Abstract
Background
The definitive local therapy options for early stage breast cancer are 1) mastectomy and 2) breast conserving surgery followed by radiation therapy. Older women and those with comorbidities frequently receive breast conserving surgery alone. The interaction of age and comorbidity with breast cancer severity and their impact on receipt of definitive therapy have not been well studied
Study Design
In a cohort of 1837 women age≥65 years receiving treatment for early stage breast cancer in 6 integrated healthcare delivery systems in 1990–1994 and followed for 10 years, we examined predictors of receiving non-definitive local therapy and assessed the impact on breast cancer recurrence within levels of severity, defined as level of risk for recurrence.
Results
Age and comorbidity were associated with receipt of non-definitive therapy. Compared to those at low risk, women at the highest risk were less likely to receive non-definitive therapy (odds ratio (OR) 0.32, 95% confidence interval (CI) 0.22, 0.47) while women at moderate risk were about half as likely (OR 0.54, CI 0.35, 0.84). Non-definitive local therapy was associated with higher rates of recurrence among women at moderate (HR 5.1, CI 1.9, 13.5) and low risk (HR 3.2, CI 1.1, 8.9). The association among women at high risk was weak (HR 1.3, CI 0.75, 2.1).
Conclusions
Among these older women with early stage breast cancer, decisions about therapy partially balanced breast cancer severity against age and comorbidity. However, even among women at low risk, omitting definitive local therapy was associated with increased recurrence.
Guidelines for definitive local therapy for women with early stage breast cancer at any age include two alternatives: mastectomy or breast conserving surgery (BCS) with radiation therapy.(1–4) Randomized trials have found these alternatives to be approximately equal in their protection against recurrence or mortality.(5–13) Prior to 1990 mastectomy was the primary alternative received by older women.(14–15) In 1990 the National Institutes of Health issued a statement recommending BCS with radiation therapy for early stage breast cancer(16) and use of BCS in the United States increased.(17) A recent analysis of SEER data for the period 1998 through 2004 found an overall drop in the rates of definitive local therapy because the steady decrease in use of mastectomy was accompanied by a rise in the rate of BCS without radiation therapy.(18) This study also found that older women, especially those age 80 and older, have substantially lower rates of definitive local therapy than other age groups. Observational studies have consistently found older women with early stage breast cancer who receive BCS to have lower rates of radiation therapy than their younger counterparts.(19–31)
A number of potential explanations have been offered for this omission. Older women are more likely to have comorbid conditions that may interact with radiation therapy, their remaining life spans may be perceived to be shorter, their tumors may be considered less aggressive and less likely to be followed by recurrence, and the women themselves (with their clinicians) may simply be making decisions based on advanced age. Decisions to provide non-definitive local therapy may reflect a considered weighing of disease severity against the risk of interactions with comorbid conditions and shortened expected life spans and may result in adequate protection against recurrence. However, if these decisions are primarily a response to patients’ age, older women may be left with unnecessarily high rates of recurrence.
We undertook an analysis of treatment and outcomes among 1837 women age 65 and older who were diagnosed with early stage breast cancer and treated in community settings. We aimed to answer two questions: did receipt of non-definitive local therapy reflect a balance between disease severity and comorbidity and, if so, was this balance effective in protecting women against recurrence?
Methods
Data for this study were derived from the Breast Cancer Treatment Effectiveness in Older Women (BOW) study. The methodology of the BOW study has been described previously.(32) The study was designed to examine outcomes among women age 65 and older diagnosed and treated with incident early stage invasive breast cancer while enrolled in one of six health systems participating in the Cancer Research Network (CRN): Group Health (Washington); Kaiser Permanente (Southern California); Lovelace (New Mexico); Henry Ford Health System (Michigan); HealthPartners (Minnesota); and Fallon Clinic (Massachusetts). Institutional Review Boards at all sites approved the study. At the time of the study, the CRN included the research programs, enrollee populations, and data systems of 11 integrated health care systems across the United States.(33)
Eligible patients were all women aged 65 years or older who were diagnosed for the first time with histologically confirmed, early-stage breast cancer between January 1, 1990 and December 31, 1994 and who received mastectomy or BCS. Eligible women were enrolled for at least 12 months before diagnosis and 12 months after or until death. We excluded women with a clinically active malignancy (except nonmelanoma skin cancer) diagnosed within the 5 years before or the 30 days after breast cancer diagnosis. Within the largest health system, we included all minority women, those 80 or older and those diagnosed at TNM stage II, and sampled 10% of non-hispanic caucasion women younger than 80 diagnosed with stage I disease. Breast cancer diagnoses were identified using tumor registries at four health systems and administrative data at two. Eligibility was verified through medical record review at all sites.
Data Collection
Using an automated data collection system(34) and trained medical record abstractors, we collected demographic, tumor, treatment, and outcome data from cancer registry, administrative, and clinical databases, as well as medical records. Data on tumors included date of diagnosis, American Joint Commission on Cancer TNM stage, tumor size, histology, and receptor status. Information about treatment included type of surgery, receipt of tamoxifen, documented discussion of radiation therapy, referral to a radiation oncologist, radiation oncology recommendations, and initiation of radiation therapy. We determined first recurrence based on the chronologically first occurrence of the following: invasive tumor in the same breast or the lymph nodes, skin or chest muscle on the same side, or metastasis.
Abstractors gathered information from medical records on date of birth and race and ethnicity as recorded by providers. We assessed diagnosis of comorbidities through medical record abstraction using a version of the Charlson Comorbidity Index for the year prior to diagnosis, weighted according to the original study.(35) We excluded cancer and categorized the remaining score as 0, 1, 2+.
For the current study, we defined disease severity as the level of risk for recurrence using criteria from the 1992 St Gallen conference which were reported during the time period when the women in this study were diagnosed with breast cancer.(36) High level of risk included positive nodes or negative nodes with any of the following: estrogen receptor negative tumor 1 centimeter or larger, estrogen positive tumor larger than 2 centimeters, or poorly- or undifferentiated tumor. Moderate risk was defined as node negative with estrogen receptor positive 1 to 2 centimeter tumor. Low risk was defined as node negative with estrogen receptor positive tumor of up to 1 centimeter. In this cohort of older women, some clinical decisions were reached without complete assessment: when axillary dissection was not performed, we categorized the case as node negative (n=344); when histologic grade was not determined or recorded, we categorized the tumor as other than poorly- or undifferentiated (n=435); when estrogen receptor status was indeterminate or not done, we categorized the tumor as estrogen receptor positive (n=223). We defined the primary outcome, non-definitive local therapy, as BCS with no radiation therapy sessions.
Analysis
We fit logistic regression models to assess the relationship of age, comorbidity and level of risk for recurrence to receipt of non-definitive local therapy. Additional models were stratified by level of risk. All models were adjusted for race/ethnicity and health system.
We used Cox proportional hazards regression to estimate the association of non-definitive local therapy with actual recurrence. Follow-up for person-time began at the date of diagnosis and ended at whichever was the earliest of the following events: the first recurrence, disenrollment from the health system, death, or completion of 10 years following diagnosis. Models were developed to include all women as well as a series stratified by level of risk. All models were adjusted for race/ethnicity and health plan. Interaction terms between age and receipt of non-definitive local therapy were assessed in all models but they had minor impacts on the effect estimates for non-definitive local therapy and were not statistically significant. We also ran models including receipt of tamoxifen to estimate the possibility of further confounding by this additional component of treatment. The addition of radiation therapy to BCS is expected to provide protection against local and regional recurrence and is unlikely to impact distant recurrences. Therefore, we also developed separate models predicting these outcomes.
Among the subgroup of women who received BCS, we followed the pathway to initiation of radiation therapy, calculating the proportion who dropped off at critical decision points.
The analyses for this study were generated using SAS software (version 9.1) of the SAS System for Windows (SAS Institute, Inc., Cary, NC)).
Results
Among the 1837 women included in the cohort, just over half received a mastectomy (Table 1). Eleven percent received non-definitive local therapy. Sixty-eight percent of the cohort had a score of zero on the Charlson Comorbidity Index and 56% were at a high level of risk for recurrence. Age, comorbidity and level of risk were all associated with receipt of non-definitive therapy. Level of risk was not strongly correlated with age or comorbidity (Table 2) with the exception of women age 80 and older who were less often at high risk.
Table 1.
Patient Characteristics Associated with Receipt of Local Therapy
| All women | Definitive local therapy | Non-definitive therapy | |||
|---|---|---|---|---|---|
| n=1,837, n (%) |
Mastectomy n=977, n (%) |
BCS with any RT, n=663, n (%) |
BCS without RT, n=197, n (%) |
p Value, definitive vs less |
|
| Age, y | |||||
| 65–69 | 625 (34) | 341 (35) | 259 (39) | 26 (13) | <0.0001 |
| 70–74 | 547 (30) | 299 (31) | 223 (34) | 25 (13) | |
| 75–79 | 304 (17) | 154 (16) | 103 (16) | 47 (24) | |
| 80+ | 360 (20) | 183 (19) | 78 (12) | 99 (50) | |
| Charlson Comorbidity Index | |||||
| 0 | 1252 (68) | 668 (68) | 477 (72) | 107 (54) | <0.0001 |
| 1 | 498 (27) | 260 (27) | 163 (25) | 75 (38) | |
| 2+ | 87 (5) | 49 (5) | 23 (3) | 15 (8) | |
| Recurrence risk | |||||
| Low | 439 (24) | 143 (15) | 218 (33) | 78 (40) | <0.0001 |
| Moderate | 378 (21) | 167 (17) | 163 (25) | 48 (24) | |
| High | 1020 (56) | 667 (68) | 282 (43) | 71 (36) | |
Table 2.
Age, Comorbidity, and Level of Risk for Recurrence
| Low risk, n (%) |
Moderate risk, n (%) |
High risk, n (%) |
|
|---|---|---|---|
| All women | 439 | 378 | 1020 |
| Age, y | |||
| 65–69 | 131 (30) | 111 (29) | 384 (38) |
| 70–74 | 139 (32) | 95 (25) | 313 (31) |
| 75–79 | 73 (17) | 66 (17) | 165 (16) |
| 80+ | 96 (22) | 106 (28) | 158 (15) |
| Charlson Comorbidity Index | |||
| 0 | 292 (67) | 250 (66) | 710 (70) |
| 1 | 126 (29) | 109 (29) | 263 (26) |
| 2+ | 21 (5) | 19 (5) | 47 (5) |
In logistic regression models that included the entire cohort, age, comorbidity, and level of risk were associated with receipt of non-definitive local therapy (Table 3), controlling for race/ethnicity and health plan. Women age 75 or older were more likely to receive non-definitive therapy, as were women with at least one comorbidity. Women at moderate or high risk were significantly less likely to receive non-definitive therapy. Within models stratified by level of risk, there was variability in the pattern of associations of age and comorbidity with non-definitive local therapy, although women age 75 or older were more likely to receive non-definitive therapy in all strata.
Table 3.
Patient Characteristics Independently Associated with Receipt of Non-Definitive Local Therapy
| All women | Women at low risk |
Women at moderate risk |
Women at high risk |
|||||
|---|---|---|---|---|---|---|---|---|
| OR* | 95% CI | OR* | 95% CI | OR* | 95% CI | OR* | 95% CI | |
| Age, y | ||||||||
| 65–69 | 1.0 | referent | 1.0 | referent | 1.0 | referent | 1.0 | referent |
| 70–74 | 1.1 | 0.59, 1.9 | 1.3 | 0.50, 3.5 | 1.6 | 0.21, 11.6 | 0.9 | 0.40, 1.9 |
| 75–79 | 4.0 | 2.4, 6.6 | 4.9 | 2.0, 12.1 | 14.6 | 3.0, 71.7 | 2.5 | 1.2, 5.1 |
| 80 + | 8.4 | 5.2, 13.5 | 9.1 | 3.9, 21.4 | 34.2 | 7.1, 165.9 | 5.1 | 2.6, 10.2 |
| Charlson Comorbidity Index | ||||||||
| 0 | 1.0 | referent | 1.0 | referent | 1.0 | referent | 1.0 | referent |
| 1 | 1.6 | 1.1, 2.3 | 1.3 | 0.70, 2.3 | 1.9 | 0.88, 4.2 | 1.9 | 1.1, 3.3 |
| 2+ | 2.0 | 1.0, 3.8 | 1.3 | 0.40, 4.1 | 1.1 | 0.24, 4.8 | 3.4 | 1.4, 8.5 |
| Recurrence Risk | ||||||||
| low | 1.0 | referent | ||||||
| moderate | 0.54 | 0.35, 0.84, | ||||||
| high | 0.32 | 0.22, 0.47 | ||||||
Controlling for health system and race/ethnicity.
The median follow-up time was 8.7 years (minimum 0.2 years, maximum 11.6 years) and 10% were lost to follow-up for recurrence by disenrolling from the participating health plan. Local, regional or distant recurrences occurred in 295 women (98 local or regional and 197 distant). In bivariate analyses, recurrence was strongly associated with level of risk (Table 4). The bivariate associations of age, comorbidity and receipt of non-definitive local therapy with recurrence were small. However, in multivariable models, receipt of non-definitive local therapy was associated with increased rate of recurrence (hazard ratio (HR) 1.6, 95% CI 1.1, 2.4) (Table 5), while age and comorbidity were not. Within analyses stratified by level of risk, receipt of non-definitive local therapy was strongly associated with recurrence among women at low (HR 3.2, CI 1.1, 8.9) and moderate (HR 5.1, CI 1.9, 13.5) risk. Among women at high risk, the association was attenuated (HR 1.3, CI 0.75, 2.1). In models controlling for receipt of tamoxifen, results were unchanged (data not shown).
Table 4.
Patient Characteristics Associated with Recurrence
| n (%) | p Value | |
|---|---|---|
| Age, y | ||
| 65–69 | 112 (18) | 0.09 |
| 70–74 | 92 (17) | |
| 75–79 | 48 (16) | |
| 80 + | 43 (12) | |
| Charlson Comorbidity Index | ||
| 0 | 207 (17) | 0.80 |
| 1 | 76 (15) | |
| 2+ | 12 (14) | |
| Recurrence risk | ||
| low | 32 (7) | <0.0001 |
| Moderate | 33 (9) | |
| High | 230 (23) | |
| Definitive local therapy | ||
| Yes | 261 (16) | 0.62 |
| No | 34 (17) | |
Table 5.
Independent Association of Patient Characteristics and Receipt of Non-Definitive Local Therapy with Recurrence
| All women | Women at low risk |
Women at moderate risk |
Women at high risk |
|||||
|---|---|---|---|---|---|---|---|---|
| HR* | 95% CI | HR* | 95% CI | HR* | 95% CI | HR* | 95% CI | |
| Definitive local therapy | 1.0 | referent | 1.0 | referent | 1.0 | referent | 1.0 | referent |
| Non-definitive local therapy | 1.6 | 1.1, 2.4 | 3.2 | 1.1, 8.9 | 5.1 | 1.9, 13.5 | 1.3 | 0.75, 2.1 |
| Age, y | ||||||||
| 65–69 | 1.0 | referent | 1.0 | referent | 1.0 | referent | 1.0 | referent |
| 70–74 | 1.0 | 0.78, 1.4 | 0.77 | 0.35, 1.7 | .78 | 0.29, 2.2 | 1.1 | 0.77, 1.4 |
| 75–79 | 1.0 | 0.71, 1.4 | 0.21 | 0.04, 1.0 | 1.3 | 0.47, 3.5 | 1.1 | 0.72, 1.6 |
| 80+ | 0.82 | 0.57, 1.2 | 0.31 | 0.09, 1.1 | .39 | 0.12, 1.2 | 0.9 | 0.60, 1.4 |
| Charlson Comorbidity Index | ||||||||
| 0 | 1.0 | referent | 1.0 | referent | 1.0 | referent | 1.0 | referent |
| 1 | 1.1 | 0.84, 1.4 | .98 | 0.43, 2.2 | 1.2 | 0.56, 2.6 | 1.1 | 0.80, 1.5 |
| 2+ | 1.1 | 0.59, 1.9 | 1.0 | .13, 7.8 | 1.2 | 0.27, 5.3 | 1.1 | 0.55, 2.1 |
| Recurrence risk | ||||||||
| low | 1.0 | referent | ||||||
| Moderate | 1.3 | 0.81, 2.2 | ||||||
| High | 3.4 | 2.3, 5.0 | ||||||
Controlling for health system and race.
In models that assessed local and regional recurrence separately from distant recurrence, receipt of non-definitive local therapy had strong and statistically significant associations with local and regional recurrence among women at all levels of risk, although the estimates had low precision and wide confidence intervals (Table 6). Associations with distant recurrence were weak and not statistically significant.
Table 6.
Independent Association of Non-Definitive Local Therapy with Local/Regional Recurrence and Distant Recurrence
| All women | Women at low risk |
Women at moderate risk |
Women at high risk |
|||||
|---|---|---|---|---|---|---|---|---|
| HR* | 95% CI | HR* | 95% CI | HR* | 95% CI | HR* | 95% CI | |
| Local/regional recurrence | 3.6 | 2.1, 6.1 | 6.7 | 1.6, 28.1 | 18.9 | 3.6, 98.4 | 2.6 | 1.3, 5.2 |
| Distant recurrence | 0.83 | 0.45, 1.5 | 1.8 | 0.35, 9.6 | 1.9 | 0.44, 7.8 | 0.72 | 0.33, 1.6 |
Controlling for age, Charlson Comorbidity Index, recurrence risk, health system, and race.
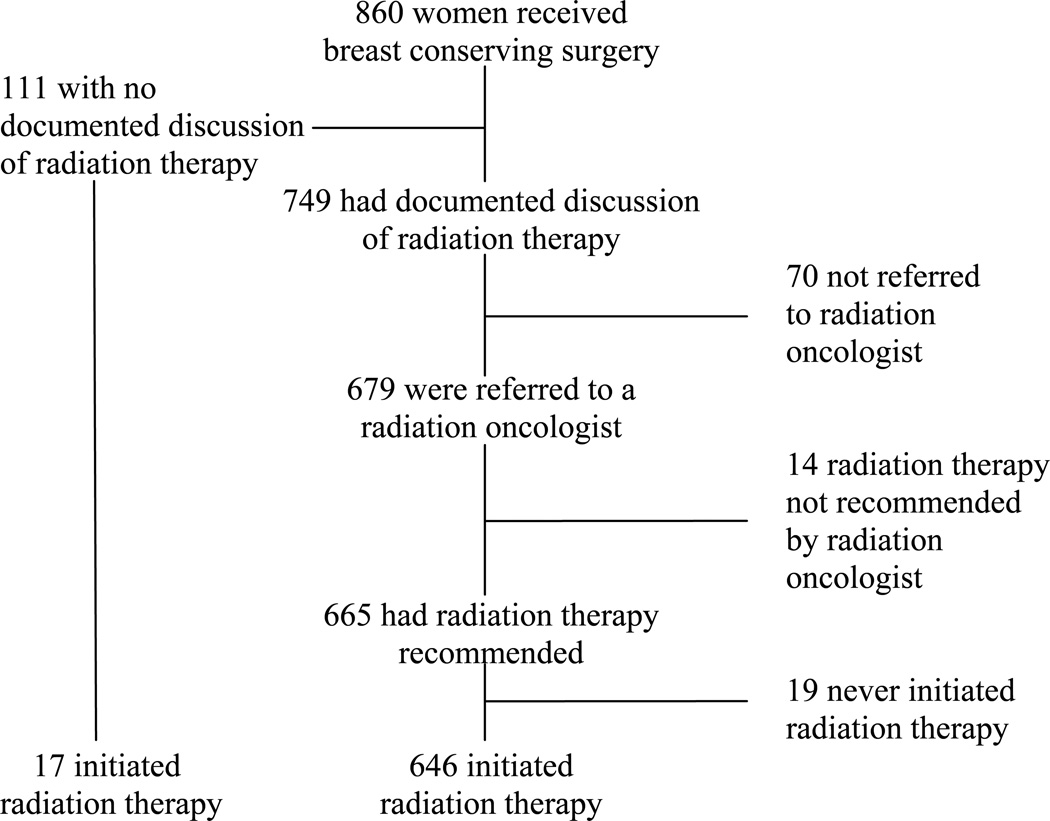
Among the 860 women who received BCS, 663 (77%) initiated radiation therapy (Figure 1). Tracking the process from diagnosis to receipt of radiation therapy, a substantial number of women with BCS had no documented discussion of radiation therapy in their medical records (111, 13% of the 860 women). Among those with a documented discussion, 70 (8%) were not referred to a radiation oncologist. Of those who saw a radiation oncologist, 14 (2%) were not recommended to receive radiation therapy. Among women with recommended radiation therapy, 19 (2%) had no treatment. For women who were not referred or not recommended, documented explanations were available for very few and were inconclusive.
Figure 1.
Pathway to initiation of radiation therapy for women with breast conserving surgery.
Discussion
Within this cohort of older women with early stage breast cancer, decisions about initial local therapy appear to have partially balanced level of risk with the presence of comorbidities. However, independent of comorbid conditions, age was strongly associated with non-definitive therapy. At low and moderate levels of risk, the associations between non-definitive therapy and rates of recurrence were substantial, particularly for local or regional recurrence. Among women whose therapy began with BCS, 23% never initiated radiation therapy; nearly half of that group had no discussion of radiation therapy documented in the medical record.
During the years when these women were diagnosed (1990–1994) mastectomy and BCS with radiation therapy were the standard of care for early stage breast cancer.(16) Despite this, many observational studies that specifically focused on BCS in this age group in the United States, Europe and Canada have found low rates of radiation therapy, particularly among women age 75 or 80 and older.(19–31) As mastectomy rates decreased and rates of BCS rose, the potential for disparities in receipt of definitive local therapy rose as well. During the years since 1990, the frequent omission of radiation therapy has produced decreasing rates of definitive local therapy among several sub-groups, including women over the age of 80.(18)
The recommendation to accompany BCS with radiation therapy is based on a series of randomized trials that consistently found lower rates of recurrence and improved mortality outcomes with the combined therapy. Since these trials rarely contained women of advanced age, the need for radiation therapy in this age group has been a matter of contention.(37) One trial that did focus specifically on older women with estrogen positive stage I breast cancer and BCS who were randomized to receive either tamoxifen plus radiation therapy or tamoxifen alone found no difference in breast cancer specific 5-year survival.(38) Within this very low risk cohort, the rate of local and regional recurrence for women without radiation therapy was higher by a small, but statistically significant amount (five-year probability of freedom from local or regional recurrence of 99% compared to 96% for women without radiation therapy). The trial’s investigators concluded that BCS without radiation therapy is a realistic choice for older women with early stage, estrogen-receptor-positive breast cancer. To date, no evidence has identified sub-groups of older women for whom definitive local therapy does not reduce recurrence rates.(39–42)
There are several potential explanations for older women receiving non-definitive therapy. The women themselves may have weighed the potential problems and outcomes associated with both mastectomy and radiation therapy against their perceptions of the impact on their future health and decided to undergo only BCS. Breast cancer surgeons and oncologists likely play major roles in these decisions, both through their recommendations and through the extent to which they frame their discussion of treatment options around the potential for recurrence.(43–46) In addition to physicians’ concerns about the women’s current comorbid conditions, they may also have considered possible risks associated with radiation therapy, which has been suspected of increasing cardiovascular outcomes.(47) This perception may have been particularly strong during the time period of this study when newer radiation therapy techniques had only recently been fully implemented.(48) Observational studies of radiation therapy have compared receipt to non receipt as well as receipt of radiation therapy for women with left vs. right breast cancer. Several studies have found higher rates of later cardiac diagnoses for left vs. right breast irradiation.(49–51) There is conflicting evidence about radiation therapy and cardiovascular outcomes; a number of studies have found no impact on long-term cardiac mortality or myocardial infarction.(52–55) If concerns about these outcomes were important issues for clinicians, mastectomy was an available alternative that would have provided women with definitive local therapy.
There are a number of limitations in this study. Medical records do not provide sufficient information to determine the reasoning related to treatment decisions, leaving open a range of possible alternative explanations. In addition, because we did not have access to the full transcripts of surgeon-patient discussions, we were unable to classify these discussions as containing recommendations for or against radiation therapy but relied on documentation of discussions and actual referrals as indicators. Our estimates are imprecise due to the small numbers of women receiving non-definitive therapy and at low level of risk. The study focuses on care during the early to mid 1990’s. Although long-term outcomes of BCS with radiation therapy continued to be published during the intervening years, recent assessments of receipt of definitive local therapy among older women have continued to identify high rates of omitted radiation therapy, particularly among those above the age of 75 or 80.(18,30) A number of women in this cohort did not have complete assessment of nodal status, histologic grade or estrogen receptor status and we followed clinical assumptions in categorizing these women. This may have led to an underestimate of their level of risk. However, the clinicians caring for them would have made similar assumptions in estimating their disease severity.
This study also has several important strengths. We identified the cohort from defined population bases, lessening the possibility of an unrepresentative sample. Because the women were diagnosed from 1990 to 1994, we were able to follow them for 10 years. They had a range of ages with a substantial number age 80 and older. The women received treatment from six integrated delivery systems spread across the country. The availability of comprehensive medical records enabled us to capture complete, detailed information about tumor characteristics, treatments, existence of comorbid conditions, and the timing of recurrences. These factors enabled us to analyze treatment, disease severity, and comorbidity and examine their impact on recurrence – a combination not available within the SEER-Medicare data and usually accessible only in single site studies of smaller cohorts.
Our findings suggest that decisions about initial treatment of older women with early stage breast cancer partially balance comorbidity with disease severity, but age remains the dominant factor, leaving women at even the lowest level of risk with increased recurrence. A number of clinical groups have suggested the need to move the focus of treatment decisions away from age and toward future life expectancy.(56–57) To accomplish this, further research will be required to delineate the decision-making process during initial breast cancer treatment for older women.
Acknowledgment
We acknowledge the contributions from the staff participating in this study. Group Health: Linda Shultz, Kristin Delaney, Margaret Farrell-Ross, Mary Sunderland, Millie Magner, and Beth Kirlin; Meyers Primary Care Institute and Fallon Clinic: Jackie Fuller, Doris Hoyer, and Janet Guilbert; Henry Ford Health System; Sharon Hensley Alford, Karen Wells, Patricia Baker, and Rita Montague; HealthPartners: Maribet McCarty and Alex Kravchik; Kaiser Permanente Southern California: Julia Stern, Janis Yao, Michelle McGuire, and Erica Hnatek-Mitchell; and Lovelace Health Plan: Judith Hurley, Hans Petersen, and Melissa Roberts
Funded by the National Cancer Institute (R01 CA093772, R Silliman, PI)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
References
- 1.National Cancer Institute: Breast cancer treatment (PDQ): health professional version 2011. [Accessed February 7, 2011]; Available at: http://www.cancer.gov/cancertopics/pdq/treatment/breast/healthprofessional.
- 2.Halberg FE, Shank BM, Haffty BG, et al. Conservative surgery and radiation in the treatment of stage I and II carcinoma of the breast. American College of Radiology. ACR Appropriateness Criteria. Radiology. 2000;215 Suppl:1193–1205. [PubMed] [Google Scholar]
- 3.Carlson RW, Allred DC, Anderson BO, et al. Breast cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2009;7:122–192. doi: 10.6004/jnccn.2009.0012. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer network: Clinical Practice Guidelines in Oncology. [Accessed February 7, 2011];Breast Cancer, version 2.2011. Available at: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 5.Veronesi U, Marubini E, Mariani L, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: long-term results of a randomized trial. Ann Oncol. 2001;12:997–1003. doi: 10.1023/a:1011136326943. [DOI] [PubMed] [Google Scholar]
- 6.Sarrazin D, Le MG, Arriagada R, et al. Ten-year results of a randomized trial comparing a conservative treatment to mastectomy in early breast cancer. Radiother Oncol. 1989;14:177–184. doi: 10.1016/0167-8140(89)90165-5. [DOI] [PubMed] [Google Scholar]
- 7.Fisher B, Anderson S, Redmond CK, et al. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1995;333:1456–1461. doi: 10.1056/NEJM199511303332203. [DOI] [PubMed] [Google Scholar]
- 8.Effects of radiotherapy and surgery in early breast cancer. An overview of the randomized trials. Early Breast Cancer Trialists' Collaborative Group. N Engl J Med. 1995;333:1444–1455. doi: 10.1056/NEJM199511303332202. [DOI] [PubMed] [Google Scholar]
- 9.Fisher B, Bryant J, Dignam JJ, et al. Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol. 2002;20:4141–4149. doi: 10.1200/JCO.2002.11.101. [DOI] [PubMed] [Google Scholar]
- 10.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 11.Poggi MM, Danforth DN, Sciuto LC, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute Randomized Trial. Cancer. 2003;98:697–702. doi: 10.1002/cncr.11580. [DOI] [PubMed] [Google Scholar]
- 12.Jatoi I, Proschan MA. Randomized trials of breast-conserving therapy versus mastectomy for primary breast cancer: a pooled analysis of updated results. Am J Clin Oncol. 2005;28:289–294. doi: 10.1097/01.coc.0000156922.58631.d7. [DOI] [PubMed] [Google Scholar]
- 13.Blichert-Toft M, Brincker H, Andersen JA, et al. A Danish randomized trial comparing breast-preserving therapy with mastectomy in mammary carcinoma. Preliminary results. Acta Oncol. 1988;27:671–677. doi: 10.3109/02841868809091767. [DOI] [PubMed] [Google Scholar]
- 14.Nattinger AB, Hoffmann RG, Kneusel RT, Schapira MM. Relation between appropriateness of primary therapy for early-stage breast carcinoma and increased use of breast-conserving surgery. Lancet. 2000;356:1148–1153. doi: 10.1016/S0140-6736(00)02757-4. [DOI] [PubMed] [Google Scholar]
- 15.Gilligan MA, Kneusel RT, Hoffmann RG, et al. Persistent differences in sociodemographic determinants of breast conserving treatment despite overall increased adoption. Med Care. 2002;40:181–189. doi: 10.1097/00005650-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 16.NIH consensus conference. Treatment of early-stage breast cancer. Jama. 1991;265:391–395. [PubMed] [Google Scholar]
- 17.Lazovich D, Solomon CC, Thomas DB, et al. Breast conservation therapy in the United States following the 1990 National Institutes of Health Consensus Development Conference on the treatment of patients with early stage invasive breast carcinoma. Cancer. 1999;86:628–637. [PubMed] [Google Scholar]
- 18.Freedman RA, He Y, Winer EP, Keating NL. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J Clin Oncol. 2009;27:713–719. doi: 10.1200/JCO.2008.17.9234. [DOI] [PubMed] [Google Scholar]
- 19.Ballard-Barbash R, Potosky AL, Harlan LC, et al. Factors associated with surgical and radiation therapy for early stage breast cancer in older women. J Natl Cancer Inst. 1996;88:716–726. doi: 10.1093/jnci/88.11.716. [DOI] [PubMed] [Google Scholar]
- 20.Joslyn SA. Radiation therapy and patient age in the survival from early-stage breast cancer. Int J Radiat Oncol Biol Phys. 1999;44:821–826. doi: 10.1016/s0360-3016(99)00071-1. [DOI] [PubMed] [Google Scholar]
- 21.Tyldesley S, Zhang-Salomons J, Groome PA, et al. Association between age and the utilization of radiotherapy in Ontario. Int J Radiat Oncol Biol Phys. 2000;47:469–480. doi: 10.1016/s0360-3016(00)00440-5. [DOI] [PubMed] [Google Scholar]
- 22.Hebert-Croteau N, Brisson J, Latreille J, et al. Compliance with consensus recommendations for the treatment of early stage breast carcinoma in elderly women. Cancer. 1999;85:1104–1113. [PubMed] [Google Scholar]
- 23.Yancik R, Wesley MN, Ries LA, et al. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 24.Giordano SH, Hortobagyi GN, Kau SW, et al. Breast cancer treatment guidelines in older women. J Clin Oncol. 2005;23:783–791. doi: 10.1200/JCO.2005.04.175. [DOI] [PubMed] [Google Scholar]
- 25.Litvak DA, Arora R. Treatment of elderly breast cancer patients in a community hospital setting. Arch Surg. 2006;141:985–990. doi: 10.1001/archsurg.141.10.985. discussion 990. [DOI] [PubMed] [Google Scholar]
- 26.Truong PT, Bernstein V, Lesperance M, et al. Radiotherapy omission after breast-conserving surgery is associated with reduced breast cancer-specific survival in elderly women with breast cancer. Am J Surg. 2006;191:749–755. doi: 10.1016/j.amjsurg.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 27.Siesling S, van de Poll-Franse LV, Jobsen JJ, et al. Explanatory factors for variation in the use of breast conserving surgery and radiotherapy in the Netherlands, 1990–2001. Breast. 2007;16:606–614. doi: 10.1016/j.breast.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Hancke K, Denkinger MD, Konig J, et al. Standard treatment of female patients with breast cancer decreases substantially for women aged 70 years and older: a German clinical cohort study. Ann Oncol. 21:748–753. doi: 10.1093/annonc/mdp364. [DOI] [PubMed] [Google Scholar]
- 29.Keating NL, Landrum MB, Brooks JM, et al. Outcomes following local therapy for early-stage breast cancer in non-trial populations. Breast Cancer Res Treat. 125:803–813. doi: 10.1007/s10549-010-0865-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schonberg MA, Marcantonio ER, Li D, et al. Breast cancer among the oldest old: tumor characteristics, treatment choices, and survival. J Clin Oncol. 2010;28:2038–2045. doi: 10.1200/JCO.2009.25.9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dragun AE, Huang B, Tucker TC, Spanos WJ. Disparities in the application of adjuvant radiotherapy after breast-conserving surgery for early stage breast cancer: impact on overall survival. Cancer. 2010 doi: 10.1002/cncr.25821. [DOI] [PubMed] [Google Scholar]
- 32.Enger SM, Thwin SS, Buist DS, et al. Breast cancer treatment of older women in integrated health care settings. J Clin Oncol. 2006;24:4377–4383. doi: 10.1200/JCO.2006.06.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner EH, Greene SM, Hart G, et al. Building a research consortium of large health systems: the Cancer Research Network. J Natl Cancer Inst Monogr. 2005:3–11. doi: 10.1093/jncimonographs/lgi032. [DOI] [PubMed] [Google Scholar]
- 34.Thwin SS, Clough-Gorr KM, McCarty MC, et al. Automated inter-rater reliability assessment and electronic data collection in a multi-center breast cancer study. BMC Med Res Methodol. 2007;7:23. doi: 10.1186/1471-2288-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 36.Glick JH, Gelber RD, Goldhirsch A, Senn HJ. Adjuvant therapy of primary breast cancer. Ann Oncol; 4th International Conference on Adjuvant Therapy of Primary Breast Cancer St; Gallen, Switzerland. 1992. pp. 801–807. [DOI] [PubMed] [Google Scholar]
- 37.Truong PT, Wong E, Bernstein V, et al. Adjuvant radiation therapy after breast-conserving surgery in elderly women with early-stage breast cancer: controversy or consensus? Clin Breast Cancer. 2004;4:407–414. doi: 10.3816/cbc.2004.n.003. [DOI] [PubMed] [Google Scholar]
- 38.Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351:971–977. doi: 10.1056/NEJMoa040587. [DOI] [PubMed] [Google Scholar]
- 39.Fisher ER, Anderson S, Tan-Chiu E, et al. Fifteen-year prognostic discriminants for invasive breast carcinoma: National Surgical Adjuvant Breast and Bowel Project Protocol-06. Cancer. 2001;91:1679–1687. [PubMed] [Google Scholar]
- 40.Kunkler IH, Williams LJ, King CC, Jack W. Breast radiotherapy: considerations in older patients. Clin Oncol (R Coll Radiol) 2009;21:111–117. doi: 10.1016/j.clon.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 41.Holli K, Hietanen P, Saaristo R, et al. Radiotherapy after segmental resection of breast cancer with favorable prognostic features: 12-year follow-up results of a randomized trial. J Clin Oncol. 2009;27:927–932. doi: 10.1200/JCO.2008.19.7129. [DOI] [PubMed] [Google Scholar]
- 42.Winzer KJ, Sauerbrei W, Braun M, et al. Radiation therapy and tamoxifen after breast-conserving surgery: updated results of a 2 × 2 randomised clinical trial in patients with low risk of recurrence. Eur J Cancer. 2010;46:95–101. doi: 10.1016/j.ejca.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 43.Maly RC, Leake B, Silliman RA. Breast cancer treatment in older women: impact of the patient-physician interaction. J Am Geriatr Soc. 2004;52:1138–1145. doi: 10.1111/j.1532-5415.2004.52312.x. [DOI] [PubMed] [Google Scholar]
- 44.Oskay-Ozcelik G, Lehmacher W, Konsgen D, et al. Breast cancer patients' expectations in respect of the physician-patient relationship and treatment management results of a survey of 617 patients. Ann Oncol. 2007;18:479–484. doi: 10.1093/annonc/mdl456. [DOI] [PubMed] [Google Scholar]
- 45.Warner E, Chow E, Fairchild A, et al. Attitudes of Canadian radiation oncologists towards post-lumpectomy radiotherapy for elderly women with stage I hormone-responsive breast cancer. Clin Oncol (R Coll Radiol) 22:97–106. doi: 10.1016/j.clon.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Halkett GK, Short M, Kristjanson LJ. How do radiation oncology health professionals inform breast cancer patients about the medical and technical aspects of their treatment? Radiother Oncol. 2009;90:153–159. doi: 10.1016/j.radonc.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 47.Senkus-Konefka E, Jassem J. Cardiovascular effects of breast cancer radiotherapy. Cancer Treat Rev. 2007;33:578–593. doi: 10.1016/j.ctrv.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Demirci S, Nam J, Hubbs JL, et al. Radiation-induced cardiac toxicity after therapy for breast cancer: interaction between treatment era and follow-up duration. Int J Radiat Oncol Biol Phys. 2009;73:980–987. doi: 10.1016/j.ijrobp.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 49.Harris EE, Correa C, Hwang WT, et al. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol. 2006;24:4100–4106. doi: 10.1200/JCO.2005.05.1037. [DOI] [PubMed] [Google Scholar]
- 50.Jagsi R, Griffith KA, Koelling T, et al. Rates of myocardial infarction and coronary artery disease and risk factors in patients treated with radiation therapy for early-stage breast cancer. Cancer. 2007;109:650–657. doi: 10.1002/cncr.22452. [DOI] [PubMed] [Google Scholar]
- 51.Correa CR, Litt HI, Hwang WT, et al. Coronary artery findings after left-sided compared with right-sided radiation treatment for early-stage breast cancer. J Clin Oncol. 2007;25:3031–3037. doi: 10.1200/JCO.2006.08.6595. [DOI] [PubMed] [Google Scholar]
- 52.Nixon AJ, Manola J, Gelman R, et al. No long-term increase in cardiac-related mortality after breast-conserving surgery and radiation therapy using modern techniques. J Clin Oncol. 1998;16:1374–1379. doi: 10.1200/JCO.1998.16.4.1374. [DOI] [PubMed] [Google Scholar]
- 53.Vallis KA, Pintilie M, Chong N, et al. Assessment of coronary heart disease morbidity and mortality after radiation therapy for early breast cancer. J Clin Oncol. 2002;20:1036–1042. doi: 10.1200/JCO.2002.20.4.1036. [DOI] [PubMed] [Google Scholar]
- 54.Patt DA, Goodwin JS, Kuo YF, et al. Cardiac morbidity of adjuvant radiotherapy for breast cancer. J Clin Oncol. 2005;23:7475–7482. doi: 10.1200/JCO.2005.13.755. [DOI] [PubMed] [Google Scholar]
- 55.Giordano SH, Kuo YF, Freeman JL, et al. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst. 2005;97:419–424. doi: 10.1093/jnci/dji067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carlson RW, Moench S, Hurria A, et al. NCCN Task Force Report: breast cancer in the older woman. J Natl Compr Canc Netw. 2008;6 Suppl 4:S1–S25. quiz S26–S27. [PubMed] [Google Scholar]
- 57.Wildiers H, Kunkler I, Biganzoli L, et al. Management of breast cancer in elderly individuals: recommendations of the International Society of Geriatric Oncology. Lancet Oncol. 2007;8:1101. doi: 10.1016/S1470-2045(07)70378-9. [DOI] [PubMed] [Google Scholar]