Abstract
Memory retrieval can involve activity in the same sensory cortical regions involved in perception of the original event, and this neural “reactivation” has been suggested as an important mechanism of memory retrieval. However, it is still unclear if fragments of experience other than sensory information are retained and later reactivated during retrieval. For example, learning in non-laboratory settings generally involves active exploration of memoranda, thus requiring the generation of action plans for behavior and the use of strategies deployed to improve subsequent memory performance. Is information pertaining to action planning and strategic processing retained and reactivated during retrieval? To address this question, we compared ERP correlates of memory retrieval for objects that had been studied in an active manner involving action planning and strategic processing to those for objects that had been studied passively. Memory performance was superior for actively studied objects, and unique ERP retrieval correlates for these objects were identified when subjects remembered the specific spatial locations at which objects were studied. Early-onset frontal shifts in ERP correlates of retrieval were noted for these objects, which parallel the recruitment of frontal cortex during learning object locations previously identified using fMRI with the same paradigm. Notably, ERPs during recall for items studied with a specific viewing strategy localized to the same supplementary motor cortex region previously identified with fMRI when this strategy was implemented during study, suggesting rapid reactivation of regions directly involved in strategic action planning. Collectively, these results implicate neural populations involved in learning in important retrieval functions, even for those populations involved in strategic control and action planning. Notably, these episodic features are not generally reported during recollective experiences, suggesting that reactivation is a more general property of memory retrieval that extends beyond those fragments of perceptual information that might be needed to re-live the past.
Keywords: active learning, passive learning, volitional control, ERP, memory retrieval, content specificity
Introduction
Many insights into the mechanisms of episodic/declarative memory have derived from cognitive neuroscience experiments showing how various parts of the brain participate in information storage and retrieval. Contributions from some regions have been found to span across multiple circumstances, including various types of stimulus materials, learning parameters, test formats etc. These “all-purpose” regions, including, for example, the hippocampus, prefrontal cortex, and lateral parietal cortex (Buckner & Wheeler, 2001; Eichenbaum & Cohen, 2004; Gabrieli, 1998; Simons & Spiers, 2003), are thought to implement various binding, executive control, and attentional processes that participate in most forms of memory. In contrast, there are also consistent reports of content-sensitivity in the neural correlates of memory retrieval, whereby modality-specific processing associated with perception is reactivated/recapitulated during retrieval. Importantly, this modality-specific reactivation occurs even when cues for memory retrieval are presented in a different modality, such as when objects are paired with odorants during study and activation of olfactory cortex occurs in response to visual object cues during test (Gottfried, Smith, Rugg, & Dolan, 2004). These modality-specific neural reactivations have also been identified for other stimulus qualities, including visual (Goldberg, Perfetti, & Schneider, 2006; Stock, Roder, Burke, Bien, & Rosler, 2009), imaginal (Gonsalves & Paller, 2000), haptic (Stock, et al., 2009), nociceptive (Kelly, Lloyd, Nurmikko, & Roberts, 2007), gustatory (Goldberg, et al., 2006), emotional (Gottfried, et al., 2004; Smith, Henson, Dolan, & Rugg, 2004), and auditory (Goldberg, et al., 2006). There are also content-specific retrieval distinctions within modalities based on anatomical specializations for different stimulus qualities (e.g., spatial vs. non-spatial visual stimuli, Khader, Burke, Bien, Ranganath, & Rosler, 2005; Khader, Heil, & Rosler, 2005).
These findings (reviewed in Danker & Anderson, 2010) provide support for the notion that information is stored in the regions of cortex associated with relevant perceptual processing (Damasio, 1989). Indeed, modality-specific information storage is a central tenet of dominant theories of declarative/episodic memory, whereby memories for complex, multimodal episodes are supported by binding of multiple memory fragments, each stored within modality-specific regions (Cohen & Eichenbaum, 1993; Damasio, 1989; Eichenbaum & Cohen, 2004; Paller, 1997; Schacter, 1987; Underwood, 1969).
However, experiences are comprised of more than purely sensory features, and include qualities such as goal states, behavioral strategies, action plans, etc. (Bernstein, 1996; Conway & Pleydell-Pearce, 2000; Eichenbaum & Cohen, 2004; Shapiro, Kennedy, & Ferbinteanu, 2006). To what extent does neural activity related to these qualities also show recapitulation during memory retrieval? Answering this question is central to determining if and how these aspects of experience are incorporated into memory. Neural recapitulation of these episodic features would suggest that they can be stored in a direct fashion and later retrieved, thus showing that the same regions involved in generation are involved in memory storage and retrieval, as is the case for perceptual stimulus qualities, thus providing converging evidence for that reactivation is a general neural property that can contribute to memory performance.1
The alternative possibility is that these features are not stored and retrieved, and that memory for this sort of episodic information, if it exists at all, is reconstructed during retrieval based on recapitulation of sensory information. Indeed, mere sensory activity can reliably cue many motor-related cognitive operations, for instance when motor-related activity is cued in response to visually presented tools, (e.g., Martin, Wiggs, Ungerleider, & Haxby, 1996). Thus, operations such as strategic action planning could become accessible as a result of memory retrieval only indirectly, in response to reactivation of the sensory information that cued them originally. Thus it is unclear if these episodic features are directly stored and later retrieved/reactivated, and evaluating this possibility will require testing for recapitulation of these episodic features independent of sensory features.
Reactivation of learned action/motor sequences has been examined rarely in humans. Activity in regions critical to the planning and execution of actions has been identified during retrieval when actions were required during study but not during retrieval (Nilsson, et al., 2000; Nyberg, et al., 2001). Similar reactivation patterns have been identified for actions that were initially performed, watched, and imagined (Heil et al., 1999; Senkfor, Van Petten, & Kutas, 2002), indicating that similar information is stored and reactivated in these conditions. However, reactivation differences have been noted between actions originally performed with objects versus pantomimed actions (Senkfor, 2008), suggesting that activity attributed to reactivation might at least partly reflect object-cued imagination of action plans rather than true reactivation of stored action plans. In rodents, activity coding for the animal’s location in space during learning is replayed later during retrieval and also during periods of behavioral quiescence such as sleep (e.g., Wilson & McNaughton, 1994), suggesting recapitulation of relatively complex episodic information concerning the original event independent from object-cued activity. However, it is still unclear whether these location reactivation patterns relate to location coding versus the control/planning of movement patterns, which is a neurobiologically relevant distinction within the structure that shows reactivation effects in rodents, the hippocampus (Foster, Castro, & McNaughton, 1989; Song, Kim, Kim, & Jung, 2005).
Likewise, very few experiments have been directed at retrieval of neural reactivation of strategies implemented during learning, and these have focused almost exclusively on verbal versus visual encoding strategies (reviewed in Danker & Anderson, 2010; for demonstration of effects of cognitive reactivation, see Foley & Foley, 2007). For example, comparisons between reactivation of words studied with a verbal rehearsal strategy versus a visual mental-imagery strategy have yielded corresponding reactivation effects in brain regions associated with linguistics versus mental imagery (Johnson & Rugg, 2007; see also Kahn, Davachi, & Wagner, 2004). However, to the extent that these strategies simply recruit region-specific neural activity (e.g., visual cortex recruitment during visual mental imagery, Kosslyn, Thompson, & Alpert, 1997), these strategic reactivation effects can actually be considered as extensions of sensory-specific reactivation effects, rather than as evidence for the storage and reactivation of the cognitive strategies per se. Strategies must be dissociated from modality-specific or stimulus-specific processing in order to provide direct evidence for their reactivation during retrieval.
To further explore the extent to which brain activity during retrieval can reflect the strategies and action plans engaged during learning, we examined event-related brain potential (ERP) correlates of object retrieval following two learning conditions: (1) active learning involving the self-generation of complex action sequences and behavioral strategies and, (2) passive learning intended to severely restrict the self-generation of these action sequences and strategies (Figure 1AB). In the active learning condition, subjects studied objects arranged on a grid layout while having full control of the visual exploration of these objects; i.e., control over the study duration for each object, the order in which objects were viewed, etc. In the passive learning condition, all of these parameters were pre-specified and thus not determined by the subject. Importantly, the pattern of visual stimulation in the passive condition was determined by the active condition of another subject (Figure 1B); i.e., the visual exploration pattern made by subject n was recorded and played as the passive condition for subject n+1. Across subjects, visual stimulation was thus equated between the active and passive learning conditions, such that any observed neural differences during study or during retrieval could be attributed to the effects of implementing particular viewing strategies or generating action plans selectively in the active condition. Thus, the active and passive conditions differentially involved the generation of action/motor plans, yet were matched for object-related processing, thus providing ideal conditions for testing for reactivation effects for action plans with reactivation of other information types held relatively constant.
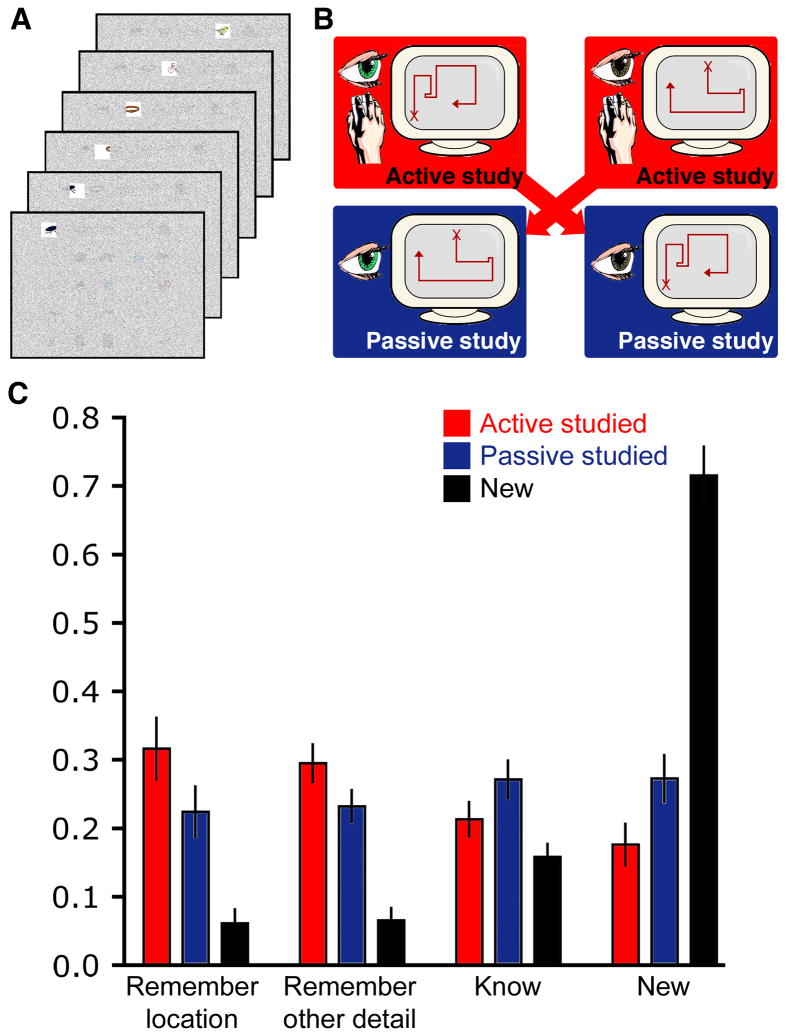
Figure 1. Effects of active learning on memory performance.
(A) Subjects studied objects arranged on a grid via a restricted-viewing paradigm that permitted study of one object at a time through a viewing window. (B) Study was controlled via a computer mouse used to move the viewing window in the active condition, and no control was provided in the passive condition (i.e., the viewing window moved and subjects merely watched). The visual information available in both conditions was matched via a subject-to-subject yoking procedure. (C) Endorsement rates are provided for the subsequent recognition memory test, separately for each stimulus type (active-studied, passive-studied, new) and response type (remember location, remember other, know, and new). Error bars indicate SE.
In a previous report (Voss, Gonsalves, Federmeier, Tranel, & Cohen, 2011) we found evidence for superior learning of objects studied in the active condition (referred to as “volitional” in the original report) relative to the passive condition, despite the fact that the same visual information was experienced in each condition by virtue of the matching of active and passive conditions described above. This active learning advantage was partly due to the implementation of simple behavioral strategies, such as viewing objects in preferred patterns, during the active condition (Voss, Warren, et al., 2011). Notably, the active learning advantage correlated with brain activity in a widespread network of prefrontal, parietal, hippocampal, and visual-processing regions. However, there were also important distinctions within this network that permitted higher levels of specificity in monitoring any potential reactivation in the current experiment. Notably, the extent of engagement of frontal cortical regions during active learning was specifically related to gains in memory for spatial information regarding the objects (i.e., object location recall).
Therefore, in the current experiment, we reasoned that retrieval-related neural activity reflecting reactivation would differentiate objects studied in the active condition from those studied in the passive condition, and would take the form of a frontal shift in ERP correlates of retrieval selectively for those objects for which spatial information was retrieved. This frontal shift would be consistent with the notion that frontal cortical networks involved in the generation of action plans and in strategic encoding factors related to the memory improvements identified for the active condition are recapitulated during retrieval. Critically, encoding activity and retrieval activity was identified based on beneficial effects of active study on memory performance. Because mere motor activity unrelated to strategic control of study in a modified active condition has been shown to have no beneficial effects on memory performance (Voss, Gonsalves, et al., 2011), it is possible to relate reactivation effects to the strategic and action-planning processing that enhanced memory performance rather than to nonspecific motor differences between active and passive study. Furthermore, we used ERP measures of retrieval that allowed assessment of the timecourse of reactivation effects on the neurophysiological timescale. This millisecond-scale precision was critical for assessing the automaticity and speed of recapitulation of complex frontally mediated action plans and behavioral strategies, i.e., as temporally independent from recapitulation of sensory processing or effortful memory search).
We attempted to provide further specificity in the link between retrieval activity and encoding activity by examining a specific strategy generated during learning. When studying objects in the current paradigm, subjects frequently spontaneously implement a strategy whereby they look back to study just-seen adjacent objects for a second time (Voss, Warren, et al., 2011). This “revisitation” strategy involves the study of objects in the order A-B-C-B-A, for example, as opposed to the linear order A-B-C with no revisitation of B-A before moving on to study other objects. We found that implementation of this revisitation strategy selectively improved memory performance and was associated with activity enhancement in a discrete region of medial supplementary motor cortex, as well as in hippocampus and cerebellum (Voss, Warren, et al., 2011). This functional neuroanatomy suggests that the revisitation strategy is based on the generation of action plans informed by hippocampal processing. In the current experiment, we examined ERP correlates of retrieval for objects studied using the revisitation strategy in order to determine if reactivation concerned strategic action plans generated during study.
Methods
Behavioral and electrophysiological data were collected from 21 individuals (15 female, ages 19–30 years, all right-handed) recruited from the University of Illinois community. Data from one participant were excluded due to excessive eyeblink artifact in the EEG record, leaving N=20 for behavioral and ERP analyses.
During the study portion of the experiment, participants attempted to memorize six 25-object arrays, each arranged as a 5 × 5 grid. Objects were common and nameable, obtained from the set described in Roission & Pourtois (2004). Each 25-object array was studied for one minute total. The entire display was occluded by a semi-transparent mask of Gaussian-distributed noise that included a small viewing window. This viewing window allowed clear viewing of one object at a time.
There were two learning conditions, active and passive, with half of the object arrays studied in each condition (i.e., 75 objects per condition). During the active learning condition, the position of the viewing window was under the continuous control of a computer mouse. Participants were instructed to move the window in any manner they intended and to memorize all of the objects and their spatial locations. In the passive condition, the window moved and was not controlled by the computer mouse, and participants were thus instructed to attempt to memorize the objects and object locations that were shown to them passively. Window movements in the passive condition were recorded from the active condition of the previous subject. Furthermore, the objects studied in the active and passive conditions alternated across participants. Thus, the same visual information was viewed in the same order, for the same duration, etc. in the active and passive conditions considered across all participants. The active and passive conditions were the same as the “volitional” and passive conditions, respectively, in Experiments 1 and 3 of Voss et al. (2011). A break of approximately 30 s was given between each 60 s period of active or passive study, and the active and passive study periods were administered in alternating order.
A recognition memory test was administered approximately 5 min. after all object arrays had been studied. In this test, participants attempted to discriminate the 150 old objects that had been studied either actively or passively from 100 similar-format objects that were not presented during the study portion of the experiment. Objects were shown in randomized order for 500 ms each, with a randomized 1,000–2,000 ms interstimulus interval. Participants were instructed to respond to each stimulus as quickly and accurately as possible using a four-choice recognition decision. Response options included: remember location, remember other, know, and new. The remember location response was used to indicate the experience of recollection of the object in addition to information regarding its location during study. The remember other response was used to indicate the experience of recollection of the object in addition to any detail other than its location during study (i.e., recollection of the specific form of the object, coloration of the object, temporal characteristics of study, or any other details). The know response was used to indicate the experience of familiarity—that is, the feeling that the object is old unsubstantiated by simultaneous retrieval of any details, contextual or otherwise, regarding the prior encounter with it. The new response was to be used to all unstudied (new) objects. This response scale was based on standard “remember/know” procedures (Gardiner & Java, 1991; Tulving, 1985), but with modification in order to subdivide the experience of recollection into two categories reflective of the type of information participants may have learned and recollected regarding the objects (location within the array versus other pertinent details). Subjects were instructed to suppress eye blinking during this test in order to improve EEG data quality.
EEG was recorded continuously during the recognition memory test from 64 scalp electrodes embedded in an elastic cap using a BioSemi Active II system (BioSemi Instrumentation, Amsterdam). Electrode locations conformed to the extended International 10–20 positioning system (Chatrian, Lettich, & Nelson, 1988). Recordings were also made from left and right mastoids, and four additional channels were used to monitor horizontal and vertical eye movements. EEG was digitized at a rate of 1,024 Hz with a bandpass of 0.01 to 120 Hz. Recordings were made with the standard Biosemi reference (CMS-DRL) and were rereferenced offline to averaged mastoids. ERPs time-locked to the onset of the visual object stimulus were calculated for each condition of interest in 1,100-ms epochs, beginning 100 ms prior to stimulus onset. Epochs contaminated by artifacts were discarded (10% on average, defined as epochs containing eye blinks, amplitude drift of over 40 μV across the epoch for any electrode, or amplitude values of over 75 μV for any electrode), and one subject was excluded due to loss of >35% of trials. For statistical assessment, ERPs were averaged over latency intervals and electrode clusters, including nine clusters that captured laterality (left, middle, right) and anterior-posterior (anterior, central, and posterior) distributions (anterior-left: AF3, AF7, F3, F5, F7; anterior-middle: Fp1, F1, Fpz, Afz, Fz, Fp2, F2; anterior-right: AF4, AF8, F4, F6, F8; central-left: FC3, FC5, FT7, C3, C5, T7, CP3, CP5, TP7; central-middle: FC1, FCz, FC2, C1, Cz, C2, CP1, CPz, CP2; central-right: FC4, FC6, FT8, C4, C6, T8, CP4, CP6, TP8; posterior-left: P3, P5, P7, PO3, PO7; posterior-middle: P1, Pz, P2, POz, O1, Oz, O2; posterior-right: P4, P6, P8, PO4, PO8). Repeated-measures ANOVAs (RM-ANOVA) incorporated Geisser-Greenhouse correction when appropriate. Post-hoc pairwise comparisons incorporated Bonferonni correction to guard against type-I error. Only significant main effects of condition and interaction effects including condition as a factor are reported (P<0.05). Any objects that were viewed for less than a total of 500 ms during either learning condition were excluded from all behavioral and ERP analyses (<1% of objects overall). Signal-to-noise ratios were compared among electrode clusters separately for each condition in order to ensure that clusters were not differentially sensitive to ERP effects. Signal-to-noise ratio was calculated using a standard approach whereby the maximum stimulus-evoked activity amplitude was divided by the pre-stimulus baseline noise level (cf. Maidhof et al., 2009; Voss & Gonsalves, 2010). Values were compared among clusters separately for each ERP condition (each old response type for active-studied and passive-studied objects, correctly rejected new objects, and objects actively studied with and without the revisitation strategy given remember-location responses, as described below) using 1-way RM-ANOVAs. No significant effects of cluster were identified for any condition [p values for F(8,104) ranged from 0.12 to 0.98], suggesting similar sensitivity among clusters.
Participants returned for a follow-up memory test one week after the initial study/test session. A test of spatial recall was administered. On each test trial, one old object, either studied actively or studied passively, appeared at a randomized central location. The display contained 25 square “placeholders” at all of the positions where objects were located originally. Participants used a computer mouse to attempt to drag the object to its original location. There was no response-time limit, but participants were encouraged to respond within approximately 10 s, and no responses of 18 s or longer were registered. The next trial began after a response was registered, and the screen was cleared of previously positioned objects on each trial. Trial order was randomized. Performance was quantified as the mean distance between the response location and the original location (distance error) as well as the proportion of objects that were returned to the correct placeholder (spatial recall hit). One subject did not return for follow-up testing.
For the analysis of the revisitation strategy, a computer algorithm was used to mark each studied item as “revisitation studied” or “other studied” based on the continuous record of viewing-window movements during study (Voss, Warren, et al., 2011). As in our previous report, the algorithm created a timeseries of visited objects, excluding partial/spurious views of less than 60 ms duration. Behavioral data from three subjects were excluded from this analysis due to a relatively large proportion of these partial views (11% on average, as opposed to 0.6% on average in other subjects). Revisitation-studied objects were coded as those that were part of revisitation sequences of between two and six objects (e.g., A-B-A to A-B-C-D-E-F-E-D-C-B-A). All objects not involved in these sequences were coded as “other studied.” As in our previous report, longer sequences (i.e., involving seven or more objects) were not considered due to their rare occurrence (only seven total in the entire dataset). Coding was performed separately for active and passive study conditions in order to determine the effects of viewing objects with the revisitation pattern when this pattern was self-generated (active study) versus when it was not self-generated (passive study). Overall, 29% of object-to-object transitions were involved in revisitation, comparable to our previously obtained value of 35% in a sample of 34 subjects of similar demographic characteristics to those in the current experiment (Voss, Warren, et al., 2011). Thirty-nine percent of all objects were revisitation-studied, with the remaining objects coded as other-studied. ERP data from an additional four subjects were excluded due to low trial counts (<5) in some conditions, leaving n=14 for ERP analysis of revisitation. These exclusions were a consequence of the post hoc nature of revisitation ERP analysis, and resulted in ERP waveforms with noticeably more noise than in primary analyses (see ERP figures below), although significant effects of revisitation on ERPs were nonetheless identified (see below).
Results
Effects of active learning on memory performance
Learning occurred for objects studied actively as well as for objects studied passively, as evidenced by above-chance performance in the recognition memory test for both. However, despite the fact that the same information was studied for the active and passive learning conditions, memory performance was significantly better for objects studied actively compared to objects studied passively. Endorsement rates of active-studied, passive-studied, and new objects for the four response types (remember location, remember other, know, and new) are provided in Figure 1C, which illustrates the significant interaction between object type and response type, F(6,114)=52.9, p<0.001.
Participants made the remember-location response significantly more for active-studied and passive-studied objects than for new objects (t(19)=7.3, p<0.001, and t(19)=6.7, p<0.001, respectively), demonstrating above-chance old/new discrimination for this response type. Critically, however, the location was remembered significantly more for active-studied than for passive-studied objects (t(19)=5.0, p<0.001), indicating higher recollection of this type of information as a result of active study. A similar pattern was observed for remember-other responses, which occurred significantly more for active-studied and passive-studied than for new objects (t(19)=7.5, p<0.001, t(19)=6.3, p<0.001, respectively), and were significantly more prevalent for active-studied than for passive-studied objects (t(19)=2.9, p=0.02).
A different pattern was observed for the know response, whereby the endorsement rate for active-studied objects was not significantly higher than for new items (t(19)=1.6, p=.12. Know responses were significantly more prevalent for passive-studied than for new objects (t(19)=3.4, p=0.003), as well as higher for passive-studied than for active-studied objects (t(19)=3.9, p=0.001). New responses were more prevalent for new items (correct rejections) than for either type of old items (misses; active-studied t(19)=10.1, p<0.001; passive-studied t(19)=8.1, p<0.001). New responses were also significantly more prevalent for passive-studied than for active-studied objects (t(19)=4.1, p=0.001).
Mean response times were computed for each stimulus type and response type (Table 1). Response times varied by response type [F(3,57)=14.8, p<0.001] but not by stimulus type [F(2,38)=0.17, p=0.84], nor was their a significant interaction of stimulus-by-response types [F(6,114)=0.79, p=0.58]. Comparisons of marginal means for each response type showed that remember-location responses were significantly faster than both remember-other and know responses (p values < 0.001). Remember-other and know response times did not differ significantly (p=0.57). Finally, new response times were significantly faster than all other response types (p values <0.002).
Table 1.
| Remember location | Remember other | Know | New | |
|---|---|---|---|---|
| Active studied | 1227 (49) | 1314 (57) | 1336 (46) | 1173 (52) |
| Passive studied | 1250 (60) | 1363 (52) | 1322 (43) | 1157 (44) |
| New | 1237 (56) | 1384 (79) | 1360 (69) | 1119 (39) |
Mean response times for each stimulus category and recognition response type (ms). Parentheses indicate SE.
To summarize, active study led to significantly more remember responses, both for location as well as for other details, than did passive study. These remember responses reflected highly successful learning in that they were more prevalent for studied than for new objects. Memory was weaker overall for passive-studied objects relative to active-studied objects, as indicated by more know responses and more misses (new responses). Recognition response times did not reflect these influences from the study phase, being roughly equated for active-studied, passive-studied, and new objects, and varied based only on the type of response that was made.
Performance on the follow-up test for spatial recall showed that the beneficial effects of active learning persisted for one week, albeit to a small extent. Very few objects were repositioned into their original locations, yet significantly more active-studied objects were correctly recalled than were passive-studied objects (6.4% versus 4.3%, respectively, t(18)=3.0, p=0.008). Likewise, the distance error between the original location and the recalled location was slightly yet significantly less overall for active-studied objects than for passive-studied objects (18.6 cm versus 19.7 cm, respectively, t(18)=2.2, p=0.04). For comparison, correct spatial recall occured for approximately 20% of passive-studied objects and the mean distance error was approximately 12 cm when tested immediately after study (Voss, Gonsalves, et al., 2011).
Performance on the follow-up test also validated the distinction between remember-location and remember-other responses made during the initial recognition memory test. To the extent that spatial information was more available for remember-location responses relative to remember-other responses, we expected superior follow-up spatial recall performance for objects given remember-location responses. Indeed, spatial distance error for the follow-up test was lower for objects initially given remember-location versus remember-other responses, for both active-studied objects (17.8 cm versus 19.7 cm, respectively, t(18)=3.1, p=0.007) and for passive-studied objects (18.3 cm versus 20.2 cm, respectively, marginal t(18)=1.8, p=0.09). Thus, spatial recall was superior for remember-location responses than for remember-other responses, supporting the notion that participants made this response when spatial information was retrieved during the recognition test.
Effects of active learning on ERP correlates of location recall
We first assessed ERP correlates of active-studied, passive-studied, and new objects as a function of response type in order to identify effects on active learning on ERPs that reflect the aforementioned effects on behavior. The focus was on ERP correlates of memory identified as significant differences between studied and new objects (“ERP old/new effects”).
For objects endorsed with remember-location responses, old/new effects differed for the active and passive learning conditions (Figure 2A). Examination of ERP waveforms suggested that old/new effects were of greater amplitude and onset earlier for active-studied objects. Moreover, ERP effects also appeared to show distributional/topographic differences based on learning condition, having a primarily anterior distribution for active-studied objects and a primarily posterior distribution for passive-studied. A three-way RM-ANOVA comparing the three conditions for the five successive latency intervals and nine electrode clusters indicated that differences between conditions differed based on latency interval and electrode cluster [F(64,1216)=2.3, p=0.02, ε=0.13]. Follow-up comparisons for each latency interval yielded significant condition-by-cluster interactions for the 200–400 ms latency interval and for all subsequent intervals [200–400ms F(16,304)=3.4, p=0.02, ε=0.21; 400–600ms F(16,304)=6.0, p=0.002, ε=0.26; 600–800ms F(16,304)=3.7, p=0.009, ε=0.24; 800–1000ms F(16,304)=3.0, p=0.02, ε=0.29].
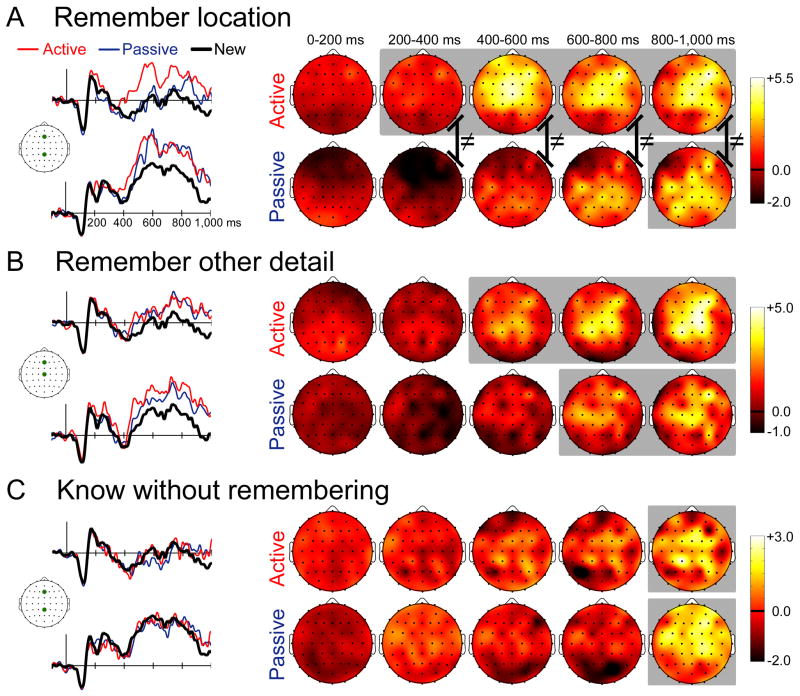
Figure 2. Electrophysiological correlates of memory retrieval.
(A) ERPs for active-studied and passive-studied objects endorsed with remember-location responses and for correctly rejected new objects are shown for two representative electrode locations marked on the cartoon plot of the head. The scalebar indicates 5μV, and positive is plotted up. Topographic plots of the old—new ERP difference are shown in 200 ms intervals starting at stimulus onset. Coloration indicates ERP difference amplitude (μV). The head is shown from a superior view, with anterior facing upward. (B) The same information is provided for active-studied and passive-studied objects endorsed with remember-other responses. (C) The same information is provided for active-studied and passive-studied objects endorsed with know responses. Gray background coloration on topographic plots indicates latency intervals for which any significant old/new differences were identified. Black lines with ≠ symbols indicate significantly different ERP topographies for the active and passive conditions.
These interactions reflected significantly more positive active-studied ERPs than either new ERPs (i.e., significant old/new effects for active-studied objects) as well as significantly more positive active-studied ERPs than passive-studied ERPs at a subset of latency intervals from 200–1000ms and at a subset of anterior and central electrode clusters. In contrast, passive-studied objects were significantly more positive than new objects only for 800–1000ms at central and posterior electrode clusters. For the 200–400 ms interval, effects of active study were observed for anterior electrode locations. Active-studied objects were more positive than new objects for the left-anterior (p=0.04) and right-anterior (p=0.04) electrode clusters. For the same interval, active-studied ERPs were also more positive than passive-studied ERPs for the left-anterior (p=0.001), mid-anterior (p=0.002), right-anterior (p=0.03), mid-central (p=0.009) and right-central (p=0.04) electrode clusters. Passive-studied ERPs were significantly less positive than new ERPs for the left-anterior (p=0.02) and mid-anterior (p=0.02) electrode clusters. Similar effects of active study were obtained for the 400–600ms interval, but positive amplitude enhancements were also observed at central electrode locations. Active-studied ERPs were significantly more positive than new ERPs at the left-, mid-, and right-anterior clusters and at the left-, mid-, and right-central clusters (p values < 0.006, 0.001, 0.001, 0.001, 0.001, and 0.006, respectively). Furthermore, active-studied ERPs were significantly more positive than passive-studied ERPs for the left-, mid-, and right-anterior electrode clusters (p values < 0.05, 0.008, and 0.05, respectively) and for the mid-central cluster (p=0.02). Similar effects of active study were also observed for the 600–800ms interval. Active-studied ERPs were significantly more positive than new ERPs for the mid-anterior, right-anterior, left-central, mid-central, and right-central electrode clusters (p values < 0.03, 0.03, 0.02, 0.001, and 0.04, respectively). Finally, similar effects were observed for the 800–1000ms interval. Active-studied ERPs were more positive than new ERPs for the mid-anterior, right-anterior, mid-central, right-central, and right-posterior electrode clusters (p values < 0.005, 0.004, 0.001, 0.003, and 0.007, respectively). Significantly positive old-new effects were also identified selectively in this latency interval for passive-studied objects. Passive-studied ERP were significantly more positive than new ERPs for the mid-central (p=0.02) and right-posterior (p=0.001) electrode clusters. In summary, active-studied ERPs tended to be significantly more positive than the other two conditions for some latency intervals 200–400ms and afterwards, and only at anterior and central electrode clusters. In contrast, passive-studied ERPs were significantly greater than new ERPs only for the latest latency interval and only at central/posterior clusters.
The aforementioned analyses suggest that ERPs for active-studied objects occur with a more anterior distribution than do passive-studied ERPs, and therefore suggest a qualitative difference between active-studied and passive-studied in terms of the relevant neural populations active during retrieval. To test for these topographic/distributional differences, we compared old minus new ERP differences for active-studied and passive-studied conditions after normalizing overall amplitude differences between conditions, given that amplitude differences could produce apparent topographic distributions as an artifact. Normalization was performed using the vector-scaling method (McCarthy & Wood, 1985), whereby a significant condition-by-electrode interaction on normalized values indicates different distributions (but not necessarily different neural generators, Urbach & Kutas, 2002). All scalp electrodes were considered. The apparent anterior vs. posterior difference for active-studied relative to passive-studied ERP old/new differences was confirmed by significant interactions for the 200–400ms latency interval and for all subsequent intervals [200–400ms F(63,1197)=3.5, p=0.002, ε=0.11; 400–600ms F(63,1197)=5.7, p<0.001, ε=0.10; 600–800ms F(63,1197)=3.1, p=0.004, ε=0.12; 800–1000ms F(63,1197)=2.2, p=0.04, ε=0.08]. These significant differences in old/new topographies are illustrated in Figure 2A. To summarize, active learning led to heightened remember-location responses that were associated with earlier-onset, greater-magnitude ERP old/new effects that occurred with a more anterior distribution relative to passive study.
Effects of active learning on ERP correlates of recalling non-location details
Next we turn to objects endorsed with remember-other responses (Figure 2B). Examination of ERP waveforms suggested that old/new effects onset earlier for active-studied objects (i.e., were of a greater amplitude at earlier latencies than were passive-studied ERP effects). However, unlike what was observed for remember-location responses, there did not appear to be topographic/distributional differences between active-studied and passive-studied effect, as both showed a mid-central topography. A three-way RM-ANOVA indicated that differences between the active-studied, passive-studied, and new conditions differed based on latency interval and electrode cluster [F(64,1216)=2.4, p=0.03, ε=0.10]. Follow-up comparisons for each latency interval yielded significant condition-by-cluster interactions for the 400–600 ms latency interval and for all subsequent intervals [400–600ms F(16,304)=4.3, p=0.04, ε=0.09; 600–800ms F(16,304)=3.7, p=0.03, ε=0.12; 800–1000ms F(16,304)=3.5, p=0.02, ε=0.16].
These interactions reflected significant positive ERP old/new effects for both active-studied and passive-studied objects at mid-central and anterior-central electrode clusters, which onset earlier for active-studied. Only active study was associated with significant old-new effects from 400–600 ms. Active-studied ERPs were significantly more positive than new ERPs for the mid-central electrode cluster for this interval (p=0.002). In contrast, both active and passive study were associated with significant old-new effects from 600–1000 ms. Active-studied and passive-studied ERPs were significantly more positive than new ERPs at the mid-central cluster for the 600–800 ms interval (p values = 0.002 and 0.04, respectively), whereas these effects were evident for both the mid-central cluster (p values < 0.001 and 0.01, respectively) and the mid-anterior cluster (p values = 0.02 and 0.008, respectively) for the 800–1000 ms interval. Unlike for objects endorsed with remember-location responses, objects endorsed with remember-other responses did not show any significant condition-by-cluster interactions on amplitude-normalized ERP values (Figure 2B), indicating that the topography/distribution of ERP old/new differences did not vary for these conditions. These findings thus suggest that the boost in remember-other responses made to active-studied vs. passive-studied objects was associated with similar old-new effects that onset earlier for the active condition.
Effects of active learning on ERP correlates of know responses
We next consider objects endorsed with know responses, which indicated a feeling of familiarity without any recollection of location or other details (Figure 2C). The three-way interaction of condition, cluster, and latency did not reach significance, nor did the main effect of condition or two way interactions involving condition. Comparisons for each latency interval separately yielded a significant condition-by-cluster interaction only for the 800–1000ms interval [F(16,304)=3.4, p=0.04, ε=0.11]. Active-studied and passive-studied ERPs were both significantly more positive than new ERPs for the mid-central cluster (p values = 0.01 and 0.007, respectively) and for the mid-anterior cluster (p values = 0.02 and 0.01, respectively). Thus, old/new effects were similar for active-studied and passive-studied objects. Topographic comparisons using the amplitude-normalized ERP values likewise failed to show distributional/topographic differences between these conditions (Figure 2C).
Cortical substrates of active-learning influences on location memory
Based on the striking anterior shift in ERP topography caused by active learning that was selective for remember-location responses (Figure 2A), we performed a source-localization analysis to identify the potential neural substrates of the distinct retrieval activity associated with active vs. passive learning. Source localization was conducted using the standardized low-resolution brain electromagnetic tomography method (LORETA; Pascual-Marqui, 2002). The ERP differences between active-studied and passive-studied objects given remember-location responses were used for source reconstruction, averaged across all participants for successive 200 ms intervals (as in the primary ERP analyses). The primary estimated neural sources (absolute maxima) are shown for each interval in Figure 3. It is noteworthy that estimated sources included dorolateral prefrontal cortex (BA 8/9) bilaterally as well as left superior parietal lobule, as we previously found that these regions show greater activity and activity that is more highly correlated with hippocampal activity during active learning than during passive learning, using the same learning paradigm as in the current experiment (Voss, Gonsalves, et al., 2011). These results therefore suggest that some of the same structures involved in active learning are also involved in the retrieval of actively learned information. Neural sources were also identified in medial aspect of BA 6, corresponding to supplementary motor area (Figure 3C). This region is strongly linked to the planning, retrieval, and execution of complex motor sequences (Forster & Brown, 2011; Wise, 1985). Modulation of activity here by active learning further suggests that location-memory retrieval for active-studied objects included information regarding the action plan executed during active learning that was relatively absent for passive-studied objects. More direct links between retrieval activity and strategic action planning were tested via the analysis of revisitation strategy that follows.
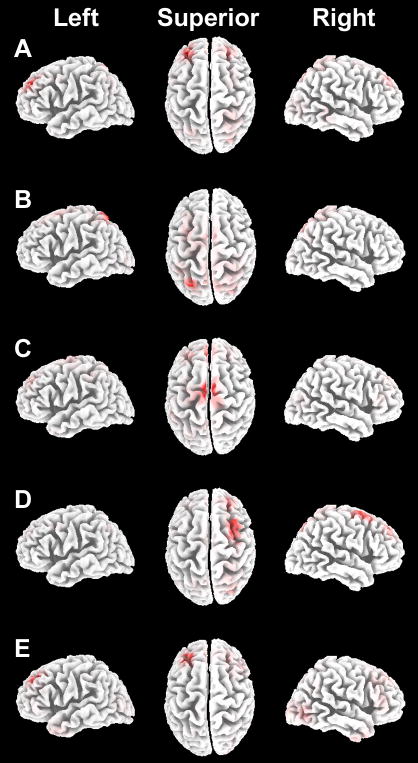
Figure 3. Estimated neural sources of ERP reactivation effects.
Estimated sources are shown for the influences of active versus passive learning on ERP correlates of remember-location responses. Results from each latency interval are shown in A—E, superimposed on a template brain shown laterally for left and right hemispheres and superiorly (with anterior oriented upward). Red coloration indicates the estimated maxima for each latency interval. Maxima for the 0–200 ms interval (A) included left and right Brodmann Area (BA) 8/9 spanning the middle and superior frontal gyri (Talairach coordinates −30, +40, +36 and +25, +38, +43, respectively). For the 200–400 ms interval (B), the maximum was left superior parietal lobule (BA 7, Talairach coordinates −24, −69, +57). For the 400–600 ms interval (C), maxima included left and right medial superior frontal gyrus (BA 6, Talairach coordinates −3, −8, +69 and +6, −11, +69, respectively) as well as more anterior left and right medial frontal gyrus (BA 8, Talairach coordinates −3, +38, +42 and +4, +39, +41, respectively). The maximum for the 600–800 ms interval (D) included right superior frontal gyrus (BA 6/8/9, Talairach coordinates +25, +39, +43). The maximum for the 800–1,000 ms interval (E) included approximately the same region of left superior frontal cortex as shown in A (BA 8/9, Talairach coordinates −27, +31, +43).
Effects of the revisitation strategy on memory performance and ERPs
Generation of the revisitation strategy during study was associated with superior later memory performance, replicating our previous demonstration of the value of this strategy for learning (Voss, Warren, et al., 2011). As in our previous experiments, self-initiated use of this strategy was necessary for performance enhancement, as no beneficial effects were produced when the same viewing pattern was merely experienced during the passive study condition. The proportion of each response type (remember location, remember other, know, and new) varied significantly with study type (active, passive) and with study strategy (revisitation, other), as indicated by a 3-way interaction [F(3,48)=3.3, p=0.03, ε=0.82; values provided in Table 2]. For the passive study condition, no response type varied for items studied with revisitation versus other items (all p values > 0.45), indicating no beneficial effects of revisitation when experienced passively. Consistent with the aforementioned effects of active study on memory, every response type for both revisitation-studied objects and other objects was indicative of superior memory for the active study condition versus the passive study condition (i.e., significantly more remember-location and remember-other responses and significantly less know and new responses, all p values < 0.04). However, the benefit of the revisitation study strategy was only apparent in remember-location responses. These responses were made more for revisitation-studied objects versus other objects in the active study condition (p=0.04), thus demonstrating a selective benefit of self-initiated revisitation on subsequent location recall. Concomitantly, new responses were less frequent for active revisitation-studied objects than other objects (p=0.001). This selective enhancement of remember-location responses associated with revisitation study was also reflected in spatial recall performance measured a week later during follow-up testing. Placement error during the spatial recall test was significantly lower for actively revisitation-studied objects (17.6 cm) versus actively other-studied objects (20.4 cm, p=0.0001). Revisitation-studied objects in the passive condition did not show the same benefit versus other-studied objects (19.1 vs. 20.1 cm, respectively, p=0.19), and error was significantly less for active revisitation-studied versus passive revisitation-studied objects (p=0.01). Thus, self-generated active revisitation selectively enhanced location recall immediately after study and also spatial recall performance measured after one week.
Table 2.
| Remember location | Remember other | Know | New | |
|---|---|---|---|---|
| Active | ||||
| Revisitation | 0.43 (0.05) | 0.32 (0.04) | 0.16 (0.03) | 0.09 (0.02) |
| Other | 0.29 (0.03) | 0.28 (0.02) | 0.22 (0.03) | 0.21 (0.03) |
| Passive | ||||
| Revisitation | 0.18 (0.03) | 0.20 (0.03) | 0.30 (0.03) | 0.32 (0.04) |
| Other | 0.19 (0.02) | 0.20 (0.02) | 0.28 (0.03) | 0.33 (0.03) |
Mean proportion of responses for each stimulus category and recognition response type in the revisitation analysis. Parentheses indicated SE.
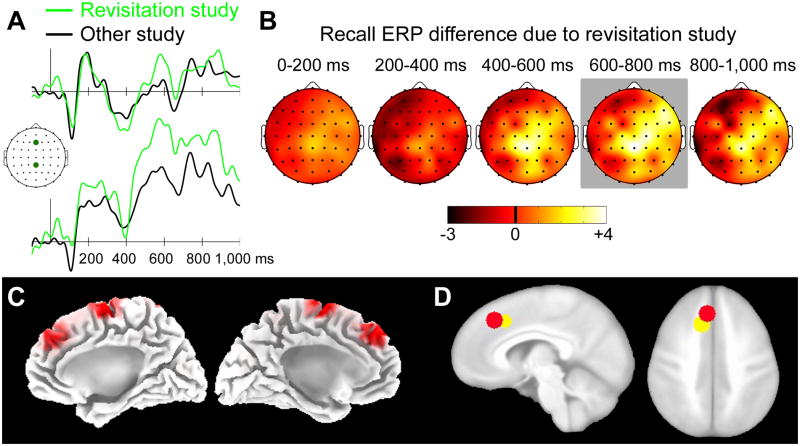
We therefore focused on effects of active revisitation on ERP correlates of remember-location responses, as revisitation did not influence other “old” response types for active study, nor did it influence performance for passive study. ERPs for this response type following active study were more positive, especially at central electrode locations, for revisitation-studied objects compared to other-studied objects (Figure 4AB). Statistical analyses of these two conditions for each of the nine electrode clusters performed separately for each 200 ms latency interval indicated that conditions differed significantly only for the 600–800 ms interval, which showed significant condition-by-electrode cluster interaction [F(8,104)=2.7, p=0.04, ε=0.01]. ERPs were more positive for revisitation-studied objects than for other objects at the mid-central and right-anterior electrode clusters (p=0.004 And p=0.03, respectively; p values for all other clusters > 0.14). Source localization of this 600–800 ms ERP difference due to active revisitation identified generators in supplementary motor cortex (BA 6) and primary motor cortex (Figure 4C). Notably, there was a high degree of overlap between the modeled supplementary motor cortex generator and the region of supplementary motor cortex identified in association with revisitation during study using fMRI in our previous report (Figure 4D; see also Figure 4 of Voss, Warren, et al., 2011). Although neural generators estimated using the LORETA technique are spatially diffuse, and thus the modeled supplementary motor generator taken in its entirety completely encompassed the supplementary motor region identified with fMRI, substantial overlap was identified even when using the more conservative approach of centering relatively small spheres on the peak voxel within each fMRI-defined and LORETA-defined region (Figure 4D). Thus, source localization indicated that nearly the same region of supplementary motor cortex activated in association with revisitation during study exhibited reactivation within 600–800 ms of stimulus onset during retrieval.
Figure 4. ERP reactivation related to the revisitation study strategy.
(A) ERPs for actively studied objects given remember-location responses are shown separately for revisitation-studied and other-studied objects for two representative electrode locations marked on the cartoon plot of the head. The scalebar indicates 5μV, and positive is plotted up. (B) Topographic plots of the revisitation—other ERP difference are shown in 200 ms intervals starting at stimulus onset. Coloration indicates ERP difference amplitude (μV). The head is shown from a superior view, with anterior facing upward. Gray background coloration indicates latency intervals for which any significant old/new differences were identified. (C) Estimates sources for the revisitation—other ERP difference from 600–800 ms are shown superimposed on the medial aspect of a template brain. Red coloration indicates the estimated maxima for each latency interval. The supplementary motor cortex (more anterior) activation maximum was identified at Talairach coordinates −6, +30, +47 for the left hemisphere and +7, +31+43 for the right hemisphere. The primary motor cortex (more posterior) activation maximum was identified at Talairach coordinates −3, −11, +70 for the left hemisphere and +4, −10, +65 for the right hemisphere. (D) A red sphere (radius = 8 mm) is shown centered on the maximum of the left-hemisphere supplementary motor cortex estimated source and a yellow sphere (same radius) is shown centered on the supplementary motor cortex maximum defined as revisitation-related fMRI activity during study (Figure 4A of Voss, Warren, et al., 2011), both superimposed on a template brain.
Discussion
As in our previous experiments using the same learning paradigm (Voss, Gonsalves, et al., 2011), we found higher recognition memory accuracy for objects studied with active control of visual exploration versus objects studied passively. This active-learning advantage for memory occurred despite the fact that visual information was matched between the active and passive study conditions. Evidence for reactivation was identified selectively when subjects claimed to recall the spatial location of objects (a claim that was validated by better spatial memory performance for these objects tested one week later relative to objects claimed to be recalled on non-spatial grounds). When these responses were made, ERP correlates of memory retrieval showed a pronounced frontal shift in distribution for objects studied actively relative to objects studied passively. This frontal shift in ERPs mirrored the pattern of activity identified during study using fMRI (Voss, Gonsalves, et al., 2011). fMRI measures showed that active study was associated with greater frontal involvement than passive study, and the degree of greater frontal involvement specifically predicted spatial memory performance, as opposed to non-spatial performance.
Analysis of ERP neural generators in the current experiment showed that many of the same frontal cortical regions associated with active learning in the fMRI study (and specifically active spatial learning) showed activity here during retrieval, selectively for when spatial information was retrieved. We thus infer that the strategic processing supported by these regions that enhanced location memory performance was encoded and retrieved in a stimulus-specific manner. Notably, the active/passive manipulation was essential for showing that the prefrontal involvement at encoding and its reactivation concerned strategic study information, rather than, for instance, nonspecific aspects of spatial encoding and retrieval. This is because spatial memory was also quite successful for the passive condition, yet the prefrontal shift in fMRI activity at encoding and concomitant prefrontal shift in ERP activity at retrieval were selective for active study, when neural measures should be expected to uniquely include active strategic processing (whereas processing related generally to spatial encoding and retrieval would be expected in both study conditions). Nonetheless, it is possible that prefrontal shifts in neural activity at both encoding and retrieval actually reflected other nonspecific processing rather than true reactivation of the same neural processing. Our analysis of the revisitation strategy provides greater specificity in linking encoding activity to retrieval activity by examining both in the context of a particular study strategy with unique neural correlates that could be examined for reactivation effects.
Subjects in the current experiment generated a revisitation study strategy, involving looking back to study some recently seen adjacent objects for a second time, with approximately the same frequency as we previously identified in a larger group of subjects using the same study paradigm (Voss, Warren, et al., 2011). As in our previous report, memory performance was significantly enhanced for objects studied using this strategy, but only when the strategy was self-generated in the active condition. No memory benefits were obtained when the same pattern was merely viewed in the passive condition. Moreover, the current findings indicate that location recall specifically was enhanced by this strategy, as was spatial recall performance measured following a one-week delay. Active generation of this strategy during study was associated with fMRI activity enhancements in left supplementary motor cortex (as well as hippocampus and cerebellum), which was taken as evidence for strategic action planning, given that the contralateral (right) hand was used to control study viewing (Voss, Warren, et al., 2011). We reasoned that activity in the same region of supplementary motor cortex during retrieval would provide strong evidence for reactivation of action-planning information, given that this region was well-delineated from other encoding-related regions and is specifically related to action-plan generation rather than to nonspecific memory encoding processes (Forster & Brown, 2011; Wise, 1985). The revisitation strategy was associated with enhanced ERP positivity for location-recall responses during retrieval, and the estimated neural generator of this enhancement overlapped substantially with the supplementary motor region identified using fMRI during study (Figure 4). These findings therefore suggest reactivation of the same region of supplementary motor cortex associated with the generation of this strategy. Moreover, the functional specificity of this region for strategic action planning is much more definitive than for other regions of frontal cortex identified in the main analysis of active versus passive study, and was specifically related to the strategic revisitation study behavior. Thus, reactivation of this region can be taken as evidence that information relevant to strategic action planning was encoded and subsequently retrieved in a stimulus-specific manner.
These findings extend previous reports of strategy-related and action-related reactivation during retrieval (e.g., Johnson & Rugg, 2007; Nilsson, et al., 2000; Nyberg, et al., 2001; Senkfor, 2008; Senkfor, et al., 2002). As noted above, the extent to which these previous experiments provided evidence for reactivation as opposed to stimulus-specific mental imagery or other more general retrieval phenomena is uncertain. For example, activity related to action sequence planning generated in response to objects studied as part of actions need not reflect storage and retrieval of specific action plans, but could instead merely reflect retrieval of the action-sequence information that comprises part of long-term object representations. Indeed, viewing objects that can be manipulated cues motor-related activity even when experiments do not involve movement (Lewis, 2006), indicating that motor related activity can reflect general object-knowledge retrieval and need not reflect reactivation. The paradigm used in the current experiment involves action sequences and strategies that produce activity in frontal cortical structures over extended periods of time (approximately 60 s) during active learning (Voss, Warren, et al., 2011), yet evidence for activity in these same structures during retrieval was identified in a stimulus-specific manner within several hundred milliseconds in the current experiment. This reactivation pattern is more consistent with the notion that elements of the strategic processing and action sequences generated during active learning were bound into the representation of some objects, and retrieved/reactivated during test. Interestingly, evidence for this reactivation occurred when subjects reported spatial recall, and this is consistent with the notion that the quality of recall can depend on the extent of reactivation (e.g., Daselaar, et al., 2008). Future research could explore why this strategy-related and action-related information was presumably more integrated with a subset of actively studied objects, thereby being expressed as reactivation for spatial recall responses as opposed to non-spatial recall responses.
It is particularly interesting that supplementary motor cortex activity was noted within 600 ms of stimulus onset, and the timecourse of ERPs reactivation effects occurred very rapidly, starting only 200 ms after the retrieval cue. This suggests that the pace of reactivation was far more rapid relative to the extended duration of relevant activity during study. This observation converges with other evidence indicating that the timeframe of reactivation is generally compressed relative to the timing of the original activity sequences. For instance, hippocampal neurons in rats fire with specific patterns during exploratory behavior, and these same activity sequences are replayed during subsequent periods of behavioral quiescence such as inactivity and sleep [(e.g., Wilson & McNaughton, 1994) reviewed in (O’Neill, Pleydell-Bouverie, Dupret, & Csicsvari, 2010)]. Notably, sequence replay during sleep occurs on a much faster timescale than the sequence observed during the original behavior (Ji & Wilson, 2007; Lee & Wilson, 2002). It is important to note that our demonstration of neural reactivation is tentative in the sense that direct evidence for reactivation would require recording the activity of individual neurons during learning and retrieval, as in experiments on hippocampal replay in rodents. Thus, although our neural-population-level findings are consistent with the rodent literature, we cannot show unequivocally that reactivation has occurred, and thus only emphasize the parallels to the timeframe of single-unit reactivation.
In conclusion, our results suggest that activity related to generating the action plans that allow one to control the intake of visual information during active learning, as well as information regarding strategic processing under such conditions (i.e., revisitation strategy) is bound into memory representations of specific objects and shows reactivation during memory retrieval. Direct evidence for true neural recapitulation will require neurophysiological recordings that permit a higher degree of certainty in the identification of overlap between encoding activity and retrieval activity (i.e., overlap in spatial and temporal dynamics). Our results nonetheless provide highly suggestive evidence that strategic and action-planning information from encoding episodes is incorporated into episodic memory representations for specific stimuli and that this information is retrieved rapidly in response to seeing these stimuli again during memory testing.
Highlights of the current research.
Memory performance was superior following active study versus passive study.
This active-learning benefit was pronounced for spatial location memory.
ERP correlates of spatial retrieval differed for active versus passive study.
Reactivation of the brain regions involved in study occurred during retrieval.
This reactivation reflected higher-order operations involved in active study.
Footnotes
Note also that a central assumption of the influential source/reality-monitoring framework (Johnson, Hashtroudi, & Lindsay, 1993) is that various cognitive operations are indeed encoded and reactivated at retrieval. These operations presumably differ for various sources, such as perceived versus imagined events, thus enabling memory decisions for the discrimination of these sources.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bernstein NA. On dexterity and its development. In: Latash ML, Turvey MT, editors. Dexterity and its development. Mahwah: Lawrence Erlbaum Associates; 1996. [Google Scholar]
- Buckner RL, Wheeler ME. The cognitive neuroscience of remembering. Nature Reviews Neuroscience. 2001;2:624–634. doi: 10.1038/35090048. [DOI] [PubMed] [Google Scholar]
- Chatrian GE, Lettich E, Nelson PL. Modified nomenclature for the “10%” electrode system. Journal of Clinical Neurophysiology. 1988;5:183–186. [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia, and the hippocampal system. Cambridge: MIT Press; 1993. [Google Scholar]
- Conway MA, Pleydell-Pearce CW. The construction of autobiographical memories in the self-memory system. Psychological Review. 2000;107:261–288. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Time-locked multiregional retroactivation: A systems-level proposal for the neural substrates of recall and recognition. Cognition. 1989;33:25–62. doi: 10.1016/0010-0277(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Danker JF, Anderson JR. The ghosts of brain states past: Remembering reactivates the brain regions engaged during encoding. Psychological Bulletin. 2010;136:87–102. doi: 10.1037/a0017937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Rice HJ, Greenberg DL, Cabeza R, LaBar KS, Rubin DC. The spatiotemporal dynamics of autobiographical memory: Neural correlates of recall, emotional intensity, and reliving. Cerebral Cortex. 2008;18:217–229. doi: 10.1093/cercor/bhm048. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: Memory systems of the brain. Oxford: Oxford University Press; 2004. [Google Scholar]
- Foley MA, Foley HJ. Source-monitoring judgments about anagrams and their solutions: Evidence for the role of cognitive operations information in memory. Memory & Cognition. 2007;35:211–221. doi: 10.3758/bf03193442. [DOI] [PubMed] [Google Scholar]
- Forster SE, Brown JW. Medial prefrontal cortex predicts and evaluates the timing of action outcomes. Neuroimage. 2011;55:253–265. doi: 10.1016/j.neuroimage.2010.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Castro CA, McNaughton BL. Spatial selectivity of rat hippocampal neurons: dependence on preparedness for movement. Science. 1989;244:1580–1582. doi: 10.1126/science.2740902. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD. Cognitive neuroscience of human memory. Annual Review of Psychology. 1998;49:87–115. doi: 10.1146/annurev.psych.49.1.87. [DOI] [PubMed] [Google Scholar]
- Gardiner JM, Java RI. Forgetting in recognition memory with and without recollective experience. Memory & Cognition. 1991;19:617–623. doi: 10.3758/bf03197157. [DOI] [PubMed] [Google Scholar]
- Goldberg RF, Perfetti CA, Schneider W. Perceptual knowledge retrieval activates sensory brain regions. Journal of Neuroscience. 2006;26:4917–4921. doi: 10.1523/JNEUROSCI.5389-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalves B, Paller KA. Brain potentials associated with recollective processing of spoken words. Memory & Cognition. 2000;28:321–330. doi: 10.3758/bf03198547. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Smith AP, Rugg MD, Dolan RJ. Remembrance of odors past: Human olfactory cortex in cross-modal recognition memory. Neuron. 2004;42:687–695. doi: 10.1016/s0896-6273(04)00270-3. [DOI] [PubMed] [Google Scholar]
- Heil M, Rolke B, Engelkamp J, Rosler F, Ozcan M, Hennighausen E. Event-related brain potentials during recognition of ordinary and bizarre action phrases following verbal and subject-performed encoding conditions. European Journal of Cognitive Psychology. 1999;11:261–280. [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nature Neuroscience. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Rugg MD. Recollection and the reinstatement of encoding-related cortical activity. Cerebral Cortex. 2007;17:2507–2515. doi: 10.1093/cercor/bhl156. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Psychological Bulletin. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Kahn I, Davachi L, Wagner AD. Functional-neuroanatomic correlates of recollection: Implications for models of recognition memory. Journal of Neuroscience. 2004;24:4172–4180. doi: 10.1523/JNEUROSCI.0624-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S, Lloyd D, Nurmikko T, Roberts N. Retrieving autobiographical memories of painful events activates the anterior cingulate cortex and inferior frontal gyrus. Journal of Pain. 2007;8:307–314. doi: 10.1016/j.jpain.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Khader P, Burke M, Bien S, Ranganath C, Rosler F. Content-specific activation during associative long-term memory retrieval. Neuroimage. 2005;27:805–816. doi: 10.1016/j.neuroimage.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Khader P, Heil M, Rosler F. Material-specific long-term memory representations of faces and spatial positions: Evidence from slow event-related brain potentials. Neuropsychologia. 2005;43:2109–2024. doi: 10.1016/j.neuropsychologia.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Thompson WL, Alpert NM. Neural systems shared by visual imagery and visual perception: A positron emission tomography study. Neuroimage. 1997;6:320–334. doi: 10.1006/nimg.1997.0295. [DOI] [PubMed] [Google Scholar]
- Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36:1183–1194. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- Lewis JW. Cortical networks related to human use of tools. Neuroscientist. 2006;12:211–231. doi: 10.1177/1073858406288327. [DOI] [PubMed] [Google Scholar]
- Maidhof C, Rieger M, Prinz W, Koelsch S. Nobody is perfect: ERP effects prior to performance errors in musicians indicate fast monitoring processes. PLoS One. 2009;4:e5032. doi: 10.1371/journal.pone.0005032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV. Neural correlates of category-specific knowledge. Nature. 1996;379:649–652. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Wood CC. Scalp distributions of event-related potentials: An ambiguity associated with analysis of variance models. Electroencephalography and Clinical Neurophysiology. 1985;62:203–208. doi: 10.1016/0168-5597(85)90015-2. [DOI] [PubMed] [Google Scholar]
- Nilsson LG, Nyberg L, Klingberg T, Aberg C, Persson J, Roland PE. Activity in motor areas while remembering action events. Neuroreport. 2000;11:2199–2201. doi: 10.1097/00001756-200007140-00027. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Petersson KM, Nilsson LG, Sandblom J, Aberg C, Ingvar M. Reactivation of motor brain areas during explicit memory for actions. Neuroimage. 2001;14:521–528. doi: 10.1006/nimg.2001.0801. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Pleydell-Bouverie B, Dupret D, Csicsvari J. Play it again: Reactivation of waking experience and memory. Trends in Neuroscience. 2010;33:220–229. doi: 10.1016/j.tins.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Paller KA. Consolidating dispersed neocortical memories: The missing link in amnesia. Memory. 1997;5:73–88. doi: 10.1080/741941150. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods & Findings in Experimental & Clinical Pharmacology. 2002;24D:5–12. [PubMed] [Google Scholar]
- Roission B, Pourtois G. Revisiting Snodgrass and Vanderwart’s object set: The role of surface detail in basic-level object recognition. Perception. 2004;3:217–236. doi: 10.1068/p5117. [DOI] [PubMed] [Google Scholar]
- Schacter DL. Implicit expressions of memory in organic amnesia: Learning of new facts and associations. Human Neurobiology. 1987;6:107–118. [PubMed] [Google Scholar]
- Senkfor AJ. Memory for pantomimed actions versus actions with real objects. Cortex. 2008;44:820–833. doi: 10.1016/j.cortex.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkfor AJ, Van Petten C, Kutas M. Episodic action memory for real objects: An ERP investigation with perform, watch, and imagine action encoding tasks versus a non-action encoding task. Journal of Cognitive Neuroscience. 2002;14:402–419. doi: 10.1162/089892902317361921. [DOI] [PubMed] [Google Scholar]
- Shapiro ML, Kennedy PJ, Ferbinteanu J. Representing episodes in the mammalian brain. Current Opinion in Neurobiology. 2006;16:701–709. doi: 10.1016/j.conb.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nature Reviews Neuroscience. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Smith AP, Henson RN, Dolan RJ, Rugg MD. fMRI correlates of the episodic retrieval of emotional contexts. Neuroimage. 2004;22:868–878. doi: 10.1016/j.neuroimage.2004.01.049. [DOI] [PubMed] [Google Scholar]
- Song EY, Kim YB, Kim YH, Jung MW. Role of active movement in place-specific firing of hippocampal neurons. Hippocampus. 2005;15:8–17. doi: 10.1002/hipo.20023. [DOI] [PubMed] [Google Scholar]
- Stock O, Roder B, Burke M, Bien S, Rosler F. Cortical activation patterns during long-term memory retrieval of visually or haptically encoded objects and locations. Journal of Cognitive Neuroscience. 2009;21:58–82. doi: 10.1162/jocn.2009.21006. [DOI] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Journal of Psychology. 1985;26:1–12. [Google Scholar]
- Underwood BJ. Attributes of memory. Psychological Review. 1969;76:559–573. [Google Scholar]
- Urbach TP, Kutas M. The intractability of scaling scalp distributions to infer neuroelectric sources. Psychophysiology. 2002;39:791–808. doi: 10.1111/1469-8986.3960791. [DOI] [PubMed] [Google Scholar]
- Voss JL, Gonsalves BD. Time to go our separate ways: Opposite effects of study duration on priming and recognition reveal distinct neural substrates. Frontiers in Human Neuroscience. 2010;4:227. doi: 10.3389/fnhum.2010.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Gonsalves BD, Federmeier KD, Tranel D, Cohen NJ. Hippocampal brain-network coordination during volitional exploratory behavior enhances learning. Nature Neuroscience. 2011;14:115–120. doi: 10.1038/nn.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Warren DE, Gonsalves BD, Federmeier KD, Tranel D, Cohen NJ. Spontaneous revisitation during visual exploration as a link among strategic behavior, learning, and the hippocampus. Proceedings of the National Academy of Sciences USA. 2011;108:E402–409. doi: 10.1073/pnas.1100225108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Wise SP. The primate premotor cortex: Past, present, and preparatory. Annual Review of Neuroscience. 1985;8:1–19. doi: 10.1146/annurev.ne.08.030185.000245. [DOI] [PubMed] [Google Scholar]