Abstract
Cellular compartmentation of Zn in the leaves of the hyperaccumulator Thlaspi caerulescens was investigated using energy-dispersive x-ray microanalysis and single-cell sap extraction. Energy-dispersive x-ray microanalysis of frozen, hydrated leaf tissues showed greatly enhanced Zn accumulation in the epidermis compared with the mesophyll cells. The relative Zn concentration in the epidermal cells correlated linearly with cell length in both young and mature leaves, suggesting that vacuolation of epidermal cells may promote the preferential Zn accumulation. The results from single-cell sap sampling showed that the Zn concentrations in the epidermal vacuolar sap were 5 to 6.5 times higher than those in the mesophyll sap and reached an average of 385 mm in plants with 20,000 μg Zn g−1 dry weight of shoots. Even when the growth medium contained no elevated Zn, preferential Zn accumulation in the epidermal vacuoles was still evident. The concentrations of K, Cl, P, and Ca in the epidermal sap generally decreased with increasing Zn. There was no evidence of association of Zn with either P or S. The present study demonstrates that Zn is sequestered in a soluble form predominantly in the epidermal vacuoles in T. caerulescens leaves and that mesophyll cells are able to tolerate up to at least 60 mm Zn in their sap.
Different mechanisms have been proposed to explain the tolerance of plants to toxic heavy metals (Baker and Walker, 1990; Verkleij and Schat, 1990). Some tolerant plant species, the so-called “excluders,” use exclusion mechanisms by which uptake and/or root-to-shoot transport of heavy metals are restricted. Other tolerant plant species are able to cope with elevated concentrations of toxic metals inside of their tissues through production of metal-binding compounds, cellular and subcellular compartmentation, or alterations of metabolism.
An extreme strategy for metal tolerance that is in sharp contrast to metal exclusion is “hyperaccumulation,” a term that was originally used by Brooks et al. (1977) to describe plants that can accumulate more than 1,000 μg Ni g−1 dry weight in their aerial parts. Approximately 400 taxa of terrestrial plants have been identified as hyperaccumulators of various heavy metals, with about 300 being Ni hyperaccumulators (Baker and Brooks 1989; Brooks, 1998). Only 16 species of Zn hyperaccumulators, which are defined as being able to accumulate more than 10,000 μg Zn g−1 in the aboveground parts on a dry weight basis in their natural habitat (Brooks, 1998), have been reported. Thlaspi caerulescens J. & C. Presl (Brassicaceae) is the best-known example of a Zn/Cd hyperaccumulator. Under hydroponic culture conditions T. caerulescens can accumulate up to 25,000 to 30,000 μg Zn g−1 dry weight in the shoots without showing any toxicity symptoms or reduction in growth (Brown et al., 1996a; Shen et al., 1997). Recently, there has been a surge of interest in the phenomenon of heavy-metal hyperaccumulation because this property may be exploited in the remediation of heavy-metal-polluted soils through phytoextraction and phytomining (McGrath et al., 1993; Brown et al., 1995b; Robinson et al., 1997).
The mechanisms for metal hyperaccumulation are not fully understood, and this is particularly true in the case of the Zn/Cd hyperaccumulators. To cope with the consequence of hyperaccumulation, plants must also be hypertolerant to the heavy metals that accumulate. Recent studies comparing the different populations of T. caerulescens have shown that hyperaccumulation of Zn is a constitutive property, although the traits are probably separate from those for tolerance (Baker et al., 1994; Meerts and Van Isacker, 1997). Compared with the nonaccumulating species, T. caerulescens possesses an enhanced capacity to take up Zn and transport it from roots to shoots (Baker et al., 1994; Brown et al., 1995a; Shen et al., 1997). Lasat et al. (1996) found that roots of T. caerulescens and the nonaccumulator Thlaspi arvense had similar apparent Km values for Zn2+, but that the Vmax in the former was 4.5-fold higher than that in the latter species, indicating that the hyperaccumulator T. caerulescens possessed more Zn2+-transport sites in the plasma membranes of root cells. Shen et al. (1997) showed that T. caerulescens was much more effective in exporting the Zn that was accumulated previously in roots to the shoots than an intermediate accumulator species, Thlaspi ochrolucum. Organic acids such as malic acid have been suggested to play a key role in shuttling Zn from cytoplasm to vacuoles (Mathys, 1977). However, the low affinity of malate to chelate Zn (stability constant pK = 3.5 at infinite dilution) does not favor this hypothesis. Moreover, high concentrations of malate found in the shoot tissues of T. caerulescens appear to be a constitutive property (Tolrà et al., 1996; Shen et al., 1997).
The extraordinary tolerance of hyperaccumulator plants must also involve compartmentation of toxic metals at the cellular and subcellular levels. Vázquez et al. (1992, 1994) studied localization of Zn in the root and leaf tissues of T. caerulescens using EDXMA. They compared two methods of sample preparation and found that Na2S fixation was not suitable for preventing the loss of metal ions from the samples. Using cryofixation and freeze substitution, they showed that Zn accumulated mainly in the vacuoles as electron-dense deposits. Many vacuoles of leaf-epidermal and subepidermal cells contained globular crystals that were very rich in Zn. However, it is not known whether the Zn-rich, globular crystal deposits occur inside of the leaf vacuoles in vivo or if they are artifacts caused by sample preparation. Also, the technique used by Vázquez et al. (1992, 1994) allows only semiquantitative determination of Zn concentrations.
In this study we used two techniques to investigate cellular compartmentation of Zn in the leaves of T. caerulescens. The first utilized EDXMA of frozen, hydrated tissue to survey the distribution patterns of Zn and other elements across different leaf cells. The second method involved sampling sap from single cells using microcapillaries, followed by fully quantitative determination of Zn and other elements using EDXMA.
MATERIALS AND METHODS
Plant Culture
Seeds of Thlaspi caerulescens (from the population at Prayon, Belgium) were sown on a mixture of perlite and vermiculite moistened with deionized water. Three weeks later, seedlings were transplanted to plastic pots each filled with 500 g of John Innes II compost (John Innes Centre, Norwich, UK). Four plants were grown in each pot. The total concentration of Zn in the unamended compost was 88 μg g−1. There were eight Zn treatments, ranging from 0 to 4000 μg g−1 compost, and each treatment was replicated in three pots. Zinc was added as ZnSO4 solution to the compost and mixed thoroughly before potting. Plants were grown for 6 weeks inside of a growth room with the following conditions: 16-h daylength with a photon flux density of 350 μmol m−1 s−1 supplied by fluorescent tubes and a 20°C/16°C day/night temperature.
EDXMA of Frozen, Hydrated Leaf Tissues
Mature and young leaves from the plants grown on 4000 μg Zn g−1 were used. The optimum conditions for sample preparation and EDXMA of frozen, hydrated plant tissues were critically reviewed by Van Stevenink and Van Steveninck (1991). A small section from the middle of young or mature leaves was excised, mounted in stainless steel stubs, and rapidly frozen in liquid nitrogen slush. The sample was transferred to a preparation chamber cooled at −180°C and fractured with a liquid nitrogen-cooled scalpel blade just above the level of the stub to reveal the surface of the cells. Ice was removed from the cell surface by exposing the sample to a high vacuum at −85°C for 2 min. After this etching process, the sample was recooled to −180°C and evaporatively coated with carbon to produce an electrically conductive surface. Carbon was used instead of a metal coating to avoid interference on the elements measured. The specimen was then transferred to a liquid- nitrogen-cooled stage (−180°C) inside of the SEM (model XL 40, Philips, Eindhoven, The Netherlands).
EDXMA was performed in the SEM using an acceleration voltage of 30 kV, a takeoff angle of 45o, and a working distance (sample to final lens of the SEM) of 10 mm. Spectra from 0 to 20 keV were collected at increments of 10 eV per channel with the electron beam focused on a rectangular area in the center of selected cells. The background and element-specific peak spectra were analyzed using the program Superquant (EDAX, San Francisco, CA), which fully deconvolutes the spectra and allows the corrections for interference between elements. Epidermal and mesophyll cells were randomly selected for EDXMA of element-specific spectra. To remove the effects of variation of surface topography between the different selected cells on the efficiency of EDXMA rate counting, counts (peak minus background) of Zn and other elements were normalized on a molar basis with the Ca counts. This was because the distribution of Ca appeared to be relatively homogenous, as revealed by EDXMA dot maps (see below). Normalization based on Ca resulted in a smaller variation between replicated determinations of the same cell type compared with that based on K.
In addition, the distribution of Zn across different cells from the upper to the lower epidermis of fractured, frozen, hydrated leaf tissue was measured semiquantitatively by collecting and analyzing the Zn spectrum within a narrow spectrum window (±20 eV) around its peak. A two-dimensional distribution pattern was also recorded by scanning an area of the specimen repeatedly for up to 2 h and integrating the counts for Zn, Ca, and K within their respective spectrum windows into dot maps. In both line and area scans the spectrum-analysis software did not allow for the separation of the element-specific x-rays (peaks) from the background counts. Therefore, area scans were performed only on the elements with an average peak-to-background ratio greater than 2, including Zn, K, and Ca.
Extraction of Sap from Single Cells and Determination of Solute Concentrations
Plants grown in pots with additions of 0, 1000, and 4000 μg Zn g−1 were used. Mature leaves were selected from T. caerulescens rosettes. Glass microcapillaries with tip outside diameters of 5 and 2 μm were used to extract sap from single epidermal and mesophyll cells, respectively. Sampling was carried out according to the method described by Tomos et al. (1994). To extract sap from mesophyll cells, the epidermis was stripped off physically and the sample was washed briefly with deionized water to remove solutes from destroyed epidermal cells. The same leaves were used for extraction of both epidermal and mesophyll sap. The extracted sap samples were transferred under water-saturated paraffin oil from the sampling microcapillary to a copper-finder grid coated with a 2% solution of Pioloform (Agar Aids, Stanstead, UK). To avoid changes in concentration due to evaporation, samples were stored for no longer than 30 min. The sap samples were then transferred from the storage to the analysis grid with a constriction pipette (approximately 10 pL). An equal volume of the internal RbF standard in mannitol was added to the sample to give a final concentration of 20 mm RbF and 100 mm mannitol. Samples were then dried according to the method described by Tomos et al. (1994) and analyzed directly, i.e. without coating, using EDXMA in the SEM.
Calibrations were established using multi-element standards containing equal molar concentrations of Zn, Ca, Cd, Cl, K, Mg, and P, as well as the internal standard RbF (Tomos et al., 1994). A reliable quantification (±10%) within the concentration range of 1 to 100 mm was achieved for the above elements with this method. The sap samples were usually diluted by 1:4 or 1:8 using constriction pipettes. Determination of S in the sap samples was not possible because the Pioloform used in the sample preparation contains S.
Determination of Zn Concentrations in Whole Shoots of T. caerulescens
Plants were harvested after growing on different treatments for 6 weeks. Shoots were cut, weighed, washed with deionized water, blotted dry, and frozen in liquid nitrogen. The samples were lyophilized for 72 h and dry weights were determined. Dried shoot samples were ground and a 0.2-g subsample was digested with a mixture of HNO3 and HClO4 (Zhao et al., 1994). Concentrations of Zn and other elements in the digests were determined using inductively coupled atomic emission spectrometry (Fisons-ARL Accuris, Ecublens, Switzerland).
RESULTS
Plant Growth and Zn Uptake
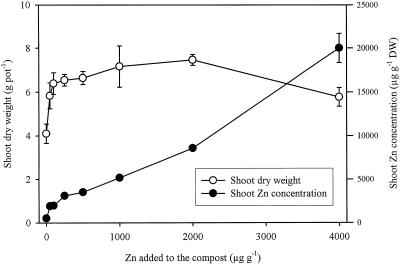
Additions of Zn to the compost that already contained a sufficient amount of Zn for the growth of normal plant species increased the dry weight of T. caerulescens shoots significantly (P < 0.01; Fig. 1), indicating a higher requirement for Zn in this species. Maximum shoot dry weight was obtained with the addition of 2,000 μg Zn g−1 and was 82% greater than that from the control (without Zn addition). Shoot dry weight was lower than the maximum with the addition of 4,000 μg Zn g−1 but was still significantly higher than that of the control. The concentration of Zn in the shoot dry matter increased from 509 μg g−1 in the control to 20,010 μg g−1 in the highest Zn treatment (Fig. 1).
Figure 1.
Effects of Zn additions on mean shoot dry weight (DW) and mean total Zn concentration in T. caerulescens. Bars represent ±se (n = 3).
The mean concentrations of K and Ca in the shoots of T. caerulescens were 4% and 2%, respectively, based on dry weight. Both K and Ca decreased generally with increasing Zn, with the effect being most pronounced at the highest Zn addition (data not shown). The concentration of P decreased significantly only at the highest Zn addition, whereas the additions of Zn as ZnSO4 beyond 1000 μg Zn g−1 increased the concentration of S in the shoots. The concentrations of the micronutrients Fe, Cu, and Mn in the shoots were all well above deficiency thresholds (Marschner, 1995).
EDXMA of Frozen, Hydrated Leaf Tissues
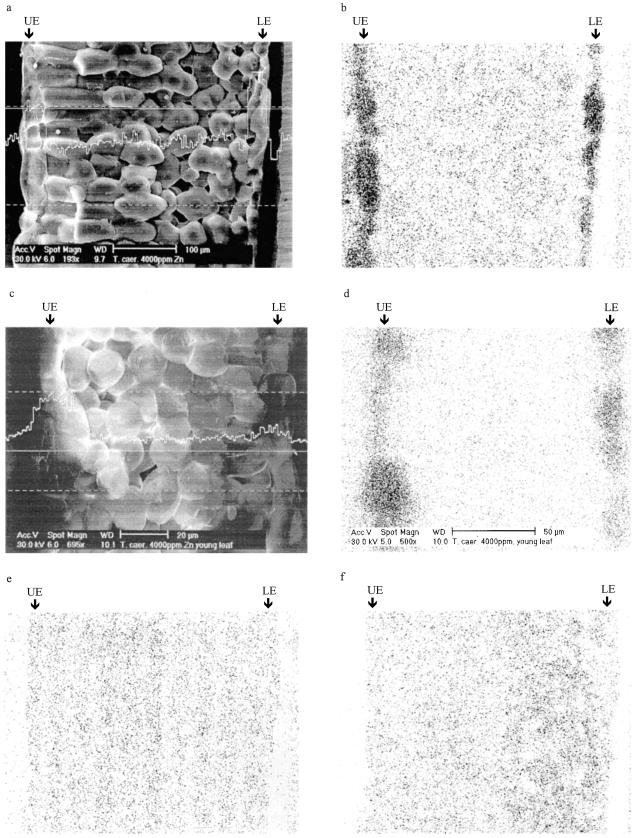
Both the EDXMA line and area scans revealed that Zn accumulated predominantly in the epidermal cells in the mature leaves of T. caerulescens (Fig. 2, a and b). The mesophyll cells, in contrast, appeared to have much lower concentrations of Zn. The Zn signal was considerably smaller in the young than in the mature leaves. Nevertheless, the accumulation of Zn in the epidermis was still apparent in the young leaves, particularly in those with enlarged epidermal cells (Fig. 2, c and d). Distribution of K and Ca was much more even across the leaf section (Fig. 2, e and f).
Figure 2.
Distribution of Zn, K, and Ca across different leaf cells of T. caerulescens as revealed by EDXMA of frozen, hydrated tissues. Plants were grown on 4000 μg Zn g−1 for 6 weeks. a, Intensity of the Zn Kα line across a mature leaf section. The measurement took place at the solid straight line. b, Dot map of Zn distribution in a mature leaf section. c, Intensity of the Zn Kα line across a young leaf section. d, Dot map of Zn distribution in a young leaf section. e, Dot map of K distribution in a mature leaf section. f, Dot map of Ca distribution in a mature leaf section. Arrows indicate epidermal cells. UE, Upper epidermis; LE, lower epidermis.
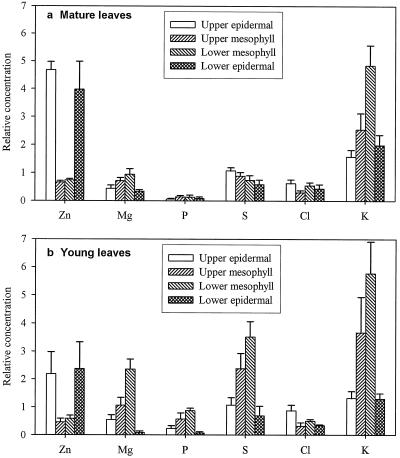
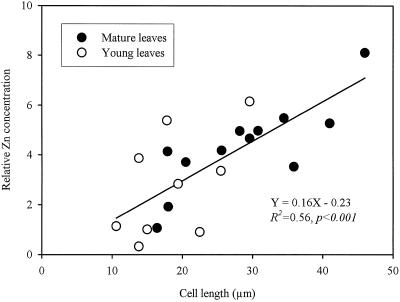
Figure 3 shows the relative concentrations of Zn, K, Mg, S, and Cl in the different cell types of young and mature leaves from the plants given 4000 μg Zn g−1. The relative concentration of Zn was 5- to 7-fold higher in the epidermal cells of mature leaves than in the mesophyll cells. In young leaves the difference was about 2 to 4 times higher. There were no consistent differences between the upper and lower epidermis or between the upper and lower mesophyll cells. In general, the variability of the relative concentration of Zn in the epidermis of young leaves was larger than in the mature leaves. The variation of the relative Zn concentration in the epidermal cells appeared to be associated with the variation in the cell size. The correlation between the relative Zn concentrations in epidermal cells and their length was highly significant (Fig. 4), and the relationship appeared to be linear and similar for both young and mature leaves.
Figure 3.
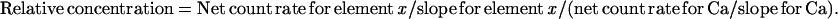 |
Figure 4.
Relationship between cell length and relative Zn concentration in the epidermal cells in frozen, hydrated leaf tissues of T. caerulescens grown on 4000 μg Zn g−1.
In contrast to the distribution pattern of Zn, higher relative concentrations of K were observed in the mesophyll cells than in the epidermis in both young and mature leaves (Fig. 3). The relative concentrations of both P and S were greater in the young than in the mature leaves, respectively, and in the former, the mesophyll cells had greater relative concentrations of P and S than the epidermal cells (Fig. 3), a pattern that was the opposite of that for Zn.
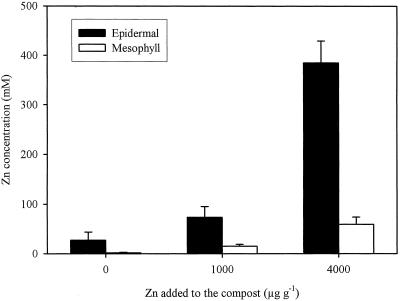
Concentrations of Zn and Other Solutes in Single-Cell Sap
Determination of the single-cell sap samples showed that the epidermis contained much greater concentrations of Zn than the mesophyll cells (Fig. 5). The mean concentration of Zn in the epidermal saps extracted from the mature leaves of T. caerulescens grown on the 4000 μg Zn g−1 treatment was 385 mm, equal to 2.5% (w/v) of soluble Zn. Even when no Zn was added to the compost, the epidermal cells still accumulated considerable concentrations of Zn in the sap, whereas the concentrations of Zn in the sap from mesophyll cells were barely detectable. In the 1000 and 4000 μg Zn g−1 treatments, the concentrations of Zn in the epidermal sap were 5 to 6.5 times greater than those in the mesophyll sap.
Figure 5.
Concentrations of Zn in the single-cell saps extracted from epidermal and mesophyll cells of mature leaves of T. caerulescens. Plants were grown on 0, 1000, or 4000 μg Zn g−1 for 6 weeks. Bars represent ses (n = 3–5).
Concentrations of K, Ca, Mg, P, and Cl are shown in Table I. In the epidermal sap extracted from the plants treated with 4000 μg Zn g−1, Zn was by far the most abundant element. In this treatment the concentrations of K, Ca, Mg, and P were lower in the epidermal than in the mesophyll sap, whereas the concentrations of Cl were similar. Different patterns for these elements were observed in the 0 and 1000 μg Zn g−1 treatments, with epidermal sap having higher concentrations of these elements than the mesophyll sap. In general, the concentrations of K, Cl, P, and Ca in the epidermal sap decreased with increasing Zn, whereas the concentrations in the mesophyll sap were less affected by the Zn treatments.
Table I.
Concentrations of K, Ca, Mg, P, and Cl in the single-cell sap extracted from the epidermal and mesophyll cells of mature leaves of T. caerulescens
| Zn Added | K
|
Ca
|
Mg
|
P
|
Cl
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal | Mesophyll | Epidermal | Mesophyll | Epidermal | Mesophyll | Epidermal | Mesophyll | Epidermal | Mesophyll | |
| μg g−1 | mm | |||||||||
| 0 | 571 ± 116 | 327 ± 62 | 516 ± 159 | 157 ± 25 | 275 ± 87 | 155 ± 45 | 151 ± 44 | 107 ± 27 | 304 ± 94 | 59 ± 15 |
| 1000 | 317 ± 61 | 305 ± 40 | 294 ± 83 | 238 ± 58 | 302 ± 70 | 195 ± 57 | 81 ± 27 | 62 ± 29 | 159 ± 24 | 87 ± 35 |
| 4000 | 193 ± 32 | 300 ± 38 | 87 ± 8 | 132 ± 24 | 122 ± 7 | 242 ± 53 | 38 ± 6 | 47 ± 10 | 78 ± 9 | 73 ± 12 |
The results are presented as means ± se (n = 3–5).
DISCUSSION
The two techniques used in this study are complimentary. EDXMA on frozen, hydrated samples provides information about the in vivo distribution of Zn and other elements in adjacent cells but is only semiquantitative and relatively insensitive (Lazof and Läuchli, 1991; Van Steveninck and Van Steveninck, 1991), whereas the single-cell-extraction technique allows quantitative determination of the sap composition. Using both techniques, we have demonstrated conclusively that the epidermal cells of the leaves of the hyperaccumulator T. caerulescens accumulated much higher concentrations of Zn than the mesophyll cells. The preferential accumulation of Zn in the epidermis was evident even when the growth medium contained no elevated concentration of Zn. Vázquez et al. (1994) also found accumulation of Zn in the epidermal cells of T. caerulescens leaves. In contrast to their results, we did not observe preferential accumulation of Zn in the subepidermal cells. Preferential distribution of Ni in the epidermal cells or trichomes has been observed in the leaves of Ni hyperaccumulator plants (Mesjasz-Przybylowicz et al., 1994; Krämer et al., 1997).
Using malate dehydrogenase as the cytoplasmic marker enzyme, Fricke et al. (1994) showed that the epidermal sap extracted from barley leaves was completely vacuolar in origin, whereas extracts from mesophyll cells also contained cytoplasmic constituents. The difference was explained by a much larger proportion in terms of intracellular volume of vacuole in the epidermal cells (approximately 99%) than in the mesophyll cells (about 60%). Thus, at least in the epidermis, the concentrations of Zn and other elements in the single-cell saps may truly represent those in the vacuolar sap. The total width of the upper and lower epidermal cells was about 15% of the thickness of the mature leaves in T. caerulescens. When the same proportions of vacuole volume in the cell volume as those for barley leaves was used, it is estimated that more than 60% of the Zn accumulated by T. caerulescens leaves was present in the epidermal vacuoles. Therefore, epidermal vacuoles are an important location for Zn sequestration in T. caerulescens leaves.
Vázquez et al. (1992, 1994) performed EDXMA of T. caerulescens leaf tissues by transmission electron microscopy. The advantage of using transmission electron microscopy is the capability of investigating the distribution pattern at the subcellular level; however, this technique requires removal of tissue water through lengthy sample preparation, which can then cause redistribution of ions and other artifacts (Van Steveninck and Van Steveninck, 1991). One possible artifact reported by Vázquez et al. (1994) is that inside the vacuoles, Zn appeared to be present in deposits as globular crystals. In contrast, our results obtained with single-cell-sap extraction indicate that Zn is present in soluble forms. We also showed in a previous study that more than 80% of the Zn accumulated in the leaves of T. caerulescens was water soluble (Zhao et al., 1998).
The decrease in the concentrations of K, Ca, Mg, and Cl in epidermal sap in response to increasing Zn concentration was probably a result of osmotic adjustment. The concentrations of Ca in both epidermal and mesophyll saps extracted from the mature leaves of T. caerulescens were much greater than those reported for barley (Leigh and Tomos, 1993; Fricke et al., 1994). This is not surprising because of the high total concentration of Ca in the shoots of T. caerulescens. The charge balance in the single-cell saps could not be calculated because major anions such as sulfate and nitrate were not determined. It is also not possible to calculate the speciation of Zn in the vacuolar saps, because organic solutes were not determined in this study, and the species of P and S were also unknown.
In the 4000 μg Zn g−1 treatment, which produced a large enrichment of Zn in the epidermis, both P and S were preferentially distributed in the mesophyll cells. Vázquez et al. (1994) also observed a low P signal in the epidermal vacuoles of T. caerulescens. Furthermore, there is little evidence that coprecipitation of Zn with P occurs in the leaves of T. caerulescens (Zhao et al., 1998). To avoid coprecipitation of P with Zn, which could induce P deficiency, T. caerulescens must be able to maintain a low Pi concentration or physically separate Zn from Pi in different leaf cells or different subcellular compartments. In the case of S, glucosinolates have been suggested to play a role in chelating Zn (Mathys, 1977), despite the lack of any evidence that Zn-glucosinolate complexes exist. The different distribution patterns of Zn and S in the leaf cells of T. caerulescens indicate that S-containing compounds are unlikely to play an important role in the sequestration of Zn in vacuoles.
The mechanisms involved in the preferential accumulation of Zn in the epidermal vacuoles in T. caerulescens leaves are not known. Several possible pathways have been proposed to explain the transfer of ions and water from xylem to leaf cells in cereal leaves (Leigh and Tomos, 1993). Different selectivity of transport at various interfaces among xylem, vein extensions, mesophyll cells, and epidermis are possibly involved, resulting in the asymmetric distribution of ions and other solutes. It is also possible that the tonoplast of the epidermis of T. caerulescens leaves may have a higher capacity for the transport of Zn into vacuoles than that of the mesophyll cells. The close relationship between cell size and the Zn concentration (relative to Ca) in the epidermal cells is also very interesting and indicates that large epidermal cells were particularly enriched with Zn. This relationship suggests that vacuolation in the epidermal cells may be an important driving force for the preferential Zn sequestration in T. caerulescens leaves.
The ability of T. caerulescens leaves to sequester Zn preferentially in the epidermal vacuoles is probably an important aspect of the hypertolerance of this species to Zn. Preferential distribution of Zn in the epidermis helps to protect mesophyll cells from the buildup and toxicity of Zn and maintain the functionality of mesophyll cells over a wide range of Zn concentrations in the leaves. Still, mesophyll cells of T. caerulescens leaves must also be able to cope with concentrations of Zn that are much higher than what might be found in most plant species (probably <1 mm). The concentration of Zn in the mesophyll sap of T. caerulescens leaves reached 60 mm when the total concentration of Zn in the shoot dry weight was about 20,000 μg g−1, a concentration that is considerably lower than the reported toxic threshold for this species (Brown et al., 1995a; Shen et al., 1997). Excess Zn in mesophyll cells is also likely to be sequestered in the vacuoles.
ACKNOWLEDGMENTS
We thank Miss Tara Breedon and Miss Sarah Dunham for assistance in setting up the pot experiment, Mr. Chris Smith and Dr. Phil Jones for assistance in using the SEM and EDXMA systems, Mrs. Janice Proud and Dr. Tracey Cuin for the help in using micropipettes, and Dr. Deri Tomos for providing some of the constriction pipettes used.
Abbreviations:
- EDXMA
energy-dispersive X-ray microanalysis
- RbF
rubidium fluoride
- SEM
scanning electron microscope
- TEM
transmission electron microscope
Footnotes
IACR-Rothamsted receives grant-aided support from the Biotechnology and Biological Sciences Research Council of the United Kingdom.
LITERATURE CITED
- Baker AJM, Brooks RR. Terrestrial higher plants which hyperaccumulate metallic elements—a review of their distribution, ecology and phytochemistry. Biorecovery. 1989;1:81–126. [Google Scholar]
- Baker AJM, Reeves RD, Hajar ASM. Heavy metal accumulation and tolerance in British populations of the metallophyte Thlaspi caerulescens J. & C. Presl (Brassicaceae) New Phytol. 1994;127:61–68. doi: 10.1111/j.1469-8137.1994.tb04259.x. [DOI] [PubMed] [Google Scholar]
- Baker AJM, Walker PL. Ecophysiology of metal uptake by tolerant plants. In: Shaw AJ, editor. Heavy Metal Tolerance in Plants: Evolutionary Aspects. Boca Raton, FL: CRC Press; 1990. pp. 155–177. [Google Scholar]
- Brooks RR (1998) Geobotany and hyperaccumulators. In RR Brooks, ed, Plants That Hyperaccumulate Heavy Metals. CAB International, Wallingford, UK, pp 55–94
- Brooks RR, Lee J, Reeves RD, Jaffre T. Detection of nickeliferous rocks by analysis of herbarium species of indicator plants. J Geochem Explor. 1977;7:49–57. [Google Scholar]
- Brown SL, Chaney RL, Angle JS, Baker AJM. Zinc and cadmium uptake by hyperaccumulator Thlaspi caerulescens grown in nutrient solution. Soil Sci Soc Am J. 1995a;59:125–133. doi: 10.1021/es00006a022. [DOI] [PubMed] [Google Scholar]
- Brown SL, Chaney RL, Angle JS, Baker AJM. Zinc and cadmium uptake by hyperaccumulator Thlaspi caerulescens and metal tolerant Silene vugaris grown on sludge-amended soils. Environ Sci Technol. 1995b;29:1581–1585. doi: 10.1021/es00006a022. [DOI] [PubMed] [Google Scholar]
- Fricke W, Leigh RA, Tomos AD. Concentrations of inorganic and organic solutes in extracts from individual epidermal, mesophyll and bundle-sheath cells of barley leaves. Planta. 1994;192:310–316. [Google Scholar]
- Krämer U, Grime GW, Smith JAC, Hawes CR, Baker AJM. Micro-PIXE as a technique for studying nickel localization in leaves of the hyperaccumulator plant Alyssum lesbiacum. Nucl Instr Meth Physics Res B. 1997;130:346–350. [Google Scholar]
- Lasat MM, Baker AJM, Kochian LV. Physiological characterization of root Zn2+ absorption and translocation to shoots in Zn hyperaccumulator and nonaccumulator species of Thlaspi. Plant Physiol. 1996;112:1715–1722. doi: 10.1104/pp.112.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazof D, Läuchli A. Complementary analysis of freeze-dried and frozen-hydrated plant tissue by electron-proble X-ray microanalysis: spectral resolution and analysis of calcium. Planta. 1991;184:327–333. doi: 10.1007/BF00195333. [DOI] [PubMed] [Google Scholar]
- Leigh RA, Tomos AD. Ion distribution in cereal leaves: pathways and mechanisms. Philos Trans R Soc Lond-Biol Sci. 1993;341:75–86. [Google Scholar]
- Marschner H. Mineral Nutrition of Higher Plants, Ed 2. London: Academic Press; 1995. [Google Scholar]
- Mathys W. The role of malate, oxalate, and mustard oil glucosides in the evolution of zinc-resistance in herbage plants. Physiol Plant. 1977;40:130–136. [Google Scholar]
- McGrath SP, Sidoli CMD, Baker AJM, Reeves RD. The potential for the use of metal-accumulating plants for the in situ decontamination of metal-polluted soils. In: Eijsackers HJP, Hamers T, editors. Integrated Soil and Sediment Research: A Basis for Proper Protection. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1993. pp. 673–677. [Google Scholar]
- Meerts P, Van Isacker N. Heavy metal tolerance and accumulation in metallicolous and non-metallicolous populations of Thlaspi caerulescens from continental Europe. Plant Ecol. 1997;133:221–231. [Google Scholar]
- Mesjasz-Przybylowicz J, Balkwill K, Przybylowicz WJ, Annegarn HJ. Proton microprobe and X-ray fluorescence investigations of nickel distribution in serpentine flora from South Africa. Nucl Instr Meth Physics Res B. 1994;89:208–212. [Google Scholar]
- Robinson BH, Brooks RR, Howes AW, Kirkman JH, Gregg PEH. The potential of the high biomass nickel hyperaccumulator Berkheya coddii for phytoremediation and phytomining. J Geochem Explor. 1997;60:115–126. [Google Scholar]
- Shen ZG, Zhao FJ, McGrath SP. Uptake and transport of zinc in the hyperaccumulator Thlaspi caerulescens and the non-hyperaccumulator Thlaspi ochroleucum. Plant Cell Environ. 1997;20:898–906. [Google Scholar]
- Tolrà RP, Poschenrieder C, Barceló J. Zinc hyperaccumulation in Thlaspi caerulescens. II. Influence on organic acids. J Plant Nutr. 1996;19:1541–1550. [Google Scholar]
- Tomos AD, Hinde P, Richardson P, Pritchard J, Fricke W. Microsampling and measurements of solutes in single cells. In: Harris N, Oparka KJ, editors. Plant Cell Biology—A Practical Approach. Oxford, UK: IRC Press; 1994. pp. 297–314. [Google Scholar]
- Van Steveninck RFM, Van Steveninck ME. Microanalysis. In: Hall JL, Hawes C, editors. Electron Microscopy of Plant Cells. London: Academic Press; 1991. pp. 415–455. [Google Scholar]
- Vázquez MD, Barceló J, Poschenrieder C, Mádico J, Hatton P, Baker AJM, Cope GH. Localization of zinc and cadmium in Thlaspi caerulescens (Brassicaceae), a metalophyte that can hyperaccumulate both metals. J Plant Physiol. 1992;140:350–355. [Google Scholar]
- Vázquez MD, Poschenrieder C, Barceló J, Baker AJM, Hatton P, Cope GH. Compartmentation of zinc in roots and leaves of the zinc hyperaccumulator Thlaspi caerulescens J & C Presl. Bot Acta. 1994;107:243–250. [Google Scholar]
- Verkleij JAC, Schat H. Mechanisms of metal tolerance in higher plants. In: Shaw AJ, editor. Heavy Metal Tolerance in Plants: Evolutionary Aspects. Boca Raton, FL: CRC Press; 1990. pp. 179–193. [Google Scholar]
- Zhao F, McGrath SP, Crosland AR. Comparison of three wet digestion methods for the determination of plant sulphur by inductively coupled plasma atomic emission spectrometry (ICP-AES) Commun Soil Sci Plant Anal. 1994;25:407–418. [Google Scholar]
- Zhao FJ, Shen ZG, McGrath SP. Solubility of zinc and interactions between zinc and phosphorus in the hyperaccumulator Thlaspi caerulescens. Plant Cell Environ. 1998;21:108–114. [Google Scholar]