Abstract
Cytochrome P4501A2 (Cyp1a2) is important in the development of uroporphyria in mice, a model of porphyria cutanea tarda in humans. Heretofore, mice homozygous for the Cyp1a2−/− mutation do not develop uroporphyria with treatment regimens that result in uroporphyria in wild-type mice. Here we report uroporphyria development in Cyp1a2−/− mice additionally null for both alleles of the hemochromatosis (Hfe) gene and heterozygous for deletion of the uroporphyrinogen decarboxylase (Urod) gene (genotype: Cyp1a2−/−; Hfe−/−;Urod+/−), demonstrating that upon adding porphyria-predisposing genetic manipulations, Cyp1a2 is not essential. Cyp1a2−/−; Hfe−/−;Urod+/− mice were treated with various combinations of an iron-enriched diet, parenteral iron-dextran, drinking water containing δ-aminolevulinic acid and intraperitoneal Aroclor 1254 (a polychlorinated biphenyl mixture) and analyzed for uroporphyrin accumulation. Animals fed an iron-enriched diet alone did not develop uroporphyria but uroporphyria developed with all treatments that included iron supplementation and δ-aminolevulinic acid, even with a regimen without Aroclor 1254. Hepatic porphyrin levels correlated with low UROD activity and high levels of an inhibitor of UROD but marked variability in the magnitude of the porphyric response was present in all treatment groups. Gene expression profiling revealed no major differences between genetically identical triple cross mice exhibiting high and low magnitude porphyric responses from iron-enriched diet and iron-dextran supplementation, and δ-aminolevulinic acid. Even though the variation in porphyric response did not parallel the hepatic iron concentration, the results are compatible with the presence of a Cyp1a2-independent, iron-dependent pathway for the generation of uroporphomethene, the UROD inhibitor required for the expression of uroporphyria in mice and PCT in humans.
Keywords: porphyria cutanea tarda, iron, δ-aminolevulinic acid, polychlorinated biphenyls, uroporphyrinogen decarboxylase
INTRODUCTION
Porphyria cutanea tarda (PCT), the most common of human porphyrias is characterized by a photodermatitis, the accumulation of uroporphyrin and hepta-carboxylporphyrin in skin, hepatocytes and other tissues and excretion of these compounds in the urine [1]. An outbreak of an estimated approximately 3,000 cases of PCT occurred in Turkey between 1956 and 1961 following widespread ingestion of seed wheat treated with hexachlorobenzene [2]. This observation led to the development of numerous rodent models of PCT induced by halogenated aromatic hydrocarbons and similar compounds that have in common the ability to induce hepatic cytochrome P450s and several other “Ah-battery” enzymes through the Ah receptor [3] and xenobiotic response element mechanism. Fur-covered rodents do not develop the typical bullous cutaneous lesions that characterize PCT in humans and the rodent models have generally been designated as uroporphyria. Iron also plays an important role in most rodent models as iron deficiency markedly attenuates the effects of porphyria–inducing compounds and iron excess magnifies the effects [4; 5; 6]. Hepatic siderosis is present in nearly all PCT cases.
We demonstrated that the mechanism responsible for the development of uroporphyria in mice and PCT in humans is the generation of a partially oxidized porphyrinogen (uroporphomethene) that inhibits the activity of uroporphyrinogen decarboxylase (UROD) in hepatocytes [7]. In humans, genetic risk factors for the development of PCT include homozygosity for loss of function mutations of the hemochromatosis gene (HFE) and heterozygosity for loss of function mutations of the UROD gene [8]. In mice these genetic factors also play a key role as Hfe−/−;Urod+/− animals develop uroporphyria at maturity in the absence of known exogenous uroporphyria-precipitating factors [9].
Oxidation of uroporphyrinogen (UROX) is catalyzed by at least five human P450s and by mouse Cyp1a2 [10] although microsomes containing induced levels of murine Cyp1a2 display increased UROX activity only when sufficient iron is present [11]. Deletion of Cyp1a2 in mice (Cyp1a2−/−) prevented the development of uroporphyria after exposure to any of the combinations of δ-aminolevulinic acid (δALA) and/or exogenous iron and 3-methylcholanthrene [12], hexachlorobenzene [10], 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) [13] or polychlorinated biphenyls (PCB) [5; 14]. It was concluded that Cyp1a2 is essential for the development uroporphyria in mice.
The important role of iron in the development of PCT in humans and uroporphyria in mice led us to hypothesize the possibility an iron-dependent pathway for the oxidation of uroporphyrinogen that could co-exist with, but be independent of, a Cyp1a2 pathway. As with the P450-catalyzed reaction, oxidation of uroporphyrinogen to uroporphomethene occurs most readily when uroporphyrinogen production is enhanced by increasing flux through the initial reactions of the heme biosynthetic pathway, either by inducing the rate-limiting enzyme in the pathway (ALA-synthase) or, as in the present experiments, by continuously administering δALA. Here we report the development of uroporphyria in Cyp1a2−/− mice that were also homozygous for a deletion at the Hfe locus and heterozygous for a deletion at the Urod locus (Cyp1a2−/−;Hfe−/−;Urod+/−). These “triple cross” mice were exposed to treatment regimens designed to produce hepatic iron overload, including a high iron diet alone; the diet plus injected iron-dextran and drinking water supplemented with δALA; the high iron diet, δALA supplemented water, and injected Aroclor 1254 (a polychlorinated biphenyl mixture); iron dextran, δALA supplemented water and Aroclor 1254; and the high iron diet, iron dextran, δALA supplemented water and Aroclor 1254. Uroporphyria developed in a subset of animals in all groups consuming δALA-supplemented water, including the group that did not receive Aroclor 1254.
MATERIALS AND METHODS
Animals
Male Cyp1a2−/−; Hfe−/−;Urod+/− and Cyp1a2−/−; Hfe−/−;Urod+/+ mice were produced by crossing C57BL/6J mice homozygous for a Cyp1a2 deletion allele [15], to our previously described C57BL/6J Urod+/−, Hfe−/− line [9]. Each parenteral strain had been backcrossed a minimum of 15 times. Mice were provided free access to Teklad 3080 mouse chow (9.6% fat) (Harlan, Indianapolis, IN) and water. Depending on the treatment regimen, some mice were fed (>14 weeks) a high iron diet containing 2 mg Fe (as carbonyl iron)/g chow from weaning, some were provided drinking water containing δALA, 2 g/L neutralized to pH 7.0, for 4 weeks; some animals also received intraperitoneal (ip) iron-dextran (10 mg iron) 4 weeks prior to sacrifice. Aroclor 1254 (4 mg), when required, was administered in corn oil vehicle and via ip injection 4 weeks prior to sacrifice. All procedures involving animals were approved by the University of Utah Animal Care and Use Committee and were in concordance with NIH guidelines for the humane care of laboratory animals.
Liver and Urine Porphyrin Analysis
Urine samples were collected from fluid voided upon handling. Liver tissue was obtained at sacrifice and immediately frozen on dry ice. Total porphyrin analysis of urine and liver samples, and the determination of total iron in liver were performed as previously described [16]. All data were recorded and reported for individual animals.
Hepatic Microsome and Cytosol Preparation
After removal of the gall bladder, the remaining liver was homogenized in four volumes of unbuffered 0.25 M sucrose. A cytosolic fraction was prepared from the homogenate with successive centrifugations of 9,000 × g (15 min), 19,000 × g (15 min) and 105,000 × g (60 min). The protein content of both microsomes (105,000 × g pellet) and cytosol (105,000 × g supernatant) was determined by the method of Lowry et al. [17].
Uroporphyrinogen Decarboxylase Activity
Cytosolic uroporphyrinogen decarboxylase activity was determined and registered as nmoles of uroporphyrinogen decarboxylated per hour per mg cytosolic protein [18]. In addition, an aliquot of the cytosol was denatured in boiling water for 5 minutes, clarified by centrifugation and assayed for inhibitory activity against 20 ng of recombinant human uroporphyrinogen decarboxylase (rhUROD) as previously described [7].
Microsomal P450 Monooxygenase Activities
The metabolism of methoxy-, ethoxy- (Sigma-Aldrich®, St. Louis, MO), pentoxy- and benzoxyresorufin (Invitrogen™, Carlsbad, CA) 7-ethoxytrifluoromethylcoumarin (Sigma-Aldrich®, St. Louis, MO) and benzyloxyquinoline were determined from the rate of fluorescence increase due to the formation of the resorufin (Ex 544 nm, Em 612 nm; [19], 7-hydroxytrifluoromethylcoumarin (Ex 409 nm, Em 550 nm) or hydroxyquinoline (Ex 410 nm, Em 538 nm, [20]) products. Data are expressed as nmoles per minute per mg microsomal protein for each individual animal.
Gene Expression Analysis by Microarray
For microarray analyses, three groups consisting of four animals per group were analyzed. Total RNA extracted from fresh liver tissue homogenized in TRIzol® reagent was analyzed for quality and quantity using a BioAnalyzer (Agilent Technologies, Foster City, CA). Total RNA was then converted to double-stranded cDNA following priming with an oligo-dT-T7 primer. A labeled cRNA pool was prepared using the Agilent Two-Color Quick Amp Labeling kit and purified with an RNeasy® column, eluted in H2O and quantified by UV spectrophotometry. The resultant cRNA was fragmented and combined with Hi-RPM hybridization buffer and added to the Agilent GE 44k v2 microarrays. Following hybridization and washing the array was scanned using an Agilent G2505C Scanner at 5 um resolution. Data were loaded into the Feature Extraction Software (version 10.5). The intensities and background measurements are used to generate a file that is subsequently normalized using the Bioconductor Package in the R statistical environment. The GeneSifter® software package was used for data analysis. Expression data for enzyme pathways of interest were extracted and compiled manually.
RESULTS
We previously reported that Cyp1a2+/+; Hfe−/−;Urod+/− C57BL/6 mice developed uroporphyria without any manipulations once hepatic iron concentrations reached high levels [9]. In our preliminary studies with triple cross animals we attempted to generate a porphyric phenotype by injecting iron dextran, adding δALA to the drinking water and administering Aroclor 1254 (Table 1, treatment 1). This three component regimen induces a robust uroporphyria in Cyp1a2+/+ animals [5; 21] but in triple cross animals, only very modest increases in hepatic porphyrin content were noted and marked variability occurred between individual animals.
Table 1.
Hepatic Porphyrin Concentrations in Different Cyp1a2, Hfe, and Urod genetic backgrounds After Treatment Regimens Consisting Variously of High Iron Diet, Iron-dextran and PCB injections and δ-Aminolevulinic acid-containing Drinking Water
| Genotype | mouse | LIVER PORPHYRINS (nmol/g tissue) | ||||
|---|---|---|---|---|---|---|
| Treatment 1 | Treatment 2 | Treatment 3 | Treatment 4 | Treatment 5 | ||
| fedex/ala/pcb | Fe-diet | Fe-diet fedex/ala | Fe-diet ala/pcb | Fe-diet fedex/ala/pcb | ||
| Cyp1A2−/− | #1 | 0.35 | 0.5 | |||
| Hfe+/+ | #2 | 0.35 | 0.36 | |||
| Urod+/+ | #3 | 0.26 | 0.34 | |||
| #4 | 0.12 | 0.33 | ||||
| #5 | 0.32 | |||||
| #6 | 0.22 | |||||
| Cyp1A2−/− | #1 | 0.65 | 0.66 | 0.50 | 2.05 | |
| Hfe−/− | #2 | 0.26 | 0.54 | 0.45 | 0.40 | |
| Urod+/+ | #3 | 0.17 | 0.44 | 0.43 | 0.38 | |
| #4 | 0.13 | 0.24 | 0.31 | 0.37 | ||
| #5 | 0.25 | 0.24 | ||||
| Cyp1A2−/− | #1 | 24.45 | 0.71 | 200.10 | 118.29 | 340.14 |
| Hfe−/− | #2 | 14.86 | 0.62 | 56.28 | 11.73 | 39.98 |
| Urod+/− | #3 | 7.46 | 0.55 | 13.06 | 11.06 | 4.50 |
| #4 | 3.42 | 0.54 | 5.06 | 3.82 | 1.80 | |
| #5 | 2.38 | 2.38 | 1.77 | |||
| #6 | 1.69 | 1.13 | ||||
Mice fed a high iron diet (fe-diet) consumed the diet after weaning for more than 14 weeks and were sacrificed 4 weeks after receiving any of additional treatments indicated. “Fedex” was a single injection of aqueous iron dextran (10 mg, ip), “ala” was d-aminolevulinic acid (2 mg/ml) provided continuously in the drinking water and “pcb” was a single injection of Aroclor 1254, (4 mg, ip in corn oil). Individual animal hepatic porphyrin levels are arranged in descending order within each group.
Because of the established role of iron in murine uroporphyria, we maximized the duration of high hepatic iron content by feeding a high iron diet from weaning onward. Mice of any genotype fed the iron-enriched diet alone (Table 1, treatment 2) did not develop elevated (> 1 nmol/g) hepatic porphyrin levels. Additional animal groups were given drinking water supplemented with δALA and either iron dextran (Table 1, treatment 3), Aroclor 1254 (Table 1, treatment 4), or iron dextran and Aroclor 1254 (Table 1, treatment 5). Four weeks later, animals were sacrificed and liver porphyrin content determined. Across all treatment regimens and mouse genotypes, the highest hepatic porphyrin levels (Table 1) were seen in Urod+/− animals. With Urod+/− mice, marked variations were seen within each treatment group.
Animals exposed to treatments 3, 4 and 5 all received drinking water supplemented with δALA in addition to components designed to produce iron overload. Mice in treatment group 3 were not exposed to Aroclor 1254 (PCBs) yet hepatic porphyrins accumulated as they did in groups 4 and 5 where it was administered. These data demonstrate that Aroclor 1254 administration is not necessary to produce an increase in hepatic porphyrin levels.
Animals on a high iron diet with Cyp1a2−/− as the sole genetic variation did not develop an increase in hepatic porphyrin levels when treated with a potent PCB-containing porphyria inducing regimen (Table 1, treatment 5). This parallels the results seen with mice on a normal diet receiving iron-dextran and PCB [14] or iron dextran, δALA and hexachlorobenzene [22].
Animals with the triple cross genotype developed both the highest and most variable hepatic porphyrin levels, regardless of treatment regimen. The hepatic porphyrin profile (the amounts of uroporphyrin, heptacarboxyl, hexacarboxyl, pentacarboxyl, and coproporphyrin) revealed an abundance of uroporphyrin and heptacarboxyl porphyrin in animals with the highest total porphyrin values (Table 2), a pattern (uro>hepta>hexa>penta<copro) characteristic of mice with uroporphyria [9] and humans with PCT [23]. A similar porphyrin profile was seen even when total liver porphyrins were not increased, likely reflecting a minor effect of the Urod+/− genotype. These data suggest that the variation in porphyrin accumulation is not a result of differences among the sequential decarboxylation steps of UROD. Development of uroporphyria in triple cross animals was monitored by analyzing urine porphyrin excretion weekly during the 4-week treatment period. Results are shown in Table 3. Individual animals in each group correspond to their presentation in Table 1. The increase in urine porphyrin excretion was most marked for animals with the highest hepatic porphyrin values but modest increases in porphyrin excretion occurred even in animals with normal or minimally elevated hepatic porphyrin levels. Of note, animals that developed the highest hepatic porphyrin levels (Table 1) had the highest urine porphyrin excretion prior to exposure to the porphyria-inducing regimen (day 0, Table 3).
Table 2.
Hepatic Porphyrins in Cyp1a2−/−, Hfe−/−, and Urod+/− Mice After Treatment Regimens Consisting Variously of High Iron Diet, Iron-dextran and PCB injections and δ-Aminolevulinic acid-containing Drinking Water
| PORPHYRIN | mouse | HEPATIC PORPHYRINS (nmol/g liver) | |||
|---|---|---|---|---|---|
| Treatment 2 | Treatment 3 | Treatment 4 | Treatment 5 | ||
| Fe-diet | Fe-diet fedex/ala | Fe-diet ala/pcb | Fe-diet fedex/ala/pcb | ||
| URO | #1 | 0.28 | 136.38 | 85.73 | 248.71 |
| #2 | 0.24 | 34.97 | 9.57 | 30.07 | |
| #3 | 0.14 | 8.47 | 8.82 | 3.36 | |
| #4 | 0.22 | 2.85 | 2.90 | 1.02 | |
| #5 | 1.60 | 1.33 | |||
| #6 | 0.77 | 0.65 | |||
| HEPTACARBOXYL | #1 | 0.43 | 60.16 | 30.99 | 86.72 |
| #2 | 0.30 | 19.82 | 1.96 | 9.33 | |
| #3 | 0.41 | 4.17 | 1.99 | 1.01 | |
| #4 | 0.26 | 1.96 | 0.73 | 0.59 | |
| #5 | 0.67 | 0.36 | |||
| #6 | 0.82 | 0.40 | |||
| HEXACARBOXYL | #1 | 0.00 | 2.39 | 1.02 | 2.96 |
| #2 | 0.08 | 0.96 | 0.13 | 0.35 | |
| #3 | 0.00 | 0.21 | 0.15 | 0.09 | |
| #4 | 0.06 | 0.09 | 0.15 | 0.08 | |
| #5 | 0.05 | 0.08 | |||
| #6 | 0.10 | 0.08 | |||
| PENTACARBOXYL | #1 | 0.00 | 0.20 | 0.14 | 0.34 |
| #2 | 0.00 | 0.12 | 0.00 | 0.05 | |
| #3 | 0.00 | 0.06 | 0.00 | 0.00 | |
| #4 | 0.00 | 0.00 | 0.00 | 0.00 | |
| #5 | 0.00 | 0.00 | |||
| #6 | 0.00 | 0.00 | |||
| COPRO | #1 | 0.00 | 0.97 | 0.42 | 1.42 |
| #2 | 0.00 | 0.41 | 0.07 | 0.17 | |
| #3 | 0.00 | 0.14 | 0.10 | 0.03 | |
| #4 | 0.00 | 0.16 | 0.04 | 0.10 | |
| #5 | 0.06 | 0.00 | |||
| #6 | 0.00 | 0.00 | |||
Mice fed a high iron diet (fe-diet, 2 mg Fe/g) after weaning > 14 weeks and were sacrificed 4 weeks after receiving any of additional treatments indicated. “Fedex” was a single injection of aqueous iron dextran (10 mg, ip), “ala” was δ-aminolevulinic acid (2 mg/ml) provided continuously in the drinking water and “pcb” was a single injection of Aroclor 1254, (4 mg, ip in corn oil). Values for animals are arranged in the same order as the male Cyp1a2−/− Hfe−/− Urod+/− group in Table 1.
Table 3.
Total and % Uro- and Heptaporhyrin in Urine of Cyp1a2−/−; Hfe−/−;Urod+/− Mice After Treatment Regimens Consisting Variously of High Iron Diet, Iron-dextran and PCB injections and δ-Aminolevulinic acid-containing Drinking Water
| URINE PORPHYRINS (uM; total/8+7) | |||||
|---|---|---|---|---|---|
| mouse | Treatment 2 | Treatment 3 | Treatment 4 | Treatment 5 | |
| Fe-diet | Fe-diet ala/fedex | Fe-diet pcb/ala | Fe-diet pcb/ala/fedex | ||
| day 0 | |||||
| #1 | 3.4/2.7 | 6.9/5.7 | 5.8/5.0 | 4.4/3.4 | |
| #2 | 3.7/2.9 | 5.2/4.3 | 4.3/3.3 | 2.2/1.6 | |
| #3 | 3.6/2.8 | - | 1.8/1.4 | 3.4/2.6 | |
| #4 | 2.5/1.9 | 4.2/3.4 | 1.8/1.3 | 3.6/2.8 | |
| #5 | 3.8/3.3 | 2.7/2.0 | |||
| #6 | 3.8/2.6 | 3.6/2.9 | |||
| at 1 week | |||||
| #1 | 2.2/1.8 | 9.6/5.4 | 5.4/2.8 | 7.6/2.9 | |
| #2 | 2.2/1.9 | 2.3/1.4 | 2.5/1.6 | 3.0/1.7 | |
| #3 | 3.9/2.9 | 4.3/2.5 | 3.8/1.8 | 3.3/1.1 | |
| #4 | 1.3/1.1 | 5.0/2.9 | 4.0/1.9 | 2.5/1.2 | |
| #5 | 5.1/3.0 | 1.6/0.9 | |||
| #6 | 3.7/2.0 | 1.5/0.8 | |||
| at 2 weeks | |||||
| #1 | 4.9/3.8 | 10.6/8.1 | 11.0/8.0 | 17.7/11.3 | |
| #2 | 2.0/1.7 | 7.7/4.4 | 4.4/2.3 | 3.5/2.4 | |
| #3 | 2.4/1.8 | 5.5/3.3 | 3.8/2.1 | 6.8/2.3 | |
| #4 | 2.1/1.6 | 8.1/4.0 | 4.2/2.7 | 4.3/2.3 | |
| #5 | 6.6/2.6 | 6.5/2.9 | |||
| #6 | 6.9/3.7 | 4.9/2.2 | |||
| at 3 weeks | |||||
| #1 | 3.5/2.8 | 21.4/13.7 | 24.8/16.7 | 23.9/19.0 | |
| #2 | - | 10.6/6.3 | 8.6/4.4 | 7.9/4.1 | |
| #3 | 2.3/1.8 | 12.0/7.5 | 8.6/5.3 | 8.5/4.6 | |
| #4 | 1.8/1.2 | 10.1/6.1 | 9.7/4.9 | 7.4/3.6 | |
| #5 | 10.5/5.3 | 6.7/3.4 | |||
| #6 | 11.9/6.5 | 9.8/4.8 | |||
| at 4 weeks | |||||
| #1 | 3.5/2.8 | 39.1/30.8 | 34.5/29.6 | 79.8/59.2 | |
| #2 | 1.4/1.2 | 10.4/4.6 | 12.5/6.3 | 14.6/9.4 | |
| #3 | 2.5/1.9 | 17.2/11.3 | 11.6/6.1 | 5.4/3.1 | |
| #4 | 2.2/1.5 | 11.2/6.3 | 11.7/4.3 | 7.9/3.0 | |
| #5 | 6.0/3.3 | 6.9/3.0 | |||
| #6 | 6.1/3.3 | 8.5/4.5 | |||
Mice fed a high iron diet (fe-diet, 2 mg Fe/g) after weaning > 14 weeks and were sacrificed 4 weeks after receiving any of additional treatments indicated. “Fedex” was a single injection of aqueous iron dextran (10 mg, ip), “ala” was δ-aminolevulinic acid (2 mg/ml) provided continuously in the drinking water and “pcb” was a single injection of Aroclor 1254, (4 mg, ip in corn oil). Values for animals are arranged in the same order as the male Cyp1a2−/− Hfe−/− Urod+/− group in Table 2.
Hepatic iron concentration was measured in triple cross animals exposed to each treatment regimen (Table 4). Iron concentrations were generally higher in animals receiving iron dextran but within any treatment regimen there was no correlation between liver iron and hepatic porphyrin levels. Lack of correlation was evident even in animals with low “outlier” iron values (#3 in treatment 3, and #2 in treatment 4).
Table 4.
Hepatic Iron Concentrations of Cyp1a2−/−;Hfe−/−;Urod+/− Mice After Treatment Regimens Consisting Variously of High Iron Diet, Iron-dextran and PCB injections and δ-Aminolevulinic acid-containing Drinking Water
| mouse | HEPATIC IRON (mg/g liver) | |||
|---|---|---|---|---|
| Treatment 2 | Treatment 3 | Treatment 4 | Treatment 5 | |
| Fe-diet | Fe-diet ala/fedex | Fe-diet pcb/ala | Fe-diet pcb/ala/fedex | |
| #1 | 2.79 | 3.10 | 3.37 | 5.24 |
| #2 | 2.32 | 4.51 | 0.89 | 4.10 |
| #3 | 2.85 | 1.83 | 2.05 | 2.48 |
| #4 | 1.75 | 3.25 | 2.35 | 4.94 |
| #5 | 4.41 | - | ||
| #6 | 7.17 | 3.93 | ||
Mice fed a high iron diet (fe-diet, 2 mg Fe/g) after weaning > 14 weeks and were sacrificed 4 weeks after receiving any of additional treatments indicated. “Fedex” was a single injection of aqueous iron dextran (10 mg, ip), “ala” was δ-aminolevulinic acid (2 mg/ml) provided continuously in the drinking water and “pcb” was a single injection of Aroclor 1254, (4 mg, ip in corn oil). Values for animals are arranged in the same order as the male Cyp1a2−/− Hfe−/− Urod+/− group in Table 2.
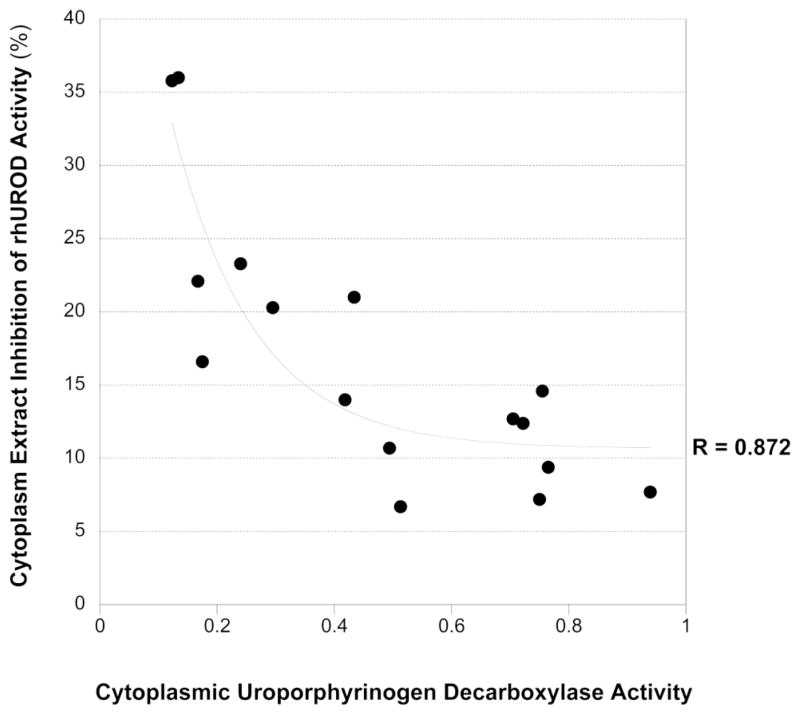
A correlation was observed between hepatic porphyrin concentration and UROD activity in all treatment groups. The highest porphyrin levels were found in animals with the lowest UROD activity (Table 5). As expected, the lowest native UROD activities were associated with the highest rhUROD inhibition and when native UROD activity was plotted against extracted inhibitor activity, a best-fit [y= 6.31-26.12logx] correlation coefficient of 0.872 could be derived (Figure 1).
Table 5.
Hepatic Uroporphyringen Decarboxylase and rhUROD-Inhibitor Activities in Cytosolic Fractions of Cyp1a2−/−;Hfe−/−;Urod+/− Mice After Treatment Regimens Consisting Variously of High Iron Diet, Iron-dextran and PCB injections and δ-Aminolevulinic acid-containing Drinking Water
| UROD Activity and Cytosolic Inhibitor | |||||
|---|---|---|---|---|---|
| mouse | Treatment 2 | Treatment 3 | Treatment 4 | Treatment 5 | |
| Fe-diet | Fe-diet ala/fedex | Fe-diet pcb/ala | Fe-diet pcb/ala/fedex | ||
| UROD activity (nmol/mg cytosolic protein/h) | #1 | 0.63 | 0.13 | 0.17 | 0.12 |
| #2 | 0.55 | 0.24 | 0.49 | 0.29 | |
| #3 | 0.87 | 0.43 | 0.17 | 0.77 | |
| #4 | 0.73 | 0.42 | 0.51 | 0.75 | |
| #5 | 0.94 | 0.76 | |||
| #6 | 0.71 | 0.72 | |||
| rhUROD inhibition (%) | #1 | 9.0 | 36.0 | 22.1 | 35.8 |
| #2 | 8.0 | 23.3 | 10.7 | 20.3 | |
| #3 | 6.6 | 21.0 | 16.6 | 9.4 | |
| #4 | 4.1 | 14.0 | 6.7 | 7.2 | |
| #5 | 7.7 | 14.6 | |||
| #6 | 12.7 | 12.4 | |||
Mice fed a high iron diet (fe-diet, 2 mg Fe/g) after weaning > 14 weeks and were sacrificed 4 weeks after receiving any of additional treatments indicated. “Fedex” was a single injection of aqueous iron dextran (10 mg, ip), “ala” was δ-aminolevulinic acid (2 mg/ml) provided continuously in the drinking water and “pcb” was a single injection of Aroclor 1254, (4 mg, ip in corn oil). Values for animals are arranged in the same order as the male Cyp1a2−/− Hfe−/− Urod+/− group in Table 2.
FIGURE 1.
Relationship between the presence of an inhibitor of recombinant human uroporphyrinogen decarboxylase and uroporphyringen decarboxylase activity in the cytosol of Cyp1a2−/−;Hfe−/−;Urod+/− mice.
To determine if a cytochrome P450 other than Cyp1a2 might play a role in the triple cross animals which developed uroporphyria, we assayed five P450 mediated dealkylase activities in hepatic microsomal fractions from mice in all treatment groups (Table 6). No dealkylase activity was elevated with treatment 3 over treatment 2 that might account for uroporphyrin development in treatment 3 and not treatment 2. Within treatment regimen 3, no P450 activity correlated with the hepatic porphyrin levels (Table 1). Three of the dealkylase activities assayed were increased in treatments 4 and 5, regimens that included Aroclor 1254 administration. Pentoxyresorufin and benzyloxyquinoline dealkylase activities did not increase with Aroclor 1254 treatment. Despite the major increases in three of the five dealkylase activities, the absence of any major increase in hepatic porphyrin levels over those seen with treatment 3 indicates that the enzyme or enzymes catalyzing these reactions are not generating the uroporphomethene inhibitor necessary for UROD inhibition and the subsequent accumulation of porphyrins.
Table 6.
Hepatic P450-dependent Dealkylase Activities in Microsomal Fractions of Cyp1a2−/−;Hfe−/−;Urod+/− Mice After Treatment Regimens Consisting Variously of High Iron Diet, Iron-dextran and PCB injections and δ-Aminolevulinic acid-containing Drinking Water
| CYP SUBSTRATE | mouse | Hepatic P450 Activities (nmol/mg microsomal protein/min) | |||
|---|---|---|---|---|---|
| Treatment 2 | Treatment 3 | Treatment 4 | Treatment 5 | ||
| Fe-diet | Fe-diet ala/fedex | Fe-diet pcb/ala | Fe-diet pcb/ala/fedex | ||
| 7-methoxyresorufin | |||||
| #1 | 0.025 | 0.020 | 0.828 | 0.263 | |
| #2 | 0.029 | 0.020 | 1.018 | 1.099 | |
| #3 | 0.015 | - | 1.142 | 0.861 | |
| #4 | 0.020 | 0.026 | 1.518 | 1.147 | |
| #5 | 0.014 | 1.216 | |||
| #6 | 0.027 | 1.133 | |||
| 7-ethoxyresorufin | |||||
| #1 | 0.100 | 0.056 | 2.259 | 1.119 | |
| #2 | 0.144 | 0.078 | 3.269 | 3.429 | |
| #3 | 0.063 | 0.052 | 3.594 | 2.609 | |
| #4 | 0.113 | 0.106 | 5.060 | 4.051 | |
| #5 | 0.051 | 4.106 | |||
| #6 | 0.085 | 3.858 | |||
| 7-ethoxy-4-trifluoro methylcoumarin | |||||
| #1 | 0.573 | 0.274 | 3.692 | 1.678 | |
| #2 | 0.605 | 0.327 | 3.970 | 4.221 | |
| #3 | 0.481 | 0.386 | 4.202 | 3.987 | |
| #4 | 0.455 | 0.389 | 5.994 | 4.795 | |
| #5 | 0.354 | 4.209 | |||
| #6 | 0.457 | 4.386 | |||
| 7-pentoxyresorufin | |||||
| #1 | 0.049 | 0.043 | 0.051 | 0.065 | |
| #2 | 0.053 | 0.039 | 0.050 | 0.065 | |
| #3 | 0.039 | 0.046 | 0.048 | 0.044 | |
| #4 | 0.046 | 0.025 | 0.060 | 0.048 | |
| #5 | 0.041 | 0.053 | |||
| #6 | 0.040 | 0.051 | |||
| 7-benzyloxy quinoline | |||||
| #1 | 0.719 | 0.462 | 0.418 | 0.229 | |
| #2 | 0.701 | 0.413 | 0.605 | 0.608 | |
| #3 | 0.520 | 0.430 | 0.560 | 0.369 | |
| #4 | 0.484 | 0.446 | 0.956 | 0.609 | |
| #5 | 0.322 | 0.624 | |||
| #6 | 0.529 | 0.699 | |||
Mice fed a high iron diet (fe-diet, 2 mg Fe/g) after weaning > 14 weeks and were sacrificed 4 weeks after receiving any of additional treatments indicated. “Fedex” was a single injection of aqueous iron dextran (10 mg, ip), “ala” was δ-aminolevulinic acid (2 mg/ml) provided continuously in the drinking water and “pcb” was a single injection of Aroclor 1254, (4 mg, ip in corn oil). Values for animals are arranged in the same order as the male Cyp1a2−/− Hfe−/− Urod+/− group in Table 2.
There was no apparent explanation for the marked variability in hepatic porphyrin accumulation in triple cross animals within treatment regimens 1, 3, 4 and 5. Genetic variations seemed highly unlikely given the number of backcrosses performed on the parenteral strains used to generate the triple cross animals which we considered syngeneic C57Bl/6J. To explore the possibility that gene expression profiles varied, we performed microarray analyses utilizing livers from triple cross animals subjected to treatments 2 and 3 and so were free from any variations in response arising from Aroclor 1254. In addition to comparing treatment groups (n = 4 in each), we compared the animal with the highest liver porphyrin value (200.1 nmol/g) with three animals with lower values (2.38, 13.06 and 56.28 nmol/g) within treatment 3. Although this 1 to 3 comparison precluded statistical evaluation, 240 gene transcripts showed greater than two-fold differences. In this comparison 65% of the genes were up-regulated including a number of cytochrome P450s and flavin monooxygenases; Cyp1a1 (3.38), Cyp2a4 (2.24), Cyp3a59 (2.09) Cyp7a1 (2.07), Fmo2 (2.06), and Fmo3 (8.44). No cytochrome P450s were down-regulated. However up-regulation of P450s and flavin monooxygenases was not detected when the four animals subjected to treatment 3 were compared (p <0.05) with the four animals subjected to treatment 2 (porphyrin values <1.0 nmol/g), although 241 genes were differentially regulated.
DISCUSSION
Uroporphyria has now been elicited in Cyp1a2−/− mice. Mice that developed porphyria were also Urod+/−, received excess δALA in their drinking water, and were genetically and experimentally iron-overloaded. Notable among the treatments that resulted in uroporphyria was a regimen without administration of polychlorinated biphenyls. Polychlorinated biphenyl- or Ah receptor agonist-independent uroporphyria has previously been observed in iron-overloaded, δALA supplemented mice, but this was in Cyp1a2+/+ animals [6; 9; 12; 24; 25; 26; 27; 28]. In Cyp1a2+/+ animals, the iron effect has been linked to a requirement for minimal levels of iron, to permit oxidation of uroporphyrinogen by Cyp1a2 [11]. Our demonstration of an iron effect in the absence of Cyp1a2, leads to the hypothesis that a true iron-dependent pathway exists for the oxidative generation of the uroporphomethene inhibitor of UROD and is most evident when high levels of uroporphyrinogen are generated by high flux through the initial steps of the heme biosynthetic pathway.
Early studies demonstrated that addition of ferrous iron to a 37,000 × g supernatant of porcine liver homogenate containing excess uroporphyrinogen led to a decrease in UROD activity [29]. It was proposed that the inhibition arose from a direct effect of ferrous iron on UROD, but the recent identification of the UROD inhibitor responsible for uroporphyria and PCT [7] makes oxidation of uroporphyrinogen to uroporphomethene by an iron dependent mechanism a more plausible explanation. In the in vivo experiments reported here, inhibition of UROD and hepatic porphyrin levels in individual animals was not proportional to the total iron content of the liver at the time of sacrifice (Table 4). We suggest that the discrepancy might arise because of variations in the level of free ferrous iron in the labile iron pool [30; 31] possibly arising from variations in the ability to export ferrous iron from hepatocytes via ferroportin-mediated efflux [32]. Elevated concentrations of ferrous iron would promote the generation of oxidizing radicals through the Fenton reaction (Fe2+ + H2O2 → Fe3+ + OH− + •OH). The requirement for ferrous rather than ferric iron to generate the inhibitor of UROD may explain the observation that the iron overload of hereditary hemochromatosis (a risk factor for PCT) where iron is largely present in the ferric form is quite common yet PCT is quite rare [8]. Phlebotomy may be effective because it is able to preferentially deplete ferrous iron, however, in with this therapy hepatocyte iron is not completely depleted [1; 33]. Alcohol abuse is a significant risk factor for PCT. High levels of reduced pyridine nucleotides are produced from alcohol and aldehyde dehydrogenase-catalyzed metabolism [34] that may elevate hepatic free thiol levels and maintain iron in the ferrous form.
The high inter-animal variability of the uroporphyria we observed may be explained by the amount of δALA consumed (ad libitum drinking water consumption) and as a consequence, the amount of uroporphyrinogen formed. However, there was no evidence of dehydration in any of the animals, suggesting a scenario of δALA limitation leading to lower levels of uroporphyria highly unlikely.
Complete oxidation of uroporphyrinogen to uroporphyrin can occur via multiple mechanisms. Whether there is complete congruency between the four-step oxidation of uroporphyrinogen to uroporphyrin, a known reaction of at least five P450s from human and mouse Cyp1a2 [10], and the one-step oxidation to the UROD-inhibitory uroporphomethene is not known. In Cyp1a2−/− mice, none of monooxygenase activities (Table 6) catalyzed to various extents [35] by the remaining P450s showed any correlation with liver porphyrin levels, either within or between treatment regimens. The lack of correlation between groups was most marked for three activities that were markedly induced by regimens that included Aroclor 1254, but did not result in any greater uroporphyrin accumulation. In addition to activities, we found no correlation between the development of uroporphyria and enhanced gene expression of any alternative cytochrome P450s, or even flavin monooxygenases, the latter consideration arising from the possibility that generation of uroporphomethene might be mediated by this family of enzymes.
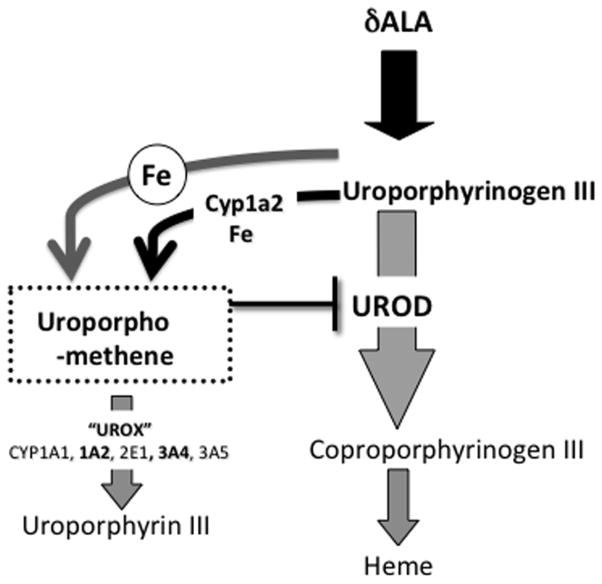
The ability to generate uroporphyria in a Cyp1a2−/− animal has allowed us to demonstrate that in addition to a Cyp1a2 dependent pathway, there co-exists an additional, separate albeit lower capacity pathway linked to iron (Figure 2). The variability in the valence state of liver iron may explain the observed high inter-animal variability in uroporphyria. The presence of such a ferrous iron-dependent, CYP1A2-independent pathway capable of generating an inhibitor of UROD in humans could explain why most cases of PCT occur without evidence of exposure to known inducers of cytochrome P4501A2.
FIGURE 2.
Summary pathways and reactions responsible for the development of uroporphyria in mice and porphyria cutanea tarda in humans.
Acknowledgments
FINANACIAL SUPPORT:
This study was supported by USPHS Grants DK020503 and DK083909 to JDP. The authors would like to thank Dr. Daniel W. Nebert, University of Cincinnati, for the generous gift of breeding stock Cyp1a2−/− mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Phillips JD, Kushner JP. The Porphyrias. In: Nathan DG, editor. Nathan and Oski’s Hematology of Infancy and Childhood. Saunders; Philadelphia: 2009. pp. 571–612. [Google Scholar]
- 2.Cam C, Nigogosyan G. Acquired Toxic Porphyria Cutanea Tarda due to Hexachlorobenzene. JAMA. 1963;183:90–93. [PubMed] [Google Scholar]
- 3.Nebert DW, Petersen DD, Fornace AJ., Jr Cellular responses to oxidative stress: the [Ah] gene battery as a paradigm. Environ Health Perspect. 1990;88:13–25. doi: 10.1289/ehp.908813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franklin MR, Phillips JD, Kushner JP. Attenuation of polychlorinated biphenyl induced uroporphyria by iron deprivation. Env Toxicol Pharmacol. 2005;20:417–423. doi: 10.1016/j.etap.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Gorman N, Ross KL, Walton HS, et al. Uroporphyria in mice: thresholds for hepatic CYP1A2 and iron. Hepatology. 2002;35:912–21. doi: 10.1053/jhep.2002.32487. [DOI] [PubMed] [Google Scholar]
- 6.Smith AG, Francis JE. Genetic variation of iron-induced uroporphyria in mice. Biochemical Journal. 1993;291:29–35. doi: 10.1042/bj2910029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips JD, Bergonia HA, Reilly CA, Franklin MR, Kushner JP. A porphomethene inhibitor of uroporphyrinogen decarboxylase causes porphyria cutanea tarda. Proc Natl Acad Sci U S A. 2007;104:5079–84. doi: 10.1073/pnas.0700547104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulaj ZJ, Phillips JD, Ajioka RS, et al. Hemochromatosis genes and other factors contributing to the pathogenesis of porphyria cutanea tarda. Blood. 2000;95:1565–1571. [PubMed] [Google Scholar]
- 9.Phillips JD, Jackson LK, Bunting M, et al. A mouse model of familial porphyria cutanea tarda. Proc Natl Acad Sci U S A. 2001;98:259–264. doi: 10.1073/pnas.011481398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinclair PR, Gorman N, Tsyrlov IB, et al. Uroporphyrinogen oxidation catalyzed by human cytochromes P450. Drug Metab Dispos. 1998;26:1019–25. [PubMed] [Google Scholar]
- 11.Nakano K, Ishizuka M, Sakamoto KQ, Fujita S. Absolute requirement for iron in the development of chemically induced uroporphyria in mice treated with 3-methylcholanthrene and 5-aminolevulinate. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2009;22:345–51. doi: 10.1007/s10534-008-9171-6. [DOI] [PubMed] [Google Scholar]
- 12.Sinclair PR, Gorman N, Dalton T, et al. Uroporphyria produced in mice by iron and 5-aminolaevulinic acid does not occur in Cyp1a2(−/−) null mutant mice. Biochem J. 1998;330:149–53. doi: 10.1042/bj3300149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith AG, Clothier B, Carthew P, et al. Protection of the Cyp1a2(−/−) null mouse against uroporphyria and hepatic injury following exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 2001;173:89–98. doi: 10.1006/taap.2001.9167. [DOI] [PubMed] [Google Scholar]
- 14.Greaves P, Clothier B, Davies R, et al. Uroporphyria and hepatic carcinogenesis induced by polychlorinated biphenyls-iron interaction: absence in the Cyp1a2(−/−) knockout mouse. Biochemical and biophysical research communications. 2005;331:147–52. doi: 10.1016/j.bbrc.2005.03.136. [DOI] [PubMed] [Google Scholar]
- 15.Liang HC, Li H, McKinnon RA, et al. Cyp1a2(−/−) null mutant mice develop normally but show deficient drug metabolism. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:1671–6. doi: 10.1073/pnas.93.4.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franklin MR, Phillips JD, Kushner JP. Cytochrome P450 induction, uroporphyrinogen decarboxylase depression, porphyrin accumulation and excretion, and gender influence in a 3-week rat model of porphyria cutanea tarda. Toxicol Appl Pharmacol. 1997;147:289–99. doi: 10.1006/taap.1997.8282. [DOI] [PubMed] [Google Scholar]
- 17.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 18.Phillips JD, Kushner JP. Measurement of Uroporphyrinogen Decarboxylase Activity. In: Maines MD, Costa LG, Reed DJ, Sassa S, Sipes IG, editors. Current Protocols in Toxicology. John Wiley & Sons, Inc; New York: 1999. pp. 8.4.1–8.4.13. [DOI] [PubMed] [Google Scholar]
- 19.Prough RA, Burke MD, Mayer RT. Direct fluorometric methods for measuring mixed function oxidase activity. Methods in enzymology. 1978;52:372–7. doi: 10.1016/s0076-6879(78)52041-7. [DOI] [PubMed] [Google Scholar]
- 20.Stresser DM, Turner SD, Blanchard AP, Miller VP, Crespi CL. Cytochrome P450 fluorometric substrates: identification of isoform-selective probes for rat CYP2D2 and human CYP3A4. Drug metabolism and disposition: the biological fate of chemicals. 2002;30:845–52. doi: 10.1124/dmd.30.7.845. [DOI] [PubMed] [Google Scholar]
- 21.Franklin MR, Phillips JD, Kushner JP. Accelerated development of uroporphyria in mice heterozygous for a deletion at the uroporphyrinogen decarboxylase locus. J Biochem Mol Toxicol. 2001;15:287–293. doi: 10.1002/jbt.10000. [DOI] [PubMed] [Google Scholar]
- 22.Sinclair PR, Gorman N, Walton HS, et al. CYP1A2 is essential in murine uroporphyria caused by hexachlorobenzene and iron. Toxicol Appl Pharmacol. 2000;162:60–7. doi: 10.1006/taap.1999.8832. [DOI] [PubMed] [Google Scholar]
- 23.Phillips JD, Anderson KE. The Porphyrias. In: Kaushansky K, Lichtman MA, Beutler E, et al., editors. Williams Hematology. McGraw Hill; New York: 2010. pp. 839–863. [Google Scholar]
- 24.Constantin D, Francis JE, Akhtar RA, Clothier B, Smith AG. Uroporphyria induced by 5-aminolaevulinic acid alone in Ahrd SWR mice. Biochem Pharmacol. 1996;52:1407–13. doi: 10.1016/s0006-2952(96)00475-3. [DOI] [PubMed] [Google Scholar]
- 25.Deam S, Elder GH. Uroporphyria produced in mice by iron and 5-aminolevulinic acid. Biochem Pharmacol. 1991;41:2019–22. doi: 10.1016/0006-2952(91)90144-t. [DOI] [PubMed] [Google Scholar]
- 26.Gorman N, Walton HS, Bement WJ, et al. Role of small differences in CYP1A2 in the development of uroporphyria produced by iron and 5-aminolevulinate in C57BL/6 and SWR strains of mice. Biochem Pharmacol. 1999;58:375–82. doi: 10.1016/s0006-2952(99)00088-x. [DOI] [PubMed] [Google Scholar]
- 27.Sinclair PR, Bement WJ, Lambrecht RW, Jacobs JM, Sinclair JF. Uroporphyria caused by acetone and 5-aminolevulinic acid in iron-loaded mice. Biochem Pharmacol. 1989;38:4341–4. doi: 10.1016/0006-2952(89)90536-4. [DOI] [PubMed] [Google Scholar]
- 28.Sinclair PR, Gorman N, Walton HS, et al. Uroporphyria in Hfe mutant mice given 5-aminolevulinate: A new model of Fe-mediated porphyria cutanea tarda. Hepatology. 2001;33:406–412. doi: 10.1053/jhep.2001.21409. [DOI] [PubMed] [Google Scholar]
- 29.Kushner JP, Lee GR, Nacht S. The role of iron in the pathogenesis of porphyria cutanea tarda. An in vitro model. J Clin Invest. 1972;51:3044–51. doi: 10.1172/JCI107131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kakhlon O, Cabantchik ZI. The labile iron pool: characterization, measurement, and participation in cellular processes(1) Free radical biology & medicine. 2002;33:1037–46. doi: 10.1016/s0891-5849(02)01006-7. [DOI] [PubMed] [Google Scholar]
- 31.Petrat F, de Groot H, Sustmann R, Rauen U. The chelatable iron pool in living cells: a methodically defined quantity. Biological chemistry. 2002;383:489–502. doi: 10.1515/BC.2002.051. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan J, Ward DM, De Domenico I. The molecular basis of iron overload disorders and iron-linked anemias. International journal of hematology. 2011;93:14–20. doi: 10.1007/s12185-010-0760-0. [DOI] [PubMed] [Google Scholar]
- 33.Di Padova C, Marchesi L, Cainelli T, et al. Effects of phlebotomy on urinary porphyrin pattern and liver histology in patients with porphyria cutanea tarda. Am J Med Sci. 1983;285:2–12. doi: 10.1097/00000441-198301000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Graham FD, Erlemann KR, Gravel S, Rokach J, Powell WS. Oxidative stress-induced changes in pyridine nucleotides and chemoattractant 5-lipoxygenase products in aging neutrophils. Free radical biology & medicine. 2009;47:62–71. doi: 10.1016/j.freeradbiomed.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLaughlin LA, Dickmann LJ, Wolf CR, Henderson CJ. Functional expression and comparative characterization of nine murine cytochromes P450 by fluorescent inhibition screening. Drug metabolism and disposition: the biological fate of chemicals. 2008;36:1322–31. doi: 10.1124/dmd.108.021261. [DOI] [PubMed] [Google Scholar]