Abstract
The appropriate regulation of intracellular calcium is a requirement for proper cell function and survival. This review focuses on the effects of proinflammatory cytokines on calcium regulation in the insulin-producing pancreatic beta-cell and how normal stimulus-secretion coupling, organelle function, and overall beta-cell viability are impacted. Proinflammatory cytokines are increasingly thought to contribute to beta-cell dysfunction not only in type 1 diabetes (T1D), but also in the progression of type 2 diabetes (T2D). Cytokine-induced disruptions in calcium handling result in reduced insulin release in response to glucose stimulation. Cytokines can alter intracellular calcium levels by depleting calcium from the endoplasmic reticulum (ER) and by increasing calcium influx from the extracellular space. Depleting ER calcium leads to protein misfolding and activation of the ER stress response. Disrupting intracellular calcium may also affect organelles, including the mitochondria and the nucleus. As a chronic condition, cytokine-induced calcium disruptions may lead to beta-cell death in T1D and T2D, although possible protective effects are also discussed. Calcium is thus central to both normal and pathological cell processes. Because the tight regulation of intracellular calcium is crucial to homeostasis, measuring the dynamics of calcium may serve as a good indicator of overall beta-cell function.
Keywords: islets, beta-cells, cytokines, interferon, tumor necrosis factor, interleukin, inflammation, inflammatory, calcium, insulin, biphasic, mitochondria, endoplasmic reticulum, ER stress, nuclear calcium
1. Overview
In the pancreatic beta-cell, cytokine-induced disruptions in calcium handling can impair insulin release in response to glucose stimulation, and more severe calcium disruptions can lead to cell death. The focus of this review is on calcium’s role in cytokine-mediated dysfunction and death of pancreatic islets and the potential role of key calcium handling organelles: the endoplasmic reticulum, mitochondria, nucleus and cytosolic spaces [3,4]. Detailed descriptions of the molecular mechanisms of cytokine action and their signaling pathways are not included but can be found in several excellent reviews [5-10].
2. Normal islet function in response to glucose stimulation
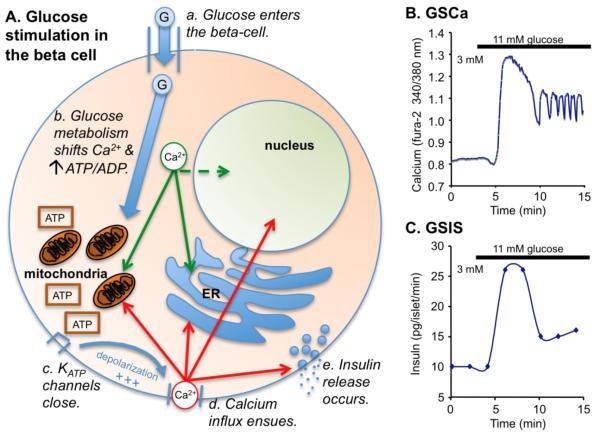
Islets of Langerhans are micro-organs within the pancreas that are responsible for regulating blood glucose and body energy metabolism [11]. The islet is composed of insulin-producing beta-cells (~60-80% of the total islet mass in rodents), glucagon-secreting alpha-cells (10-30%), somatostatin-secreting delta-cells (5-10%), and others [15,16]. At the level of the individual beta-cell, the ‘Consensus Model’ provides a detailed description of the cellular response to glucose stimulation [17-19]. As shown in Figure 1A (a-e), glucose is taken up through glucose transporters [20,21] and then metabolized in the beta-cell through glycolysis and the tricarboxylic acid (TCA) cycle to increase ATP production (for a more detailed description of these processes, see [22,23]). During this time, calcium levels in the mitochondria and ER increase in response to glucose stimulation, which causes an overall dip in cytosolic calcium as shown in Figure 1B. The resulting increase in the ATP-to-ADP ratio closes ATP-sensitive potassium channels (KATP-channels), which initiates a large spike in calcium influx and the first phase of insulin release. Following this initial burst, calcium and insulin release rates remain elevated throughout the second phase response, which continues as long as glucose remains elevated.
Figure 1.
Stimulus-secretion coupling in the pancreatic beta-cell. (A) The processes involved with glucose uptake to insulin release are described in steps a-e. Note that green arrows denote the redistribution of intracellular calcium, and red arrows indicate the targets of calcium influx. (B-C) This process can be recorded in real-time by the changes in calcium (B) and insulin release (C). Panel (C) was modified with permission from the Am Physiol Soc (Nunemaker et al, Am J Physiol-Endo and Metab, 2006 ([197]).
Because calcium is a strong trigger of exocytosis, both glucose-stimulated calcium (GSCa, Figure 1B) and glucose-stimulated insulin secretion (GSIS, Figure 1C) show similar trajectories under these conditions. GSCa can thus be used to assess the physiological response of islets to glucose stimulation. Calcium imaging is advantageous because it provides high temporal precision of real-time changes in response to stimuli at the level of the individual beta-cell. Changes in the latency, trajectory, and amplitude of the triphasic GSCa response may indicate specific defects in stimulus-secretion coupling or other aspects of islet dysfunction. To be complete, amplifying processes also operate in parallel with the pathways of the Consensus Model to couple glucose uptake and metabolism with insulin exocytosis [24-27]. These processes allow additional insulin release to occur independently of changes in calcium. GSCa thus provides a reasonable first approximation of GSIS and can provide a good estimate of overall islet health and function.
3. Cytokines, calcium, and islet dysfunction
Pro-inflammatory cytokines are broadly considered immunomodulators that consist of several families of signaling molecules, including interleukins and interferons. Cytokines play a prominent role in the pathophysiology of type 1 diabetes (T1D) [28-32], but increasing evidence suggests a significant role for cytokines in the loss of beta-cell mass in T2D as well [34,35]. There are notable similarities and differences in the action of cytokines on the beta-cell between T1D versus T2D [35-37]. First, in T1D, beta-cells are the direct target of an autoimmune invasion that results in insulitis and beta-cell death [37]. Whereas, in T2D metabolic stress is thought to activate the innate immune system, resulting in a chronic inflammatory state marked by increased cytokines, increased islet-associated macrophages, and beta-cell apoptosis [37-39]. Second, a number of factors contribute to beta-cell decline and destruction in T2D including glucotoxicity, lipotoxicity, ER stress, oxidative stress, and amyloid deposition [40-46], any of which can additionally trigger an inflammatory response. In fact, evidence is emerging that all of these factors may even be centrally linked with inflammasome activation [47-49]. Third, the most dominant pro-inflammatory cytokines in T1D are tumor necrosis factor alpha (TNF-a), interleukin (IL)-1B, and interferon-gamma (IFN-g). These cytokines are secreted by infiltrating immune cells at high concentrations in close proximity to the beta-cell and act synergistically to inflict direct inhibitory and cytotoxic effects [32,33,50,51]. In T2D, a chronic low-grade inflammatory state is thought to involve lower levels of cytokines that are produced by adipose tissue [52,53], islet-associated macrophages [54,55], and the islets themselves [7].
These differences in disease progression between T1D and T2D result in islet exposure to different extracellular cytokine concentrations, durations of exposure, and cytokine milieu, which can markedly affect cytokine signaling and net effects on the beta-cell. For example, short-term treatment with low-dose IL-1B may improve the function of rodent islets, whereas long-term, high-dose treatment impairs islet function [56-58] and accelerates the development of T1D [59]. Low-dose TNF-a treatment has been shown to inhibit the autoimmune response in models of T1D to slow the disease process [60-62], whereas TNF-a and IFN-g produce dose- and duration-dependent islet dysfunction in vitro when acting in concert [63,64]. In the context of T2D, the duration of low-grade inflammation may be a key factor, as cytokines could have compensatory/protective effects initially that become deleterious under chronic conditions. Differences among duration and dose of cytokine treatment [58,65], species being tested [66-68], and combination of cytokines [32,66,69,70] are thus all important factors that contribute to both positive or negative effects on the beta-cell. In addition, synergistic activity among multiple cytokines can alter or amplify signaling pathways [32,70-72], adding an additional layer of complexity to cytokine action.
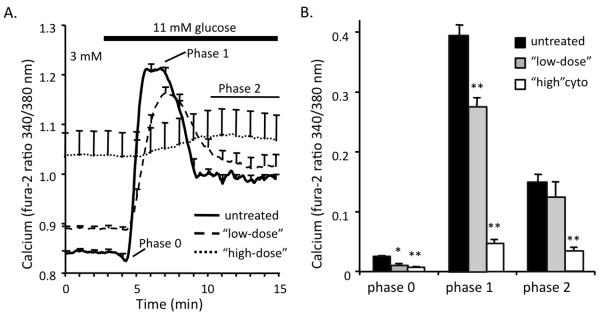
A number of proinflammatory cytokines are found at serum levels 2-5x higher in obese individuals compared to lean individuals, and these increased levels are associated with increased risk of developing T2D [73-75]. This low-grade systemic inflammation may also play a direct role in triggering beta-cell dysfunction, particularly in T2D. We have previously reported that cytokines can directly affect calcium handling in rodent islets at circulating concentrations in vitro [76,77]. As shown in Figure 2, we present similar findings comparing islets treated overnight with a ‘low-dose’ cytokine treatment to mimic circulating cytokine levels in rodents (slightly higher than found in human serum) or a ‘high-dose’ cytokine treatment to mimic concentrations associated with direct immune assault in T1D [67,70,78], which is thought to be ~100-1000 fold higher than found in serum. First note that treatment with cytokines dose-dependently elevated the basal level of calcium in low glucose prior to stimulation. Prolonged excess calcium is a well-established trigger of beta-cell death [79]. Following glucose stimulation, the phase 0 response associated with increased calcium uptake by the endoplasmic reticulum was dose-dependently reduced by cytokines. The low-dose cytokine treatment also attenuated phase 1 response without affecting the phase 2 response as measured relative to basal calcium levels. Of interest, loss of first phase insulin secretion without the loss of second phase secretion is an early sign of islet dysfunction in both T1D and T2D [80]. The high-dose cytokine treatment substantially impaired all phases of the calcium response, as well as producing significant cell death in overnight culture (see also [76]). These data suggest that cytokines can harm beta-cells in a graded manner by impairing ER calcium handling and calcium flux across the plasma membrane. We note, however, inasmuch as stimulatory and protective effects have been observed with lower concentrations as discussed above, cytokine action in the beta-cell results in more complex and multi-faceted interactions than understood at present. The remaining sections of this review focus on four major calcium handling organelles (the plasma membrane, the ER, mitochondria, and nucleus) and how each may be involved with cytokine-induced changes in [Ca2+]i.
Figure 2.
(A) Glucose-stimulated [Ca2+]i responses from islets after overnight treatment with “high” (0.5 ng/ml IL1-B + 1 ng/ml TNF-a + 10 ng/ml IFN-g, n=10), “low” (5 pg/ml IL1-B + 10 pg/ml TNF-a + 100 pg/ml IFN-g, n=11), or left untreated (n=18). (B) Dose-dependent cytokine effects were observed in phases 0, 1, and 2. *P<0.01, **P<0.001. These data were collected from islets that were isolated by our published protocol [198] and are in agreement with our published findings as described previously [76,77].
4. Cytokines and cell membrane sources of [Ca2+]i
The influx of calcium through various ion channels and exchangers in the cell membrane provides a key source of [Ca2+]i for the signaling cascades of proinflammatory cytokines [46,79,81,82]. Although the L-type calcium channel is dominant and considered critical to normal islet function, several other subtypes may also be physiologically relevant including the N-type and T-type calcium-channels [83,84]. The subunits that form these various channels can also vary, thus affecting voltage-sensing, Ca2+-conducting channel activation, inactivation, and current amplitude [85,86]. In addition, changes in the expression, regulation, and function of these calcium-channels, as well as the insertion and removal of the channels from the membrane, and the association of the channel with protein complexes in the membrane (the exocytotic machinery for example [87]) can all affect calcium flux into the beta-cell. Further, modulation of other voltage-gated ion channels, including the KATP-channel and Kv-channels, will alter the membrane potential to make calcium influx more or less favorable. A number of K+-channels, including BK [88,89], SK [90] and the KSlow current [91,92] are also calcium-sensitive, and their activity can affect calcium flux via their effects on the membrane potential. Finally, the distribution and movement of calcium upon entering the beta-cell is intricate and crucial to normal islet function [93]. Thus, cytokines and other factors could indirectly regulate calcium flux in many ways. This is particularly pertinent to cytokine action since effects on calcium occur over a period of hours as opposed to the comparatively rapid effects of glucose-stimulated calcium changes on the order of seconds to minutes. No acute cytokine effects on the order of seconds have been observed on calcium-channels to our knowledge.
With regard to cytokines, several studies in the late-1980s provided evidence that cytokine action was linked with calcium influx, suggesting that the cytokine IL-1 may alter calcium flux across the beta-cell membrane to produce beta-cell dysfunction or toxicity [94,95]. Subsequent studies have suggested an important permissive role of calcium influx in cytokine-mediated cell death. For example, blocking L-type calcium channels prevents cell death caused by IL-1B/IFN-g treatment [64] in insulin cell lines and prevents IL-1B-induced apoptosis in rodent [97,98] and human islets [99]. Several signaling pathways related to IL-1B are also dependent on calcium influx through L-type calcium channels, including ERK1/2, p38, and JNK [97,99,100].
Sodium/calcium exchangers (NCXs), plasma-membrane Ca2+ATPases (PMCAs), and other calcium regulators may also contribute to cytokine effects. Under normal conditions, NCXs play an active role in calcium homeostasis, accounting for up to 70% of calcium extrusion and also participating in calcium influx in response to membrane depolarization [101]. Overexpression of NCX can deplete ER calcium and induce apoptosis in insulin-secreting BRIN-BD11 cells [101]. Similarly, PMCAs play a key role in calcium homeostasis, with over expression resulting in apoptosis by calcium depletion from both the ER and mitochondria [102]. Cytokines appear to downregulate PMCA expression in RINm5F cells [103], and reduced PMCA expression decreases TNF-induced cell death in L929 cells [104]. Insulin resistance also appears to downregulate PMCA expression and increase [Ca2+]i in rat islets [105]. Collectively, these data present a complex picture regarding the role of NCXs and PMCAs warranting further study. Other ion channels may also influence beta-cell [Ca2+]i [84], although little is known about cytokine sensitivity.
Even without inducing cell death, cytokine-mediated changes in calcium flux can impact beta-cell function in numerous ways. Early studies showed that IL-1 inhibits glucose-stimulated calcium influx [94], but others have shown stimulatory effects of IL-1 on insulin secretion that may depend on diacylglycerol production and protein kinase C activation [106,107] rather than changes in [Ca2+]i [107]. Interleukin-6 (IL-6) has been shown to increase insulin secretion and preproinsulin mRNA expression by mechanisms that rely on calcium influx [108]. A recent study suggests that IL-6 enhances insulin secretion through involvement of the PLC-IP3 pathway [109]. In contrast, numerous studies have also shown inhibitory effects of IL-6 in combination with other inflammatory cytokines [110,111]. We recently reported that the combination of IL-6 and IL-1B at relatively low concentrations can elevate calcium levels in low glucose and attenuate glucose-stimulated calcium responses in mouse islets [77]. Our previous work suggests that calcium influx through L-type calcium channels is at least partly responsible for these cytokine-mediated effects[76]. As mentioned previously, differing effects on islet function may depend upon a variety of factors, chiefly the dose and combination of cytokines used [32,66,67,70].
Calcium influx also appears to be central to beta-cell dysfunction in several models of diabetes. In the NOD mouse model of T1D, increased expression of the low-voltage activated (LVA) T-type calcium channel was observed in beta-cells, resulting in elevated basal [Ca2+]i [112]. In addition, chronic treatment of beta-cells from control mice with a combination of cytokines upregulated expression of this channel [112]. Also, exposing beta-cells to serum isolated from T1D patients induced apoptosis, but not when the L-type channel blocker nifedipine was included in the media [113,114]. Hyperglycemia is a known contributing factor to beta-cell loss in the latter stages of both T1D and T2D, and evidence suggests that this pathway is also inhibited by blocking L-type calcium channels [115]. These findings collectively suggest that numerous cytokine-mediated signals require calcium influx for their full effects.
5. Cytokines, ER Calcium homeostasis, and ER Stress
The ER plays an important role in calcium storage and signaling. The resting intra-ER calcium concentration is three to four orders of magnitude higher than cytosolic calcium [46]. When cytoplasmic calcium is high, the ER will sequester calcium, while when it is low, the ER will release calcium [116-118]. ER dysfunction is manifested by deficiencies in normal ER calcium handling due primarily to the changes in the sarco(endo)plasmic reticulum calcium ATP-ase (SERCA2b) pump in insulin-secreting cells [119]. Because ER calcium is required for protein binding and chaperone activity, severe ER calcium depletion will impair the quality of ER protein folding and assembly and cause ER stress [120-123]. Cells attempt to alleviate ER stress by means of the unfolded protein response (UPR), which includes increasing ER chaperone proteins to increase protein folding activity, degrading misfolded proteins, and decreasing protein translation to prevent accumulation of unfolded protein [124,125]. Failure to relieve ER stress or a prolonged UPR may lead to cell death through the activation of apoptotic signals by c-Jun N-terminal kinase (JNK) [124-127], CCAAT/enhancer binding protein homologous protein (CHOP)[124,125,128], Bcl-2-associated X protein (Bax) [125], and nuclear factor kappaB (NFkB) [74,124,126].
There is conflicting evidence as to how proinflammatory cytokines induce ER stress. One hypothesis is that proinflammatory cytokines activate inducible nitric oxide synthase (iNOS) leading to excessive nitric oxide (NO) production [129]. NO then acts as a signal to various intracellular components including the ER. By down-regulation of SERCA2b pump activity, NO causes a decrease or even depletion of ER calcium [130], leading to increased cytosolic calcium concentrations and ER stress [123,131,132]. Conversely, there is also evidence suggesting that cytokines induce ER stress through an undetermined pathway independent of nitric oxide production [133,134].
Non-cytokine induced ER stress and cytokine treatment share many of the same features in terms of disruptions in calcium handling. Similarly to cytokines, thapsigargin, a drug that inhibits SERCA activity, has been shown to deplete ER calcium and lead to ER stress [135]. Not surprisingly, cytokine and thapsigargin treatment both produce similar effects on calcium handling in response to glucose stimulation: (a) elevated basal calcium prior to stimulation, (b) attenuated phase 1 response to glucose stimulation, (c) disruptions in phase 2 oscillations [76,116,136]. However, unlike the SERCA inhibitor, cytokines do not appear to trigger key UPR genes, such as activating transcription factor 6 (ATF-6) and ER chaperone BiP, thereby depriving beta-cells of a mechanism for cell survival during ER stress [132] and promoting apoptosis. Two ER membrane calcium-releasing channels that should be noted as possible regulators of ER stress are inositol triphosphate receptors and ryanodine receptors. Inhibition of these receptors has been shown to alleviate ER stress and apoptosis resulting from impaired SERCA function, whereas activation of the receptors while inhibiting the SERCA pump has been shown to increase ER stress and cell death [137].
With regard to diabetes, emerging evidence suggests that ER stress linked to ER calcium depletion may play a key role in beta-cell failure, at least in T2D. The db/db mouse, a leptin-receptor-deficient model of (T2D) and obesity, has an increased activation of genes related to ER stress [138], and in the db/db mouse, it is evident that ER calcium mishandling plays a role in the onset of T2D. Compared to their controls, db/db islets lack the phase 0 dip in [Ca2+]i and the subsequent [Ca2+]i oscillations following glucose stimulation [76,139,140]. When control islets are treated with thapsigargin, they show basal and glucose-stimulated insulin secretion levels similar to those in db/db islets, suggesting that aberrant ER calcium sequestration underlies the impaired glucose responses in the db/db mouse and may play a role in defective insulin secretion [139]. While there is evidence that human islet ER stress exists for T2D [138], there is little evidence to date for ER stress in T1D in humans [141]. Two pieces of evidence, that NO is less important for cytokine-induced human β-cell death [67], and that islets from mice lacking an inducible iNOS (iNOS −/−) are only partially protected against cytokines [142,143], suggest a non-NO/ER stress cell death pathway in T1D [134,144-146].
6. Mitochondrial calcium and cytokine action
Mitochondrial metabolism and cytosolic calcium have a dynamic and often reciprocal relationship. Nutrient-stimulated changes in metabolism initially hyperpolarize the mitochondrial membrane potential (ΔΨm), but increases in [Ca2+]i tend to increase mitochondrial calcium, which depolarizes the ΔΨm and can reduce ATP production [147-150]. Mitochondria can thus act as calcium buffers that compensate for temporary increases in [Ca2+]i levels; however, an overload in mitochondrial calcium can lead to dysfunction or downstream activation of cell death pathways [151-153]. Mitochondrial-mediated cell death pathways are initiated by depolarization of the ΔΨm, onset of the mitochondrial permeability transition via opening of mitochondrial permeability transition pores causing osmotic changes that lead to mitochondrial swelling, and increased reactive oxygen species (ROS) production [137,151,152,154].
In the beta-cell, damaged or missing portions of mitochondrial DNA have been shown to disrupt expression of mitochondrial respiratory enzymes, leading to decreased insulin release, reduced calcium response to glucose stimulation, and reduced overall function [155-158]. This suggests that mitochondrial mutations could increase susceptibility to beta-cell dysfunction, the activation of mitochondrial-mediated cell death pathways, and to the development of diabetes. In fact, mutations in mitochondrial DNA are the direct cause of certain rare forms of diabetes [157]. Further, chronic conditions of high glucose, free fatty acids, proinflammatory cytokines, or other diabetes-associated triggers can impair [Ca2+]i signaling and mitochondrial function in several tissues including islets [159-161] and may lead to mitochondrial calcium overload and cell death [162,163].
Exposure to proinflammatory cytokines is known to cause beta-cell dysfunction, and changes in mitochondrial calcium handling may play a role [64,164-167]. Grunnet et al. 2009 demonstrated that the proinflammatory cytokines IL-1β, IFN-γ, and TNF-α led to a calcium-activated, calcineurin-dependent dephosphorylation of the pro-apoptotic protein Bad, which caused beta-cells to undergo apoptosis [164]. Parkash et al 2005 demonstrated that TNF-α-treated RIN beta-cells expressed significantly less calbindin-D and a greater [Ca2+]i response to increasing concentrations of ionomycin when compared to controls [168]. Further, TNF-α induced localization of the proapoptotic protein, Bax, to the perinuclear regions of RIN cells that contain the highest density of mitochondria, implying a calcium-dependent link between TNF-α and activation of the mitochondrial apoptotic pathway [168]. The uncoupling protein (UCP2) is another established effector of mitochondrial-mediated beta-cell death in several models of immune-mediated diabetes [169-171], and UCP2 is also known to regulate mitochondrial calcium sequestration [172]. Finally, the mitochondrial sodium-calcium exchanger, calcium uniporter or other anion channels can also affect islet function and could potentially play a role in cytokine action [173-176], although little is known at present. Cytokine action thus appears to be linked with mitochondrial dysfunction, at least in part, via calcium-dependent mechanisms.
7. Nuclear calcium and the effects of cytokines on the nucleus
Calcium’s most important role is in the nucleus due to its involvement in the transcription of genes regulating proliferation, apoptosis, and other key components of basic cellular life [1,2,177]. In the beta-cell, nutrients such as glucose are known to modulate the expression of many immediate early genes involved in these key processes in a [Ca2+]i dependent manner [178]. In addition, nutrient-driven increases in calcium are thought to be the greatest in the nucleus. As demonstrated by Quesada et al., both cytoplasmic and nuclear calcium responses to depolarization by KCl are similar, however, the nuclear [Ca2+]i response is significantly greater than that of the cytoplasm following glucose or oleate stimulation [179,180]. This effect is likely due to nuclear KATP channels and suggests that nutrients could produce nuclear-specific signals by generating transitory [Ca2+]i levels within the nucleoplasm in excess of what is required for activation of cytosolic functions [180]. Raising nuclear calcium may indirectly regulate gene transcription as well as activate certain kinases, phosphatases and [Ca2+]i binding transcription factors [2,181]. Also, the role of nuclear calcium may vary amongst particular cell types [2,181]. For instance, deep nuclear envelope invaginations have been observed in the nucleoplasm of GH3 pituitary cells and may even contain cytoplasmic organelles [150]. Thus, cell-specific morphological differences in the nucleoplasmic reticulum could potentially increase calcium release and aid in calcium homeostasis within the nucleoplasm [182]. In addition, recent studies indicate the possibility that [Ca2+]i release from microstores into subnuclear microdomains could regulate the expression of restricted areas of the genome [181].
While cytokines are known to have adverse effects on calcium signaling [132], very little is known about cytokine effects on nuclear calcium signaling. Various proinflammatory cytokines affect many signal transduction pathways such as NF-kB, p38MAPK, JNK, and JAK-STAT, which are heavily involved in nuclear signaling and can induce apoptosis [183-186]. However, the extent to which changes in nuclear calcium can influence these pathways is not known. Numerous channels and receptors regulate nuclear calcium including ryanodine receptors, IP3Rs, and NAADPRs [187], which could potentially be prime targets of cytokine action. Considering the complex interconnectivity and the correlation of sizeable changes in nuclear calcium with nutrient availability, it stands to reason that cytokine-induced disruptions in nuclear calcium handling could lead to significant dysfunction in beta-cells and possibly induce cell death.
8. Final thoughts
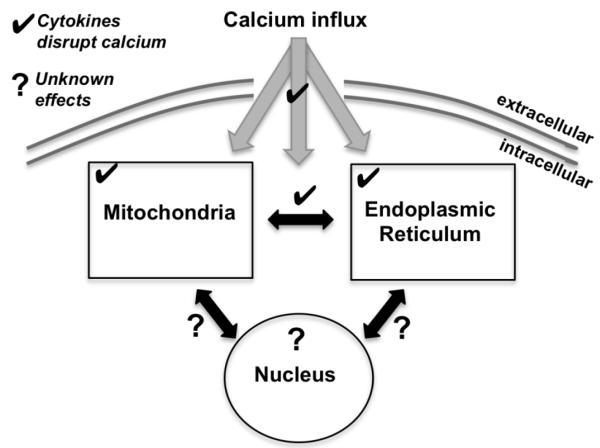
As we have reviewed, glucose stimulates dynamic changes in cytosolic calcium that occur through extracellular influx and uptake/release from the ER, mitochondria, and nucleus (see Figure 3). The potential for calcium-mediated interplay among these organelles suggests that tremendously intricate communication may be necessary to carryout the dynamic events involved with the triphasic response to glucose stimulation and subsequent oscillations in [Ca2+]i and insulin release [188,189]. The flipside of this intricate give-and-take is that disruptions in any of these calcium-regulating organelles can have consequences on all aspects of the cellular response. Proinflammatory cytokines utilize calcium in their signaling pathways and can also impact calcium handling in each of these organelles. The ER is widely appreciated for its dynamic calcium regulation through action of the SERCA pump, a direct cytokine target. Likewise, the mitochondrial permeability transition pore triggers apoptosis in response to mitochondrial calcium overload, which also may occur in response to cytokine action. Given the impact of cytokines on gene expression and nuclear signaling, elucidating the effect of cytokines on nuclear calcium is a particularly intriguing area for future research.
Figure 3.
Calcium interactions. Cytokines are known to affect calcium handling within the mitochondria and ER (✔) and may disrupt nuclear calcium (?). Cytokines may also influence calcium signaling between these organelles (arrows).
With regard to diabetes therapy, several anti-inflammatory compounds are being considered in clinical trials. For example, a recent pilot study showed that the TNF inhibitor, etanercept, improved A1C and islet function in new-onset T1D patients [190]. The IL-1 receptor antagonist, Anakinra, has shown similar success in improving glycemia and beta-cell secretory function in patients with T2D [191] and in mouse models of high-fat-diet-induced hyperglycemia [192]. Since calcium is important to both IL-1B and TNF-a signaling, and their respective cytokine receptor antagonists improve islet function, it is reasonable to suspect that calcium also plays a role in these improvements. To our knowledge, however, no studies have examined calcium changes directly. Anti-hypertensive drugs, including calcium-channel blockers, are also being examined for their anti-diabetic and anti-inflammatory effects. A recent meta-analysis of clinical studies showed that calcium-channel blockers were neutral in patients with hypertension with regard to new-onset diabetes [193], although it should be noted that beta-cell function was not a specific endpoint. This study also showed that the risk of developing diabetes was increased with diuretics and decreased with ARBs or ACE inhibitors [193]. ARBs are particularly intriguing because recent studies suggest they have anti-inflammatory properties and can improve beta-cell function [194,195].
Although it is not clear whether targeting calcium channels would be therapeutically beneficial to the beta-cell in diabetes patients, it is clear that the role of calcium, though not fully understood, is nevertheless central to many mechanisms of cytokine-induced beta-cell dysfunction and death. Measuring these dynamic changes in [Ca2+]i can thus provide valuable insight into beta-cell function under normal and pathological circumstances and may even serve as a biomarker of islet function in vivo. A recently developed technique involving manganese-enhanced magnetic resonance imaging can be used to detect pancreatic responses to glucose stimulation by measuring changes in the activity of voltage-gated calcium channels [196] as a surrogate measurement of insulin secretion. This technique may one day be used clinically to identify functional beta-cell mass in diabetic patients.
Acknowledgments
This work was supported NIH K01 DK081621 to C.S.N. Mouse islets were acquired through the UVA Cell and Islet Isolation Core facility. A special thanks to Kathryn Corbin for technical support for this project. Thanks also to colleagues for their support and critiques, especially Drs. K. Maedler and D. Luciani.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- [2].Alonso MT, Manjarres IM, Garcia-Sancho J. Modulation of calcium signalling by intracellular organelles seen with targeted aequorins. Acta Physiol (Oxf) 2009;195:37–49. doi: 10.1111/j.1748-1716.2008.01920.x. [DOI] [PubMed] [Google Scholar]
- [3].Rutter GA, Tsuboi T, Ravier MA. Ca2+ microdomains and the control of insulin secretion. Cell Calcium. 2006;40:539–551. doi: 10.1016/j.ceca.2006.08.015. [DOI] [PubMed] [Google Scholar]
- [4].Chad JE, Eckert R. Calcium domains associated with individual channels can account for anomalous voltage relations of CA-dependent responses. Biophys J. 1984;45:993–999. doi: 10.1016/S0006-3495(84)84244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Thomas HE, Graham KL, Angstetra E, McKenzie MD, Dudek NL, Kay TW. Interferon signalling in pancreatic beta cells. Front Biosci. 2009;14:644–656. doi: 10.2741/3270. [DOI] [PubMed] [Google Scholar]
- [6].Kim KA, Lee MS. Recent progress in research on beta-cell apoptosis by cytokines. Front Biosci. 2009;14:657–664. doi: 10.2741/3271. [DOI] [PubMed] [Google Scholar]
- [7].Donath MY, Boni-Schnetzler M, Ellingsgaard H, Halban PA, Ehses JA. Cytokine production by islets in health and diabetes: cellular origin, regulation and function. Trends Endocrinol Metab. 2010;21:261–267. doi: 10.1016/j.tem.2009.12.010. [DOI] [PubMed] [Google Scholar]
- [8].Donath MY, Storling J, Berchtold LA, Billestrup N, Mandrup-Poulsen T. Cytokines and beta-cell biology: from concept to clinical translation. Endocr Rev. 2008;29:334–350. doi: 10.1210/er.2007-0033. [DOI] [PubMed] [Google Scholar]
- [9].Gysemans C, Callewaert H, Overbergh L, Mathieu C. Cytokine signalling in the beta-cell: a dual role for IFNgamma. Biochem Soc Trans. 2008;36:328–333. doi: 10.1042/BST0360328. [DOI] [PubMed] [Google Scholar]
- [10].Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1beta in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:314–321. doi: 10.1097/MED.0b013e32833bf6dc. [DOI] [PubMed] [Google Scholar]
- [11].Meglasson MD, Matschinsky FM. Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab Rev. 1986;2:163–214. doi: 10.1002/dmr.5610020301. [DOI] [PubMed] [Google Scholar]
- [12].Henderson JR. Why are the islets of Langerhans? Lancet. 1969;2:469–470. doi: 10.1016/s0140-6736(69)90171-8. [DOI] [PubMed] [Google Scholar]
- [13].Bonner-Weir S, Orci L. New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes. 1982;31:883–889. doi: 10.2337/diab.31.10.883. [DOI] [PubMed] [Google Scholar]
- [14].Zanone MM, Favaro E, Camussi G. From endothelial to beta cells: insights into pancreatic islet microendothelium. Curr Diabetes Rev. 2008;4:1–9. doi: 10.2174/157339908783502415. [DOI] [PubMed] [Google Scholar]
- [15].Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kim A, Miller K, Jo J, Kilimnik G, Wojcik P, Hara M. Islet architecture: A comparative study. Islets. 2009;1:129–136. doi: 10.4161/isl.1.2.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ashcroft FM, Rorsman P. ATP-sensitive K+ channels: a link between B-cell metabolism and insulin secretion. Biochem Soc Trans. 1990;18:109–111. doi: 10.1042/bst0180109. [DOI] [PubMed] [Google Scholar]
- [18].Maechler P, Wollheim CB. Role of mitochondria in metabolism-secretion coupling of insulin release in the pancreatic beta-cell. Biofactors. 1998;8:255–262. doi: 10.1002/biof.5520080313. [DOI] [PubMed] [Google Scholar]
- [19].Henquin JC. Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia. 2009;52:739–751. doi: 10.1007/s00125-009-1314-y. [DOI] [PubMed] [Google Scholar]
- [20].Mueckler M. Facilitative glucose transporters. Eur J Biochem. 1994;219:713–725. doi: 10.1111/j.1432-1033.1994.tb18550.x. [DOI] [PubMed] [Google Scholar]
- [21].De Vos A, Heimberg H, Quartier E, et al. Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J Clin Invest. 1995;96:2489–2495. doi: 10.1172/JCI118308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Deeney JT, Prentki M, Corkey BE. Metabolic control of beta-cell function. Semin Cell Dev Biol. 2000;11:267–275. doi: 10.1006/scdb.2000.0175. [DOI] [PubMed] [Google Scholar]
- [23].Jensen MV, Joseph JW, Ronnebaum SM, Burgess SC, Sherry AD, Newgard CB. Metabolic cycling in control of glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab. 2008;295:E1287–97. doi: 10.1152/ajpendo.90604.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Aizawa T, Sato Y, Komatsu M. Importance of nonionic signals for glucose-induced biphasic insulin secretion. Diabetes. 2002;51(Suppl 1):S96–8. doi: 10.2337/diabetes.51.2007.s96. [DOI] [PubMed] [Google Scholar]
- [25].Straub SG, James RF, Dunne MJ, Sharp GW. Glucose activates both K(ATP) channel-dependent and K(ATP) channel-independent signaling pathways in human islets. Diabetes. 1998;47:758–763. doi: 10.2337/diabetes.47.5.758. [DOI] [PubMed] [Google Scholar]
- [26].Sato Y, Henquin JC. The K+-ATP channel-independent pathway of regulation of insulin secretion by glucose: in search of the underlying mechanism. Diabetes. 1998;47:1713–1721. doi: 10.2337/diabetes.47.11.1713. [DOI] [PubMed] [Google Scholar]
- [27].Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49:1751–1760. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- [28].Mandrup-Poulsen T, Bendtzen K, Nielsen JH, Bendixen G, Nerup J. Cytokines cause functional and structural damage to isolated islets of Langerhans. Allergy. 1985;40:424–429. doi: 10.1111/j.1398-9995.1985.tb02681.x. [DOI] [PubMed] [Google Scholar]
- [29].Bendtzen K, Mandrup-Poulsen T, Nerup J, Nielsen JH, Dinarello CA, Svenson M. Cytotoxicity of human pI 7 interleukin-1 for pancreatic islets of Langerhans. Science. 1986;232:1545–1547. doi: 10.1126/science.3086977. [DOI] [PubMed] [Google Scholar]
- [30].Mandrup-Poulsen T, Bendtzen K, Nerup J, Dinarello CA, Svenson M, Nielsen JH. Affinity-purified human interleukin I is cytotoxic to isolated islets of Langerhans. Diabetologia. 1986;29:63–67. doi: 10.1007/BF02427283. [DOI] [PubMed] [Google Scholar]
- [31].Pujol-Borrell R, Todd I, Doshi M, et al. HLA class II induction in human islet cells by interferon-gamma plus tumour necrosis factor or lymphotoxin. Nature. 1987;326:304–306. doi: 10.1038/326304a0. [DOI] [PubMed] [Google Scholar]
- [32].Pukel C, Baquerizo H, Rabinovitch A. Destruction of rat islet cell monolayers by cytokines. Synergistic interactions of interferon-gamma, tumor necrosis factor, lymphotoxin, and interleukin 1. Diabetes. 1988;37:133–136. doi: 10.2337/diab.37.1.133. [DOI] [PubMed] [Google Scholar]
- [33].Rabinovitch A, Suarez-Pinzon WL. Cytokines and their roles in pancreatic islet beta-cell destruction and insulin-dependent diabetes mellitus. Biochem Pharmacol. 1998;55:1139–1149. doi: 10.1016/s0006-2952(97)00492-9. [DOI] [PubMed] [Google Scholar]
- [34].Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- [35].Greenberg AS, McDaniel ML. Identifying the links between obesity, insulin resistance and beta-cell function: potential role of adipocyte-derived cytokines in the pathogenesis of type 2 diabetes. Eur J Clin Invest. 2002;32(Suppl 3):24–34. doi: 10.1046/j.1365-2362.32.s3.4.x. [DOI] [PubMed] [Google Scholar]
- [36].Donath MY, Storling J, Maedler K, Mandrup-Poulsen T. Inflammatory mediators and islet beta-cell failure: a link between type 1 and type 2 diabetes. J Mol Med. 2003;81:455–470. doi: 10.1007/s00109-003-0450-y. [DOI] [PubMed] [Google Scholar]
- [37].Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54(Suppl 2):S97–107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- [38].Ehses JA, Perren A, Eppler E, et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56:2356–2370. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- [39].Donath MY, Boni-Schnetzler M, Ellingsgaard H, Ehses JA. Islet inflammation impairs the pancreatic beta-cell in type 2 diabetes. Physiology (Bethesda) 2009;24:325–331. doi: 10.1152/physiol.00032.2009. [DOI] [PubMed] [Google Scholar]
- [40].Poitout V, Robertson RP. Minireview: Secondary beta-cell failure in type 2 diabetes--a convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002;143:339–342. doi: 10.1210/endo.143.2.8623. [DOI] [PubMed] [Google Scholar]
- [41].Robertson RP, Harmon J, Tran PO, Poitout V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53(Suppl 1):S119–24. doi: 10.2337/diabetes.53.2007.s119. [DOI] [PubMed] [Google Scholar]
- [42].Porte D, Jr, Kahn SE. Beta-Cell Dysfunction and Failure in Type 2 Diabetes: Potential Mechanisms. Diabetes. 2001;50(Suppl 1):S160–3. doi: 10.2337/diabetes.50.2007.s160. [DOI] [PubMed] [Google Scholar]
- [43].Haataja L, Gurlo T, Huang CJ, Butler PC. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev. 2008;29:303–316. doi: 10.1210/er.2007-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Duchen MR. Roles of mitochondria in health and disease. Diabetes. 2004;53(Suppl 1):S96–102. doi: 10.2337/diabetes.53.2007.s96. [DOI] [PubMed] [Google Scholar]
- [45].Araki E, Oyadomari S, Mori M. Impact of endoplasmic reticulum stress pathway on pancreatic beta-cells and diabetes mellitus. Exp Biol Med (Maywood) 2003;228:1213–1217. doi: 10.1177/153537020322801018. [DOI] [PubMed] [Google Scholar]
- [46].Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- [47].Martinon F, Gaide O, Petrilli V, Mayor A, Tschopp J. NALP inflammasomes: a central role in innate immunity. Semin Immunopathol. 2007;29:213–229. doi: 10.1007/s00281-007-0079-y. [DOI] [PubMed] [Google Scholar]
- [48].Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010;327:296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- [49].Masters SL, Dunne A, Subramanian SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mandrup-Poulsen T, Bendtzen K, Dinarello CA, Nerup J. Human tumor necrosis factor potentiates human interleukin 1-mediated rat pancreatic beta-cell cytotoxicity. J Immunol. 1987;139:4077–4082. [PubMed] [Google Scholar]
- [51].Campbell IL, Iscaro A, Harrison LC. IFN-gamma and tumor necrosis factor-alpha. Cytotoxicity to murine islets of Langerhans. J Immunol. 1988;141:2325–2329. [PubMed] [Google Scholar]
- [52].Lee YH, Pratley RE. The evolving role of inflammation in obesity and the metabolic syndrome. Curr Diab Rep. 2005;5:70–75. doi: 10.1007/s11892-005-0071-7. [DOI] [PubMed] [Google Scholar]
- [53].Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb. 2010;17:332–341. doi: 10.5551/jat.3939. [DOI] [PubMed] [Google Scholar]
- [54].Boni-Schnetzler M, Ehses JA, Faulenbach M, Donath MY. Insulitis in type 2 diabetes. Diabetes Obes Metab. 2008;10(Suppl 4):201–204. doi: 10.1111/j.1463-1326.2008.00950.x. [DOI] [PubMed] [Google Scholar]
- [55].Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. Islet-associated macrophages in type 2 diabetes. Diabetologia. 2009;52:1686–1688. doi: 10.1007/s00125-009-1410-z. [DOI] [PubMed] [Google Scholar]
- [56].Spinas GA, Hansen BS, Linde S, et al. Interleukin 1 dose-dependently affects the biosynthesis of (pro)insulin in isolated rat islets of Langerhans. Diabetologia. 1987;30:474–480. doi: 10.1007/BF00279615. [DOI] [PubMed] [Google Scholar]
- [57].Spinas GA, Mandrup-Poulsen T, Molvig J, et al. Low concentrations of interleukin-1 stimulate and high concentrations inhibit insulin release from isolated rat islets of Langerhans. Acta Endocrinol (Copenh) 1986;113:551–558. doi: 10.1530/acta.0.1130551. [DOI] [PubMed] [Google Scholar]
- [58].Spinas GA, Palmer JP, Mandrup-Poulsen T, Andersen H, Nielsen JH, Nerup J. The bimodal effect of interleukin 1 on rat pancreatic beta-cells--stimulation followed by inhibition--depends upon dose, duration of exposure, and ambient glucose concentration. Acta Endocrinol (Copenh) 1988;119:307–311. doi: 10.1530/acta.0.1190307. [DOI] [PubMed] [Google Scholar]
- [59].Wilson CA, Jacobs C, Baker P, et al. IL-1 beta modulation of spontaneous autoimmune diabetes and thyroiditis in the BB rat. J Immunol. 1990;144:3784–3788. [PubMed] [Google Scholar]
- [60].Satoh J, Seino H, Abo T, et al. Recombinant human tumor necrosis factor alpha suppresses autoimmune diabetes in nonobese diabetic mice. J Clin Invest. 1989;84:1345–1348. doi: 10.1172/JCI114304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Satoh J, Seino H, Shintani S, et al. Inhibition of type 1 diabetes in BB rats with recombinant human tumor necrosis factor-alpha. J Immunol. 1990;145:1395–1399. [PubMed] [Google Scholar]
- [62].Jacob CO, Aiso S, Michie SA, McDevitt HO, Acha-Orbea H. Prevention of diabetes in nonobese diabetic mice by tumor necrosis factor (TNF): similarities between TNF-alpha and interleukin 1. Proc Natl Acad Sci U S A. 1990;87:968–972. doi: 10.1073/pnas.87.3.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Dunger A, Cunningham JM, Delaney CA, et al. Tumor necrosis factor-alpha and interferon-gamma inhibit insulin secretion and cause DNA damage in unweaned-rat islets. Extent of nitric oxide involvement. Diabetes. 1996;45:183–189. doi: 10.2337/diab.45.2.183. [DOI] [PubMed] [Google Scholar]
- [64].Chang I, Cho N, Kim S, et al. Role of calcium in pancreatic islet cell death by IFN-gamma/TNF-alpha. J Immunol. 2004;172:7008–7014. doi: 10.4049/jimmunol.172.11.7008. [DOI] [PubMed] [Google Scholar]
- [65].Scarim AL, Heitmeier MR, Corbett JA. Irreversible inhibition of metabolic function and islet destruction after a 36-hour exposure to interleukin-1beta. Endocrinology. 1997;138:5301–5307. doi: 10.1210/endo.138.12.5583. [DOI] [PubMed] [Google Scholar]
- [66].Eizirik DL, Pipeleers DG, Ling Z, Welsh N, Hellerstrom C, Andersson A. Major species differences between humans and rodents in the susceptibility to pancreatic beta-cell injury. Proc Natl Acad Sci U S A. 1994;91:9253–9256. doi: 10.1073/pnas.91.20.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Eizirik DL, Sandler S, Welsh N, et al. Cytokines suppress human islet function irrespective of their effects on nitric oxide generation. J Clin Invest. 1994;93:1968–1974. doi: 10.1172/JCI117188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kawahara DJ, Kenney JS. Species differences in human and rat islet sensitivity to human cytokines. Monoclonal anti-interleukin-1 (IL-1) influences on direct and indirect IL-1-mediated islet effects. Cytokine. 1991;3:117–124. doi: 10.1016/1043-4666(91)90031-8. [DOI] [PubMed] [Google Scholar]
- [69].Corbett JA, Kwon G, Turk J, McDaniel ML. IL-1 beta induces the coexpression of both nitric oxide synthase and cyclooxygenase by islets of Langerhans: activation of cyclooxygenase by nitric oxide. Biochemistry. 1993;32:13767–13770. doi: 10.1021/bi00213a002. [DOI] [PubMed] [Google Scholar]
- [70].Corbett JA, Sweetland MA, Wang JL, Lancaster JR, Jr, McDaniel ML. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proc Natl Acad Sci U S A. 1993;90:1731–1735. doi: 10.1073/pnas.90.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ablamunits V, Baranova F, Mandrup-Poulsen T, Nerup J. In vitro inhibition of insulin release by blood mononuclear cells from insulin-dependent diabetic and healthy subjects: synergistic action of IL-1 and TNF. Cell Transplant. 1994;3:55–60. doi: 10.1177/096368979400300109. [DOI] [PubMed] [Google Scholar]
- [72].Cetkovic-Cvrlje M, Eizirik DL. TNF-alpha and IFN-gamma potentiate the deleterious effects of IL-1 beta on mouse pancreatic islets mainly via generation of nitric oxide. Cytokine. 1994;6:399–406. doi: 10.1016/1043-4666(94)90064-7. [DOI] [PubMed] [Google Scholar]
- [73].Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- [74].Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- [75].Spranger J, Kroke A, Mohlig M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- [76].Dula SB, Jecmenica M, Wu R, et al. Evidence that low-grade systemic inflammation can induce islet dysfunction as measured by impaired calcium handling. Cell Calcium. 2010;48:133–142. doi: 10.1016/j.ceca.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Corbin KL, Hall TE, Haile R, Nunemaker CS. A novel fluorescence imaging approach for comparative measurements of pancreatic islet function in vitro. Islets. 2011;3:14–20. doi: 10.4161/isl.3.1.14133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Karlsen AE, Ronn SG, Lindberg K, et al. Suppressor of cytokine signaling 3 (SOCS-3) protects beta -cells against interleukin-1beta - and interferon-gamma -mediated toxicity. Proc Natl Acad Sci U S A. 2001;98:12191–12196. doi: 10.1073/pnas.211445998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Chandra J, Zhivotovsky B, Zaitsev S, Juntti-Berggren L, Berggren PO, Orrenius S. Role of apoptosis in pancreatic beta-cell death in diabetes. Diabetes. 2001;50(Suppl 1):S44–7. doi: 10.2337/diabetes.50.2007.s44. [DOI] [PubMed] [Google Scholar]
- [80].Boitard C. Insulin secretion in type 2 diabetes: clinical aspects. Diabetes Metab. 2002;28:4S33–8. [PubMed] [Google Scholar]
- [81].Lee MS, Chang I, Kim S. Death effectors of beta-cell apoptosis in type 1 diabetes. Mol Genet Metab. 2004;83:82–92. doi: 10.1016/j.ymgme.2004.08.002. [DOI] [PubMed] [Google Scholar]
- [82].Levy J. Abnormal cell calcium homeostasis in type 2 diabetes mellitus: a new look on old disease. Endocrine. 1999;10:1–6. doi: 10.1385/ENDO:10:1:1. [DOI] [PubMed] [Google Scholar]
- [83].Braun M, Ramracheya R, Bengtsson M, et al. Voltage-gated ion channels in human pancreatic beta-cells: electrophysiological characterization and role in insulin secretion. Diabetes. 2008;57:1618–1628. doi: 10.2337/db07-0991. [DOI] [PubMed] [Google Scholar]
- [84].Drews G, Krippeit-Drews P, Dufer M. Electrophysiology of islet cells. Adv Exp Med Biol. 2010;654:115–163. doi: 10.1007/978-90-481-3271-3_7. [DOI] [PubMed] [Google Scholar]
- [85].Davies A, Hendrich J, Van Minh AT, Wratten J, Douglas L, Dolphin AC. Functional biology of the alpha(2)delta subunits of voltage-gated calcium channels. Trends Pharmacol Sci. 2007;28:220–228. doi: 10.1016/j.tips.2007.03.005. [DOI] [PubMed] [Google Scholar]
- [86].Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol. 2003;13:298–307. doi: 10.1016/s0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- [87].Collins SC, Hoppa MB, Walker JN, et al. Progression of diet-induced diabetes in C57BL6J mice involves functional dissociation of Ca2(+) channels from secretory vesicles. Diabetes. 2010;59:1192–1201. doi: 10.2337/db09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Houamed KM, Sweet IR, Satin LS. BK channels mediate a novel ionic mechanism that regulates glucose-dependent electrical activity and insulin secretion in mouse pancreatic beta-cells. J Physiol. 2010;588:3511–3523. doi: 10.1113/jphysiol.2009.184341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Dufer M, Neye Y, Horth K, et al. BK channels affect glucose homeostasis and cell viability of murine pancreatic beta cells. Diabetologia. 2011;54:423–432. doi: 10.1007/s00125-010-1936-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Jacobson DA, Mendez F, Thompson M, Torres J, Cochet O, Philipson LH. Calcium-activated and voltage-gated potassium channels of the pancreatic islet impart distinct and complementary roles during secretagogue induced electrical responses. J Physiol. 2010;588:3525–3537. doi: 10.1113/jphysiol.2010.190207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Gopel SO, Kanno T, Barg S, et al. Activation of Ca(2+)-dependent K(+) channels contributes to rhythmic firing of action potentials in mouse pancreatic beta cells. J Gen Physiol. 1999;114:759–770. doi: 10.1085/jgp.114.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Zhang M, Houamed K, Kupershmidt S, Roden D, Satin LS. Pharmacological properties and functional role of Kslow current in mouse pancreatic beta-cells: SK channels contribute to Kslow tail current and modulate insulin secretion. J Gen Physiol. 2005;126:353–363. doi: 10.1085/jgp.200509312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Satin LS. Localized calcium influx in pancreatic beta-cells: its significance for Ca2+-dependent insulin secretion from the islets of Langerhans. Endocrine. 2000;13:251–262. doi: 10.1385/ENDO:13:3:251. [DOI] [PubMed] [Google Scholar]
- [94].Wolf BA, Hughes JH, Florholmen J, Turk J, McDaniel ML. Interleukin-1 inhibits glucose-induced Ca2+ uptake by islets of Langerhans. FEBS Lett. 1989;248:35–38. doi: 10.1016/0014-5793(89)80426-0. [DOI] [PubMed] [Google Scholar]
- [95].Helqvist S, Bouchelouche P, Andersen HU, Nerup J. Modulation of calcium flux influences interleukin 1 beta effects on insulin release from isolated islets of Langerhans. Acta Endocrinol (Copenh) 1989;121:447–455. doi: 10.1530/acta.0.1210447. [DOI] [PubMed] [Google Scholar]
- [96].McDaniel ML, Hughes JH, Wolf BA, Easom RA, Turk JW. Descriptive and mechanistic considerations of interleukin 1 and insulin secretion. Diabetes. 1988;37:1311–1315. doi: 10.2337/diab.37.10.1311. [DOI] [PubMed] [Google Scholar]
- [97].Fei H, Zhao B, Zhao S, Wang Q. Requirements of calcium fluxes and ERK kinase activation for glucose- and interleukin-1beta-induced beta-cell apoptosis. Mol Cell Biochem. 2008;315:75–84. doi: 10.1007/s11010-008-9791-8. [DOI] [PubMed] [Google Scholar]
- [98].Zaitsev SV, Appelskog IB, Kapelioukh IL, et al. Imidazoline compounds protect against interleukin 1beta-induced beta-cell apoptosis. Diabetes. 2001;50(Suppl 1):S70–6. doi: 10.2337/diabetes.50.2007.s70. [DOI] [PubMed] [Google Scholar]
- [99].Maedler K, Storling J, Sturis J, et al. Glucose- and interleukin-1beta-induced beta-cell apoptosis requires Ca2+ influx and extracellular signal-regulated kinase (ERK) 1/2 activation and is prevented by a sulfonylurea receptor 1/inwardly rectifying K+ channel 6.2 (SUR/Kir6.2) selective potassium channel opener in human islets. Diabetes. 2004;53:1706–1713. doi: 10.2337/diabetes.53.7.1706. [DOI] [PubMed] [Google Scholar]
- [100].Storling J, Zaitsev SV, Kapelioukh IL, et al. Calcium has a permissive role in interleukin-1beta-induced c-jun N-terminal kinase activation in insulin-secreting cells. Endocrinology. 2005;146:3026–3036. doi: 10.1210/en.2005-0036. [DOI] [PubMed] [Google Scholar]
- [101].Herchuelz A, Diaz-Horta O, van Eylen F. Na/Ca exchange and Ca2+ homeostasis in the pancreatic beta-cell. Diabetes Metab. 2002;28:3S54–60. discussion 3S108-12. [PubMed] [Google Scholar]
- [102].Jiang L, Allagnat F, Nguidjoe E, et al. Plasma membrane Ca2+-ATPase overexpression depletes both mitochondrial and endoplasmic reticulum Ca2+ stores and triggers apoptosis in insulin-secreting BRIN-BD11 cells. J Biol Chem. 2010;285:30634–30643. doi: 10.1074/jbc.M110.116681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Souza KL, Elsner M, Mathias PC, Lenzen S, Tiedge M. Cytokines activate genes of the endocytotic pathway in insulin-producing RINm5F cells. Diabetologia. 2004;47:1292–1302. doi: 10.1007/s00125-004-1435-2. [DOI] [PubMed] [Google Scholar]
- [104].Ono K, Wang X, Han J. Resistance to tumor necrosis factor-induced cell death mediated by PMCA4 deficiency. Mol Cell Biol. 2001;21:8276–8288. doi: 10.1128/MCB.21.24.8276-8288.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Alzugaray ME, Garcia ME, Del Zotto HH, et al. Changes in islet plasma membrane calcium-ATPase activity and isoform expression induced by insulin resistance. Arch Biochem Biophys. 2009;490:17–23. doi: 10.1016/j.abb.2009.07.022. [DOI] [PubMed] [Google Scholar]
- [106].Eizirik DL, Sandler S, Welsh N, Juntti-Berggren L, Berggren PO. Interleukin-1 beta-induced stimulation of insulin release in mouse pancreatic islets is related to diacylglycerol production and protein kinase C activation. Mol Cell Endocrinol. 1995;111:159–165. doi: 10.1016/0303-7207(95)03561-k. [DOI] [PubMed] [Google Scholar]
- [107].Welsh N, Nilsson T, Hallberg A, Arkhammar P, Berggren PO, Sandler S. Human interleukin 1 beta stimulates islet insulin release by a mechanism not dependent on changes in phospholipase C and protein kinase C activities or Ca2+ handling. Acta Endocrinol (Copenh) 1989;121:698–704. doi: 10.1530/acta.0.1210698. [DOI] [PubMed] [Google Scholar]
- [108].Shimizu H, Ohtani K, Kato Y, Mori M. Interleukin-6 increases insulin secretion and preproinsulin mRNA expression via Ca2+-dependent mechanism. J Endocrinol. 2000;166:121–126. doi: 10.1677/joe.0.1660121. [DOI] [PubMed] [Google Scholar]
- [109].Suzuki T, Imai J, Yamada T, et al. Interleukin-6 enhances glucose-stimulated insulin secretion from pancreatic beta-cells: potential involvement of the PLC-IP3-dependent pathway. Diabetes. 2011;60:537–547. doi: 10.2337/db10-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Southern C, Schulster D, Green IC. Inhibition of insulin secretion from rat islets of Langerhans by interleukin-6. An effect distinct from that of interleukin-1. Biochem J. 1990;272:243–245. doi: 10.1042/bj2720243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005;54(Suppl 2):S114–24. doi: 10.2337/diabetes.54.suppl_2.s114. [DOI] [PubMed] [Google Scholar]
- [112].Wang L, Bhattacharjee A, Fu J, Li M. Abnormally expressed low-voltage-activated calcium channels in beta-cells from NOD mice and a related clonal cell line. Diabetes. 1996;45:1678–1683. doi: 10.2337/diab.45.12.1678. [DOI] [PubMed] [Google Scholar]
- [113].Juntti-Berggren L, Larsson O, Rorsman P, et al. Increased activity of L-type Ca2+ channels exposed to serum from patients with type I diabetes. Science. 1993;261:86–90. doi: 10.1126/science.7686306. [DOI] [PubMed] [Google Scholar]
- [114].Conroy SJ, Green I, Dixon G, et al. Evidence for a sustained increase in clonal beta-cell basal intracellular Ca2+ levels after incubation in the presence of newly diagnosed Type-1 diabetic patient sera. Possible role in serum-induced inhibition of insulin secretion. J Endocrinol. 2002;173:53–62. doi: 10.1677/joe.0.1730053. [DOI] [PubMed] [Google Scholar]
- [115].Efanova IB, Zaitsev SV, Zhivotovsky B, et al. Glucose and tolbutamide induce apoptosis in pancreatic beta-cells. A process dependent on intracellular Ca2+ concentration. J Biol Chem. 1998;273:33501–33507. doi: 10.1074/jbc.273.50.33501. [DOI] [PubMed] [Google Scholar]
- [116].Jahanshahi P, Wu R, Carter JD, Nunemaker CS. Evidence of diminished glucose stimulation and endoplasmic reticulum function in nonoscillatory pancreatic islets. Endocrinology. 2009;150:607–615. doi: 10.1210/en.2008-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Gilon P, Arredouani A, Gailly P, Gromada J, Henquin JC. Uptake and release of Ca2+ by the endoplasmic reticulum contribute to the oscillations of the cytosolic Ca2+ concentration triggered by Ca2+ influx in the electrically excitable pancreatic B-cell. J Biol Chem. 1999;274:20197–20205. doi: 10.1074/jbc.274.29.20197. [DOI] [PubMed] [Google Scholar]
- [118].Tengholm A, Hellman B, Gylfe E. The endoplasmic reticulum is a glucose-modulated high-affinity sink for Ca2+ in mouse pancreatic beta-cells. J Physiol. 2001;530:533–540. doi: 10.1111/j.1469-7793.2001.0533k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Varadi A, Rutter GA. Dynamic imaging of endoplasmic reticulum Ca2+ concentration in insulin-secreting MIN6 Cells using recombinant targeted cameleons: roles of sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)-2 and ryanodine receptors. Diabetes. 2002;51(Suppl 1):S190–201. doi: 10.2337/diabetes.51.2007.s190. [DOI] [PubMed] [Google Scholar]
- [120].Zhang K, Kaufman RJ. Protein folding in the endoplasmic reticulum and the unfolded protein response. Handb Exp Pharmacol. 2006;(172):69–91. doi: 10.1007/3-540-29717-0_3. [DOI] [PubMed] [Google Scholar]
- [121].Patil C, Walter P. Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr Opin Cell Biol. 2001;13:349–355. doi: 10.1016/s0955-0674(00)00219-2. [DOI] [PubMed] [Google Scholar]
- [122].Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- [123].Ni M, Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641–3651. doi: 10.1016/j.febslet.2007.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Austin RC. The unfolded protein response in health and disease. Antioxid Redox Signal. 2009;11:2279–2287. doi: 10.1089/ars.2009.2686. [DOI] [PubMed] [Google Scholar]
- [125].Fonseca SG, Lipson KL, Urano F. Endoplasmic reticulum stress signaling in pancreatic beta-cells. Antioxid Redox Signal. 2007;9:2335–2344. doi: 10.1089/ars.2007.1790. [DOI] [PubMed] [Google Scholar]
- [126].Kaneko M, Niinuma Y, Nomura Y. Activation signal of nuclear factor-kappa B in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol Pharm Bull. 2003;26:931–935. doi: 10.1248/bpb.26.931. [DOI] [PubMed] [Google Scholar]
- [127].Urano F, Wang X, Bertolotti A, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- [128].Ortsater H, Sjoholm A. A busy cell--endoplasmic reticulum stress in the pancreatic beta-cell. Mol Cell Endocrinol. 2007;277:1–5. doi: 10.1016/j.mce.2007.06.006. [DOI] [PubMed] [Google Scholar]
- [129].Chambers KT, Unverferth JA, Weber SM, Wek RC, Urano F, Corbett JA. The role of nitric oxide and the unfolded protein response in cytokine-induced beta-cell death. Diabetes. 2008;57:124–132. doi: 10.2337/db07-0944. [DOI] [PubMed] [Google Scholar]
- [130].Wang Q, Zhang H, Zhao B, Fei H. IL-1beta caused pancreatic beta-cells apoptosis is mediated in part by endoplasmic reticulum stress via the induction of endoplasmic reticulum Ca2+ release through the c-Jun N-terminal kinase pathway. Mol Cell Biochem. 2009;324:183–190. doi: 10.1007/s11010-008-9997-9. [DOI] [PubMed] [Google Scholar]
- [131].Oyadomari S, Takeda K, Takiguchi M, et al. Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci U S A. 2001;98:10845–10850. doi: 10.1073/pnas.191207498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Cardozo AK, Ortis F, Storling J, et al. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes. 2005;54:452–461. doi: 10.2337/diabetes.54.2.452. [DOI] [PubMed] [Google Scholar]
- [133].Chan JY, Cooney GJ, Biden TJ, Laybutt DR. Differential regulation of adaptive and apoptotic unfolded protein response signalling by cytokine-induced nitric oxide production in mouse pancreatic beta cells. Diabetologia. 2011 doi: 10.1007/s00125-011-2139-z. [DOI] [PubMed] [Google Scholar]
- [134].Akerfeldt MC, Howes J, Chan JY, et al. Cytokine-induced beta-cell death is independent of endoplasmic reticulum stress signaling. Diabetes. 2008;57:3034–3044. doi: 10.2337/db07-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Zhou YP, Teng D, Dralyuk F, et al. Apoptosis in insulin-secreting cells. Evidence for the role of intracellular Ca2+ stores and arachidonic acid metabolism. J Clin Invest. 1998;101:1623–1632. doi: 10.1172/JCI1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Roe MW, Mertz RJ, Lancaster ME, Worley JF, 3rd, Dukes ID. Thapsigargin inhibits the glucose-induced decrease of intracellular Ca2+ in mouse islets of Langerhans. Am J Physiol. 1994;266:E852–62. doi: 10.1152/ajpendo.1994.266.6.E852. [DOI] [PubMed] [Google Scholar]
- [137].Luciani DS, Gwiazda KS, Yang TL, et al. Roles of IP3R and RyR Ca2+ channels in endoplasmic reticulum stress and beta-cell death. Diabetes. 2009;58:422–432. doi: 10.2337/db07-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Laybutt DR, Preston AM, Akerfeldt MC, et al. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007;50:752–763. doi: 10.1007/s00125-006-0590-z. [DOI] [PubMed] [Google Scholar]
- [139].Roe MW, Philipson LH, Frangakis CJ, et al. Defective glucose-dependent endoplasmic reticulum Ca2+ sequestration in diabetic mouse islets of Langerhans. J Biol Chem. 1994;269:18279–18282. [PubMed] [Google Scholar]
- [140].Evans-Molina C, Robbins RD, Kono T, et al. Peroxisome proliferator-activated receptor gamma activation restores islet function in diabetic mice through reduction of endoplasmic reticulum stress and maintenance of euchromatin structure. Mol Cell Biol. 2009;29:2053–2067. doi: 10.1128/MCB.01179-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Huang CJ, Lin CY, Haataja L, et al. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes. 2007;56:2016–2027. doi: 10.2337/db07-0197. [DOI] [PubMed] [Google Scholar]
- [142].Liu D, Pavlovic D, Chen MC, Flodstrom M, Sandler S, Eizirik DL. Cytokines induce apoptosis in beta-cells isolated from mice lacking the inducible isoform of nitric oxide synthase (iNOS−/−) Diabetes. 2000;49:1116–1122. doi: 10.2337/diabetes.49.7.1116. [DOI] [PubMed] [Google Scholar]
- [143].Zumsteg U, Frigerio S, Hollander GA. Nitric oxide production and Fas surface expression mediate two independent pathways of cytokine-induced murine beta-cell damage. Diabetes. 2000;49:39–47. doi: 10.2337/diabetes.49.1.39. [DOI] [PubMed] [Google Scholar]
- [144].Eizirik DL, Darville MI. Beta-Cell Apoptosis and Defense Mechanisms: Lessons from Type 1 Diabetes. Diabetes. 2001;50(Suppl 1):S64–9. doi: 10.2337/diabetes.50.2007.s64. [DOI] [PubMed] [Google Scholar]
- [145].Nakata M, Uto N, Maruyama I, Yada T. Nitric oxide induces apoptosis via Ca2+-dependent processes in the pancreatic beta-cell line MIN6. Cell Struct Funct. 1999;24:451–455. doi: 10.1247/csf.24.451. [DOI] [PubMed] [Google Scholar]
- [146].Tonnesen MF, Grunnet LG, Friberg J, et al. Inhibition of nuclear factor-kappaB or Bax prevents endoplasmic reticulum stress- but not nitric oxide-mediated apoptosis in INS-1E cells. Endocrinology. 2009;150:4094–4103. doi: 10.1210/en.2009-0029. [DOI] [PubMed] [Google Scholar]
- [147].Duchen MR, Smith PA, Ashcroft FM. Substrate-dependent changes in mitochondrial function, intracellular free calcium concentration and membrane channels in pancreatic beta-cells. Biochem J. 1993;294(Pt 1):35–42. doi: 10.1042/bj2940035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Krippeit-Drews P, Dufer M, Drews G. Parallel oscillations of intracellular calcium activity and mitochondrial membrane potential in mouse pancreatic B-cells. Biochem Biophys Res Commun. 2000;267:179–183. doi: 10.1006/bbrc.1999.1921. [DOI] [PubMed] [Google Scholar]
- [149].Kindmark H, Kohler M, Brown G, Branstrom R, Larsson O, Berggren PO. Glucose-induced oscillations in cytoplasmic free Ca2+ concentration precede oscillations in mitochondrial membrane potential in the pancreatic beta-cell. J Biol Chem. 2001;276:34530–34536. doi: 10.1074/jbc.M102492200. [DOI] [PubMed] [Google Scholar]
- [150].Alonso MT, Villalobos C, Chamero P, Alvarez J, Garcia-Sancho J. Calcium microdomains in mitochondria and nucleus. Cell Calcium. 2006;40:513–525. doi: 10.1016/j.ceca.2006.08.013. [DOI] [PubMed] [Google Scholar]
- [151].Murgia M, Giorgi C, Pinton P, Rizzuto R. Controlling metabolism and cell death: at the heart of mitochondrial calcium signalling. J Mol Cell Cardiol. 2009;46:781–788. doi: 10.1016/j.yjmcc.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Szabadkai G, Duchen MR. Mitochondria mediated cell death in diabetes. Apoptosis. 2009;14:1405–1423. doi: 10.1007/s10495-009-0363-5. [DOI] [PubMed] [Google Scholar]
- [153].Szabadkai G, Duchen MR. Mitochondria: the hub of cellular Ca2+ signaling. Physiology (Bethesda) 2008;23:84–94. doi: 10.1152/physiol.00046.2007. [DOI] [PubMed] [Google Scholar]
- [154].Busija DW, Gaspar T, Domoki F, Katakam PV, Bari F. Mitochondrial-mediated suppression of ROS production upon exposure of neurons to lethal stress: mitochondrial targeted preconditioning. Adv Drug Deliv Rev. 2008;60:1471–1477. doi: 10.1016/j.addr.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Kennedy ED, Maechler P, Wollheim CB. Effects of depletion of mitochondrial DNA in metabolism secretion coupling in INS-1 cells. Diabetes. 1998;47:374–380. doi: 10.2337/diabetes.47.3.374. [DOI] [PubMed] [Google Scholar]
- [156].Noda M, Yamashita S, Takahashi N, et al. Switch to anaerobic glucose metabolism with NADH accumulation in the beta-cell model of mitochondrial diabetes. Characteristics of betaHC9 cells deficient in mitochondrial DNA transcription. J Biol Chem. 2002;277:41817–41826. doi: 10.1074/jbc.M207690200. [DOI] [PubMed] [Google Scholar]
- [157].Maassen JA, ’t Hart LM, Janssen GM, Reiling E, Romijn JA, Lemkes HH. Mitochondrial diabetes and its lessons for common Type 2 diabetes. Biochem Soc Trans. 2006;34:819–823. doi: 10.1042/BST0340819. [DOI] [PubMed] [Google Scholar]
- [158].Tsuruzoe K, Araki E, Furukawa N, et al. Creation and characterization of a mitochondrial DNA-depleted pancreatic beta-cell line: impaired insulin secretion induced by glucose, leucine, and sulfonylureas. Diabetes. 1998;47:621–631. doi: 10.2337/diabetes.47.4.621. [DOI] [PubMed] [Google Scholar]
- [159].Silva JP, Kohler M, Graff C, et al. Impaired insulin secretion and beta-cell loss in tissue-specific knockout mice with mitochondrial diabetes. Nat Genet. 2000;26:336–340. doi: 10.1038/81649. [DOI] [PubMed] [Google Scholar]
- [160].Lu H, Koshkin V, Allister EM, Gyulkhandanyan AV, Wheeler MB. Molecular and metabolic evidence for mitochondrial defects associated with beta-cell dysfunction in a mouse model of type 2 diabetes. Diabetes. 2010;59:448–459. doi: 10.2337/db09-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Gao CL, Zhu C, Zhao YP, et al. Mitochondrial dysfunction is induced by high levels of glucose and free fatty acids in 3T3-L1 adipocytes. Mol Cell Endocrinol. 2010;320:25–33. doi: 10.1016/j.mce.2010.01.039. [DOI] [PubMed] [Google Scholar]
- [162].van den Oever IA, Raterman HG, Nurmohamed MT, Simsek S. Endothelial dysfunction, inflammation, and apoptosis in diabetes mellitus. Mediators Inflamm. 2010;2010:792393. doi: 10.1155/2010/792393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Marchetti P, Bugliani M, Lupi R, et al. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia. 2007;50:2486–2494. doi: 10.1007/s00125-007-0816-8. [DOI] [PubMed] [Google Scholar]
- [164].Grunnet LG, Aikin R, Tonnesen MF, et al. Proinflammatory cytokines activate the intrinsic apoptotic pathway in beta-cells. Diabetes. 2009;58:1807–1815. doi: 10.2337/db08-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Barbu A, Welsh N, Saldeen J. Cytokine-induced apoptosis and necrosis are preceded by disruption of the mitochondrial membrane potential (Deltapsi(m)) in pancreatic RINm5F cells: prevention by Bcl-2. Mol Cell Endocrinol. 2002;190:75–82. doi: 10.1016/s0303-7207(02)00009-6. [DOI] [PubMed] [Google Scholar]
- [166].Papaccio G, Graziano A, D’Aquino R, Valiante S, Naro F. A biphasic role of nuclear transcription factor (NF)-kappaB in the islet beta-cell apoptosis induced by interleukin (IL)-1beta. J Cell Physiol. 2005;204:124–130. doi: 10.1002/jcp.20276. [DOI] [PubMed] [Google Scholar]
- [167].Wang L, Bhattacharjee A, Zuo Z, et al. A low voltage-activated Ca2+ current mediates cytokine-induced pancreatic beta-cell death. Endocrinology. 1999;140:1200–1204. doi: 10.1210/endo.140.3.6556. [DOI] [PubMed] [Google Scholar]
- [168].Parkash J, Chaudhry MA, Rhoten WB. Tumor necrosis factor-alpha-induced changes in insulin-producing beta-cells. Anat Rec A Discov Mol Cell Evol Biol. 2005;286:982–993. doi: 10.1002/ar.a.20229. [DOI] [PubMed] [Google Scholar]
- [169].Emre Y, Hurtaud C, Karaca M, Nubel T, Zavala F, Ricquier D. Role of uncoupling protein UCP2 in cell-mediated immunity: how macrophage-mediated insulitis is accelerated in a model of autoimmune diabetes. Proc Natl Acad Sci U S A. 2007;104:19085–19090. doi: 10.1073/pnas.0709557104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Lee SC, Robson-Doucette CA, Wheeler MB. Uncoupling protein 2 regulates reactive oxygen species formation in islets and influences susceptibility to diabetogenic action of streptozotocin. J Endocrinol. 2009;203:33–43. doi: 10.1677/JOE-09-0117. [DOI] [PubMed] [Google Scholar]
- [171].Emre Y, Nubel T. Uncoupling protein UCP2: when mitochondrial activity meets immunity. FEBS Lett. 2010;584:1437–1442. doi: 10.1016/j.febslet.2010.03.014. [DOI] [PubMed] [Google Scholar]
- [172].Trenker M, Malli R, Fertschai I, Levak-Frank S, Graier WF. Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat Cell Biol. 2007;9:445–452. doi: 10.1038/ncb1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Lee B, Miles PD, Vargas L, et al. Inhibition of mitochondrial Na+-Ca2+ exchanger increases mitochondrial metabolism and potentiates glucose-stimulated insulin secretion in rat pancreatic islets. Diabetes. 2003;52:965–973. doi: 10.2337/diabetes.52.4.965. [DOI] [PubMed] [Google Scholar]
- [174].Luciani DS, Ao P, Hu X, Warnock GL, Johnson JD. Voltage-gated Ca(2+) influx and insulin secretion in human and mouse beta-cells are impaired by the mitochondrial Na(+)/Ca(2+) exchange inhibitor CGP-37157. Eur J Pharmacol. 2007;576:18–25. doi: 10.1016/j.ejphar.2007.07.055. [DOI] [PubMed] [Google Scholar]
- [175].Hoppe UC. Mitochondrial calcium channels. FEBS Lett. 2010;584:1975–1981. doi: 10.1016/j.febslet.2010.04.017. [DOI] [PubMed] [Google Scholar]
- [176].Wiederkehr A, Wollheim CB. Impact of mitochondrial calcium on the coupling of metabolism to insulin secretion in the pancreatic beta-cell. Cell Calcium. 2008;44:64–76. doi: 10.1016/j.ceca.2007.11.004. [DOI] [PubMed] [Google Scholar]
- [177].Bootman MD, Thomas D, Tovey SC, Berridge MJ, Lipp P. Nuclear calcium signalling. Cell Mol Life Sci. 2000;57:371–378. doi: 10.1007/PL00000699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Villalobos C, Nadal A, Nunez L, et al. Bioluminescence imaging of nuclear calcium oscillations in intact pancreatic islets of Langerhans from the mouse. Cell Calcium. 2005;38:131–139. doi: 10.1016/j.ceca.2005.06.029. [DOI] [PubMed] [Google Scholar]
- [179].Quesada I, Martin F, Roche E, Soria B. Nutrients induce different Ca(2+) signals in cytosol and nucleus in pancreatic beta-cells. Diabetes. 2004;53(Suppl 1):S92–5. doi: 10.2337/diabetes.53.2007.s92. [DOI] [PubMed] [Google Scholar]
- [180].Quesada I, Rovira JM, Martin F, Roche E, Nadal A, Soria B. Nuclear KATP channels trigger nuclear Ca(2+) transients that modulate nuclear function. Proc Natl Acad Sci U S A. 2002;99:9544–9549. doi: 10.1073/pnas.142039299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Villalobos C, Alonso MT, Garcia-Sancho J. Bioluminescence imaging of calcium oscillations inside intracellular organelles. Methods Mol Biol. 2009;574:203–214. doi: 10.1007/978-1-60327-321-3_17. [DOI] [PubMed] [Google Scholar]
- [182].Echevarria W, Leite MF, Guerra MT, Zipfel WR, Nathanson MH. Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum. Nat Cell Biol. 2003;5:440–446. doi: 10.1038/ncb980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Eizirik DL, Mandrup-Poulsen T. A choice of death--the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia. 2001;44:2115–2133. doi: 10.1007/s001250100021. [DOI] [PubMed] [Google Scholar]
- [184].Sprinkel AM, Andersen NA, Mandrup-Poulsen T. Glucose potentiates interleukin-1 beta (IL-1 beta)-induced p38 mitogen-activated protein kinase activity in rat pancreatic islets of Langerhans. Eur Cytokine Netw. 2001;12:331–339. [PubMed] [Google Scholar]
- [185].Scarim AL, Nishimoto SY, Weber SM, Corbett JA. Role for c-Jun N-terminal kinase in beta-cell recovery from nitric oxide-mediated damage. Endocrinology. 2003;144:3415–3422. doi: 10.1210/en.2002-0112. [DOI] [PubMed] [Google Scholar]
- [186].Storling J, Binzer J, Andersson AK, et al. Nitric oxide contributes to cytokine-induced apoptosis in pancreatic beta cells via potentiation of JNK activity and inhibition of Akt. Diabetologia. 2005;48:2039–2050. doi: 10.1007/s00125-005-1912-2. [DOI] [PubMed] [Google Scholar]
- [187].Bootman MD, Fearnley C, Smyrnias I, MacDonald F, Roderick HL. An update on nuclear calcium signalling. J Cell Sci. 2009;122:2337–2350. doi: 10.1242/jcs.028100. [DOI] [PubMed] [Google Scholar]
- [188].Bertram R, Sherman A, Satin LS. Metabolic and electrical oscillations: partners in controlling pulsatile insulin secretion. Am J Physiol Endocrinol Metab. 2007;293:E890–900. doi: 10.1152/ajpendo.00359.2007. [DOI] [PubMed] [Google Scholar]
- [189].Fridlyand LE, Tamarina N, Philipson LH. Bursting and calcium oscillations in pancreatic beta-cells: specific pacemakers for specific mechanisms. Am J Physiol Endocrinol Metab. 2010;299:E517–32. doi: 10.1152/ajpendo.00177.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Mastrandrea L, Yu J, Behrens T, et al. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care. 2009;32:1244–1249. doi: 10.2337/dc09-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- [192].Sauter NS, Schulthess FT, Galasso R, Castellani LW, Maedler K. The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology. 2008;149:2208–2218. doi: 10.1210/en.2007-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Grimm C, Koberlein J, Wiosna W, Kresimon J, Kiencke P, Rychlik R. New-onset diabetes and antihypertensive treatment. GMS Health Technol Assess. 2010;6 doi: 10.3205/hta000081. Doc03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Abuissa H, Jones PG, Marso SP, O’Keefe JH., Jr Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes: a meta-analysis of randomized clinical trials. J Am Coll Cardiol. 2005;46:821–826. doi: 10.1016/j.jacc.2005.05.051. [DOI] [PubMed] [Google Scholar]
- [195].Cole BK, Keller SR, Wu R, Carter JD, Nadler JL, Nunemaker CS. Valsartan protects pancreatic islets and adipose tissue from the inflammatory and metabolic consequences of a high-fat diet in mice. Hypertension. 2010;55:715–721. doi: 10.1161/HYPERTENSIONAHA.109.148049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Antkowiak PF, Tersey SA, Carter JD, et al. Noninvasive assessment of pancreatic beta-cell function in vivo with manganese-enhanced magnetic resonance imaging. Am J Physiol Endocrinol Metab. 2009;296:E573–8. doi: 10.1152/ajpendo.90336.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Nunemaker CS, Wasserman DH, McGuinness OP, Sweet IR, Teague JC, Satin LS. Insulin secretion in the conscious mouse is biphasic and pulsatile. Am J Physiol Endocrinol Metab. 2006;290:E523–9. doi: 10.1152/ajpendo.00392.2005. [DOI] [PubMed] [Google Scholar]
- [198].Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS. A practical guide to rodent islet isolation and assessment. Biol Proced Online. 2009;11:3–31. doi: 10.1007/s12575-009-9021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]