Abstract
The current study investigated the relationship between bilingual language proficiency and onset of probable Alzheimer’s disease (AD) in 44 Spanish-English bilinguals at the UCSD Alzheimer’s Disease Research Center. Degree of bilingualism along a continuum was measured using Boston Naming Test (BNT) scores in each language. Higher degrees of bilingualism were associated with increasingly later age-of-diagnosis (and age of onset of symptoms), but this effect was driven by participants with low education level (a significant interaction between years of education and bilingualism) most of whom (73%) were also Spanish-dominant. Additionally, only objective measures (i.e., BNT scores), not self-reported degree of bilingualism, predicted age-of-diagnosis even though objective and self-reported measures were significantly correlated. These findings establish a specific connection between knowledge of two languages and delay of AD onset, and demonstrate that bilingual effects can be obscured by interactions between education and bilingualism, and by failure to obtain objective measures of bilingualism. More generally, these data support analogies between the effects of bilingualism and “cognitive reserve” and suggest an upper limit on the extent to which reserve can function to delay dementia.
The Alzheimer’s Association (2004) warns “During the first half of the 21st century, the number of Hispanic elders suffering from Alzheimer’s and related dementias could increase more than six-fold, from fewer than 200,000 today to as many as 1.3 million by 2050.” Age is the single greatest risk factor for Alzheimer’s disease (AD; from age 65 prevalence doubles every 5 years reaching 47% of people ≥85). The proportion of people 65 or older in the US is projected to increase from 12% to 20% in 2030. In addition, the Hispanic population is predicted to triple in size, and will account for an increasingly larger proportion of the total population over 65. As the population ages and becomes more ethnically diverse, the number of Hispanics with AD is also rising rapidly.
One factor that may provide some protection against AD in Hispanics is bilingualism. Many Hispanics speak both Spanish and English, and recent research suggests that bilingualism may delay the onset of AD. Bialystok and colleagues, recruited patients from a memory clinic in Toronto, Canada comparing people who “…spent the majority of life, at least from early adulthood, regularly using at least two languages” (Bialystok, Craik, & Freedman, 2007; Craik, Bialystok, & Freedman, 2010, pg. 1727) to monolinguals, and found that bilinguals reported onset of first symptoms 4–5 years later than monolinguals. A second study in Montreal, Canada, found a delay in age of diagnosis of AD for immigrant bilinguals when compared with less educated immigrants who remained monolingual, but no significant bilingual advantage when comparing native Canadian bilinguals to monolinguals (although trends in this direction were reported for participants with French as the first-learned language; Chertkow, Whitehead, Phillips, Wolfson, Atherton, & Bergman, 2010). In this same study, there was a clear delay in diagnosis for multilinguals whether Canadian natives or immigrants.
These pioneering studies complement recent reports that bilingualism enhances executive function (Costa, Hernández, Costa-Faidella, Sebastián-Gallés, 2009; for review see Bialystok, Craik, Green, & Gollan, 2009), imply a beneficial effect of bilingualism on cognitive reserve (Stern, 2009; Valenzuela & Sachdev, 2006a&b), and raise interesting questions regarding mechanisms underlying these effects. What about bilingualism introduces the advantage, and why is it sometimes difficult to observe? Is the advantage only found in highly proficient bilinguals, and only in bilinguals who learn both languages from birth? Will delay of AD also be observed in Spanish-English bilinguals in the USA? Is the effect dependent on an extreme contrast between bilinguals and monolinguals? We investigated if degree of bilingualism is related to onset of AD within a uniform bilingual population using a continuous and objective measure of bilingual language proficiency.
Methods
Participants
The UCSD Alzheimer’s Disease Research Center (ADRC) follows about 100 Hispanic participants about equally divided between patients with dementia and healthy non-demented controls. Clinical diagnoses are made by two neurologists based on medical, neurological, and neuropsychological exams, laboratory tests, and neuroimaging. We focused on 44 bilinguals with a diagnosis of probable AD (excluding those with possible AD, Parkinson’s disease, dementia with Lewy bodies, and non-amnestic MCI). Half preferred to be tested in English (n=22) and half in Spanish (n=22). Table 1 shows participant characteristics (language history questionnaire data were missing for a handful of participants who could not be contacted).
Table 1.
Mean and standard deviation of participant characteristics for 44 bilinguals divided by language preference on the left, and by education level on the right.
| Characteristic | Prefer English (n=22) | Prefer Spanish (n=22) | High Education (n=22) | Low Education (n=22) | ||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | |
| Age of Diagnosis | 75.1 | (8.6) | 77.1 | (7.0) | 75.1 | (8.1) | 77.1 | (6.8) |
| Age of Onset | 72.5 | (9.4) | 74.6 | (7.8) | 72.1 | (9.5) | 75.0 | (7.5) |
| Education | 12.9 | (4.2) | 7.4** | (4.7) | 14.6 | (2.6) | 5.6†† | (2.7) |
| MMSE at Diagnosis | 23.8 | (4.3) | 23.8 | (4.1) | 23.4 | (4.0) | 24.2 | (4.4) |
| DRS at Diagnosisb | 117.7 | (11.5) | 110.3* | (12.7) | 119.3 | (9.9) | 108.7†† | (12.9) |
| BNT-based Bilingual Indexa | .59 | (.25) | .47 | (.31) | .64 | (.24) | .42†† | (.28) |
| Self-rated Bilingual Indexab | .72 | (.24) | .55* | (.27) | .74 | (.21) | .52†† | (.27) |
| Percent daily use of Englishc | 86.2 | (15.1) | 13.0* | (24.5) | 75.6 | (30.6) | 21.1†† | (33.9) |
| Age of acquisition of Englishc | 1.5 | (2.7) | 22.7** | (17.2) | 2.9 | (3.9) | 19.9†† | (18.8) |
| English self-rated speaking cd | 6.7 | (0.5) | 3.7** | (1.9) | 6.3 | (0.9) | 4.0†† | (2.3) |
| Spanish self-rated speakingcd | 4.8 | (1.6) | 6.5** | (0.8) | 5.4 | (1.7) | 5.9 | (1.4) |
| Years in Spanish-speaking Country c | 4.1 | (9.2) | 34.2** | (18.6) | 15.0 | (21.7) | 23.2 | (19.7) |
| Dominant language BNT scoree | .64 | (.21) | .56 | (.22) | .67 | (.16) | .53† | (.24) |
| Nondominant language BNT scoree | .36 | (.20) | .29 | (.22) | .43 | (.20) | .22†† | (.17) |
| English BNT scoree | .63 | (.21) | .30** | (.23) | .62 | (.16) | .30†† | (.27) |
| Spanish BNT scoree | .37 | (.21) | .56** | (.22) | .48 | (.24) | .45 | (.23) |
Significant difference between prefer English and prefer Spanish groups at p < .05 level
Significant difference between prefer English and prefer Spanish groups at p < .01 level
Significant difference between low education and high education groups at p < .05 level
Significant difference between low education and high education groups at p < .01 level
Index scores calculated by dividing the nondominant score by the dominant score (see text).
DRS scores are a bit low relative to scores obtained in highly-educated cognitively intact monolingual English speakers. This could be due to a bilingual disadvantage, translation of the test (Peña, 2007) for bilinguals who preferred to be tested in Spanish, and the relatively low education level for some bilinguals in this cohort (the correlation between education level and DRS scores in all 43 bilinguals was robust, r = .497, p < .01; by contrast MMSE scores were not influenced by education level, r = −.069, p = .66).
Degrees of freedom for this comparison were less than 42 but at least 35.
Proficiency level based on self-ratings using a scale of 1–7 with 1 being “little to no knowledge” and 7 being “like a native speaker.”
Proportion of pictures named correctly.
Materials & Procedure
Participant age when given the diagnosis of probable AD was obtained from medical records. Age-of-onset of first symptoms was obtained from an informant (spouse or adult-child) through structured interview by the neurologist during the first year of ADRC participation. During annual evaluations participants were tested on the Boston Naming Test (BNT) first in their self-reported dominant language, and then in their non-dominant language. To measure the degree of bilingualism objectively we calculated bilingual index scores by dividing the proportion of pictures named correctly in whichever language produced a lower naming score by the proportion named correctly in whichever language produced the higher naming score (Gollan, Weissberger, Runnqvist, Montoya, & Cera, in press). The index provides an objective but also intuitive estimate of relative ability in each language without being influenced by overall naming ability. To illustrate, a bilingual who names 3 pictures in one language and 6 in the other would be 50% bilingual, as would a person who names 30 in one and 60 in the other. Alternative responses were allowed (e.g., galleta, rosquilla, or pretzel) in Spanish to adjust for the problem that the BNT was not designed for use with bilinguals or Spanish speakers (Allegri, Mangone, Fernandez Villavicencio, Rymberg, Taragano, & Baumann, 1997; Gollan et al., in press; Gollan, Fennema-Notestine, Montoya, & Jernigan, 2007; Kohnert, Hernandez, & Bates, 1998).
Testing in both languages at the ADRC began for some participants in 2002 and became annual for all Hispanic participants in 2007. We used naming scores from the first year of dual-language testing. On average bilinguals were tested in both languages for the first time under a year after being diagnosed (M=0.82 years, SD=3.01). Delay between dual-language testing and diagnosis did not vary with language preference (English versus Spanish), or with education level (both ts< 1), an important consideration given that the dominant language may decline more rapidly than the nondominant language for some bilinguals with AD (Gollan, Salmon, Montoya, & da Pena, 2010).
Results
Initially we correlated age-of-diagnosis and age-of-onset with the BNT based bilingual index, and other variables which we thought might vary with language preference. Given the exploratory nature of this initial analysis we used an unadjusted alpha level of p=.05. To evaluate the utility of the objective versus subjective measures we compared the BNT index to an index calculated using bilinguals’ self-rated spoken proficiency in each language. Previous studies have used age-of-onset (Bialystok et al., 2007) or both age-of-onset and age-of-diagnosis (Chertkow et al., 2010) as dependent variables. Both outcome variables are inherently flawed in some ways; age of onset assumes different families will notice onset of symptoms at a similar level of impairment (which may or may not be true), and age of diagnosis may be subject to variability in access to healthcare (e.g., Spanish-dominant bilinguals in the USA may be more reluctant to seek healthcare because the majority of healthcare givers will speak English only). We focus our discussion primarily on age of diagnosis because of our preference for using an objective and professionally determined clinical classification, but note that the pattern of results and significance reported was the same when we repeated our analyses with age of onset as the measure (see also Chertkow et al., 2010).
Language-dominance subgroups
In Spanish-dominant bilinguals age at diagnosis was increasingly older in bilinguals who were objectively more bilingual. Table 2 shows the results separated by language preference. Subjective and objective bilingual index scores were significantly correlated with each other, but only the objective measure significantly predicted age-of-diagnosis. Bilingualism was also correlated with education level such that higher levels of bilingualism (on both objective and subjective index scores) were found in more educated bilinguals. At the time of diagnosis, Mini-Mental Status Exam (MMSE; Folstein & Folstein, 1975) scores were not correlated with age-of-diagnosis, education level, or bilingual index scores, but were strongly correlated with Dementia Rating Scale (DRS; Mattis, 1988) scores. DRS scores appeared to be more sensitive (than MMSE) and were significantly correlated with index scores, and marginally correlated with education and age-of-diagnosis such that better educated and more bilingual individuals obtained higher DRS scores, and those diagnosed at a later age had marginally higher DRS scores.
Table 2.
Pearson bivariate correlations between predictor variables and age-of-diagnosis by language group (n=22 in each correlation except where noted otherwise.
| Bilinguals who prefer Spanish | age at diagnosis | age of onset | BNT index | self-rating index | education | DRS at diagnosis | |
|---|---|---|---|---|---|---|---|
| BNT based bilingual index | r | .47 | .42 | ||||
| p-value | .03 | .05 | |||||
| self-rating based bilingual index | r | .27a | .12a | .65a | |||
| p-value | .26 | .63 | <.01 | ||||
| Education | r | .19 | .14 | .74 | .64a | ||
| p-value | .40 | .523 | <.01 | <.01 | |||
| DRS Score at Diagnosis | r | .40 | .40 | .50 | .49a | .42 | |
| p-value | .07 | .07 | .02 | .04 | .05 | ||
| MMSE Score at Diagnosis | r | .15 | .27 | −.06 | <.01a | −.24 | .55 |
| p-value | .51 | .22 | .78 | 1.0 | .28 | <.01 | |
| Bilinguals who prefer English | age at diagnosis | age of onset | BNT index | self-rating index | education | DRS at diagnosis | |
| BNT based bilingual index | r | −.33 | −.33 | ||||
| p-value | .13 | .13 | |||||
| self-rating based bilingual index | r | −.19b | −.18b | .46b | |||
| p-value | .40 | .43 | .04 | ||||
| education | r | −.30 | −.34 | −.01 | .13b | ||
| p-value | .18 | .12 | .98 | .59 | |||
| DRS Score at Diagnosis | r | −.03 | −.11 | −.16 | −<.01b | .31 | |
| p-value | .89 | .63 | .47 | .99 | .17 | ||
| MMSE Score at Diagnosis | r | .05 | .02 | −.09 | −.06b | .03 | .66 |
| p-value | .82 | .93 | .68 | .79 | .89 | <.01 | |
Number of subjects in this correlation was 19;
Number of subjects in this correlation was 21
In striking contrast to the many significant correlations observed in the Spanish-dominant bilinguals, the English-dominant bilinguals’ data revealed only two significant correlations. MMSE and DRS scores were strongly correlated, and subjective and objective bilingual index scores were significantly correlated. However, degree of bilingualism was not associated with age-of-diagnosis.
These exploratory analyses implied (but see below) that degree of bilingualism predicts age-of-diagnosis for Spanish-dominant but not for English-dominant bilinguals. A regression analysis seemed to confirm this conclusion (but again see below); for this analysis we coded language-dominance categorically (with 1s and −1s), entered BNT index scores as a continuous predictor, and created an interaction term by centering the continuous predictor (Aiken & West, 1991), and then multiplying the centered predictor by the categorical codes. In this analysis language-dominance and BNT index scores were not significant predictors (both ps≥.42), but the interaction term was a significant predictor of age of diagnosis, β=−.39, p=.01. The total model was marginally significant, F(3, 40)=2.70, p=.06. However, Table 1 reveals that Spanish-dominant bilinguals were significantly less educated (averaging just over a primary school education), than English-dominant bilinguals (who averaged more than a high-school education).
Low-education versus High-Education subgroups
To explore the possible effects of education we repeated the regression analysis this time entering education level (as a continuous predictor) instead of language-dominance, and creating an interaction term by centering the education variable, and then multiplying the two centered variables (education and BNT index). In this analysis, years of education and BNT index scores were not significant predictors (both ps .13), but the interaction term was a significant predictor of age-of-diagnosis, β=−.42, p=.01. In this case, the total model was also significant, F(3, 40)=3.37, p=.03, thus the contrast between groups seemed to be more robust when entering education (instead of language preference) as a predictor.
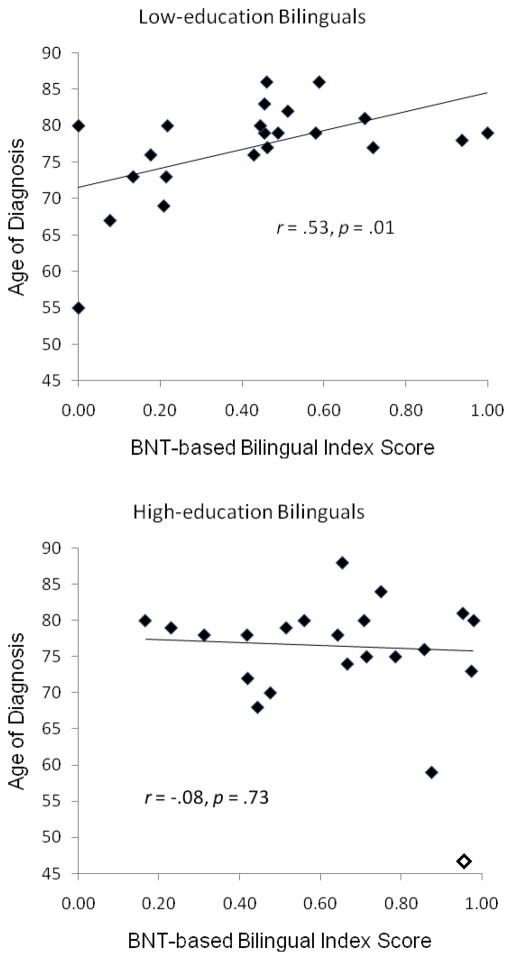
To illustrate the interaction between education and degree of bilingualism on age-of-diagnosis we divided the participants into high (≥12) and low (≤11) education groups (with 22 bilinguals in each group). The participant characteristics for education subgroups are shown in Table 1, and Figure 1 shows the relationship between degree of bilingualism and age-of-diagnosis in each group. In bilinguals with 2–11 years of education age-of-diagnosis increases with increasing levels of bilingualism as measured by bilingual index scores. In contrast, in bilinguals with 12–20 years of education there seems to be no association between BNT index scores and age-of-diagnosis; if anything, the relationship trends in the wrong direction. This negative trend was driven in part by one highly balanced and highly educated bilingual with an early age-of-diagnosis (a lawyer with a 95% BNT index; she named 70% pictures in English and 73% in Spanish). The interaction between education level and BNT index score was still significant after removing this bilingual from the data, β=−.31, p<.05).
Figure 1.
The relationship between age-of-diagnosis of Alzheimer’s disease and relative ability to name pictures in each language (BNT-based index = nondominant language naming score/dominant language naming score) in 16 Spanish-dominant and 6 English-dominant low education (top panel) and 6 Spanish-dominant and 16 English-dominant high education (bottom panel) Spanish-English bilinguals. One bilingual with very early-age of onset (open instead of filled diamond) was excluded from calculation of regression line (in the bottom panel).
Comparison of language dominance to education subgroups (see Table 1) provides further clues as to the nature of the effects observed here. The low education group is on average significantly less bilingual than the high education group; Figure 1 also shows the range to be more restricted (e.g., note that several data points are at or below 20% bilingual in the low-education group, but only one data point is in this range for the high-education group). The low education group index scores covered the full possible range (0–1). The correlation between BNT index scores and age-of-diagnosis remained significant, r=.517, p=.02, after removing two participants who could not name any pictures in a second language (i.e., these participants are effectively monolingual), and trended in the same direction (though it was no longer significant) after removing all index scores outside the range of scores found for high education bilinguals, r=.38, p=.13. Other differences emerged as significant when comparing low to high education subgroups (that had not been significant when comparing by language preference). Most notably, BNT naming scores in both the dominant and nondominant languages were significantly higher in high than in low education groups, as well as other differences likely associated with education level (e.g., the higher BNT index scores in high versus low education bilinguals). Conversely, some differences that were significant in the contrast between the two language preference groups were no longer significant in the education groups contrast, including years spent in a Spanish-speaking country and ability to speak Spanish.
Discussion
The current results build on and extend existing data on the relationship between bilingualism and diagnosis of AD in a number of ways. Most notably, in prior studies bilinguals were not well characterized in terms of degree of proficiency in each language. This left open several questions about the nature of the effect, and the possibility that some factor that correlated with bilingualism, but not specifically related to knowledge of two languages, was critical for introducing the observed effects. The current study used an objective measure of ability to produce picture names in each language, and showed that age of diagnosis of AD increased with increasing similarity of naming scores in each language. These data establish an explicit connection between knowledge of two languages and onset of AD (both measured as age-of-diagnosis and when we repeated our analyses using age of onset as the measure). Because all participants belonged to the same ethnic group (Hispanics in the USA) the current data reduce possible concerns that previously reported bilingual advantages were introduced by confounds inherent to comparing across language groups (i.e., bilinguals versus monolinguals).
In previous studies, bilinguals spoke a variety of different languages (Bialystok et al., 2007; Craik et al., 2010; Chertkow et al., 2010; Kavé, Eyal, Shorek, Cohen-Mansfield, 2008). The inclusion of a variety of bilingual types is a strength which implies that the benefits of bilingualism generalize broadly. On the other hand, it is very difficult to measure and compare proficiency levels across multiple languages. Thus, that approach left several questions unanswered with respect to how bilingual people must be before advantages begin to emerge. The current data provide additional clues as to the nature of bilingual effects, revealing them to be continuous in nature with greater benefits accruing with increasing proficiency levels.
Another important finding was that the benefit associated with bilingualism was robust only in bilinguals with low education level. This result implies an upper limit on the amount of benefit that bilingualism can confer for delaying diagnosis of AD. In bilinguals with low education level, the benefit can come from increasing bilingual language proficiency, but at higher levels of education the power of cognitive reserve for delaying AD is already at a maximum level and bilingualism does have any further effect. This interpretation increases confidence in prior conclusions of the cognitive advantages associated with bilingualism, and analogies between the effects of bilingualism and cognitive reserve (Bialystok et al., 2007; Craik et al., 2010).
Initial analyses suggested that the relationship between bilingual language proficiency and diagnosis of AD was present only for bilinguals who preferred to be tested in Spanish. This was a surprising result given that most Spanish-dominant bilinguals were not life-long bilinguals whereas English-dominant bilinguals had been bilingual since an early age. It might seem that this result resembles that of Chertkow et al, (2010) who found a trend suggesting delay in age of onset of AD associated with bilingualism for native-French speakers but not for native-English speakers in Montreal. However, in that study education levels were relatively high (ranging from 10.3 to 12.8 in the native French groups; see Supplementary Materials in Chertkow et al.), and comparable for those who did versus didn’t show the bilingual advantage (i.e., French versus English natives). In addition, native-French study participants actually had higher SES than native-English study participants a puzzling result given that historically native-French speakers were disadvantaged in Montreal. In contrast, in the current study Spanish-dominant bilinguals who exhibited the advantage also tended to be less educated (5.7 years on average; see Table 1), and many were immigrants. In this respect our results resemble more the immigrant participants studied in Chertkow et al. who also had low education levels (although in this case it was monolinguals who had low education levels, 6.3 years on average, versus bilinguals who had 10.5 years). Indeed in our study, analyses considering education as a continuous variable suggested that this was a critical difference between groups determining where the benefit of bilingualism is found (see Figure 1).
A number of limitations in the current report call for caution in any conclusions drawn. As in previous work bilingualism and education level were confounded with other factors (see Table 1). For example, bilinguals with lower education levels had significantly lower DRS scores than bilinguals with high education levels. In previous studies, MMSE scores were used to measure the level of impairment at age-of-diagnosis and did not reveal any difference between bilinguals and monolinguals implying that bilingualism delays the onset but not the progression of AD (Bialystok et al., 2007; Craik et al., 2010). Consistent with this finding MMSE scores were not correlated with age-of-diagnosis (see Table 2), and low and high education groups did not differ in MMSE scores at age-of-diagnosis (see Table 1). However, the MMSE is a very brief measure, and it is possible that more sensitive measures of cognitive status like the DRS, and longitudinal data, will alter slightly the conclusions drawn to date with respect to disease progression.
Perhaps the most notable limitation in the current report is that although the results appear to be robust statistically, the number of bilinguals tested here is relatively small. A useful avenue to explore in further research is whether previously reported bilingual advantages remain, or if they are driven largely by participants with relatively lower education levels. Previously, an advantage was found comparing bilinguals to monolinguals even though bilinguals were significantly less educated than monolinguals (Craik et al., 2010; but see Chertkow et al., 2010). In one study, education level was not significant when included as a covariate in the comparison of bilinguals to monolinguals (Bialystok et al., 2007). However, the inclusion of education as a covariate is not the same as specifying an interaction between education and bilingualism in a regression model. Our use of a continuous and objective measure of bilingualism in the current study, and a more rigorous approach to possible education effects, revealed relationships between these factors, and allowed significant effects to emerge despite the relatively small number of participants tested. Indeed although self-report and objective measures of degree of bilingualism are significantly correlated (see Table 2; Gollan et al., in press) self-report measures in the current study were not sufficiently sensitive to reveal the continuous nature of the effects of bilingualism.
A question that arises is why might there be an upper limit on the amount of cognitive reserve that can accumulate. Although our data do not provide an answer to this question, and our explanation of the interaction between bilingualism and education level is admittedly speculative at this point, the data we report bear striking resemblance to previous findings reported from the Nun Study. Mortimer and colleagues measured the relationship between brain size (inferred from head circumference) and dementia risk, and found a significant interaction such that larger brain size was associated with lower incidence of dementia but only in nuns with lower education level (Mortimer, Snowdon, & Markesbery, 2003). This result supports the hypothesis that cognitive reserve is modulated by education level. The cross-study similarity could suggest that in addition to bilingualism increasing cognitive reserve, people who manage to become bilingual despite low education levels (or low SES) may also be better able to accumulate reserve. Additionally, this cross-study similarity provides converging evidence which lessens concerns about possible confounds in the current study (e.g., language-dominance), and possible problems with using onset and age of diagnosis or onset as the measure (incidence of AD was the outcome of interest in the Nun Study).
A practically important aspect of the current data is the extension of benefits associated with bilingualism to a relatively homogenous group of bilinguals, and to the most common type of bilingual in the USA. Previous studies included a majority of bilinguals who were immigrants from Europe (especially Eastern Europe speaking Yiddish, Polish, Hungarian and Romanian (Bialystok et al., 2007; Chertkow et al., 2010; Craik et al., 2010); one study did not report the individual languages spoken by participants (Kavé et al., 2008), but the majority originated from Israel and Europe/America). As pointed out by Chertkow et al., (2010) people who lived in Europe during World War II likely had very different life experiences including possible risk factors for dementia. The present data suggest that this particular set of life experiences, and also early-bilingualism, are not necessary to find an effect of bilingualism on dementia onset.
Highlights.
Objectively measured degree of bilingualism predicts age-of-diagnosis of Alzheimer’s disease
Spanish-English bilinguals with probable AD
Significant effect obtained only in low education groups
Bilingualism along a continuum increases cognitive reserve
Acknowledgments
The authors thank Ellen Bialystok who provided honest and constructive criticism with humor and refreshing spunk on an earlier version of this manuscript. This research was supported by NICHD (R01 HD050287), NIDCD (R01 DC011492) and by NIA (P50, AG05131).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiken L, West S. Testing Interactions in Multiple Regression. Lawrence Erlbaum; 1991. [Google Scholar]
- Allegri RF, Mangone CA, Fernandez Villavicencio A, Rymberg S, Taragano FE, Baumann D. Spanish Boston Naming Test norms. The Clinical Neuropsychologist. 1997;11:416–420. [Google Scholar]
- Alzheimer’s Association. Hispanics/Latinos and Alzheimer’s disease. 2004 http://www.alz.org/national/documents/reporthispanic.pdf.
- Bialystok E, Craik FIM, Freedman M. Bilingualism as a protection against the onset of symptoms of dementia. Neuropsychologia. 2007;45:459–464. doi: 10.1016/j.neuropsychologia.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Bialystok E, Craik FIM, Green DW, Gollan TH. Bilingual minds. Psychological Science in the Public Interest. 2009;10:89–129. doi: 10.1177/1529100610387084. [DOI] [PubMed] [Google Scholar]
- ChertkowHWhitehead V, Phillips N, Wolfson C, Atherton J, Bergman H. Multilingualism (but not always bilingualism) delays the onset of Alzheimer disease: evidence from a bilingual community. Alzheimer Dis Assoc Disord. 2010;24:118–125. doi: 10.1097/WAD.0b013e3181ca1221. [DOI] [PubMed] [Google Scholar]
- Costa A, Hernández M, Costa-Faidella J, Sebastián-Gallés N. On the bilingual advantage in conflict processing: Now you see it, now you don’t. Cognition. 2009;113:135–149. doi: 10.1016/j.cognition.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Bialystok E, Freedman M. Delaying the onset of Alzheimer disease Bilingualism as a form of cognitive reserve. Neurology. 2010;75:1726–1729. doi: 10.1212/WNL.0b013e3181fc2a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gollan TH, Fennema-Notestine C, Montoya RI, Jernigan TL. The Bilingual Effect on Boston Naming Test performance. The Journal of the International Neuropsychological Society. 2007;13:197–208. doi: 10.1017/S1355617707070038. [DOI] [PubMed] [Google Scholar]
- Gollan TH, Salmon DP, Montoya RI, Da Pena E. Accessibility of the nondominant language in picture naming: A counterintuitive effect of dementia on bilingual language production. Neuropsychologia. 2010;48:1356–1366. doi: 10.1016/j.neuropsychologia.2009.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan TH, Weissberger G, Runnqvist E, Montoya RI, Cera CM. Self-ratings of spoken language dominance: A multi-lingual naming test (MINT) and preliminary norms for young and aging Spanish-English bilinguals. Bilingualism: Language and Cognition. doi: 10.1017/S1366728911000332. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavé G, Eyal N, Shorek A, Cohen-Mansfield J. Multilingualism and cognitive state in the oldest old. Psychology and Aging. 2008;23:70–78. doi: 10.1037/0882-7974.23.1.70. [DOI] [PubMed] [Google Scholar]
- Kohnert KJ, Hernandez AE, Bates E. Bilingual performance on the Boston Naming Test: Preliminary norms in Spanish and English. Brain and Language. 1998;65:422–440. doi: 10.1006/brln.1998.2001. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale: Professional Manual. Odessa, Florida: Psychological Assessment Resources; 1988. [Google Scholar]
- Mortimer JA, Snowdon DA, Markesbery WR. Head circumference, education and risk of dementia: Findings from the Nun Study. Journal of Clinical and Experimental Neuropsychology. 2003;25:671–679. doi: 10.1076/jcen.25.5.671.14584. [DOI] [PubMed] [Google Scholar]
- Peña ED. Lost in translation: Methodological considerations in cross-cultural research. Child Development. 2007;78:1255–1264. doi: 10.1111/j.1467-8624.2007.01064.x. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychololgical Medicine. 2006a;36:441–454. doi: 10.1017/S0033291705006264. [DOI] [PubMed] [Google Scholar]
- Valenzuela MJ, Sachdev P. Brain reserve and cognitive decline: A non-parametric systematic review. Psychololgical Medicine. 2006b;36:1065–1073. doi: 10.1017/S0033291706007744. [DOI] [PubMed] [Google Scholar]