Abstract
The mechanistic (or mammalian) target of rapamycin (mTOR), an evolutionarily conserved protein kinase, orchestrates cellular responses to growth, metabolic and stress signals. mTOR processes various extracellular and intracellular inputs as part of two mTOR protein complexes, mTORC1 or mTORC2. The mTORCs have numerous cellular targets but members of a family of protein kinases, the protein kinase (PK)A/PKG/PKC (AGC) family are the best characterized direct mTOR substrates. The AGC kinases control multiple cellular functions and deregulation of many members of this family underlies numerous pathological conditions. mTOR phosphorylates conserved motifs in these kinases to allosterically augment their activity, influence substrate specificity, and promote protein maturation and stability. Activation of AGC kinases in turn triggers the phosphorylation of diverse, often overlapping, targets that ultimately control cellular response to a wide spectrum of stimuli. This review will highlight recent findings on how mTOR regulates AGC kinases and how mTOR activity is feedback regulated by these kinases. We will discuss how this regulation can modulate downstream targets in the mTOR pathway that could account for the varied cellular functions of mTOR.
I: Introduction
mTOR regulates diverse cellular and physiological functions that ultimately control cell and body growth (Wullschleger et al., 2006, Polak and Hall, 2009, Zoncu et al., 2011). In whole organisms, mTOR plays a role in metabolism, growth, differentiation and aging. At the cellular level, mTOR responds to the changes in nutrient status and many other cellular or stress cues that ultimately impact cell growth and division. At the molecular level, it functions to regulate translation initiation, ribosome biogenesis, protein maturation, autophagy, actin cytoskeleton reorganization, and transcription. Abnormal mTOR signaling has been associated with numerous pathological conditions including cancer, immune disorders, diabetes, cardiovascular and neurological diseases. mTOR integrates multiple intracellular signaling pathways but the direct cellular targets of mTOR and exactly how it regulates its targets at the molecular level remain to be fully elucidated.
Our knowledge about mTOR functions and signaling emerged with the use of rapamycin. When rapamycin is associated with a conserved cellular protein, FKBP12, it binds and allosterically inhibits mTOR (Jacinto and Hall, 2003). Rapamycin was originally identified from the bacteria, Streptomyces hygroscopicus, isolated from soil samples collected from Easter Island, known to locals as Rapa nui. Although it has later been revealed that not all functions of mTOR are inhibited by rapamycin, this drug has remained instrumental in defining the downstream targets and functions of mTOR. The new generation of mTOR active site inhibitors is now unraveling important clues on both the rapamycin sensitive and insensitive functions of mTOR (Guertin and Sabatini, 2009).
More recent studies using gene deficient mice that impair functions of mTOR and its regulators are expanding our knowledge about how mTORCs function in the development and homeostasis of different tissues (Polak and Hall, 2009, Alessi et al., 2009). Tissue-specific knockouts and mTORC2-disrupted cell lines have further advanced our understanding of mTOR in regulation of AGC kinases and have also led to the identification of new mTOR downstream targets. This review will focus on the recent advances in our understanding of how mTOR and its best characterized substrates, the AGC kinases, coregulate each other. mTOR is critical for the optimal activation of several well known AGC kinases. Reciprocally, the AGC kinases not only mediate mTOR signals via phosphorylation of downstream intracellular targets but also fine tune the mTOR response through feedback regulation of mTOR activity, which is accomplished by phosphorylating upstream mTOR activators.
II: mTOR, an atypical protein kinase
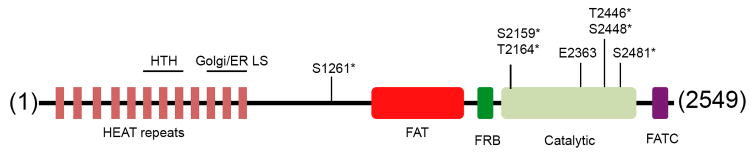
Mammalian TOR is a 290 kDa protein and a member of a subgroup of the atypical protein kinases termed phosphoinositide 3-kinase-related kinases (PIKKs) (Manning et al., 2002b). Members of this family share homology with lipid kinases but possess Ser/Thr protein kinase activity. The kinase (catalytic) domain of mTOR is located near the C-terminus and is flanked by the FAT (FRAP, ATM, TRRAP) and FATC (FAT C-terminus) domains that are common to PIKKs (Bosotti et al., 2000)(Figure 1). The N-terminal region consists of HEAT (Huntingtin, Elongation factor 3, A subunit of protein phosphatase 2A, TOR1) repeats, most likely to mediate protein-protein interactions (Figure 1). The N-terminal region also contains the helix turn helix (HTH) motif that was shown to bind to the promoter of 36S rDNA, and an ER and Golgi localization sequence (LS) (Liu and Zheng, 2007). An alternatively spliced isoform, mTOR-β, that is mostly devoid of the HEAT and FAT domains but retains the capacity to phosphorylate its substrates has been reported (Panasyuk et al., 2009). Many PIKKs form multi-protein complexes and seem to recognize their targets via their binding partners (Lovejoy and Cortez, 2009).
Figure 1. Schematic structure of human mTORprotein.
Human mTOR is a 2549 amino acid protein. It contains multiple HEAT repeats, a helix turn helix (HTH) motif, and a Golgi/ER localization signal sequence (LS) at its N-terminus. Its catalytic domain is flanked by a FAT and FATC sequences. Immediate upstream to the catalytic domain is the FKBP12-rapamycin binding (FRB) motif. A phosphomimetic residue Asp (E2363) at its putative activation loop, three known phosphorylation sites, T2446, S2448, and S2481, and three other less characterized phosphorylation sites, S1261, S2159, T2164, are shown.
Most PIKKs phosphorylate Ser/Thr followed by Gln residues (Abraham, 2004) but mTOR displays preference toward substrates with Pro, hydrophobic (Leu, Val), and aromatic residues (Phe, Trp, Tyr) at the +1 position. Some of the canonical mTOR target phosphorylation sites are shown in Table 1. Recently, a phosphoproteomic screen identified hundreds of possible direct targets of mTOR and a positional scanning peptide library assay validated the motif preferences of mTOR (Hsu et al., 2011, Yu et al., 2011).
Table 1.
Best characterized mTOR substrates and sequence motifs
| mTORC1 substrates | mTORC2 substrates | ||
|---|---|---|---|
| S6K1 | S371PDD (TM) | Akt/PKB | T450PPD (TM) |
| FLGFT389YVAPS (HM) | FPQFS473YSASG (HM) | ||
| EKFS401FEP | cPKCα | T638PPD (TM) | |
| IRS411PRRFIGS418PRT421PVS424PVK | FEGFS657YVNPQ (HM) | ||
| 4E-BP1 | DYSTT37PGG | ||
| FSTT46PGG | SGK1 | S397IGKS401PD (TM) | |
| CRNS65PVTKT70PPR | FLGFS422YAPP(HM) | ||
Mammalian TOR can be directly regulated via phosphorylation. Some of these phosphosites, such as Thr2446, Ser2448, and Ser2481, have been shown or suggested to be modulated by growth signals (Figure 1) (Cheng et al., 2004, Copp et al., 2009). Thr2446 and Ser2448 lie in a repressor domain region of mTOR (Sekulic et al., 2000) and whereas the former is linked to nutrient stimulation, the latter site responds to growth factor stimulation (Cheng et al., 2004). More recent studies demonstrated that these sites can be phosphorylated by S6K (Holz and Blenis, 2005, Chiang and Abraham, 2005). mTOR becomes autophosphorylated at Ser2481 and this phosphorylation was earlier shown to be insensitive to amino acid or serum-withdrawal but was abolished upon wortmannin treatment (Peterson et al., 2000). More recently, Ser2481 phosphorylation was demonstrated to be induced by insulin in a PI3K-dependent manner within both mTORC1 and mTORC2 (Copp et al., 2009, Soliman et al., 2010). In addition, the mTORC1-associated mTOR S2481 autophosphorylation, similar to mTORC1 signaling, were regulated not only by serum but also by amino acids and energy stress (Soliman et al., 2010). Phosphorylation of this site, along with Ser2448 is conserved among vertebrate species but absent in invertebrates (Copp et al., 2009), hence regulation of this site may have evolved in vertebrates in response to growth factors. Consistent with the earlier finding that Ser2481 phosphorylation is insensitive to conditions that inhibit mTORC1 activity (Peterson et al., 2000), this phosphosite was not sensitive to acute rapamycin treatment in mTORC2 immunoprecipitates (Soliman et al., 2010). Furthermore, prolonged rapamycin treatment, which can also disrupt mTORC2 (Sarbassov et al., 2006), can abolish Ser2481 phosphorylation, suggesting that phosphorylation of this site could be a marker for intact mTORC2 (Copp et al., 2009). However, Ser2481 phosphorylated mTOR was also found in the raptor complex and was sensitive to acute rapamycin treatment, indicating it may also have mTORC1 associated function under certain conditions (Soliman et al., 2010). Nevertheless, this phosphorylation site can be used as a marker of mTOR intrinsic kinase activity, either as part of mTORC1 or mTORC2. Together, the mTORC-regulatory signals must play a role to control mTORC intrinsic catalytic activity.
Other mTOR phosphorylation sites could regulate how mTOR complexes may be formed and how it may interact with their respective substrates. Ser1261 is also phosphorylated in an insulin/PI3K-dependent manner to promote mTORC1-mediated substrate phosphorylation and cell growth (Acosta-Jaquez et al., 2009). It is not known if phosphorylation of this site can modulate a specific mTORC2 function. Two sites within the N-terminus of the kinase domain, Ser2159 and Thr2164, are also phosphorylated and their phosphorylation correlates with increased mTORC1 signaling, cell growth and cell cycle progression (Ekim et al., 2011). Phosphorylation at these sites modulates the mTOR-raptor interaction while attenuating raptor-PRAS40 association. It remains unclear what phosphorylates these sites and if they are regulated by growth signals. Other mTOR autophosphorylation sites such as Ser2454, Thr2473/Thr2474 were identified by mass spectrometry but their function remains unknown (Tao et al., 2010).
In addition to phosphorylation regulation, mutations of specific amino acid residues in the mTOR kinase domain can confer hyperactivity to mTOR (Ohne et al., 2008). Two mutations (V2198A and L2216H), located on the αC-helix, a domain that plays a role in conformational changes in the kinase catalytic core, can enhance mTOR kinase activity. Other mutations were found in the repressor domain of mTOR that spans residues from amino acid 2430–2450. Interestingly, whereas these hyperactivating mutations can augment mTORC1 signaling, they do not appear to significantly enhance the mTORC2-mediated Akt phosphorylation. It would be interesting to determine how these mutations affect mTORC assembly and localization, which could explain their distinct effects on mTOR complex activity. Thus, the recent identification of regulatory and localization sites and motifs in mTOR would facilitate a more detailed analysis of how its activity can be controlled by numerous signals.
III: mTOR forms distinct multi-protein complexes: mTORC1 and mTORC2
Many PIKKs form multi-protein complexes and seem to recognize their targets via their binding partners (Lovejoy and Cortez, 2009). mTOR forms at least two distinct protein complexes termed mTOR complex (mTORC) 1 and mTORC2 (Figure 2). While there is likely extensive signal crosstalk between mTORC1 and mTORC2, these two complexes perform distinct functions. The availability of pharmacological inhibitors that inhibit both mTORC1 and mTORC2, along with the generation of animal models that disrupt mTORCs in specific tissues have allowed more detailed characterization of mTORCs.
Figure 2. Two mTOR complexes in activation of AGC kinases and cellular functions.
In response to amino acids and growth factors, mTORC1 and mTORC2 phosphorylate several AGC kinases at conserved motifs in their carboxyl-terminal tail. Phosphorylation at these sites, in conjunction with PDK1 phosphorylation of the AGC kinase activation loop, confers optimal activities to these kinases. In turn, the activated AGC kinases could serve to positively or negatively regulate mTORC signaling to control various cellular functions as indicated.
mTORC1 consists of mTOR, and the evolutionarily conserved proteins, regulatory-associated protein of mTOR (raptor) and mLST8 (also called GβL) (Hara et al., 2002, Loewith et al., 2002, Kim et al., 2002). Other less-conserved proteins have also been found associated with mTORC1 including PRAS40 and Deptor (Kim et al., 2003, Sancak et al., 2007) (Figure 2). Raptor contains four internal HEAT repeats and seven C-terminal WD40 repeats. Raptor association with mTOR is sensitive to the presence of rapamycin and nutrient status (Kim et al., 2002). It serves as a scaffold to bring substrates to mTOR without affecting the intrinsic catalytic activity of mTOR (Hara et al., 2002). Similar to the mTOR knockout (KO) mice, raptor KO mice die in the uterus, indicating that raptor is an essential gene (Guertin et al., 2006). Raptor is phosphorylated at multiple sites. It is phosphorylated by Rsk1/2 both in vivo and in vitro (Carriere et al., 2008). In response to mitogens, Rsk phosphorylates raptor at three sites, Ser719, Ser721, and Ser722, within a Rsk consensus motif upon activation of the Ras/MAPK pathway (Carriere et al., 2008). Mutation of these sites to Ala significantly reduced mTORC1 activity without affecting the interaction of raptor with mTOR or mTORC1 substrates. Thus, in addition to the Ras/MAPK/Rsk regulation of TSC2 (Roux et al., 2004), Ras can directly signal to mTORC1 via the Rsk-mediated phosphorylation of raptor although how these raptor phosphorylations modify mTORC1 activity is unclear at the moment. Raptor is also phosphorylated at Ser859 and Ser863 by mTOR, and the Ser863 phosphorylation can modulate mTORC1 activity (Wang et al., 2009, Foster et al., 2010). In addition, ERK1/2 was shown to directly phosphorylate raptor at Ser863 upon activation of the Ras/MAPK pathway suggesting that the insulin/PI3K/Akt and Ras/MAPK pathways converge on raptor to control mTORC1 signaling via this phosphorylation (Carriere et al., 2011). Other raptor phosphosites, such as Ser696, that positively regulate mTORC1 activity have also been identified including sites that are mediated by the ERK1/2 MAPK (Foster et al., 2010, Langlais et al., 2011, Carriere et al., 2011). During mitosis, raptor becomes hyperphosphorylated at sites including Ser696, Thr706, Ser722, Ser855, Ser859, Ser863, and Ser877 (Ramirez-Valle et al., 2010). Mitotic raptor promotes mRNA translation by internal ribosome entry sites (IRES). Inhibitors of cdc2 and GSK3 pathways can block the mitosis-specific phosphorylation of raptor, suggesting possible involvement of these kinases. mTORC1 can also be negatively regulated by phosphorylation of raptor by AMPK (Gwinn et al., 2008) and the autophagy regulator ULK1 (Dunlop et al., 2011). Thus, several pathways can converge on mTORC1 to regulate its activity temporally and most likely spatially as well via phosphorylation of raptor.
mLST8 is also an essential protein made up entirely of seven WD40 repeats. Unlike raptor, it is found in both mTORC1 and mTORC2, and binds to the kinase domain of mTOR to stimulate mTOR catalytic activity although how it can do so is still unclear. It has been speculated that mLST8 may contribute to the stability and folding of the mTOR kinase domain (Guertin et al., 2006). Alternatively, it may play a role in the recognition and recruitment of substrates to mTOR. The association of mLST8 with mTOR is insensitive to nutrients, but is required for the nutrient-sensitive interaction of raptor with mTOR. Whereas mLST8 is not essential for S6K1 and 4E-BP1 phosphorylation, its ablation in mice abolishes mTORC2 functions (Guertin et al., 2006). mTORC1 additionally interacts with PRAS40, FKBP38 and Deptor to control the activity of mTORC1 under different growth conditions (Dunlop and Tee, 2009, Laplante and Sabatini, 2009).
mTORC1 activity is regulated by diverse stimuli such as amino acid availability, oxygen, energy and redox status, growth factors, mitogens and is acutely inhibited by rapamycin. In the presence of amino acids, small GTPases Rag (heterodimers of either RagA or RagB with either RagC or RagD) become active, bind to raptor and anchors mTORC1 at the surface of late endosomes and lysosomes (Sancak et al., 2008). A protein complex, termed the Ragulator consisting of MAPK scaffold protein 1, p14, and p18, localizes Rag to these membrane compartments and subsequently promote the interaction between mTORC1 and Rheb, a small GTPase that positively regulates mTORC1 (Saucedo et al., 2003, Sancak et al., 2008, Sancak et al., 2010). In contrast, growth factors induce mTORC1 activation via the PI3K-Akt signaling pathway. PI3K activation leads to PDK1 phosphorylation of Akt at Thr308 in the catalytic T-loop. Activated Akt then inactivates the tuberous sclerosis complex (TSC1/TSC2) proteins by phosphorylation of TSC2, which functions as a GTPase-activating protein (GAP) for Rheb. Precisely how TSC2 phosphorylation by Akt inhibits TSC function remains controversial and several models have been proposed including altered TSC2 subcellular localization and protein stability (Huang and Manning, 2008). Inhibition of TSC allows the active, GTP-bound form of Rheb to interact with mTORC1 to stimulate its activity (Manning and Cantley, 2003). The cellular energy level, as determined by intracellular ATP/ADP ratio, also controls mTORC1 activity via modulation of TSC. A reduction in ATP intracellular concentration activates AMPK, which also phosphorylates TSC2.but in contrast to the Akt mediated TSC2 phosphorylation, the AMPK mediated phosphorylation reduces the GTP-bound form of Rheb, leading to the inhibition of mTORC1 activity (Inoki et al., 2003b, Shaw et al., 2004). Similarly, oxygen availability can also regulate mTOR function. Under hypoxic conditions, a concomitant decrease in ATP leads to AMPK activation. Alternatively, hypoxia can inhibit mTORC1 activity by enhancing the expression of DNA damage response 1 (REDD1). REDD1 inhibits mTORC1 by causing TSC2 to dissociate from 14-3-3 proteins and enhancing its ability to block Rheb (Brugarolas et al., 2004, DeYoung et al., 2008). Cellular redox status also regulates mTORC1 activity. Cysteine oxidants can enhance mTORC1 signaling despite the absence of amino acids (Sarbassov and Sabatini, 2005). It has been postulated that mTOR or an mTORC1 component can sense redox potential (Dames et al., 2005, Sarbassov and Sabatini, 2005). GRp58/ERp57, an mTORC1 interactor and a disulfide isomerase, may also sense redox status to regulate mTORC1 signaling (Ramirez-Rangel et al., 2011). The TSC complex and Rheb appear to mediate the redox-sensitive mTORC1 signaling (Yoshida et al., 2011). Mitogens can also increase mTORC1 activity via phospholipase D1 (PLD1) (Sun and Chen, 2008). PLD can promote the hydrolysis of phosphatidylcholine to generate phosphatidic acid (PA). PA can associate with the FRB domain of mTOR, compete with binding of FKBP12-rapamycin to this region, and enhances mTORC1 signaling. How PA activates mTOR remains obscure but recent studies demonstrate that TSC, via Rheb, is an upstream regulator of PLD (Sun et al., 2008). Thus in mammals, several pathways convey the growth and nutritional signals to mTORC1 via regulation of TSC1/TSC2.
Mammalian TORC2 contains the conserved core components rictor, Sin1 and mLST8 and also the less-conserved proteins PRR5/Protor, PRR5L (Pearce et al., 2007, Woo et al., 2007, Thedieck et al., 2007) and Deptor (Peterson et al., 2009). Rictor lacks known common motifs but contains a C-terminus that is conserved among vertebrates. Sin1 is another conserved adaptor protein with at least five isoforms due to alternative splicing of a single gene (Cheng et al., 2005, Frias et al., 2006). The two longest isoforms contain a pleckstrin homology (PH) like domain that has been shown to bind lipids and membranes, and a Ras binding domain (RBD) that can bind activated Ras (Schroder et al., 2007). At least three distinct mTORC2 complexes could be formed independently by three of these isoforms, but unique functions for each of the isoforms in mTOR signaling in vivo remain to be fully characterized.
Protor/PRR5 associates with rictor even under mTORC2-disrupted conditions. The expression of PRR5 is also regulated by rictor. In some cancers such as colorectal and breast carcinomas, protor mRNA levels were found elevated (Johnstone et al., 2005). Deptor contains two DEP (disheveled, egl-10, pleckstrin) domains and a PDZ (postsynaptic density 95, discs large, zonula occludens-1) domain (Peterson et al., 2009). It interacts at the vicinity of the N-terminal portion of the mTOR kinase domain and is associated with either mTORC1 or mTORC2. Deptor expression is regulated by both mTORCs at the transcriptional and post-translational level. Loss of Deptor activates mTORC kinase activities, indicating that it negatively regulates both mTORCs. However, Deptor is found to be highly overexpressed in a subset of multiple myelomas and that this overexpression maintains PI3K and Akt activation, suggesting that in these cancers, the overexpressed Deptor can activate mTORC2 signaling.
The stability and integrity of mTORC2 depends on both rictor and Sin1. Deficiency in rictor attenuates Sin1 levels and vice versa, whereas the level of mTOR is unaffected (Jacinto et al., 2006, Guertin et al., 2006, Yang et al., 2006, Frias et al., 2006). The tight interaction of rictor and Sin1 suggests they require each other for stability (Yang et al., 2006). However in established cell lines, such regulation appears lost since abundant rictor levels are observed in immortalized Sin1 deficient MEFs (Jacinto et al., 2006, Facchinetti et al., 2008). Deficiency in mLST8 impairs the association of rictor with mTOR but does not affect raptor and mTOR (Guertin et al., 2006) nor rictor and Sin1 association (Garcia-Martinez and Alessi, 2008). Thus, mLST8 may not be required for protein stability of rictor and Sin1. The rictor and Sin1 that associates with mTOR appears phosphorylated suggesting that their interaction with mTOR could be regulated and mTOR itself or yet to be determined kinases may phosphorylate them (Yang et al., 2006, Akcakanat et al., 2007).
Rictor is also a phosphoprotein and up to 37 potential phosphosites that mostly reside in the C-terminal half of rictor were identified (Dibble et al., 2009, Julien et al., 2010, Treins et al., 2009). One of these sites, Thr1135, that lies within an AGC kinase recognition motif, is phosphorylated by S6K1 and binds 14-3-3 proteins when phosphorylated (Dibble et al., 2009, Julien et al., 2010, Treins et al., 2009). Phosphorylation of Thr1135 is amino acid-, and growth factor-regulated, and is acutely sensitive to rapamycin but does not affect mTORC2 assembly nor in vitro kinase activity. Expression of a Thr1135 to Ala mutant does not significantly alter the growth factor-dependent phosphorylation of Akt at Ser473, nor does it affect other mTORC2 targets such as SGK1, conventional (c) PKCα, and Akt-Thr450 phosphorylation (Dibble et al., 2009, Julien et al., 2010, Treins et al., 2009, Boulbes et al., 2010). Since rictor Thr1135 phosphorylation is not abolished in Sin1-null murine embryonic fibroblasts (MEFs), this suggests that it may involve an mTORC2-independent function of rictor (Boulbes et al., 2010). Indeed, Thr1135 was recently shown to be likewise phosphorylated by AGC kinases Akt and SGK1 in addition to S6K, and such phosphorylation disrupted the interaction of rictor with cullin-1 and reduced the ability of the rictor/cullin complex to ubiquitinate and degrade SGK1 (Gao et al., 2010). This rictor/cullin E3 ligase activity appeared to be independent of mTORC activity. Rictor may also be phosphorylated and regulated by non-AGC kinases as it was found to associate with integrin-linked kinase (ILK) (McDonald et al., 2008), and was phosphorylated at Ser1235 by GSK3β during endoplasmic reticulum (ER) stress (Chen et al., 2011). The Ser1235 phosphorylation interfered with Akt-mTORC2 binding and mTORC2 downstream signaling. Thus, numerous cellular inputs can converge on rictor to regulate mTORC2 activity.
Sin1 is also likely phosphorylated but the identity of the phosphosites remains to be identified. Whether AGC kinases are involved in phosphorylating Sin1 is also unclear. Based on mobility shift on SDS-PAGE gels, it was suggested that hypophosphorylated Sin1 may have low affinity for mTOR (Yang et al., 2006) but could still bind rictor (Rosner and Hengstschlager, 2008). Sin1 also associates with other proteins independently of mTOR and rictor. Remarkably, these proteins are involved in stress responses including ras, MEKK2, JNK, p38, ATF2, and the stress-related cytokine receptors IFNAR2, TNFR1 and TNFR2 (Schroder et al., 2007, Cheng et al., 2005, Makino et al., 2006, Schroder et al., 2005, Ghosh et al., 2008). Whether such interactions could impact the mTORC2-dependent functions and regulation remains to be investigated.
TOR complexes can also undergo further oligomerization. Cryo-electron microscopy confirmed that mTORC1 forms a dimer (Yip et al., 2010). The dimerization state is sensitive to nutrients, but insensitive to growth factors (Zhang et al., 2006b, Takahara et al., 2006). In yeast, TORC2 is oligomeric and likely forms TORC2-TORC2 dimers (Wullschleger et al., 2005) but there are conflicting reports as to whether mTORC2 can form dimers/multimers (Takahara et al., 2006, Frias et al., 2006). Dimerization may facilitate inter- and intramolecular phosphorylation of other complex components or substrates.
In addition, other factors that are not unique to mTOR complexes have been reported to affect mTORC activity or assembly. For instance, Hsp70 interacts with rictor and its knockdown reduces rictor level as well as mTOR-rictor interaction, resulting in impaired mTORC2 formation and activity (Martin et al., 2008). The maturation and assembly of mTORCs was also shown to be dependent on Tel2 and Tti (Takai et al., 2007, Kaizuka et al., 2010). Hsp90 was shown to mediate the formation of both TORCs, as well as other PIKKs (Horejsi et al., 2010, Takai et al., 2010). Whether mTORC signaling can be modulated by these interactors remains to be examined.
Chronic exposure to rapamycin, which binds to mTOR as part of mTORC1, also reduces mTORC2 levels in many cell lines resulting in attenuation of phosphorylation of Akt, possibly by preventing de novo assembly of mTORC2 (Sarbassov et al., 2006). Under this condition, rictor and Sin1 levels are not perturbed, perhaps due to undisrupted interaction between rictor and Sin1. The mTOR inhibitors that target the active site, such as Torin1, have no effect on the stability of either mTORC1 or mTORC2 (Thoreen et al., 2009).
IV: The prototypical AGC kinases
The AGC kinase family, whose 63 members in the human genome share high homology (roughly 40% sequence identity) in their kinase domain, belongs to the conventional eukaryotic protein kinases (ePKs) (Gold et al., 2006). AGC kinases are Ser/Thr kinases and are known to play key roles in multiple important intracellular signaling pathways. The prototypical kinase structure was first resolved using an AGC kinase, the cAMP-dependent protein kinase (PKA) (Taylor et al., 2008). The X-ray structure of PKA revealed a bilobal architecture (N and C lobes) that is common to all protein kinases. Activation of AGC kinases, like other protein kinases, involves conformational changes in the key regulatory C-helix in the N-lobe (Biondi and Nebreda, 2003). Phosphorylation of an AGC kinase at the activation loop (T-loop) either by itself or by another AGC kinase, PDK1, confers optimal conformation for substrate binding. The C-terminal tail (C-tail) of AGC kinases, a hallmark of this kinase family, repositions the C-helix via phosphorylation regulation (Kannan et al., 2007). The C-tail consists of three segments: N-lobe tether (NLT) that includes the hydrophobic motif (HM), C-lobe tether (CLT) that interacts with the C-lobe and the interlobe linker connecting the N and C lobes, and the active-site tether (AST) that interacts with the ATP binding pocket (Kannan et al., 2007). The C-tail serves as a docking site for trans-acting factors, in addition to its cis-acting function, and is thereby essential for acquiring optimal conformation and activity.
The HM plays a pivotal role in regulating AGC kinase activity. Based on the PKA structure, the HM folds back into a hydrophobic pocket in the N-lobe. The HM is present in most AGC kinases (with the notable exception of PDK1) and contains a Ser/Thr residue that may or may not be phosphorylated. Phosphorylation of this conserved residue is important in regulating some AGC members whereas a phosphomimetic residue is found in other AGC kinases. In the absence of a phosphorylatable residue or if the HM is truncated, the AGC kinase adopts other mechanisms for additional regulation (Gold et al., 2006). When phosphorylated, the HM serves as the docking site for PDK1, which phosphorylates the T-loop and consequently activates the AGC kinase.
Another highly conserved motif in the C-tail of AGC kinases that precedes the HM is the turn motif (TM). One or more phosphorylated or phosphomimetic residues are nestled in this motif in most of the AGC kinases (Newton, 2003). The phosphorylated residue sits at the apex of a tight turn in PKA, hence the name. The structures of PKA and PKCι hint that the TM phosphorylation can stabilize the active conformation of the kinase domain by anchoring the C-terminus on top of this domain (Messerschmidt et al., 2005). Molecular remodeling, genetic and biochemical studies of the AGC kinases Akt and conventional PKCs reveal that the TM phosphorylation promotes the association between the C-tail and the kinase domain, which stabilizes the newly synthesized Akt and cPKC. This mode of regulation is likely shared by a number of other AGC kinases (Hauge et al., 2007, Facchinetti et al., 2008).
A conserved sequence motif (PXXP; where P is Pro and X is any amino acid) that is not phosphorylated lies within the CLT (Kannan et al., 2007). Initial studies show that this motif acts as an SH3 binding site in Akt (Jiang and Qiu, 2003). In PKC, sequences adjacent to this motif are important for binding Hsp90, which plays a role in the maturation of the protein kinase (Gould et al., 2009). Given the high conservation of this motif and Hsp90 binding to several AGC kinases, this motif along with Hsp90 could play a role in kinase maturation and/or sustaining kinase activity.
The AGC kinase domain displays high homology between members of this family. Not surprisingly, there is significant overlapping substrate specificity among these kinases. For instance, the minimal substrate recognition sequence for Akt was determined as RXRXXS/TB where X is any amino acid and B is a bulky hydrophobic residue (Alessi et al., 1996b). However, this sequence is also recognized by SGK, S6K and Rsk. As these AGC kinases also have their preferred substrates, the common minimal recognition sequence suggests that additional substrate determination factors are utilized by different AGC kinases. In PKC, the consensus motif was determined earlier to be RXXS/TXRX but a more detailed analysis has revealed further specific sequence preference among different PKC isozymes (Nishikawa et al., 1997).
The AGC kinases contain divergent sequences outside the catalytic domain and C-tail, hence adding another layer of specificity in their regulation and function. They could be regulated at different levels such as by transcription, post-translational modification, subcellular localization, protein stability, and protein-protein interaction.
V: Phosphorylation of AGC kinases by mTOR
S6K
S6 kinase (S6K) was the first rapamycin-sensitive target of mTOR to be identified (Pearson et al., 1995, Burnett et al., 1998). S6K is phosphorylated by mTORC1 and its activity is potently inhibited by rapamycin (Kim et al., 2002, Choo et al., 2008). There are two S6K homologues in mammals, S6K1 and S6K2, with divergent N- and C-terminal regulatory sequences (Fenton and Gout, 2011). S6K contains a TOR signaling (TOS) motif in the N-terminus, a conserved five amino acid sequence (FDIDL) that is essential for S6K activation by mTOR (Schalm and Blenis, 2002). Truncation of the N-terminus including the TOS motif prevents S6K1 activation by mTOR. The kinase domain is followed by a linker domain that includes the TM and HM. At the end of the C-tail is an autoinhibitory pseudosubstrate domain that is unique to S6K among AGC kinases. Like most other AGC kinases, the T-loop of S6K is phosphorylated by PDK1. However, T-loop phosphorylation occurs only when a cluster of Ser/Thr residues in the C-terminal regulatory domain are also phosphorylated. Although the kinase(s) that phosphorylates these sites in vivo remain to be identified, proline-directed kinases including ERK1/2, JNK1/2 and CDK1 can mediate phosphorylation in vitro (Mukhopadhyay et al., 1992).
S6K is phosphorylated at Thr389 of the HM and Ser404 of the linker domain (Pullen and Thomas, 1997). Both sites are highly sensitive to rapamycin treatment. Phosphorylation of the HM site of S6K1 was shown to occur only after sequential phosphorylation of the TM site followed by the T-loop (Keshwani et al., 2011). Previous studies have demonstrated that the phosphorylated HM serves as a PDK1 docking site to enhance T-loop phosphorylation (Biondi et al., 2001). Knockdown of mTORC1 components leads to defective HM site phosphorylation (Kim et al., 2002). In vivo, the adipose tissue-specific knockout of mTORC1 component raptor led to defective Thr389 phosphorylation and shared similar phenotype with germline KO of S6K, highlighting the importance of mTORC1 in S6K regulation (Polak et al., 2008). Phosphorylation of the HM site has become a marker for mTORC1 activation and plays a role in cell growth (Fenton and Gout, 2011).
S6K is also phosphorylated at seven other residues with a Ser/Thr-Pro motif. The TM Ser371 is one of these sites and has been shown to be phosphorylated constitutively prior to the T-loop and HM site (Keshwani et al., 2011). Its phosphorylation has also been reported to be mitogen-inducible, and like the HM and T-loop phosphorylation, is required for kinase activity (Saitoh et al., 2002). Although the S6K TM is phosphorylated by mTOR in vitro, there are conflicting reports as to whether this phosphorylation is rapamycin-sensitive (Saitoh et al., 2002, Moser et al., 1997, Shah and Hunter, 2004, Schalm et al., 2005). Whereas the loss of the mTOR inhibitors TSC1 or TSC2 led to hyperphosphorylation of Thr389 and the T-loop site Thr229, TM site (Ser371) phosphorylation was not significantly altered (Shah and Hunter, 2004). These findings suggest that the HM and TM sites in S6K may be differentially regulated.
Four other phosphorylation sites (Ser411, Ser418, Thr421, and Ser424) situated in the autoinhibitory domain of S6K contain a Pro residue at the +1 position and a hydrophobic residue at the -2 position (Pullen and Thomas, 1997). Phosphorylation of these sites is induced upon serum stimulation and is rapamycin sensitive indicating that they are mTORC1 targets. Loss of either TSC1 or TSC2 increased the basal phosphorylation of Ser411 but not the adjacent Ser421/Ser424 (Shah and Hunter, 2004). Both PDK1 and NEK6/7 also promote phosphorylation of Ser411 (Ser412 in human) (Balendran et al., 1999b, Belham et al., 2001). Mutation of these sites to Ala suppresses S6K activation. Deletion of the autoinhibitory region or mutation of all four residues into acidic residues reverse the inhibitory effect of N-terminal truncation of S6K1 resulting in its activation (Dennis et al., 1996, Cheatham et al., 1995, Weng et al., 1995). This active S6K1 mutant becomes rapamycin-insensitive and appears to depend on mTORC2 for T389 phosphorylation under this non-physiological condition (Ali and Sabatini, 2005). It is interesting to note that this autoinhibitory region is unique to S6K and could confer mTORC1 specificity.
Although it is well established that the HM site of S6K is regulated by mTORC1, other kinases such as PDK1, and S6K itself have been implicated in the phosphorylation of this motif (Balendran et al., 1999a, Romanelli et al., 2002). Atypical PKC has also been reported to enhance S6K activation (Romanelli et al., 1999). As discussed below for Akt and PKC, the HM site can be autophosphorylated or trans-phosphorylated by other kinases. The functional significance of the non-mTOR mediated versus the mTOR-mediated HM phosphorylation is still unclear.
Akt/PKB
Akt/PKB (Protein Kinase B) plays numerous roles in cell proliferation, survival, motility, growth, and differentiation. Abnormal Akt activation has been widely associated with many diseases such as cancer. Akt phosphorylation and activation requires membrane translocation. Upon growth factor stimulation that activates PI3K to produce the lipid products PtdIns (3,4,5)P3 and PtdIns(3,4)P2, Akt is recruited to a membrane compartment by binding to the lipids via its PH domain. Akt is catalytically activated by phosphorylation of the T-loop at Thr308 by PDK1. A phosphomimetic at the Thr308 site showed diminished membrane association, suggesting a role of this phosphorylation for promoting dissociation of activated Akt from the membrane (Ananthanarayanan et al., 2007).
Phosphorylation at the T-loop site is insufficient for optimal reorganization of the catalytic site (Yang et al., 2002). Akt is also phosphorylated by mTORC2 at the conserved motifs in the C-tail including the TM site Thr450 and the HM site Ser473 (Facchinetti et al., 2008, Ikenoue et al., 2008, Sarbassov et al., 2005, Hresko and Mueckler, 2005, Jacinto et al., 2006, Shiota et al., 2006, Guertin et al., 2006). HM site phosphorylation, which increases the binding affinity of the HM to the hydrophobic groove in the catalytic center, allosterically activates Akt. In mTORC2-disrupted cells such as Sin1−/−, rictor−/−, or mLST8−/− MEFs, Akt HM phosphorylation becomes abrogated (Jacinto et al., 2006, Shiota et al., 2006, Guertin et al., 2006). However, Akt in the mTORC2 deficient MEFs undergo T-loop phosphorylation, sometimes even slightly enhanced, demonstrating that Akt HM phosphorylation is not a prerequisite for the PDK1-mediated T-loop phosphorylation in this particular AGC kinase (Jacinto et al., 2006). HM site phosphorylation is robustly induced by stimuli such as growth factors/hormones (Alessi et al., 1996a) and depending on starvation conditions, amino acids as well (Tato et al., 2011). HM phosphorylation requires the recruitment of Akt to the plasma membrane via its PH domain. This would imply that mTORC2 and Akt would colocalize at the membrane. Indeed, mTORC2 has been localized to membrane compartments (Liu and Zheng, 2007, Hresko and Mueckler, 2005). Recently, mTORC2 was shown to be recruited to lipid rafts in a syndecan-4 and PKCα-dependent manner in endothelial cells for Akt activation (Partovian et al., 2008). Consistently, we and others have also found that the long isoforms of Sin1α and Sin1β are also localized to the plasma membrane (B. Su, unpublished results) (Schroder et al., 2007).
Akt HM site phosphorylation not only allosterically activates Akt but also leads to increased specificity towards its many substrates including FoxO1/3. In mTORC2-deficient MEFs in which the HM phosphorylation is absent, FoxO1/3 phosphorylation becomes defective whereas other Akt substrates such as GSK3 and TSC2 remain phosphorylated (Jacinto et al., 2006, Guertin et al., 2006). These findings suggest that HM phosphorylation by mTORC2 may confer substrate specificity to Akt. Alternatively, other related AGC kinases that do not depend on mTORC2 for activation may compensate for the loss of Akt activity to phosphorylate these substrates. However, the effect of mTORC2 deficiency on the AGC kinase substrates may be cell type specific since in Sin1-deficient embryos or Sin1-deficient B cells, the extent and kinetics of TSC and S6K phosphorylation were also impaired in addition to the FOXO1/3a phosphorylation (A. Lazorchak and B. Su, unpublished data). In addition to positively regulating Akt activity and specificity, Akt HM phosphorylation can also negatively regulate the levels of active, phosphorylated Akt through K48-linked ubiquitination and proteasome degradation (Wu et al., 2011). This would serve to terminate Akt signaling and thus prevent overactivation of this pathway that can lead to tumor development. Given that Akt is a highly stable protein with numerous targets and cellular functions, this mode of regulation would allow cells to downregulate a specific branch of Akt signaling without having impact on the overall Akt functions, thus providing another layer of specificity in regulation.
Phosphorylation of the HM site has been shown to be elevated in a number of cancers, indicating that the regulation of Akt by mTORC2 could play a significant role in cancer progression. In mouse genetic and xenograft studies, development of prostate cancer caused by PTEN deletion is dependent on mTORC2 (Guertin et al., 2009). When several cancer cells were treated with rapamycin for prolonged periods, a subset of these cell types have attenuated HM phosphorylation that correlated with disruption of mTORC2 (Sarbassov et al., 2006). Why other cancer cells remain insensitive to this treatment is not clear but other protein kinases have been linked to phosphorylation of the HM site and could therefore phosphorylate the HM under specific cellular conditions. Other kinases that could phosphorylate the HM include the double-stranded DNA-dependent protein kinase (DNA-PK), ataxia telangiectasia mutated (ATM) gene product, integrin-linked kinase (ILK), TANK-binding kinase 1 (TBK1) (Bozulic et al., 2008, Viniegra et al., 2005, Persad et al., 2001, Xie et al., 2011, Ou et al., 2011, Joung et al., 2011) and protein kinase C alpha (PKC alpha) (Dong and Liu, 2005). Thus, abrogation of HM phosphorylation accompanied by disruption of mTORC2 could serve as good biomarkers for tumors that can respond to mTOR inhibitors.
Akt TM site phosphorylation is highly dependent on mTORC2 but its regulation is distinct from that of the HM. The TM was undetected in the early crystal structure of Akt2 (Yang et al., 2002) and its phosphorylation was demonstrated to be constitutive (Alessi et al., 1996a), hence its significance was unclear for quite a long time. Its phosphorylation is an early event in the processing of Akt leading to T-loop and HM site phosphorylation (Bellacosa et al., 1998). The HM and TM phosphorylation for a number of the AGC kinases was reported to play cooperative roles in enhancing kinase activity (Hauge et al., 2007). Using Sin1−/− or rictor−/− MEFs, TM site phosphorylation of Akt and cPKC has been shown to absolutely require mTORC2 (Facchinetti et al., 2008, Ikenoue et al., 2008). When a myristylated form of Akt in mTORC2-disrupted cells was expressed, TM phosphorylation remained absent, whereas HM phosphorylation is partially restored (Facchinetti et al., 2008). Most striking difference between the HM and TM site phosphorylation is the inducibility of HM site phosphorylation upon growth factor stimulation. In contrast, TM site phosphorylation is largely unaffected by withdrawal of growth cues and inhibition of PI3K and other kinases. Thus, TM site phosphorylation is quite stable (Facchinetti et al., 2008, Feldman et al., 2009). These initial findings presented a puzzle since they would suggest that mTORC2 regulates phosphorylation of these two sites under different conditions. Indeed, more recent findings revealed that the TM site is controlled by mTORC2 exclusively during translation. Phosphorylation of the Akt TM site by mTORC2 is a one-off event that occurs during the synthesis of nascent Akt while the polypeptide is still attached to the ribosomes (Oh et al., 2010). It is therefore not surprising that TM phosphorylation is absent only in newly synthesized Akt. The association of mTORC2 with ribosomal proteins that line the rim of the ribosomal exit tunnel further supports that mTORC2 could phosphorylate this site as it emerges from the tunnel. TM phosphorylation serves as an anchor for the C-tail to attach to the kinase domain and is therefore vital for Akt stability, and perhaps also modulates the conformation of active Akt when the HM site is also phosphorylated (Facchinetti et al., 2008, Hauge et al., 2007). The lack of TM phosphorylation causes cotranslational ubiquitination of nascent Akt (Oh et al., 2010). A slight but consistent reduction of Akt expression was observed in mTORC2-deficient cells but remarkably, Akt levels were rapidly downregulated upon inhibition of the folding chaperone Hsp90 suggesting that Hsp90 was able to rescue substantial amount of Akt that is not phosphorylated at the TM site (Facchinetti et al., 2008, Ikenoue et al., 2008).
Phosphorylation at this site was also proposed to negatively regulate T-loop phosphorylation although the mechanism is obscure (Hiraoka et al., 2011). TM phosphorylation is likely solely mediated by TORC2 from yeast to man, and well conserved during evolution (Kamada et al., 2005, Facchinetti et al., 2008).
PKC
There are three classes of PKC (Protein Kinase C) in mammals: the conventional (c)PKC that includes α, β1, βII, and γ; the novel PKC (nPKC) that consists of δ, ε, θ, and η/Λ; and the atypical PKC (aPKC) that comprisesζ and ι/λ (Newton, 2003, Cameron et al., 2007). Members of the PKC family have overlapping substrate specificities with a variety of cellular functions but appears to primarily control the spatial distribution of signals (Rosse et al., 2010). Like other AGC kinases, the T-loop site of PKC is phosphorylated by PDK1 but this phosphorylation is constitutive and does not require phosphoinositides (Sonnenburg et al., 2001).
HM phosphorylation at Ser657/660 of PKCα/βII is constitutive and was shown to be an autophosphorylation event (Behn-Krappa and Newton, 1999). Knockdown of rictor diminished cPKCα HM site phosphorylation, suggesting that this phosphorylation also requires mTORC2 (Sarbassov et al., 2004). In mTORC2-disrupted cells, cPKC expression was dramatically reduced and although HM phosphorylation was also severely diminished, it was not abolished (Ikenoue et al., 2008, Facchinetti et al., 2008). Interestingly, deletion of raptor in rictor null muscle cells, which rescued the expression of cPKCα, also restored the cPKCα HM site phosphorylation (Bentzinger et al., 2008). Thus, HM site phosphorylation is closely linked to cPKC expression. Indeed, phosphorylation of the HM site is important for cPKC maturation and stability and depends on interactions with Hsp90 (Gould et al., 2009). It is noteworthy that the TORC2-dependent constitutive phosphorylation of sites in mammalian Akt and cPKC along with yeast PKC1 is crucial for kinase maturation and stability (Facchinetti et al., 2008, Oh et al., 2010, Ikenoue et al., 2008, Newton, 2010). Thus, stabilization of these AGC kinases via constitutive phosphorylation could be a primitive function of TORC2 that has been conserved from yeast to mammals.
In contrast to HM site phosphorylation, phosphorylation of the TM (Thr638/641 of PKCα/βII) sites of all conventional PKC (cPKC) and some novel PKCs (nPKC) requires mTORC2 and is insensitive to rapamycin (Facchinetti et al., 2008, Sarbassov et al., 2004, Ikenoue et al., 2008, Lee et al., 2010). Thus, TM site phosphorylation was completely abrogated in mTORC2-deficient cells (Facchinetti et al., 2008, Ikenoue et al., 2008). In vitro studies have shown that TM phosphorylation plays a role in the stabilization of the protein (Bornancin and Parker, 1996). Molecular modeling revealed that the TM phosphorylation of PKC induces a closed conformation and contributes to PKC stabilization (Hauge et al., 2007, Facchinetti et al., 2008). Thus, the lack of TM phosphorylation could account for the decreased PKC protein levels. Consistently, mutation of the TM site specifically increased the binding of cPKC to the folding chaperone Hsp70. This binding of Hsp70 to the TM-site mutant PKC stabilizes the protein and prolongs the signaling capacity of the kinase (Gao and Newton, 2002). Furthermore, inhibition of Hsp90 led to rapid degradation of remaining cPKC in mTORC2-disrupted cells. Why the absence of TM site phosphorylation has a more dramatic effect on cPKC but not Akt expression levels is unclear. Given that both Akt and cPKC are heavily dependent on chaperones for stability in the absence of TM phosphorylation, it is possible that they each have distinct modes of interaction with folding chaperones (Facchinetti et al., 2008, Ikenoue et al., 2008). Alternatively, it is also plausible that the cPKC levels may be attenuated at the level of transcription or translation in mTORC2-deficient cells. Although conventional kinase assays failed to reconstitute PKC TM phosphorylation (Ikenoue et al., 2008), addition of mTORC2 during in vitro translation of cPKC, enabled detection phosphorylation at this site (Oh et al., 2010). Whether this phosphorylation occurs cotranslationally, as in Akt TM site phosphorylation, remains to be investigated.
The activation of different PKC isoforms is followed by their degradation (Roffey et al., 2009). Defects in downregulating PKC could lead to pathological conditions. In cancer cells, elevated PKC levels have been observed. For example, increased PKCα expression in breast and ovarian cancer (Lahn et al., 2004) and upregulated PKCβ in diffuse large B cell lymphoma have been reported (Roffey et al., 2009). In primary human glioblastoma tumors where EGFR is upregulated, increased PKCα but not Akt phosphorylation correlated with the EGFR levels, suggesting that inhibiting PKC could be an important therapeutic target in malignant glioma (Fan et al., 2009). It is not clear in this case what led to the increased PKCα phosphorylation although another study has shown that rictor levels and mTORC2 activity are elevated in glioma and glioblastoma cell lines (Masri et al., 2007). Whether there is a correlation between the mTORC2 component levels and cPKC expression remains to be determined.
SGK
The serum- and glucocorticoid-induced kinases (including SGK1, SGK2, SGK3), which display about 55% identity in the kinase domain with Akt, are stimulated by growth factors and are involved in the regulation of sodium homeostasis (Lang et al., 2006, Tessier and Woodgett, 2006). Like Akt, SGK1 is phosphorylated at the T-loop by PDK1 (Kobayashi and Cohen, 1999). There has been controversy as to whether the HM site phosphorylation of SGK1 is mediated by mTORC1 or mTORC2. In melanoma and MCF7 cells, SGK1 HM phosphorylation is abolished upon rapamycin treatment implicating mTORC1 as the HM site regulator (Hong et al., 2008). Consistently, activation of SGK1 correlated with p27 phosphorylation, which is also rapamycin sensitive. However, another study showed that the SGK1 HM site phosphorylation was not rapamycin sensitive (Park et al., 1999). Notably, knockdown of rictor in melanoma cell lines appeared to decrease more p27-Thr157 phosphorylation and SGK1 phosphorylation than knockdown of raptor (Hong et al., 2008). The SGK1 HM site (Ser422) is phosphorylated by mTOR in vitro and in vivo. However, rapamycin did not abrogate this phosphorylation. Together with the finding that the SGK1 HM site was not phosphorylated in mTORC2-deficient cells, these results point to mTORC2 as the relevant kinase for the HM site (Garcia-Martinez and Alessi, 2008). In support of this, phosphorylation of the previously identified SGK1 substrate, NDRG1, was also abolished in mTORC2-disrupted cells. In addition, Protor-1/PRR5 functions as an adaptor or enhancer of mTORC2 activity to phosphorylate the HM of SGK1 (Pearce et al., 2007). More recently, mTORC2 was demonstrated to activate epithelial sodium channel (ENaC)-dependent Na+ transport in kidney epithelial cells (Lu et al., 2010). Furthermore, SGK1 could interact specifically with mTOR-rictor, supporting the view that mTORC2 is essential for SGK1 activation and function.
SGK1/2 also contain conserved TM sites (Ser397 and Ser401 in SGK1). Phosphorylation of these sites is required for maximum SGK1 activity in response to growth factors and stress stimuli (Chen et al., 2009). Phosphorylation at these sites involves active Akt and WNK1 (with no lysine kinase 1), a kinase that is overexpressed in a rare form of hypertension (Chen et al., 2009). Whether mTORC2 is required for phosphorylation of these sites remains to be investigated. A potential mTORC2-target site would be Thr368 that is part of a Thr-Pro-Pro motif based on similarity with the sites recognized in Akt and cPKC and is conserved in SGK1-3. Whether mTORC2 is required for phosphorylation of these sites directly or indirectly would need to be verified.
SGK1 could also interact with Hsp90 and pharmacological inhibition of Hsp90 impairs phosphorylation of SGK1 (Belova et al., 2008). Since the effect of Hsp90 inhibition on PDK1 levels occur at much later time points than the acute dephosphorylation of SGK1 (Fujita et al., 2002, Basso et al., 2002), it is likely that optimal SGK1 conformation to allow HM site phosphorylation is affected upon Hsp90 inhibition. Whether Hsp90 may protect SGK1 from degradation in mTORC2 deficient cells is unclear.
SGK1 is a short-lived protein and its N-terminus contains a hydrophobic degradation (HD)/PEST domain that mediates constitutive degradation via the ubiquitin-proteasome pathway (Kobayashi et al., 1999, Bogusz et al., 2006). In contrary to Akt and cPKC, the expression of SGK1 is elevated in rictor null cells (Gao et al., 2010). Rictor, but not the other mTORC2 components, co-precipitates with Cullin-1 and is proposed to form a functional E3 ubiquitin ligase complex to degrade SGK1 independent of mTOR. These studies indicate that SGK1 expression, along with its activity, may be critically monitored by both mTOR dependent and independent mechanisms.
VI: Phosphorylation of other mTOR regulators by AGC kinases
Many signaling molecules that control mTOR activity are also phosphorylated by the AGC kinases. Phosphorylation of the proline-rich Akt substrate of 40 kDa (PRAS40) at Ser246 by Akt relieves its inhibition of mTORC1 (Sancak et al., 2007, Vander Haar et al., 2007). Insulin stimulated Akt activation can also promote mTORC1 activity via direct phosphorylation and inhibition of TSC2, (Manning et al., 2002a, Inoki et al., 2002, Dan et al., 2002). Five phosphorylation sites in TSC2 with canonical Akt recognition motif are likely targeted by Akt (Huang and Manning, 2008). Mutations of different combination of these sites to Ala blocked the Akt-mediated mTORC1 activation when these TSC2 mutants were overexpressed. How Akt inhibits the TSC1-TSC2 function to promote mTORC1 activation is still not fully understood. Speculatively, Akt-mediated phosphorylation could promote degradation of TSC proteins or disrupt the TSC1-TSC2 complex. In addition, binding to 14-3-3 of phosphorylated TSC was shown to alter TSC2’s subcellular localization thus serving as another potential Akt-dependent regulation of TSC1/2 function (Cai et al., 2006, Rosner et al., 2007).
TSC2 is also phosphorylated by Rsk, an AGC kinase that is activated by members of the extracellular signal-regulated kinase (ERK)1/2. Rsk phosphorylates Ser1798 and also overlaps with Akt in the phosphorylation of Ser939 and Thr1462 (Roux et al., 2004). Phosphorylation of TSC2 by Rsk at the above sites plus the ERK1/2-targeted sites Ser540 and Ser664, contribute to the ERK-mediated activation of mTORC1 signaling (Ma et al., 2005).
TSC2 is putatively phosphorylated also by PKC at Ser1364 based on phosphorylation profiling studies and the induction of this phosphorylation by phorbol esters (Ballif et al., 2005). This phosphorylation was sensitive to a selective PKC inhibitor but is insensitive to a MAPK inhibitor, implicating PKC as the kinase instead of Rsk.
TSC1 is likely regulated by phosphorylation as well although it is not clear if there are AGC kinase-targeted sites. In Drosophila, Ser533 of dTSC1 is phosphorylated by dAkt. However this site is not conserved in mammals. Notably, flies expressing single dTsc1 mutant or dTsc1 and dTsc2 double mutants that lack the dAkt phosphorylation sites, are viable and are normal in size (Schleich and Teleman, 2009), suggesting that dAkt may not be involved in regulating dTORC1 activity via the dTSC complex.
Another upstream regulator of mTORCs that is subject to regulation by numerous kinases including several AGC kinases is the insulin receptor substrate (IRS). IRS1, the extensively studied member of the IRS family of adaptor molecules, is tyrosine phosphorylated in response to insulin, IGF-1 and cytokines (Taniguchi et al., 2006). Tyrosine phosphorylation recruits a number of SH2-containing proteins including PI3K. IRS1 is also phosphorylated potentially at over 70 Ser/Thr residues that either positively or negatively regulate insulin signaling (Gual et al., 2005). Chronic hyperphosphorylation of Ser/Thr residues of IRS1 is linked to insulin resistance. It is suggested that serine phosphorylation may allosterically inhibit the interactions between IRS1 and the insulin receptor (IR), making IRS1 a poorer substrate for the IR; alternatively phosphorylation could initiate IRS1 degradation or prevent IRS1 interaction with its downstream effectors.
IRS1 is phosphorylated within the phosphotyrosine-binding (PTB) domain by Akt at several sites following insulin stimulation. Phosphorylation at these sites could either enhance or inhibit insulin signaling (Paz et al., 1999). S6K can phosphorylate Ser302 (human Ser307) in vitro and knockdown of S6K inhibits this phosphorylation (Harrington et al., 2004). S6K (as well as mTOR) has been shown to phosphorylate IRS1 on Ser612 and Ser632, both proximal to tyrosine residues that could promote binding of PI3K. In adipose tissues of various obese mice, activation of S6K correlates with elevated Ser632 phosphorylation. This phosphorylation is abrogated in S6K1 null mice (Um et al., 2004). S6K1 also directly phosphorylates Ser1101 in vitro. Phosphorylation of this site depends on insulin stimulation and is modulated by the presence of amino acids. Mutation of this residue to Ala led to increased insulin signaling to Akt despite the presence of phosphorylation at other insulin resistance-associated sites (Ser307, Ser312, Ser636/639) (Tremblay et al., 2007). However, in TSC2-deficient MEFs, there was no apparent elevation in Ser1101 phosphorylation despite increased S6K activation. This is probably because of constitutive activation of mTOR/S6K, which promotes hyperphosphorylation of IRS1 on other serine residues to consequently trigger IRS-1 degradation (Shah and Hunter, 2006).
Different PKC isoforms also contribute to insulin signaling and resistance. In the diabetic fat tissue the conventional cPKCβII, which display elevated expression and kinase activity, plays a role in the phosphorylation of IRS1 at Ser336 (Liberman et al., 2008). Knockout of cPKCβII show that this isoform is not essential for insulin-stimulated glucose transport and overall glucose homeostasis (Standaert et al., 1999). Another cPKC isoform, cPKCα, may also downregulate IRS1. Knockout of PKCα led to enhanced insulin signaling in skeletal muscles and adipocytes (Leitges et al., 2002). A possible site of phosphorylation by cPKCα is Ser24 based on bioinformatics and biochemical analyses (Nawaratne et al., 2006). Whether this site plays a role in insulin resistance remains to be validated.
The novel PKCs have also been implicated in impaired insulin signaling. Inhibition of nPKCε prevents insulin resistance in the liver (Samuel et al., 2007). nPKCθ was also shown to inhibit insulin signaling via phosphorylation of IRS1 at Ser1101 (Li et al., 2004). Furthermore, nPKCθ activity is higher in muscles from obese diabetic patients. nPKCδ phosphorylates Ser357. Phosphorylation of this site by active nPKCδ are important for the insulin-induced Akt stimulation (Waraich et al., 2008). The atypical PKCs have been considered as positive modulators of insulin signaling, however more recent evidence suggest that they can also inhibit insulin signals. aPKCζ phosphorylates Ser318 of IRS1 and this phosphorylation decreases the tyrosine phosphorylation of IRS1 and interaction of IRS1 with the IR (Moeschel et al., 2004). Although some novel and atypical PKCs have been implicated in mTOR signaling (Lee et al., 2010, Leseux et al., 2008, Parekh et al., 1999), whether they are directly phosphorylated by either mTORCs is unclear at the moment (Viniegra et al., 2005).
Another upstream regulator of mTORC1 that has been shown to be regulated by the AGC kinase Rsk is LKB1 (Sapkota et al., 2001). LKB1 is a serine/threonine protein kinase mutated in autosomal dominantly inherited Peutz-Jeghers syndrome (PJS), a disease characterized by increased risk of benign and malignant tumors in multiple tissues, harmartomatous polyps in the gastrointestinal tract, and mucocutaneous pigmentation (Katajisto et al., 2007, Alessi et al., 2006). LKB1 regulates mTORC1 activity by directly phosphorylating and activating the mTOR upstream inhibitor AMPK (Hawley et al., 2003, Shaw et al., 2004, Woods et al., 2003). AMPK suppresses mTOR signaling in response to energy stress by phosphorylation of TSC2 and raptor (Shaw et al., 2004, Gwinn et al., 2008, Inoki et al., 2003a). In addition to Rsk, oncogenic B-RAF kinase also regulates LKB1 and inhibits its ability to bind to and phosphorylate AMPK in mediating the oncogenic activity of B-RAF (Zheng et al., 2009).
In addition to the AGC kinases, mTOR can itself phosphorylate signaling molecules that are proximal to growth factor receptors. As mentioned above, mTORC1 phosphorylates IRS-1 and this phosphorylation can downregulate insulin/IRS-1 signals (Tzatsos and Kandror, 2006). More recently, mTORC1 was demonstrated to phosphorylate growth factor receptor-bound protein 10 (Grb10) at multiple sites. Phosphorylation of Grb10 by mTORC1 serves as a feedback mechanism to negatively regulate growth factor signaling (Hsu et al., 2011, Yu et al., 2011). More mTOR and AGC kinase targets that act as feedback regulatory mechanisms would likely emerge from these recent phosphoproteomic studies on mTOR substrates.
VII: mTORC functions mediated by AGC kinases
The discovery of the AGC kinase recognition motif and the development of antibodies that recognize such motif have facilitated the identification of AGC kinase substrates. Phosphorylation of many AGC kinase substrates has been shown to be dependent on the mTOR complexes. Here, we will discuss cellular functions that have been linked to mTOR and its regulation of AGC kinases and the AGC-kinase-dependent substrate phosphorylation.
Translation and folding
The function of mTORC1 in translation initiation is supported by its role in the phosphorylation and activation of S6K1 and 4EBP, two critical translation regulators. Early studies show that S6K1 (p70S6K/p85S6K) phosphorylates the 40S ribosomal protein (rp) S6 at several sites (Fingar and Blenis, 2004) (Table 2). The significance of this phosphorylation is currently controversial since neither S6K1 activity nor phosphorylation of S6 is required to drive 5′-terminal oligopyrimidine (TOP) translation (Ruvinsky and Meyuhas, 2006). While S6K1 may not directly regulate 5′-TOP translation, it may have other translation-related functions. For example, it phosphorylates and activates the eukaryotic initiation factor 4B (eIF4B) at Ser422, a process that is sensitive to PI3K and mTOR inhibitors (Raught et al., 2004, Proud, 2007). S6K1 also phosphorylates the elongation factor 2 kinase (eEF2K) at Ser366 (Wang et al., 2001) (Table 2). Phosphorylation of eEF2K inactivates its kinase activity towards eEF2 and consequently enhances elongation. Another mTORC1 target, 4E-BP1 also functions in translation. Recent findings suggest that whereas the mTORC1-mediated phosphorylation of 4E-BP1 promotes cell cycle progression, S6K1 phosphorylation is more closely linked to promotion of cell growth (Dowling et al., 2010). The difference in regulation between these two mTORC1 substrates is also supported by findings that rapamycin can differentially inhibit the phosphorylation of these two proteins (Choo et al., 2008). Hence mRNA translation could be distinctly controlled by these two mTORC1 substrates.
Table 2.
Putative and known AGC kinase substrates of mTORC and non-mTORC
| Substrates | Phosphorylation sites | AGC kinases |
|---|---|---|
| mTOR | RSRTRT2446DS2448YS | S6K |
| PESIHS2481FIGDG(*) | S6K, (mTOR) | |
| KKLHVS1261TINLQ(**) | ND | |
| Raptor | RLRS719VS721S722YG | Rsk, S6K |
| Rictor | RIRTLT1135EPSVD | S6K, SGK, Akt |
| PRAS40 | RPRLNT246SDF | Akt |
| elF4B | RSRTGS422ESS | S6K, Rsk |
| eEF2K | RVRTLSS366GSR | S6K, Rsk |
| p27Kip1 | KPGLRRRQT198 | Akt, Rsk, SGK |
| FOXO1 | RPRSCT24WPL | Akt, SGK |
| PDCD4 | RLRKNS67SRDS71GRGDS76VS | S6K |
| GSK3a | RARTSS21FAE | Akt, Rsk, S6K |
| S6 | RRRLS235S236LRAS240TSKS244E | S6K |
| TSC2 | RARSTS939LNE | Akt, Rsk |
| RARSTS LNE RCRSIS981VSE | Akt | |
| RDRVRS1130MS1132GGH | Akt | |
| RPRGYT1462ISD | Akt, Rsk | |
| RKRLIS1798SVE | Rsk | |
| RVVS1364SEGGRP | PKC | |
| IRS1 | RKPKS24MHK | PKCa |
| RPRSKS265QS267SS | S6K | |
| RSRTES302ITATS307PAS | S6K | |
| GGKPGS318FRVR | aPKCζ | |
| MSRPAS336VDGS | cPKCβII | |
| RHRGS357SRL | nPKCδ | |
| GYMPMS612PGVA | S6K, (mTOR) | |
| GDYMPMS632PKSV | S6K, (mTOR) | |
| RRRHSS1101ETF (H.S.) | S6K, nPKCθ |
S6K is also recruited to newly synthesized RNA by SKAR (S6K1/Aly/REF-like substrate) and promotes activation of the initiation complex through a series of phosphorylation events (Ma et al., 2008). S6K1 phosphorylates and promotes degradation of the tumor suppressor, PDCD4, an inhibitor of RNA helicase eIF4A (Dorrello et al., 2006). Together with the recruitment of eIF4B to eIF4A, the degradation of PDCD4 greatly enhances eIF4A helicase activity and facilitates 40S ribosomal subunit scanning to the initiation codon. Given these multiple roles of S6K in translation, it may seem puzzling why S6K null mice do not exhibit a more severe translation defect (Pende et al., 2004). However, Rsk, another AGC kinase that shares a number of substrates with S6K, may compensate for the loss of S6K. Rsk phosphorylates eIF4B on Ser422, the same residue that is targeted by S6K (Shahbazian et al., 2006). Rsk also phosphorylates rpS6 at Ser235/236 and promotes translation (Roux et al., 2007). It remains to be determined if the mTORCs may also regulate Rsk.
Akt and PKC also play roles in translation both directly or indirectly. cPKCβII may regulate translation via interaction with the scaffold protein RACK1 (receptor for activated protein kinase C), a component of the small ribosomal subunit which could recruit PKC to the translation machinery. Purified ribosomes harbor PKC activity and stimulation of PKC results in increased polysomes, suggesting that PKC can modulate translation (Grosso et al., 2008a, Grosso et al., 2008b). Indeed, cPKCβII phosphorylates eIF6, a protein proposed to regulate the joining of 40S and 60S subunits in the assembly of 80S ribosomes (Grosso et al., 2008a). The role of cPKCβII in translation control was shown to be independent of mTORC1 (Grosso et al., 2008a). Whether mTORC2 may play a role in the cPKC-mediated function during translation needs to be further elucidated.
Although numerous studies have largely focused on the rapamycin-sensitive and – insensitive functions of mTORC1 in protein translation (Ma and Blenis, 2009, Sonenberg and Hinnebusch, 2009), mTORC2 is emerging as a player in this process. Using inhibitors targeting mTOR active site, thus blocking both mTORC1 and mTORC2, more severe impairment of protein synthesis as compared to rapamycin treatment was observed (Yu et al., 2009). For example, OSI-027, an inhibitor of mTORC1 and mTORC2, decreased the assembly of polysomal complex in rapamycin-insensitive leukemia cells (Carayol et al., 2010). OSI-027 also impaired mRNA translation, suppressed proliferation, and induced apoptosis, resulting in antileukemic responses. PP242, another mTOR active site inhibitor also impaired translation and displayed potent anti-leukemic activity (Janes et al., 2010, Evangelisti et al., 2011). This mTOR catalytic inhibitor could further suppress translation via inhibition of the rapamycin-sensitive and -insensitive mTORC1 targets (Feldman et al., 2009, Choo et al., 2008, Thoreen et al., 2009). In addition, blocking mTORC2 functions in protein synthesis could also contribute to these more pronounced defects. Consistent with the function of mTORC2 in translation, disruption of mTORC2 led to defective phosphorylation of eEF2 (Oh et al., 2010). An intact mTORC2 was found in polysome fractions associating with ribosomal proteins. Moreover, mTORC2 components could directly interact with the 60S large ribosome subunit (Oh et al., 2010, Zinzalla et al., 2011).
The interaction of mTORC2 with the large ribosomal subunit revealed that mTOR could also play a role in cotranslational protein folding or maturation. Nascent Akt is phosphorylated by mTORC2 to promote proper folding of the kinase domain and inhibit premature degradation of the nascent chain (Oh et al., 2010). Whether there are other cotranslational targets of mTORC2 remains to be identified. Other correlative findings have been reported that support a role for mTOR and AGC kinases in protein folding. CCTβ, a component of the chaperonin complex TCP-1 or TRiC, was shown to be phosphorylated at Ser260 by S6K1 and p90Rsk (Abe et al., 2009, Jastrzebski et al., 2011). This phosphorylation has been linked to chaperonin folding activity (Jastrzebski et al., 2011) and cell proliferation (Abe et al., 2009). The rapamycin-sensitivity of this phosphorylation remains to be resolved since conflicting results have been reported by these two studies. The availability of folding chaperones during conditions that lead to enhanced protein misfolding can limit mTORC1 signaling (Qian et al., 2010). Conversely, increased mTORC1 signaling, such as upon TSC deletion, that lead to enhanced translation promotes misfolding and thereby increases the unfolded protein response (Ozcan et al., 2008). Thus, mTOR plays a significant role in keeping the balance between protein synthesis and quality control.
Transcription and cell survival
mTOR can control gene transcription in response to growth and stress signals via the AGC kinases, which in turn can phosphorylate a number of transcription factors. The transcription factor FOXO1 is phosphorylated by Akt at Thr24, Ser256, and Ser319 under growth-promoting conditions (Manning and Cantley, 2007). Such phosphorylation prevents FOXO-mediated transcription of genes that could either promote cell death or reprogram cell differentiation. FOXO is also phosphorylated by SGK1 at identical sites targeted by Akt. In mTORC2-deficient cells including the Sin1−/−, rictor−/−, or mLST8−/− MEFs, the phosphorylation of FOXO1/3a at the Akt target sites Thr24/32 was greatly diminished. In line with this, Sin1−/− cells have decreased cell survival in the presence of stress-inducing agents (Jacinto et al., 2006). In addition to regulating the expression of genes involved in cell death, FOXO1/3a also plays an important role in regulating genes involved in cell differentiation and development. Recently, it has been shown that in Sin1-mTORC2 deficient B cells, two FOXO1 regulated genes, il7r and rag1/2, are upregulated (Lazorchak et al., 2010). This is due to the defect in Akt mediated phosphorylation and down regulation of FOXO1. mTORC2 also controls peripheral T cell differentiation via Akt and PKC. Whereas active Akt can rescue T-bet transcription factor expression and T helper 1 (Th1) differentiation, active nPKCθ can restore GATA3 transcription factor and Th2 defect in T cells with disrupted mTORC2 (Lee et al., 2010). In another study, it was shown that rictor deficient T cells were impaired in Th1, Treg and Th17 cell differentiation but not Th2 differentiation (Delgoffe et al., 2011)
Transcription of ribosomal genes can also be mediated by mTOR. The rDNA transcription factor UBF1 is dephosphorylated upon rapamycin treatment, providing a mechanism for mTOR control of ribosome biogenesis (Hannan et al., 2003). Whether UBF1 can be directly phosphorylated by S6K1 or other AGC kinases remains to be demonstrated.
In the presence of insulin stimulation, Akt phosphorylates and inhibits GSK3, a protein kinase that is involved in promoting apoptosis, among its many other cellular functions. The same sites in GSK3 (GSK3α at Ser21 and GSK3β at Ser9) arealso phosphorylated by Rsk in response to phorbol esters and growth factors (Frame and Cohen, 2001) and by S6K upon induction by amino acids (Armstrong et al., 2001). In TSC1 or TSC2 deficient cells, GSK3 is mainly phosphorylated by S6K1. This S6K mediated phosphorylation and inhibition of GSK3 contributes to proliferative defects in these TSC-deficient cells (Zhang et al., 2006a). Thus, under conditions where Akt activity is attenuated and S6K activity is elevated, S6K becomes a predominant kinase for GSK3. In mTORC2-disrupted MEFs where Akt activity is also attenuated, GSK3 phosphorylation at the same sites remained normal although S6K activity is not significantly elevated in these cells (Jacinto et al., 2006, Shiota et al., 2006). However, in other cell types such as B cells or in Sin1-deficient embryos, GSK3 phosphorylation was attenuated (B. Su, unpublished data). Thus, the suboptimal Akt activity may be sufficient to phosphorylate GSK3 only in specific cell types. Although SGK1 was reported to mediate GSK3 phosphorylation (Sakoda et al., 2003), it is unlikely that it will phosphorylate GSK3 in mTORC2-disrupted cells since SGK1 activation is highly defective in these cells (Garcia-Martinez and Alessi, 2008). Alternatively S6K or Rsk may contribute to GSK3 phosphorylation in mTORC2 deficient cells (Eldar-Finkelman et al., 1995, Frame and Cohen, 2001, Armstrong et al., 2001).
Cell cycle
mTOR has been linked to G1/S and G2/M check point control during cell cycle progression (Fingar and Blenis, 2004). Studies that have used rapamycin demonstrate that mTORC1 can control the expression of cyclin dependent kinase inhibitor (CDKI) proteins at the transcriptional and translational levels. AGC kinases, presumably activated in an mTOR-dependent manner, can phosphorylate cell cycle regulators. In TSC2-deficient cells where mTORC1 is upregulated, the CDKI p27Kip1, which blocks cyclin E-CDK2 activity during the G0 to G1 transition of the cell cycle, becomes inactivated (Rosner et al., 2006). Akt phosphorylates p27on Thr157, while both Akt and Rsk can phosphorylate p27 on Thr198 (Besson et al., 2008). Phosphorylation at these sites prevents p27 localization to the nucleus by sequestering it in the cytosol via 14-3-3 binding and thereby attenuates the cell-cycle inhibitory effects of p27. SGK1 phosphorylates p27 at the same sites as Akt in vitro and this phosphorylation was inhibited by rapamycin and in SGK1 knockdown cells (Hong et al., 2008). However, since SGK1 is phosphorylated and activated by mTORC2 (Garcia-Martinez and Alessi, 2008), it remains to be resolved how p27 can be regulated by both mTORC1 and mTORC2. Regulation of cyclin levels has also been shown to be mTORC1-controlled at the G1/S and G2/M phases of the cell cycle (Wang and Proud, 2009) but the involvement of AGC kinases in this function remains to be further elucidated.
Conclusion
Recent studies based on knockout mouse models and mTOR active site inhibitors have revealed numerous functions for mTOR in regulating various cellular targets. Proteomic screening for mTOR substrates identified a plethora of mTOR targets that play various roles not only in protein synthesis but also in vesicular trafficking, lipid biogenesis, RNA processing, protein folding and maturation and more (Yu et al., 2011, Hsu et al., 2011, Jastrzebski et al., 2011). Future studies should reveal how mTOR can phosphorylate these substrates directly or whether they can be indirectly mediated by AGC-type or other protein kinases. Defining specific substrates of mTOR in different cell types and conditions would provide novel insights on how mTOR can control the development and physiological functions of specific tissues.
The mTOR mediated phosphorylation of its targets is highly influenced by where it takes place in the cell. In fact, even the same protein can be differentially modulated at different cellular locations by mTOR complex. The best example of this is the regulation of the TM versus HM sites of Akt wherein the former is exclusively phosphorylated in translating ribosomes whereas the latter occurs in the membrane. The mTOR complexes have been localized in different organelles and membrane compartments. Whether specific mTOR partners can regulate the core complex components to provide specificity in these different compartments would need to be further addressed. This would provide a mechanism as to how mTORCs can regulate AGC kinases not only temporally but spatially.
Finally, it is apparent that AGC kinases are not the only cellular mTORC substrates. Furthermore, the mTOR complexes not only allosterically activate these kinases but also promote their maturation and stability. It is therefore possible that phosphorylation of these novel mTORC targets can control not only their activity but also protein maturation, stability and localization. Understanding how mTORCs can regulate these numerous targets would illuminate how we can tap these mTOR functions for developing new modes and targets for therapy against cancer and other growth-related disorders.
Acknowledgments
We thank the members of Su and Jacinto laboratories for helpful discussions and comments on this manuscript, and Ms. Rong Tan for help with figure illustration.
Footnotes
Declaration of Interest
The authors report no declarations of interest. This work was supported in part by grants from NIH (AI063348) and DOD (PR093728) to BS, and the American Cancer Society RSG0721601TBE, Cancer Research Institute and NIH (GM079176) to EJ.
References
- ABE Y, YOON SO, KUBOTA K, MENDOZA MC, GYGI SP, BLENIS J. p90 ribosomal S6 kinase and p70 ribosomal S6 kinase link phosphorylation of the eukaryotic chaperonin containing TCP-1 to growth factor, insulin, and nutrient signaling. J Biol Chem. 2009;284:14939–48. doi: 10.1074/jbc.M900097200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ABRAHAM RT. PI 3-kinase related kinases: ‘big’ players in stress-induced signaling pathways. DNA Repair (Amst) 2004;3:883–7. doi: 10.1016/j.dnarep.2004.04.002. [DOI] [PubMed] [Google Scholar]
- ACOSTA-JAQUEZ HA, KELLER JA, FOSTER KG, EKIM B, SOLIMAN GA, FEENER EP, BALLIF BA, FINGAR DC. Site-specific mTOR phosphorylation promotes mTORC1-mediated signaling and cell growth. Mol Cell Biol. 2009;29:4308–24. doi: 10.1128/MCB.01665-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AKCAKANAT A, SINGH G, HUNG MC, MERIC-BERNSTAM F. Rapamycin regulates the phosphorylation of rictor. Biochem Biophys Res Commun. 2007;362:330–3. doi: 10.1016/j.bbrc.2007.07.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALESSI DR, ANDJELKOVIC M, CAUDWELL B, CRON P, MORRICE N, COHEN P, HEMMINGS BA. Mechanism of activation of protein kinase B by insulin and IGF-1. Embo J. 1996a;15:6541–51. [PMC free article] [PubMed] [Google Scholar]
- ALESSI DR, CAUDWELL FB, ANDJELKOVIC M, HEMMINGS BA, COHEN P. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 1996b;399:333–8. doi: 10.1016/s0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- ALESSI DR, PEARCE LR, GARCIA-MARTINEZ JM. New insights into mTOR signaling: mTORC2 and beyond. Sci Signal. 2009;2:pe27. doi: 10.1126/scisignal.267pe27. [DOI] [PubMed] [Google Scholar]
- ALESSI DR, SAKAMOTO K, BAYASCAS JR. LKB1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–63. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- ALI SM, SABATINI DM. Structure of S6 kinase 1 determines whether raptor-mTOR or rictor-mTOR phosphorylates its hydrophobic motif site. J Biol Chem. 2005;280:19445–8. doi: 10.1074/jbc.C500125200. [DOI] [PubMed] [Google Scholar]
- ANANTHANARAYANAN B, FOSBRINK M, RAHDAR M, ZHANG J. Live-cell molecular analysis of Akt activation reveals roles for activation loop phosphorylation. J Biol Chem. 2007;282:36634–41. doi: 10.1074/jbc.M706227200. [DOI] [PubMed] [Google Scholar]
- ARMSTRONG JL, BONAVAUD SM, TOOLE BJ, YEAMAN SJ. Regulation of glycogen synthesis by amino acids in cultured human muscle cells. J Biol Chem. 2001;276:952–6. doi: 10.1074/jbc.M004812200. [DOI] [PubMed] [Google Scholar]
- BALENDRAN A, CASAMAYOR A, DEAK M, PATERSON A, GAFFNEY P, CURRIE R, DOWNES CP, ALESSI DR. PDK1 acquires PDK2 activity in the presence of a synthetic peptide derived from the carboxyl terminus of PRK2. Curr Biol. 1999a;9:393–404. doi: 10.1016/s0960-9822(99)80186-9. [DOI] [PubMed] [Google Scholar]
- BALENDRAN A, CURRIE R, ARMSTRONG CG, AVRUCH J, ALESSI DR. Evidence that 3-phosphoinositide-dependent protein kinase-1 mediates phosphorylation of p70 S6 kinase in vivo at Thr-412 as well as Thr-252. J Biol Chem. 1999b;274:37400–6. doi: 10.1074/jbc.274.52.37400. [DOI] [PubMed] [Google Scholar]
- BALLIF BA, ROUX PP, GERBER SA, MACKEIGAN JP, BLENIS J, GYGI SP. Quantitative phosphorylation profiling of the ERK/p90 ribosomal S6 kinase-signaling cassette and its targets, the tuberous sclerosis tumor suppressors. Proc Natl Acad Sci U S A. 2005;102:667–72. doi: 10.1073/pnas.0409143102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASSO AD, SOLIT DB, CHIOSIS G, GIRI B, TSICHLIS P, ROSEN N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J Biol Chem. 2002;277:39858–66. doi: 10.1074/jbc.M206322200. [DOI] [PubMed] [Google Scholar]
- BEHN-KRAPPA A, NEWTON AC. The hydrophobic phosphorylation motif of conventional protein kinase C is regulated by autophosphorylation. Curr Biol. 1999;9:728–37. doi: 10.1016/s0960-9822(99)80332-7. [DOI] [PubMed] [Google Scholar]
- BELHAM C, COMB MJ, AVRUCH J. Identification of the NIMA family kinases NEK6/7 as regulators of the p70 ribosomal S6 kinase. Curr Biol. 2001;11:1155–67. doi: 10.1016/s0960-9822(01)00369-4. [DOI] [PubMed] [Google Scholar]
- BELLACOSA A, CHAN TO, AHMED NN, DATTA K, MALSTROM S, STOKOE D, MCCORMICK F, FENG J, TSICHLIS P. Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene. 1998;17:313–25. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- BELOVA L, BRICKLEY DR, KY B, SHARMA SK, CONZEN SD. Hsp90 regulates the phosphorylation and activity of serum- and glucocorticoid-regulated kinase-1. J Biol Chem. 2008;283:18821–31. doi: 10.1074/jbc.M803289200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENTZINGER CF, ROMANINO K, CLOETTA D, LIN S, MASCARENHAS JB, OLIVERI F, XIA J, CASANOVA E, COSTA CF, BRINK M, ZORZATO F, HALL MN, RUEGG MA. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 2008;8:411–24. doi: 10.1016/j.cmet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- BESSON A, DOWDY SF, ROBERTS JM. CDK inhibitors: cell cycle regulators and beyond. Dev Cell. 2008;14:159–69. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- BIONDI RM, KIELOCH A, CURRIE RA, DEAK M, ALESSI DR. The PIF-binding pocket in PDK1 is essential for activation of S6K and SGK, but not PKB. Embo J. 2001;20:4380–90. doi: 10.1093/emboj/20.16.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIONDI RM, NEBREDA AR. Signalling specificity of Ser/Thr protein kinases through docking-site-mediated interactions. Biochem J. 2003;372:1–13. doi: 10.1042/BJ20021641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOGUSZ AM, BRICKLEY DR, PEW T, CONZEN SD. A novel N-terminal hydrophobic motif mediates constitutive degradation of serum- and glucocorticoid-induced kinase-1 by the ubiquitin-proteasome pathway. Febs J. 2006;273:2913–28. doi: 10.1111/j.1742-4658.2006.05304.x. [DOI] [PubMed] [Google Scholar]
- BORNANCIN F, PARKER PJ. Phosphorylation of threonine 638 critically controls the dephosphorylation and inactivation of protein kinase Calpha. Curr Biol. 1996;6:1114–23. doi: 10.1016/s0960-9822(02)70678-7. [DOI] [PubMed] [Google Scholar]
- BOSOTTI R, ISACCHI A, SONNHAMMER EL. FAT: a novel domain in PIK-related kinases. Trends Biochem Sci. 2000;25:225–7. doi: 10.1016/s0968-0004(00)01563-2. [DOI] [PubMed] [Google Scholar]
- BOULBES D, CHEN CH, SHAIKENOV T, AGARWAL NK, PETERSON TR, ADDONA TA, KESHISHIAN H, CARR SA, MAGNUSON MA, SABATINI DM, SARBASSOV DOS D. Rictor phosphorylation on the Thr-1135 site does not require mammalian target of rapamycin complex 2. Mol Cancer Res. 2010;8:896–906. doi: 10.1158/1541-7786.MCR-09-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOZULIC L, SURUCU B, HYNX D, HEMMINGS BA. PKBalpha/Akt1 acts downstream of DNA-PK in the DNA double-strand break response and promotes survival. Mol Cell. 2008;30:203–13. doi: 10.1016/j.molcel.2008.02.024. [DOI] [PubMed] [Google Scholar]
- BRUGAROLAS J, LEI K, HURLEY RL, MANNING BD, REILING JH, HAFEN E, WITTERS LA, ELLISEN LW, KAELIN WG., JR Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNETT PE, BARROW RK, COHEN NA, SNYDER SH, SABATINI DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci U S A. 1998;95:1432–7. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAI SL, TEE AR, SHORT JD, BERGERON JM, KIM J, SHEN J, GUO R, JOHNSON CL, KIGUCHI K, WALKER CL. Activity of TSC2 is inhibited by AKT-mediated phosphorylation and membrane partitioning. J Cell Biol. 2006;173:279–89. doi: 10.1083/jcb.200507119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMERON AJ, DE RYCKER M, CALLEJA V, ALCOR D, KJAER S, KOSTELECKY B, SAURIN A, FAISAL A, LAGUERRE M, HEMMINGS BA, MCDONALD N, LARIJANI B, PARKER PJ. Protein kinases, from B to C. Biochem Soc Trans. 2007;35:1013–7. doi: 10.1042/BST0351013. [DOI] [PubMed] [Google Scholar]
- CARAYOL N, VAKANA E, SASSANO A, KAUR S, GOUSSETIS DJ, GLASER H, DRUKER BJ, DONATO NJ, ALTMAN JK, BARR S, PLATANIAS LC. Critical roles for mTORC2- and rapamycin-insensitive mTORC1-complexes in growth and survival of BCR-ABL-expressing leukemic cells. Proc Natl Acad Sci U S A. 2010;107:12469–74. doi: 10.1073/pnas.1005114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARRIERE A, CARGNELLO M, JULIEN LA, GAO H, BONNEIL E, THIBAULT P, ROUX PP. Oncogenic MAPK signaling stimulates mTORC1 activity by promoting RSK-mediated raptor phosphorylation. Curr Biol. 2008;18:1269–77. doi: 10.1016/j.cub.2008.07.078. [DOI] [PubMed] [Google Scholar]
- CARRIERE A, ROMEO Y, ACOSTA-JAQUEZ HA, MOREAU J, BONNEIL E, THIBAULT P, FINGAR DC, ROUX PP. ERK1/2 phosphorylate Raptor to promote Ras-dependent activation of mTOR complex 1 (mTORC1) J Biol Chem. 2011;286:567–77. doi: 10.1074/jbc.M110.159046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEATHAM L, MONFAR M, CHOU MM, BLENIS J. Structural and functional analysis of pp70S6k. Proc Natl Acad Sci U S A. 1995;92:11696–700. doi: 10.1073/pnas.92.25.11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN CH, SHAIKENOV T, PETERSON TR, AIMBETOV R, BISSENBAEV AK, LEE SW, WU J, LIN HK, SARBASSOV DOS D. ER stress inhibits mTORC2 and Akt signaling through GSK-3beta-mediated phosphorylation of rictor. Sci Signal. 2011;4:ra10. doi: 10.1126/scisignal.2001731. [DOI] [PubMed] [Google Scholar]
- CHEN W, CHEN Y, XU BE, JUANG YC, STIPPEC S, ZHAO Y, COBB MH. Regulation of a third conserved phosphorylation site in SGK1. J Biol Chem. 2009;284:3453–60. doi: 10.1074/jbc.M807502200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHENG J, ZHANG D, KIM K, ZHAO Y, SU B. Mip1, an MEKK2-interacting protein, controls MEKK2 dimerization and activation. Mol Cell Biol. 2005;25:5955–64. doi: 10.1128/MCB.25.14.5955-5964.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHENG SW, FRYER LG, CARLING D, SHEPHERD PR. Thr2446 is a novel mammalian target of rapamycin (mTOR) phosphorylation site regulated by nutrient status. J Biol Chem. 2004;279:15719–22. doi: 10.1074/jbc.C300534200. [DOI] [PubMed] [Google Scholar]
- CHIANG GG, ABRAHAM RT. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J Biol Chem. 2005;280:25485–90. doi: 10.1074/jbc.M501707200. [DOI] [PubMed] [Google Scholar]
- CHOO AY, YOON SO, KIM SG, ROUX PP, BLENIS J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci U S A. 2008;105:17414–9. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COPP J, MANNING G, HUNTER T. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res. 2009;69:1821–7. doi: 10.1158/0008-5472.CAN-08-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAMES SA, MULET JM, RATHGEB-SZABO K, HALL MN, GRZESIEK S. The solution structure of the FATC domain of the protein kinase target of rapamycin suggests a role for redox-dependent structural and cellular stability. J Biol Chem. 2005;280:20558–64. doi: 10.1074/jbc.M501116200. [DOI] [PubMed] [Google Scholar]
- DAN HC, SUN M, YANG L, FELDMAN RI, SUI XM, YEUNG RS, HALLEY DJ, NICOSIA SV, PLEDGER WJ, CHENG JQ. PI3K/AKT pathway regulates TSC tumor suppressor complex by phosphorylation of tuberin. J Biol Chem. 2002;11:11. doi: 10.1074/jbc.M205838200. [DOI] [PubMed] [Google Scholar]
- DELGOFFE GM, POLLIZZI KN, WAICKMAN AT, HEIKAMP E, MEYERS DJ, HORTON MR, XIAO B, WORLEY PF, POWELL JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENNIS PB, PULLEN N, KOZMA SC, THOMAS G. The principal rapamycin-sensitive p70(s6k) phosphorylation sites, T-229 and T-389, are differentially regulated by rapamycin-insensitive kinase kinases. Mol Cell Biol. 1996;16:6242–51. doi: 10.1128/mcb.16.11.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEYOUNG MP, HORAK P, SOFER A, SGROI D, ELLISEN LW. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 2008;22:239–51. doi: 10.1101/gad.1617608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIBBLE CC, ASARA JM, MANNING BD. Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol Cell Biol. 2009;29:5657–70. doi: 10.1128/MCB.00735-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONG LQ, LIU F. PDK2: the missing piece in the receptor tyrosine kinase signaling pathway puzzle. Am J Physiol Endocrinol Metab. 2005;289:E187–96. doi: 10.1152/ajpendo.00011.2005. [DOI] [PubMed] [Google Scholar]
- DORRELLO NV, PESCHIAROLI A, GUARDAVACCARO D, COLBURN NH, SHERMAN NE, PAGANO M. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science. 2006;314:467–71. doi: 10.1126/science.1130276. [DOI] [PubMed] [Google Scholar]
- DOWLING RJ, TOPISIROVIC I, ALAIN T, BIDINOSTI M, FONSECA BD, PETROULAKIS E, WANG X, LARSSON O, SELVARAJ A, LIU Y, KOZMA SC, THOMAS G, SONENBERG N. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science. 2010;328:1172–6. doi: 10.1126/science.1187532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNLOP EA, HUNT DK, ACOST A-J, AQUEZ HA, FINGAR DC, TEE AR. ULK1 inhibits mTORC1 signaling, promotes multisite Raptor phosphorylation and hinders substrate binding. Autophagy. 2011:7. doi: 10.4161/auto.7.7.15491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNLOP EA, TEE AR. Mammalian target of rapamycin complex 1: signalling inputs, substrates and feedback mechanisms. Cell Signal. 2009;21:827–35. doi: 10.1016/j.cellsig.2009.01.012. [DOI] [PubMed] [Google Scholar]
- EKIM B, MAGNUSON B, ACOSTA-JAQUEZ HA, KELLER JA, FEENER EP, FINGAR DC. mTOR kinase domain phosphorylation promotes mTORC1 signaling, cell growth, and cell cycle progression. Mol Cell Biol. 2011 doi: 10.1128/MCB.05437-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELDAR-FINKELMAN H, SEGER R, VANDENHEEDE JR, KREBS EG. Inactivation of glycogen synthase kinase-3 by epidermal growth factor is mediated by mitogen-activated protein kinase/p90 ribosomal protein S6 kinase signaling pathway in NIH/3T3 cells. J Biol Chem. 1995;270:987–90. doi: 10.1074/jbc.270.3.987. [DOI] [PubMed] [Google Scholar]
- EVANGELISTI C, RICCI F, TAZZARI P, TABELLINI G, BATTISTELLI M, FALCIERI E, CHIARINI F, BORTUL R, MELCHIONDA F, PAGLIARO P, PESSION A, MCCUBREY JA, MARTELLI AM. Targeted inhibition of mTORC1 and mTORC2 by active-site mTOR inhibitors has cytotoxic effects in T-cell acute lymphoblastic leukemia. Leukemia. 2011 doi: 10.1038/leu.2011.20. [DOI] [PubMed] [Google Scholar]
- FACCHINETTI V, OUYANG W, WEI H, SOTO N, LAZORCHAK A, GOULD C, LOWRY C, NEWTON AC, MAO Y, MIAO RQ, SESSA WC, QIN J, ZHANG P, SU B, JACINTO E. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J. 2008;27:1932–43. doi: 10.1038/emboj.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAN QW, CHENG C, KNIGHT ZA, HAAS-KOGAN D, STOKOE D, JAMES CD, MCCORMICK F, SHOKAT KM, WEISS WA. EGFR signals to mTOR through PKC and independently of Akt in glioma. Sci Signal. 2009;2:ra4. doi: 10.1126/scisignal.2000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDMAN ME, APSEL B, UOTILA A, LOEWITH R, KNIGHT ZA, RUGGERO D, SHOKAT KM. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENTON TR, GOUT IT. Functions and regulation of the 70kDa ribosomal S6 kinases. Int J Biochem Cell Biol. 2011;43:47–59. doi: 10.1016/j.biocel.2010.09.018. [DOI] [PubMed] [Google Scholar]
- FINGAR DC, BLENIS J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–71. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- FOSTER KG, ACOSTA-JAQUEZ HA, ROMEO Y, EKIM B, SOLIMAN GA, CARRIERE A, ROUX PP, BALLIF BA, FINGAR DC. Regulation of mTOR complex 1 (mTORC1) by raptor Ser863 and multisite phosphorylation. J Biol Chem. 2010;285:80–94. doi: 10.1074/jbc.M109.029637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAME S, COHEN P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIAS MA, THOREEN CC, JAFFE JD, SCHRODER W, SCULLEY T, CARR SA, SABATINI DM. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16:1865–70. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- FUJITA N, SATO S, ISHIDA A, TSURUO T. Involvement of Hsp90 in signaling and stability of 3-phosphoinositide-dependent kinase-1. J Biol Chem. 2002;277:10346–53. doi: 10.1074/jbc.M106736200. [DOI] [PubMed] [Google Scholar]
- GAO D, WAN L, INUZUKA H, BERG AH, TSENG A, ZHAI B, SHAIK S, BENNETT E, TRON AE, GASSER JA, LAU A, GYGI SP, HARPER JW, DECAPRIO JA, TOKER A, WEI W. Rictor forms a complex with Cullin-1 to promote SGK1 ubiquitination and destruction. Mol Cell. 2010;39:797–808. doi: 10.1016/j.molcel.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAO T, NEWTON AC. The turn motif is a phosphorylation switch that regulates the binding of Hsp70 to protein kinase C. J Biol Chem. 2002;277:31585–92. doi: 10.1074/jbc.M204335200. [DOI] [PubMed] [Google Scholar]
- GARCIA-MARTINEZ JM, ALESSI DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Biochem J. 2008;416:375–85. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- GHOSH D, SRIVASTAVA GP, XU D, SCHULZ LC, ROBERTS RM. A link between SIN1 (MAPKAP1) and poly(rC) binding protein 2 (PCBP2) in counteracting environmental stress. Proc Natl Acad Sci U S A. 2008;105:11673–8. doi: 10.1073/pnas.0803182105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLD MG, BARFORD D, KOMANDER D. Lining the pockets of kinases and phosphatases. Curr Opin Struct Biol. 2006;16:693–701. doi: 10.1016/j.sbi.2006.10.006. [DOI] [PubMed] [Google Scholar]
- GOULD CM, KANNAN N, TAYLOR SS, NEWTON AC. The chaperones Hsp90 and Cdc37 mediate the maturation and stabilization of protein kinase C through a conserved PXXP motif in the C-terminal tail. J Biol Chem. 2009;284:4921–35. doi: 10.1074/jbc.M808436200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSSO S, VOLTA V, SALA LA, VIETRI M, MARCHISIO PC, RON D, BIFFO S. PKCbetaII modulates translation independently from mTOR and through RACK1. Biochem J. 2008a;415:77–85. doi: 10.1042/BJ20080463. [DOI] [PubMed] [Google Scholar]
- GROSSO S, VOLTA V, VIETRI M, GORRINI C, MARCHISIO PC, BIFFO S. Eukaryotic ribosomes host PKC activity. Biochem Biophys Res Commun. 2008b;376:65–9. doi: 10.1016/j.bbrc.2008.08.118. [DOI] [PubMed] [Google Scholar]
- GUAL P, LE MARCHAND-BRUSTEL Y, TANTI JF. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie. 2005;87:99–109. doi: 10.1016/j.biochi.2004.10.019. [DOI] [PubMed] [Google Scholar]
- GUERTIN DA, SABATINI DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- GUERTIN DA, STEVENS DM, SAITOH M, KINKEL S, CROSBY K, SHEEN JH, MULLHOLLAND DJ, MAGNUSON MA, WU H, SABATINI DM. mTOR complex 2 is required for the development of prostate cancer induced by Pten loss in mice. Cancer Cell. 2009;15:148–59. doi: 10.1016/j.ccr.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUERTIN DA, STEVENS DM, THOREEN CC, BURDS AA, KALAANY NY, MOFFAT J, BROWN M, FITZGERALD KJ, SABATINI DM. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–71. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- GWINN DM, SHACKELFORD DB, EGAN DF, MIHAYLOVA MM, MERY A, VASQUEZ DS, TURK BE, SHAW RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANNAN KM, BRANDENBURGER Y, JENKINS A, SHARKEY K, CAVANAUGH A, ROTHBLUM L, MOSS T, POORTINGA G, MCARTHUR GA, PEARSON RB, HANNAN RD. mTOR-dependent regulation of ribosomal gene transcription requires S6K1 and is mediated by phosphorylation of the carboxy-terminal activation domain of the nucleolar transcription factor UBF. Mol Cell Biol. 2003;23:8862–77. doi: 10.1128/MCB.23.23.8862-8877.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARA K, MARUKI Y, LONG X, YOSHINO K, OSHIRO N, HIDAYAT S, TOKUNAGA C, AVRUCH J, YONEZAWA K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–89. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- HARRINGTON LS, FINDLAY GM, GRAY A, TOLKACHEVA T, WIGFIELD S, REBHOLZ H, BARNETT J, LESLIE NR, CHENG S, SHEPHERD PR, GOUT I, DOWNES CP, LAMB RF. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–23. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUGE C, ANTAL TL, HIRSCHBERG D, DOEHN U, THORUP K, IDRISSOVA L, HANSEN K, JENSEN ON, JORGENSEN TJ, BIONDI RM, FRODIN M. Mechanism for activation of the growth factor-activated AGC kinases by turn motif phosphorylation. Embo J. 2007;26:2251–61. doi: 10.1038/sj.emboj.7601682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAWLEY SA, BOUDEAU J, REID JL, MUSTARD KJ, UDD L, MAKELA TP, ALESSI DR, HARDIE DG. Complexes between the LKB1 tumor suppressor, STRADalpha/beta and MO25alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRAOKA D, OKUMURA E, KISHIMOTO T. Turn motif phosphorylation negatively regulates activation loop phosphorylation in Akt. Oncogene. 2011 doi: 10.1038/onc.2011.155. [DOI] [PubMed] [Google Scholar]
- HOLZ MK, BLENIS J. Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. J Biol Chem. 2005;280:26089–93. doi: 10.1074/jbc.M504045200. [DOI] [PubMed] [Google Scholar]
- HONG F, LARREA MD, DOUGHTY C, KWIATKOWSKI DJ, SQUILLACE R, SLINGERLAND JM. mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol Cell. 2008;30:701–11. doi: 10.1016/j.molcel.2008.04.027. [DOI] [PubMed] [Google Scholar]
- HOREJSI Z, TAKAI H, ADELMAN CA, COLLIS SJ, FLYNN H, MASLEN S, SKEHEL JM, DE LANGE T, BOULTON SJ. CK2 phospho-dependent binding of R2TP complex to TEL2 is essential for mTOR and SMG1 stability. Mol Cell. 2010;39:839–50. doi: 10.1016/j.molcel.2010.08.037. [DOI] [PubMed] [Google Scholar]
- HRESKO RC, MUECKLER M. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem. 2005;280:40406–16. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- HSU PP, KANG SA, RAMESEDER J, ZHANG Y, OTTINA KA, LIM D, PETERSON TR, CHOI Y, GRAY NS, YAFFE MB, MARTO JA, SABATINI DM. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 2011;332:1317–22. doi: 10.1126/science.1199498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG J, MANNING BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412:179–90. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IKENOUE T, INOKI K, YANG Q, ZHOU X, GUAN KL. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 2008;27:1919–31. doi: 10.1038/emboj.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOKI K, LI Y, XU T, GUAN KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003a;17:1829–34. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOKI K, LI Y, ZHU T, WU J, GUAN KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–57. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- INOKI K, ZHU T, GUAN KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003b;115:577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- JACINTO E, FACCHINETTI V, LIU D, SOTO N, WEI S, JUNG SY, HUANG Q, QIN J, SU B. SIN1/MIP1 Maintains rictor-mTOR Complex Integrity and Regulates Akt Phosphorylation and Substrate Specificity. Cell. 2006;127:125–37. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- JACINTO E, HALL MN. Tor signalling in bugs, brain and brawn. Nat Rev Mol Cell Biol. 2003;4:117–26. doi: 10.1038/nrm1018. [DOI] [PubMed] [Google Scholar]
- JANES MR, LIMON JJ, SO L, CHEN J, LIM RJ, CHAVEZ MA, VU C, LILLY MB, MALLYA S, ONG ST, KONOPLEVA M, MARTIN MB, REN P, LIU Y, ROMMEL C, FRUMAN DA. Effective and selective targeting of leukemia cells using a TORC1/2 kinase inhibitor. Nat Med. 2010;16:205–13. doi: 10.1038/nm.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JASTRZEBSKI K, HANNAN KM, HOUSE CM, HUNG SS, PEARSON RB, HANNAN RD. A phospho-proteomic screen identifies novel S6K1 and mTORC1 substrates revealing additional complexity in the signaling network regulating cell growth. Cell Signal. 2011;23:1338–47. doi: 10.1016/j.cellsig.2011.03.016. [DOI] [PubMed] [Google Scholar]
- JIANG T, QIU Y. Interaction between Src and a C-terminal proline-rich motif of Akt is required for Akt activation. J Biol Chem. 2003;278:15789–93. doi: 10.1074/jbc.M212525200. [DOI] [PubMed] [Google Scholar]
- JOHNSTONE CN, CASTELLVI-BEL S, CHANG LM, SUNG RK, BOWSER MJ, PIQUE JM, CASTELLS A, RUSTGI AK. PRR5 encodes a conserved proline-rich protein predominant in kidney: analysis of genomic organization, expression, and mutation status in breast and colorectal carcinomas. Genomics. 2005;85:338–51. doi: 10.1016/j.ygeno.2004.11.002. [DOI] [PubMed] [Google Scholar]
- JOUNG SM, PARK ZY, RANI S, TAKEUCHI O, AKIRA S, LEE JY. Akt contributes to activation of the TRIF-dependent signaling pathways of TLRs by interacting with TANK-binding kinase 1. J Immunol. 2011;186:499–507. doi: 10.4049/jimmunol.0903534. [DOI] [PubMed] [Google Scholar]
- JULIEN LA, CARRIERE A, MOREAU J, ROUX PP. mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling. Mol Cell Biol. 2010;30:908–21. doi: 10.1128/MCB.00601-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAIZUKA T, HARA T, OSHIRO N, KIKKAWA U, YONEZAWA K, TAKEHANA K, IEMURA S, NATSUME T, MIZUSHIMA N. Tti1 and Tel2 are critical factors in mammalian target of rapamycin complex assembly. J Biol Chem. 2010;285:20109–16. doi: 10.1074/jbc.M110.121699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMADA Y, FUJIOKA Y, SUZUKI NN, INAGAKI F, WULLSCHLEGER S, LOEWITH R, HALL MN, OHSUMI Y. Tor2 directly phosphorylates the AGC kinase Ypk2 to regulate actin polarization. Mol Cell Biol. 2005;25:7239–48. doi: 10.1128/MCB.25.16.7239-7248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANNAN N, HASTE N, TAYLOR SS, NEUWALD AF. The hallmark of AGC kinase functional divergence is its C-terminal tail, a cis-acting regulatory module. Proc Natl Acad Sci U S A. 2007;104:1272–7. doi: 10.1073/pnas.0610251104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATAJISTO P, VALLENIUS T, VAAHTOMERI K, EKMAN N, UDD L, TIAINEN M, MAKELA TP. The LKB1 tumor suppressor kinase in human disease. Biochim Biophys Acta. 2007;1775:63–75. doi: 10.1016/j.bbcan.2006.08.003. [DOI] [PubMed] [Google Scholar]
- KESHWANI MM, VON DAAKE S, NEWTON AC, HARRIS TK, TAYLOR SS. Hydrophobic Motif Phosphorylation Is Not Required for Activation Loop Phosphorylation of p70 Ribosomal Protein S6 Kinase 1 (S6K1) J Biol Chem. 2011;286:23552–8. doi: 10.1074/jbc.M111.258004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM DH, SARBASSOV DOS D, ALI SM, KING JE, LATEK RR, ERDJUMENT-BROMAGE H, TEMPST P, SABATINI DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–75. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- KIM DH, SARBASSOV DOS D, ALI SM, LATEK RR, GUNTUR KV, ERDJUMENT-BROMAGE H, TEMPST P, SABATINI DM. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003;11:895–904. doi: 10.1016/s1097-2765(03)00114-x. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI T, COHEN P. Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem J. 1999;339 (Pt 2):319–28. [PMC free article] [PubMed] [Google Scholar]
- KOBAYASHI T, DEAK M, MORRICE N, COHEN P. Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem J. 1999;344(Pt 1):189–97. [PMC free article] [PubMed] [Google Scholar]
- LAHN M, KOHLER G, SUNDELL K, SU C, LI S, PATERSON BM, BUMOL TF. Protein kinase C alpha expression in breast and ovarian cancer. Oncology. 2004;67:1–10. doi: 10.1159/000080279. [DOI] [PubMed] [Google Scholar]
- LANG F, BOHMER C, PALMADA M, SEEBOHM G, STRUTZ-SEEBOHM N, VALLON V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151–78. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- LANGLAIS P, YI Z, MANDARINO LJ. The identification of raptor as a substrate for p44/42 MAPK. Endocrinology. 2011;152:1264–73. doi: 10.1210/en.2010-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAPLANTE M, SABATINI DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–94. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAZORCHAK AS, LIU D, FACCHINETTI V, DI LORENZO A, SESSA WC, SCHATZ DG, SU B. Sin1-mTORC2 suppresses rag and il7r gene expression through Akt2 in B cells. Mol Cell. 2010;39:433–43. doi: 10.1016/j.molcel.2010.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE K, GUDAPATI P, DRAGOVIC S, SPENCER C, JOYCE S, KILLEEN N, MAGNUSON MA, BOOTHBY M. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–53. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEITGES M, PLOMANN M, STANDAERT ML, BANDYOPADHYAY G, SAJAN MP, KANOH Y, FARESE RV. Knockout of PKC alpha enhances insulin signaling through PI3K. Mol Endocrinol. 2002;16:847–58. doi: 10.1210/mend.16.4.0809. [DOI] [PubMed] [Google Scholar]
- LESEUX L, LAURENT G, LAURENT C, RIGO M, BLANC A, OLIVE D, BEZOMBES C. PKC zeta mTOR pathway: a new target for rituximab therapy in follicular lymphoma. Blood. 2008;111:285–91. doi: 10.1182/blood-2007-04-085092. [DOI] [PubMed] [Google Scholar]
- LI Y, SOOS TJ, LI X, WU J, DEGENNARO M, SUN X, LITTMAN DR, BIRNBAUM MJ, POLAKIEWICZ RD. Protein kinase C Theta inhibits insulin signaling by phosphorylating IRS1 at Ser(1101) J Biol Chem. 2004;279:45304–7. doi: 10.1074/jbc.C400186200. [DOI] [PubMed] [Google Scholar]
- LIBERMAN Z, PLOTKIN B, TENNENBAUM T, ELDAR-FINKELMAN H. Coordinated phosphorylation of insulin receptor substrate-1 by glycogen synthase kinase-3 and protein kinase C betaII in the diabetic fat tissue. Am J Physiol Endocrinol Metab. 2008;294:E1169–77. doi: 10.1152/ajpendo.00050.2008. [DOI] [PubMed] [Google Scholar]
- LIU X, ZHENG XF. Endoplasmic reticulum and Golgi localization sequences for mammalian target of rapamycin. Mol Biol Cell. 2007;18:1073–82. doi: 10.1091/mbc.E06-05-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOEWITH R, JACINTO E, WULLSCHLEGER S, LORBERG A, CRESPO JL, BONENFANT D, OPPLIGER W, JENOE P, HALL MN. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- LOVEJOY CA, CORTEZ D. Common mechanisms of PIKK regulation. DNA Repair (Amst) 2009;8:1004–8. doi: 10.1016/j.dnarep.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LU M, WANG J, JONES KT, IV, ES HE, FELDMAN ME, YAO LJ, SHOKAT KM, ASHRAFI K, PEARCE D. mTOR complex-2 activates ENaC by phosphorylating SGK1. J Am Soc Nephrol. 2010;21:811–8. doi: 10.1681/ASN.2009111168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MA L, CHEN Z, ERDJUMENT-BROMAGE H, TEMPST P, PANDOLFI PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–93. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- MA XM, BLENIS J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–18. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- MA XM, YOON SO, RICHARDSON CJ, JULICH K, BLENIS J. SKAR links pre-mRNA splicing to mTOR/S6K1-mediated enhanced translation efficiency of spliced mRNAs. Cell. 2008;133:303–13. doi: 10.1016/j.cell.2008.02.031. [DOI] [PubMed] [Google Scholar]
- MAKINO C, SANO Y, SHINAGAWA T, MILLAR JB, ISHII S. Sin1 binds to both ATF-2 and p38 and enhances ATF-2-dependent transcription in an SAPK signaling pathway. Genes Cells. 2006;11:1239–51. doi: 10.1111/j.1365-2443.2006.01016.x. [DOI] [PubMed] [Google Scholar]
- MANNING BD, CANTLEY LC. Rheb fills a GAP between TSC and TOR. Trends Biochem Sci. 2003;28:573–6. doi: 10.1016/j.tibs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- MANNING BD, CANTLEY LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANNING BD, TEE AR, LOGSDON MN, BLENIS J, CANTLEY LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002a;10:151–62. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- MANNING G, WHYTE DB, MARTINEZ R, HUNTER T, SUDARSANAM S. The protein kinase complement of the human genome. Science. 2002b;298:1912–34. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- MARTIN J, MASRI J, BERNATH A, NISHIMURA RN, GERA J. Hsp70 associates with Rictor and is required for mTORC2 formation and activity. Biochem Biophys Res Commun. 2008;372:578–83. doi: 10.1016/j.bbrc.2008.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASRI J, BERNATH A, MARTIN J, JO OD, VARTANIAN R, FUNK A, GERA J. mTORC2 activity is elevated in gliomas and promotes growth and cell motility via overexpression of rictor. Cancer Res. 2007;67:11712–20. doi: 10.1158/0008-5472.CAN-07-2223. [DOI] [PubMed] [Google Scholar]
- MCDONALD PC, OLOUMI A, MILLS J, DOBREVA I, MAIDAN M, GRAY V, WEDERELL ED, BALLY MB, FOSTER LJ, DEDHAR S. Rictor and integrin-linked kinase interact and regulate Akt phosphorylation and cancer cell survival. Cancer Res. 2008;68:1618–24. doi: 10.1158/0008-5472.CAN-07-5869. [DOI] [PubMed] [Google Scholar]
- MESSERSCHMIDT A, MACIEIRA S, VELARDE M, BADEKER M, BENDA C, JESTEL A, BRANDSTETTER H, NEUEFEIND T, BLAESSE M. Crystal structure of the catalytic domain of human atypical protein kinase C-iota reveals interaction mode of phosphorylation site in turn motif. J Mol Biol. 2005;352:918–31. doi: 10.1016/j.jmb.2005.07.060. [DOI] [PubMed] [Google Scholar]
- MOESCHEL K, BECK A, WEIGERT C, LAMMERS R, KALBACHER H, VOELTER W, SCHLEICHER ED, HARING HU, LEHMANN R. Protein kinase C-zeta-induced phosphorylation of Ser318 in insulin receptor substrate-1 (IRS-1) attenuates the interaction with the insulin receptor and the tyrosine phosphorylation of IRS-1. J Biol Chem. 2004;279:25157–63. doi: 10.1074/jbc.M402477200. [DOI] [PubMed] [Google Scholar]
- MOSER BA, DENNIS PB, PULLEN N, PEARSON RB, WILLIAMSON NA, WETTENHALL RE, KOZMA SC, THOMAS G. Dual requirement for a newly identified phosphorylation site in p70s6k. Mol Cell Biol. 1997;17:5648–55. doi: 10.1128/mcb.17.9.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUKHOPADHYAY NK, PRICE DJ, KYRIAKIS JM, PELECH S, SANGHERA J, AVRUCH J. An array of insulin-activated, proline-directed serine/threonine protein kinases phosphorylate the p70 S6 kinase. J Biol Chem. 1992;267:3325–35. [PubMed] [Google Scholar]
- NAWARATNE R, GRAY A, JORGENSEN CH, DOWNES CP, SIDDLE K, SETHI JK. Regulation of insulin receptor substrate 1 pleckstrin homology domain by protein kinase C: role of serine 24 phosphorylation. Mol Endocrinol. 2006;20:1838–52. doi: 10.1210/me.2005-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem J. 2003;370:361–71. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON AC. Protein kinase C: poised to signal. Am J Physiol Endocrinol Metab. 2010;298:E395–402. doi: 10.1152/ajpendo.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIKAWA K, TOKER A, JOHANNES FJ, SONGYANG Z, CANTLEY LC. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J Biol Chem. 1997;272:952–60. doi: 10.1074/jbc.272.2.952. [DOI] [PubMed] [Google Scholar]
- OH WJ, WU CC, KIM SJ, FACCHINETTI V, JULIEN LA, FINLAN M, ROUX PP, SU B, JACINTO E. mTORC2 can associate with ribosomes to promote cotranslational phosphorylation and stability of nascent Akt polypeptide. Embo J. 2010;29:3939–51. doi: 10.1038/emboj.2010.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHNE Y, TAKAHARA T, HATAKEYAMA R, MATSUZAKI T, NODA M, MIZUSHIMA N, MAEDA T. Isolation of hyperactive mutants of mammalian target of rapamycin. J Biol Chem. 2008;283:31861–70. doi: 10.1074/jbc.M801546200. [DOI] [PubMed] [Google Scholar]
- OU YH, TORRES M, RAM R, FORMSTECHER E, ROLAND C, CHENG T, BREKKEN R, WURZ R, TASKER A, POLVERINO T, TAN SL, WHITE MA. TBK1 directly engages Akt/PKB survival signaling to support oncogenic transformation. Mol Cell. 2011;41:458–70. doi: 10.1016/j.molcel.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZCAN U, OZCAN L, YILMAZ E, DUVEL K, SAHIN M, MANNING BD, HOTAMISLIGIL GS. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell. 2008;29:541–51. doi: 10.1016/j.molcel.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANASYUK G, NEMAZANYY I, ZHYVOLOUP A, FILONENKO V, DAVIES D, ROBSON M, PEDLEY RB, WATERFIELD M, GOUT I. mTORbeta splicing isoform promotes cell proliferation and tumorigenesis. J Biol Chem. 2009;284:30807–14. doi: 10.1074/jbc.M109.056085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAREKH D, ZIEGLER W, YONEZAWA K, HARA K, PARKER PJ. Mammalian TOR controls one of two kinase pathways acting upon nPKCdelta and nPKCepsilon. J Biol Chem. 1999;274:34758–64. doi: 10.1074/jbc.274.49.34758. [DOI] [PubMed] [Google Scholar]
- PARK J, LEONG ML, BUSE P, MAIYAR AC, FIRESTONE GL, HEMMINGS BA. Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway. Embo J. 1999;18:3024–33. doi: 10.1093/emboj/18.11.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARTOVIAN C, JU R, ZHUANG ZW, MARTIN KA, SIMONS M. Syndecan-4 regulates subcellular localization of mTOR Complex2 and Akt activation in a PKCalpha-dependent manner in endothelial cells. Mol Cell. 2008;32:140–9. doi: 10.1016/j.molcel.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAZ K, LIU YF, SHORER H, HEMI R, LEROITH D, QUAN M, KANETY H, SEGER R, ZICK Y. Phosphorylation of insulin receptor substrate-1 (IRS-1) by protein kinase B positively regulates IRS-1 function. J Biol Chem. 1999;274:28816–22. doi: 10.1074/jbc.274.40.28816. [DOI] [PubMed] [Google Scholar]
- PEARCE LR, HUANG X, BOUDEAU J, PAWLOWSKI R, WULLSCHLEGER S, DEAK M, IBRAHIM AF, GOURLAY R, MAGNUSON MA, ALESSI DR. Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem J. 2007;405:513–22. doi: 10.1042/BJ20070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEARSON RB, DENNIS PB, HAN JW, WILLIAMSON NA, KOZMA SC, WETTENHALL RE, THOMAS G. The principal target of rapamycin-induced p70s6k inactivation is a novel phosphorylation site within a conserved hydrophobic domain. Embo J. 1995;14:5279–87. doi: 10.1002/j.1460-2075.1995.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENDE M, UM SH, MIEULET V, STICKER M, GOSS VL, MESTAN J, MUELLER M, FUMAGALLI S, KOZMA SC, THOMAS G. S6K1(−/−)/S6K2(−/−) mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol Cell Biol. 2004;24:3112–24. doi: 10.1128/MCB.24.8.3112-3124.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERSAD S, ATTWELL S, GRAY V, MAWJI N, DENG JT, LEUNG D, YAN J, SANGHERA J, WALSH MP, DEDHAR S. Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343. J Biol Chem. 2001;276:27462–9. doi: 10.1074/jbc.M102940200. [DOI] [PubMed] [Google Scholar]
- PETERSON RT, BEAL PA, COMB MJ, SCHREIBER SL. FKBP12-rapamycin-associated protein (FRAP) autophosphorylates at serine 2481 under translationally repressive conditions. J Biol Chem. 2000;275:7416–23. doi: 10.1074/jbc.275.10.7416. [DOI] [PubMed] [Google Scholar]
- PETERSON TR, LAPLANTE M, THOREEN CC, SANCAK Y, KANG SA, KUEHL WM, GRAY NS, SABATINI DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–86. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLAK P, CYBULSKI N, FEIGE JN, AUWERX J, RUEGG MA, HALL MN. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab. 2008;8:399–410. doi: 10.1016/j.cmet.2008.09.003. [DOI] [PubMed] [Google Scholar]
- POLAK P, HALL MN. mTOR and the control of whole body metabolism. Curr Opin Cell Biol. 2009;21:209–18. doi: 10.1016/j.ceb.2009.01.024. [DOI] [PubMed] [Google Scholar]
- PROUD CG. Signalling to translation: how signal transduction pathways control the protein synthetic machinery. Biochem J. 2007;403:217–34. doi: 10.1042/BJ20070024. [DOI] [PubMed] [Google Scholar]
- PULLEN N, THOMAS G. The modular phosphorylation and activation of p70s6k. FEBS Lett. 1997;410:78–82. doi: 10.1016/s0014-5793(97)00323-2. [DOI] [PubMed] [Google Scholar]
- QIAN SB, ZHANG X, SUN J, BENNINK JR, YEWDELL JW, PATTERSON C. mTORC1 links protein quality and quantity control by sensing chaperone availability. J Biol Chem. 2010;285:27385–95. doi: 10.1074/jbc.M110.120295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMIREZ-RANGEL I, BRACHO-VALDES I, VAZQUEZ-MACIAS A, CARRETERO-ORTEGA J, REYES-CRUZ G, VAZQUEZ-PRADO J. Regulation of mTORC1 complex assembly and signaling by GRp58/ERp57. Mol Cell Biol. 2011;31:1657–71. doi: 10.1128/MCB.00824-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMIREZ-VALLE F, BADURA ML, BRAUNSTEIN S, NARASIMHAN M, SCHNEIDER RJ. Mitotic raptor promotes mTORC1 activity, G(2)/M cell cycle progression, and internal ribosome entry site-mediated mRNA translation. Mol Cell Biol. 2010;30:3151–64. doi: 10.1128/MCB.00322-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAUGHT B, PEIRETTI F, GINGRAS AC, LIVINGSTONE M, SHAHBAZIAN D, MAYEUR GL, POLAKIEWICZ RD, SONENBERG N, HERSHEY JW. Phosphorylation of eucaryotic translation initiation factor 4B Ser422 is modulated by S6 kinases. Embo J. 2004;23:1761–1769. doi: 10.1038/sj.emboj.7600193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROFFEY J, ROSSE C, LINCH M, HIBBERT A, MCDONALD NQ, PARKER PJ. Protein kinase C intervention: the state of play. Curr Opin Cell Biol. 2009;21:268–79. doi: 10.1016/j.ceb.2009.01.019. [DOI] [PubMed] [Google Scholar]
- ROMANELLI A, DREISBACH VC, BLENIS J. Characterization of phosphatidylinositol 3-kinase-dependent phosphorylation of the hydrophobic motif site Thr(389) in p70 S6 kinase 1. J Biol Chem. 2002;277:40281–9. doi: 10.1074/jbc.M205168200. [DOI] [PubMed] [Google Scholar]
- ROMANELLI A, MARTIN KA, TOKER A, BLENIS J. p70 S6 kinase is regulated by protein kinase Czeta and participates in a phosphoinositide 3-kinase-regulated signalling complex. Mol Cell Biol. 1999;19:2921–8. doi: 10.1128/mcb.19.4.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSNER M, FREILINGER A, HENGSTSCHLAGER M. The tuberous sclerosis genes and regulation of the cyclin-dependent kinase inhibitor p27. Mutat Res. 2006;613:10–6. doi: 10.1016/j.mrrev.2006.03.001. [DOI] [PubMed] [Google Scholar]
- ROSNER M, FREILINGER A, HENGSTSCHLAGER M. Akt regulates nuclear/cytoplasmic localization of tuberin. Oncogene. 2007;26:521–31. doi: 10.1038/sj.onc.1209812. [DOI] [PubMed] [Google Scholar]
- ROSNER M, HENGSTSCHLAGER M. Cytoplasmic and nuclear distribution of the protein complexes mTORC1 and mTORC2: rapamycin triggers dephosphorylation and delocalization of the mTORC2 components rictor and sin1. Hum Mol Genet. 2008;17:2934–48. doi: 10.1093/hmg/ddn192. [DOI] [PubMed] [Google Scholar]
- ROSSE C, LINCH M, KERMORGANT S, CAMERON AJ, BOECKELER K, PARKER PJ. PKC and the control of localized signal dynamics. Nat Rev Mol Cell Biol. 2010;11:103–12. doi: 10.1038/nrm2847. [DOI] [PubMed] [Google Scholar]
- ROUX PP, BALLIF BA, ANJUM R, GYGI SP, BLENIS J. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci U S A. 2004;101:13489–94. doi: 10.1073/pnas.0405659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROUX PP, SHAHBAZIAN D, VU H, HOLZ MK, COHEN MS, TAUNTON J, SONENBERG N, BLENIS J. RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J Biol Chem. 2007;282:14056–64. doi: 10.1074/jbc.M700906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUVINSKY I, MEYUHAS O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci. 2006;31:342–8. doi: 10.1016/j.tibs.2006.04.003. [DOI] [PubMed] [Google Scholar]
- SAITOH M, PULLEN N, BRENNAN P, CANTRELL D, DENNIS PB, THOMAS G. Regulation of an activated s6 kinase 1 variant reveals a novel Mammalian target of rapamycin phosphorylation site. J Biol Chem. 2002;277:20104–12. doi: 10.1074/jbc.M201745200. [DOI] [PubMed] [Google Scholar]
- SAKODA H, GOTOH Y, KATAGIRI H, KUROKAWA M, ONO H, ONISHI Y, ANAI M, OGIHARA T, FUJISHIRO M, FUKUSHIMA Y, ABE M, SHOJIMA N, KIKUCHI M, OKA Y, HIRAI H, ASANO T. Differing roles of Akt and serum- and glucocorticoid-regulated kinase in glucose metabolism, DNA synthesis, and oncogenic activity. J Biol Chem. 2003;278:25802–7. doi: 10.1074/jbc.M301127200. [DOI] [PubMed] [Google Scholar]
- SAMUEL VT, LIU ZX, WANG A, BEDDOW SA, GEISLER JG, KAHN M, ZHANG XM, MONIA BP, BHANOT S, SHULMAN GI. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 2007;117:739–45. doi: 10.1172/JCI30400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANCAK Y, BAR-PELED L, ZONCU R, MARKHARD AL, NADA S, SABATINI DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANCAK Y, PETERSON TR, SHAUL YD, LINDQUIST RA, THOREEN CC, BAR-PELED L, SABATINI DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANCAK Y, THOREEN CC, PETERSON TR, LINDQUIST RA, KANG SA, SPOONER E, CARR SA, SABATINI DM. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–15. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- SAPKOTA GP, KIELOCH A, LIZCANO JM, LAIN S, ARTHUR JS, WILLIAMS MR, MORRICE N, DEAK M, ALESSI DR. Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser431 by p90(RSK) and cAMP-dependent protein kinase, but not its farnesylation at Cys(433), is essential for LKB1 to suppress cell vrowth. J Biol Chem. 2001;276:19469–82. doi: 10.1074/jbc.M009953200. [DOI] [PubMed] [Google Scholar]
- SARBASSOV D, ALI SM, KIM DH, GUERTIN DA, LATEK RR, ERDJUMENT-BROMAGE H, TEMPST P, SABATINI DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- SARBASSOV DD, ALI SM, SENGUPTA S, SHEEN JH, HSU PP, BAGLEY AF, MARKHARD AL, SABATINI DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–68. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- SARBASSOV DD, GUERTIN DA, ALI SM, SABATINI DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- SARBASSOV DD, SABATINI DM. Redox regulation of the nutrient-sensitive raptor-mTOR pathway and complex. J Biol Chem. 2005;280:39505–9. doi: 10.1074/jbc.M506096200. [DOI] [PubMed] [Google Scholar]
- SAUCEDO LJ, GAO X, CHIARELLI DA, LI L, PAN D, EDGAR BA. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat Cell Biol. 2003;5:566–71. doi: 10.1038/ncb996. [DOI] [PubMed] [Google Scholar]
- SCHALM SS, BLENIS J. Identification of a Conserved Motif Required for mTOR Signaling. Curr Biol. 2002;12:632–9. doi: 10.1016/s0960-9822(02)00762-5. [DOI] [PubMed] [Google Scholar]
- SCHALM SS, TEE AR, BLENIS J. Characterization of a conserved C-terminal motif (RSPRR) in ribosomal protein S6 kinase 1 required for its mammalian target of rapamycin-dependent regulation. J Biol Chem. 2005;280:11101–6. doi: 10.1074/jbc.M413995200. [DOI] [PubMed] [Google Scholar]
- SCHLEICH S, TELEMAN AA. Akt phosphorylates both Tsc1 and Tsc2 in Drosophila, but neither phosphorylation is required for normal animal growth. PLoS One. 2009;4:e6305. doi: 10.1371/journal.pone.0006305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHRODER W, BUSHELL G, SCULLEY T. The human stress-activated protein kinase-interacting 1 gene encodes JNK-binding proteins. Cell Signal. 2005;17:761–7. doi: 10.1016/j.cellsig.2004.10.015. [DOI] [PubMed] [Google Scholar]
- SCHRODER WA, BUCK M, CLOONAN N, HANCOCK JF, SUHRBIER A, SCULLEY T, BUSHELL G. Human Sin1 contains Ras-binding and pleckstrin homology domains and suppresses Ras signalling. Cell Signal. 2007;19:1279–89. doi: 10.1016/j.cellsig.2007.01.013. [DOI] [PubMed] [Google Scholar]
- SEKULIC A, HUDSON CC, HOMME JL, YIN P, OTTERNESS DM, KARNITZ LM, ABRAHAM RT. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60:3504–13. [PubMed] [Google Scholar]
- SHAH OJ, HUNTER T. Critical role of T-loop and H-motif phosphorylation in the regulation of S6 kinase 1 by the tuberous sclerosis complex. J Biol Chem. 2004;279:20816–23. doi: 10.1074/jbc.M400957200. [DOI] [PubMed] [Google Scholar]
- SHAH OJ, HUNTER T. Turnover of the active fraction of IRS1 involves raptor-mTOR- and S6K1-dependent serine phosphorylation in cell culture models of tuberous sclerosis. Mol Cell Biol. 2006;26:6425–34. doi: 10.1128/MCB.01254-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAHBAZIAN D, ROUX PP, MIEULET V, COHEN MS, RAUGHT B, TAUNTON J, HERSHEY JW, BLENIS J, PENDE M, SONENBERG N. The mTOR/PI3K and MAPK pathways converge on eIF4B to control its phosphorylation and activity. Embo J. 2006;25:2781–91. doi: 10.1038/sj.emboj.7601166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAW RJ, BARDEESY N, MANNING BD, LOPEZ L, KOSMATKA M, DEPINHO RA, CANTLEY LC. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–9. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- SHIOTA C, WOO JT, LINDNER J, SHELTON KD, MAGNUSON MA. Multiallelic Disruption of the rictor Gene in Mice Reveals that mTOR Complex 2 Is Essential for Fetal Growth and Viability. Dev Cell. 2006;11:583–9. doi: 10.1016/j.devcel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- SOLIMAN GA, ACOSTA-JAQUEZ HA, DUNLOP EA, EKIM B, MAJ NE, TEE AR, FINGAR DC. mTOR Ser-2481 autophosphorylation monitors mTORC-specific catalytic activity and clarifies rapamycin mechanism of action. J Biol Chem. 2010;285:7866–79. doi: 10.1074/jbc.M109.096222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONENBERG N, HINNEBUSCH AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–45. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONNENBURG ED, GAO T, NEWTON AC. The phosphoinositide-dependent kinase, PDK-1, phosphorylates conventional protein kinase C isozymes by a mechanism that is independent of phosphoinositide 3-kinase. J Biol Chem. 2001;276:45289–97. doi: 10.1074/jbc.M107416200. [DOI] [PubMed] [Google Scholar]
- STANDAERT ML, BANDYOPADHYAY G, GALLOWAY L, SOTO J, ONO Y, KIKKAWA U, FARESE RV, LEITGES M. Effects of knockout of the protein kinase C beta gene on glucose transport and glucose homeostasis. Endocrinology. 1999;140:4470–7. doi: 10.1210/endo.140.10.7073. [DOI] [PubMed] [Google Scholar]
- SUN Y, CHEN J. mTOR signaling: PLD takes center stage. Cell Cycle. 2008;7:3118–23. doi: 10.4161/cc.7.20.6881. [DOI] [PubMed] [Google Scholar]
- SUN Y, FANG Y, YOON MS, ZHANG C, ROCCIO M, ZWARTKRUIS FJ, ARMSTRONG M, BROWN HA, CHEN J. Phospholipase D1 is an effector of Rheb in the mTOR pathway. Proc Natl Acad Sci U S A. 2008;105:8286–91. doi: 10.1073/pnas.0712268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHARA T, HARA K, YONEZAWA K, SORIMACHI H, MAEDA T. Nutrient-dependent multimerization of the mammalian target of rapamycin through the N-terminal HEAT repeat region. J Biol Chem. 2006;281:28605–14. doi: 10.1074/jbc.M606087200. [DOI] [PubMed] [Google Scholar]
- TAKAI H, WANG RC, TAKAI KK, YANG H, DE LANGE T. Tel2 regulates the stability of PI3K-related protein kinases. Cell. 2007;131:1248–59. doi: 10.1016/j.cell.2007.10.052. [DOI] [PubMed] [Google Scholar]
- TAKAI H, XIE Y, DE LANGE T, PAVLETICH NP. Tel2 structure and function in the Hsp90-dependent maturation of mTOR and ATR complexes. Genes Dev. 2010;24:2019–30. doi: 10.1101/gad.1956410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANIGUCHI CM, EMANUELLI B, KAHN CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- TAO Z, BARKER J, SHI SD, GEHRING M, SUN S. Steady-state kinetic and inhibition studies of the mammalian target of rapamycin (mTOR) kinase domain and mTOR complexes. Biochemistry. 2010;49:8488–98. doi: 10.1021/bi100673c. [DOI] [PubMed] [Google Scholar]
- TATO I, BARTRONS R, VENTURA F, ROSA JL. Amino acids activate mammalian target of rapamycin complex 2 (mTORC2) via PI3K/Akt signaling. J Biol Chem. 2011;286:6128–42. doi: 10.1074/jbc.M110.166991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR SS, KIM C, CHENG CY, BROWN SH, WU J, KANNAN N. Signaling through cAMP and cAMP-dependent protein kinase: diverse strategies for drug design. Biochim Biophys Acta. 2008;1784:16–26. doi: 10.1016/j.bbapap.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TESSIER M, WOODGETT JR. Serum and glucocorticoid-regulated protein kinases: variations on a theme. J Cell Biochem. 2006;98:1391–407. doi: 10.1002/jcb.20894. [DOI] [PubMed] [Google Scholar]
- THEDIECK K, POLAK P, KIM ML, MOLLE KD, COHEN A, JENO P, ARRIEUMERLOU C, HALL MN. PRAS40 and PRR5-Like Protein Are New mTOR Interactors that Regulate Apoptosis. PLoS ONE. 2007;2:e1217. doi: 10.1371/journal.pone.0001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOREEN CC, KANG SA, CHANG JW, LIU Q, ZHANG J, GAO Y, REICHLING LJ, SIM T, SABATINI DM, GRAY NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–32. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREINS C, WARNE PH, MAGNUSON MA, PENDE M, DOWNWARD J. Rictor is a novel target of p70 S6 kinase-1. Oncogene. 2009 doi: 10.1038/onc.2009.401. [DOI] [PubMed] [Google Scholar]
- TREMBLAY F, BRULE S, HEE UM S, LI Y, MASUDA K, RODEN M, SUN XJ, KREBS M, POLAKIEWICZ RD, THOMAS G, MARETTE A. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2007;104:14056–61. doi: 10.1073/pnas.0706517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TZATSOS A, KANDROR KV. Nutrients suppress phosphatidylinositol 3-kinase/Akt signaling via raptor-dependent mTOR-mediated insulin receptor substrate 1 phosphorylation. Mol Cell Biol. 2006;26:63–76. doi: 10.1128/MCB.26.1.63-76.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UM SH, FRIGERIO F, WATANABE M, PICARD F, JOAQUIN M, STICKER M, FUMAGALLI S, ALLEGRINI PR, KOZMA SC, AUWERX J, THOMAS G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–5. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- VANDER HAAR E, LEE SI, BANDHAKAVI S, GRIFFIN TJ, KIM DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–23. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- VINIEGRA JG, MARTINEZ N, MODIRASSARI P, LOSA JH, PARADA COBO C, LOBO VJ, LUQUERO CI, ALVAREZ-VALLINA L, RAMON Y CAJAL S, ROJAS JM, SANCHEZ-PRIETO R. Full activation of PKB/Akt in response to insulin or ionizing radiation is mediated through ATM. J Biol Chem. 2005;280:4029–36. doi: 10.1074/jbc.M410344200. [DOI] [PubMed] [Google Scholar]
- WANG L, LAWRENCE JC, JR, STURGILL TW, HARRIS TE. Mammalian target of rapamycin complex 1 (mTORC1) activity is associated with phosphorylation of raptor by mTOR. J Biol Chem. 2009;284:14693–7. doi: 10.1074/jbc.C109.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG X, LI W, WILLIAMS M, TERADA N, ALESSI DR, PROUD CG. Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase. Embo J. 2001;20:4370–9. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG X, PROUD CG. Nutrient control of TORC1, a cell-cycle regulator. Trends Cell Biol. 2009;19:260–7. doi: 10.1016/j.tcb.2009.03.005. [DOI] [PubMed] [Google Scholar]
- WARAICH RS, WEIGERT C, KALBACHER H, HENNIGE AM, LUTZ SZ, HARING HU, SCHLEICHER ED, VOELTER W, LEHMANN R. Phosphorylation of Ser357 of rat insulin receptor substrate-1 mediates adverse effects of protein kinase C-delta on insulin action in skeletal muscle cells. J Biol Chem. 2008;283:11226–33. doi: 10.1074/jbc.M708588200. [DOI] [PubMed] [Google Scholar]
- WENG QP, ANDRABI K, KOZLOWSKI MT, GROVE JR, AVRUCH J. Multiple independent inputs are required for activation of the p70 S6 kinase. Mol Cell Biol. 1995;15:2333–40. doi: 10.1128/mcb.15.5.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOO SY, KIM DH, JUN CB, KIM YM, HAAR EV, LEE SI, HEGG JW, BANDHAKAVI S, GRIFFIN TJ. PRR5, a novel component of mTOR complex 2, regulates platelet-derived growth factor receptor beta expression and signaling. J Biol Chem. 2007;282:25604–12. doi: 10.1074/jbc.M704343200. [DOI] [PubMed] [Google Scholar]
- WOODS A, JOHNSTONE SR, DICKERSON K, LEIPER FC, FRYER LG, NEUMANN D, SCHLATTNER U, WALLIMANN T, CARLSON M, CARLING D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–8. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- WU YT, OUYANG W, LAZORCHAK AS, LIU D, SHEN HM, SU B. mTOR Complex 2 Targets Akt for Proteasomal Degradation via Phosphorylation at the Hydrophobic Motif. J Biol Chem. 2011 doi: 10.1074/jbc.M111.219923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WULLSCHLEGER S, LOEWITH R, HALL MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- WULLSCHLEGER S, LOEWITH R, OPPLIGER W, HALL MN. Molecular organization of target of rapamycin complex 2. J Biol Chem. 2005;280:30697–704. doi: 10.1074/jbc.M505553200. [DOI] [PubMed] [Google Scholar]
- XIE X, ZHANG D, ZHAO B, LU MK, YOU M, CONDORELLI G, WANG CY, GUAN KL. I{kappa}B kinase {varepsilon} and TANK-binding kinase 1 activate AKT by direct phosphorylation. Proc Natl Acad Sci U S A. 2011;108:6474–9. doi: 10.1073/pnas.1016132108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG J, CRON P, THOMPSON V, GOOD VM, HESS D, HEMMINGS BA, BARFORD D. Molecular mechanism for the regulation of protein kinase B/Akt by hydrophobic motif phosphorylation. Mol Cell. 2002;9:1227–40. doi: 10.1016/s1097-2765(02)00550-6. [DOI] [PubMed] [Google Scholar]
- YANG Q, INOKI K, IKENOUE T, GUAN KL. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006;20:2820–32. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YIP CK, MURATA K, WALZ T, SABATINI DM, KANG SA. Structure of the human mTOR complex I and its implications for rapamycin inhibition. Mol Cell. 2010;38:768–74. doi: 10.1016/j.molcel.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIDA S, HONG S, SUZUKI T, NADA S, MANNAN AM, WANG J, OKADA M, GUAN KL, INOKI K. REDOX REGULATES mTORC1 ACTIVITY BY MODULATING THE TSC1/TSC2-Rheb PATHWAY. J Biol Chem. 2011 doi: 10.1074/jbc.M111.238014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YU K, TORAL-BARZA L, SHI C, ZHANG WG, LUCAS J, SHOR B, KIM J, VERHEIJEN J, CURRAN K, MALWITZ DJ, COLE DC, ELLINGBOE J, AYRAL-KALOUSTIAN S, MANSOUR TS, GIBBONS JJ, ABRAHAM RT, NOWAK P, ZASK A. Biochemical, cellular, and in vivo activity of novel ATP-competitive and selective inhibitors of the mammalian target of rapamycin. Cancer Res. 2009;69:6232–40. doi: 10.1158/0008-5472.CAN-09-0299. [DOI] [PubMed] [Google Scholar]
- YU Y, YOON SO, POULOGIANNIS G, YANG Q, MA XM, VILLEN J, KUBICA N, HOFFMAN GR, CANTLEY LC, GYGI SP, BLENIS J. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science. 2011;332:1322–6. doi: 10.1126/science.1199484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG HH, LIPOVSKY AI, DIBBLE CC, SAHIN M, MANNING BD. S6K1 regulates GSK3 under conditions of mTOR-dependent feedback inhibition of Akt. Mol Cell. 2006a;24:185–97. doi: 10.1016/j.molcel.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG Y, BILLINGTON CJ, JR, PAN D, NEUFELD TP. Drosophila target of rapamycin kinase functions as a multimer. Genetics. 2006b;172:355–62. doi: 10.1534/genetics.105.051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHENG B, JEONG JH, ASARA JM, YUAN YY, GRANTER SR, CHIN L, CANTLEY LC. Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Mol Cell. 2009;33:237–47. doi: 10.1016/j.molcel.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINZALLA V, STRACKA D, OPPLIGER W, HALL MN. Activation of mTORC2 by Association with the Ribosome. Cell. 2011;144:757–68. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]
- ZONCU R, EFEYAN A, SABATINI DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]