The authors have no relevant conflicts of interests to disclose
REFERENCES
- 1.Kim WR. The burden of hepatitis C in the United States. Hepatology. 2002;36(5) Suppl 1:S30. doi: 10.1053/jhep.2002.36791. [DOI] [PubMed] [Google Scholar]
- 2.Buti M, San Miguel R, Brosa M, et al. Estimating the impact of hepatitis C virus therapy on future liver-related morbidity, mortality and costs related to chronic hepatitis C. J Hepatol. 2005;42(5):639. doi: 10.1016/j.jhep.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 3.Organ Procurement and Transplant Network. Online Data. 2011 [Google Scholar]
- 4.Nocito A, El-Badry AM, Clavien PA. When is steatosis too much for transplantation? J Hepatol. 2006;45(4):494. doi: 10.1016/j.jhep.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Doyle MB, Vachharajani N, Wellen JR, et al. Short- and long-term outcomes after steatotic liver transplantation. Arch Surg. 2010;145(7):653. doi: 10.1001/archsurg.2010.119. [DOI] [PubMed] [Google Scholar]
- 6.Verran D, Kusyk T, Painter D, et al. Clinical experience gained from the use of 120 steatotic donor livers for orthotopic liver transplantation. Liver Transpl. 2003;9(5):500. doi: 10.1053/jlts.2003.50099. [DOI] [PubMed] [Google Scholar]
- 7.Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21(1):105. doi: 10.1055/s-2001-12933. [DOI] [PubMed] [Google Scholar]
- 8.Angulo P. Nonalcoholic fatty liver disease and liver transplantation. Liver Transpl. 2006;12(4):523. doi: 10.1002/lt.20738. [DOI] [PubMed] [Google Scholar]
- 9.Gane EJ. The natural history of recurrent hepatitis C and what influences this. Liver Transpl. 2008;14 Suppl 2:S36. doi: 10.1002/lt.21646. [DOI] [PubMed] [Google Scholar]
- 10.Feray C, Samuel D, Thiers V, et al. Reinfection of liver graft by hepatitis C virus after liver transplantation. J Clin Invest. 1992;89(4):1361. doi: 10.1172/JCI115723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berenguer M, Lopez-Labrador FX, Wright TL. Hepatitis C and liver transplantation. J Hepatol. 2001;35(5):666. doi: 10.1016/s0168-8278(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 12.Hassoba H, Leheta O, Sayed A, et al. IL-10 and IL-12p40 in Egyptian patients with HCV-related chronic liver disease. Egypt J Immunol. 2003;10(1):1. [PubMed] [Google Scholar]
- 13.Sobue S, Nomura T, Ishikawa T, et al. Th1/Th2 cytokine profiles and their relationship to clinical features in patients with chronic hepatitis C virus infection. J Gastroenterol. 2001;36(8):544. doi: 10.1007/s005350170057. [DOI] [PubMed] [Google Scholar]
- 14.Bharat A, Barros F, Narayanan K, et al. Characterization of virus-specific T-cell immunity in liver allograft recipients with HCV-induced cirrhosis. Am J Transplant. 2008;8(6):1214. doi: 10.1111/j.1600-6143.2008.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basha HI, Subramanian V, Seetharam A, et al. Characterization of HCV-Specific CD4+Th17 Immunity in Recurrent Hepatitis C-Induced Liver Allograft Fibrosis. Am J Transplant. 2011;11(4):775. doi: 10.1111/j.1600-6143.2011.03458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borg BB, Seetharam A, Subramanian V, et al. Immune response to extracellular matrix collagen in chronic hepatitis C-induced liver fibrosis. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2011;17(7):814. doi: 10.1002/lt.22303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiesner RH, Sorrell M, Villamil F. Report of the first International Liver Transplantation Society expert panel consensus conference on liver transplantation and hepatitis C. Liver Transpl. 2003;9(11):S1. doi: 10.1053/jlts.2003.50268. [DOI] [PubMed] [Google Scholar]
- 18.Briceno J, Ciria R, Pleguezuelo M, et al. Impact of donor graft steatosis on overall outcome and viral recurrence after liver transplantation for hepatitis C virus cirrhosis. Liver Transpl. 2009;15(1):37. doi: 10.1002/lt.21566. [DOI] [PubMed] [Google Scholar]
- 19.Burra P, Loreno M, Russo FP, et al. Donor livers with steatosis are safe to use in hepatitis C virus-positive recipients. Liver Transpl. 2009;15(6):619. doi: 10.1002/lt.21761. [DOI] [PubMed] [Google Scholar]
- 20.Anderson CD, Upadhya G, Conzen KD, et al. Endoplasmic reticulum stress is a mediator of posttransplant injury in severely steatotic liver allografts. Liver Transpl. 17(2):189. doi: 10.1002/lt.22220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selzner M, Rudiger HA, Sindram D, Madden J, Clavien PA. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology. 2000;32(6):1280. doi: 10.1053/jhep.2000.20528. [DOI] [PubMed] [Google Scholar]
- 22.McCormack L, Petrowsky H, Jochum W, Mullhaupt B, Weber M, Clavien PA. Use of severely steatotic grafts in liver transplantation: a matched case-control study. Ann Surg. 2007;246(6):940. doi: 10.1097/SLA.0b013e31815c2a3f. [DOI] [PubMed] [Google Scholar]
- 23.Baron PW, Sindram D, Higdon D, et al. Prolonged rewarming time during allograft implantation predisposes to recurrent hepatitis C infection after liver transplantation. Liver Transpl. 2000;6(4):407. doi: 10.1053/jlts.2000.7581. [DOI] [PubMed] [Google Scholar]
- 24.Watt KD, Lyden ER, Gulizia JM, McCashland TM. Recurrent hepatitis C posttransplant: early preservation injury may predict poor outcome. Liver Transpl. 2006;12(1):134. doi: 10.1002/lt.20583. [DOI] [PubMed] [Google Scholar]
- 25.Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25(3):658. doi: 10.1002/hep.510250328. [DOI] [PubMed] [Google Scholar]
- 26.Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19(12):1409. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Gruener NH, Jung MC, Schirren CA. Recurrent hepatitis C virus infection after liver transplantation: natural course, therapeutic approach and possible mechanisms of viral control. J Antimicrob Chemother. 2004;54(1):17. doi: 10.1093/jac/dkh297. [DOI] [PubMed] [Google Scholar]
- 28.Ploeg RJ, D'Alessandro AM, Knechtle SJ, et al. Risk factors for primary dysfunction after liver transplantation--a multivariate analysis. Transplantation. 1993;55(4):807. doi: 10.1097/00007890-199304000-00024. [DOI] [PubMed] [Google Scholar]
- 29.Strasberg SM, Howard TK, Molmenti EP, Hertl M. Selecting the donor liver: risk factors for poor function after orthotopic liver transplantation. Hepatology. 1994;20(4 Pt 1):829. doi: 10.1002/hep.1840200410. [DOI] [PubMed] [Google Scholar]
- 30.Charlton M, Seaberg E. Impact of immunosuppression and acute rejection on recurrence of hepatitis C: results of the National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Liver Transpl Surg. 1999;5(4) Suppl 1:S107. doi: 10.1053/JTLS005s00107. [DOI] [PubMed] [Google Scholar]
- 31.Papatheodoridis GV, Davies S, Dhillon AP, et al. The role of different immunosuppression in the long-term histological outcome of HCV reinfection after liver transplantation for HCV cirrhosis. Transplantation. 2001;72(3):412. doi: 10.1097/00007890-200108150-00009. [DOI] [PubMed] [Google Scholar]
- 32.Ghobrial RM, Steadman R, Gornbein J, et al. A 10-year experience of liver transplantation for hepatitis C: analysis of factors determining outcome in over 500 patients. Ann Surg. 2001;234(3):384. doi: 10.1097/00000658-200109000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teramoto K, Bowers JL, Khettry U, Palombo JD, Clouse ME. A rat fatty liver transplant model. Transplantation. 1993;55(4):737. doi: 10.1097/00007890-199304000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Chui AK, Shi LW, Rao AR, et al. Donor fatty (steatotic) liver allografts in orthotopic liver transplantation: a revisit. Transplantation proceedings. 2000;32(7):2101. doi: 10.1016/s0041-1345(00)01587-6. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Retortillo M, Forns X, Feliu A, et al. Hepatitis C virus kinetics during and immediately after liver transplantation. Hepatology. 2002;35(3):680. doi: 10.1053/jhep.2002.31773. [DOI] [PubMed] [Google Scholar]
- 36.Gane EJ, Naoumov NV, Qian KP, et al. A longitudinal analysis of hepatitis C virus replication following liver transplantation. Gastroenterology. 1996;110(1):167. doi: 10.1053/gast.1996.v110.pm8536853. [DOI] [PubMed] [Google Scholar]
- 37.Rosen HR. Hepatitis C virus in the human liver transplantation model. Clin Liver Dis. 2003;7(1):107. doi: 10.1016/s1089-3261(02)00073-9. [DOI] [PubMed] [Google Scholar]
- 38.Oyanagi Y, Takahashi T, Matsui S, et al. Enhanced expression of interleukin-6 in chronic hepatitis C. Liver. 1999;19(6):464. doi: 10.1111/j.1478-3231.1999.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 39.Bortolami M, Kotsafti A, Cardin R, Farinati F. Fas / FasL system, IL-1beta expression and apoptosis in chronic HBV and HCV liver disease. J Viral Hepat. 2008;15(7):515. doi: 10.1111/j.1365-2893.2008.00974.x. [DOI] [PubMed] [Google Scholar]
- 40.Lemmers A, Moreno C, Gustot T, et al. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 2009;49(2):646. doi: 10.1002/hep.22680. [DOI] [PubMed] [Google Scholar]
- 41.Zhang JY, Zhang Z, Lin F, et al. Interleukin-17-producing CD4(+) T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology. 51(1):81. doi: 10.1002/hep.23273. [DOI] [PubMed] [Google Scholar]
- 42.Bharat A, Saini D, Steward N, et al. Antibodies to self-antigens predispose to primary lung allograft dysfunction and chronic rejection. Ann Thorac Surg. 90(4):1094. doi: 10.1016/j.athoracsur.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fedoseyeva EV, Zhang F, Orr PL, Levin D, Buncke HJ, Benichou G. De novo autoimmunity to cardiac myosin after heart transplantation and its contribution to the rejection process. J Immunol. 1999;162(11):6836. [PubMed] [Google Scholar]
- 44.Bhogal RK, Bona CA. B cells: no longer bystanders in liver fibrosis. J Clin Invest. 2005;115(11):2962. doi: 10.1172/JCI26845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abou-Shady M, Friess H, Zimmermann A, et al. Connective tissue growth factor in human liver cirrhosis. Liver. 2000;20(4):296. doi: 10.1034/j.1600-0676.2000.020004296.x. [DOI] [PubMed] [Google Scholar]