Abstract
Introduction
Despite proven benefits of regular physical activity, estimates indicate that few cancer survivors meet physical activity guidelines. The purpose of this paper is to identify and compare exercise barriers among cancer survivors, both cross-sectionally and longitudinally as they undergo home-based behavioral interventions.
Methods
Data on a sample of 452 breast and prostate cancer survivors who completed the FRESH START trial were analyzed collectively, as well as separately by cancer-type.
Results
More total barriers (3.5 vs. 2.4, p < 0.01) were reported among breast cancer survivors compared to prostate cancer survivors. Commonly reported baseline exercise barriers among both groups were “too busy” (breast 52%; prostate 45%) and “no willpower” (breast 51%; prostate 44%). At baseline, breast cancer survivors who reported “no willpower” also reported 18.7 fewer minutes of physical activity compared to those not reporting this barrier (p < 0.01). Among prostate cancer survivors, this difference was 39.5 minutes (p < 0.01). Change in barriers was not associated with change in minutes of physical activity from baseline to post-intervention in either cancer survivor group.
Conclusions
This is the largest study evaluating barriers and physical activity over time among cancer survivors. There are both similarities and differences that need to be taken into consideration when promoting physical activity among subgroups of survivors.
Implications for cancer survivors
Knowledge concerning barriers associated with reported physical activity may be helpful in designing optimally-targeted physical activity interventions among breast and prostate cancer survivors.
Keywords: breast neoplasms, prostatic neoplasms, barriers, physical activity, exercise
Introduction
Approximately 1.5 million Americans are diagnosed with cancer annually [1]. Breast and prostate cancer are among the most commonly-diagnosed malignancies among women and men, and 5-year survival rates for both early-stage cancers now exceed 90% [1,2]. Among roughly 12 million cancer survivors in the U.S., the largest segment is composed of these two cancer subtypes.
Cancer survivors face many physical and emotional challenges throughout their treatment and recovery. Participation in physical activity has been suggested to reduce undesirable sequelae associated with diagnosis and treatment, and to reduce comorbidities [3]. Physical activity has been shown to improve quality-of-life, vitality, self-esteem, and physical functioning among cancer survivors [4–7].
The American Cancer Society recommends at least 150 minutes/week of moderate-to-vigorous physical activity among adult cancer survivors [8]. However, the percent of survivors meeting these guidelines is reportedly as low as 29.6% [9]. Thus, more research is necessary to understand barriers to being physically active in this growing population. An understanding of the specific factors limiting physical activity in cancer survivors will help in designing effective interventions to ultimately reduce cancer recurrence and comorbidities in this population [10,11].
Previous investigations report the following exercise barriers among cancer survivors: lack of time and enjoyment, treatment side effects, and fatigue [12–14]. Investigations have generally been cross-sectional and have focused on breast cancer survivors. Less is known about barriers in other cancer survivors and how these barriers change during recovery and over the course of intervention.
The primary research aims of this study were to: 1) identify and compare baseline exercise barriers among breast and prostate cancer survivors, 2) evaluate the association between baseline barriers and levels of physical activity in both groups, and 3) identify the change in exercise barriers and reported physical activity longitudinally in response to a home-based physical activity intervention. Based on strong gender-related differences in the practice of physical activity [15], it was hypothesized that breast cancer survivors (women) would report more barriers and less physical activity, compared to prostate cancer survivors (men). It was also hypothesized exercise barriers would decrease among participants in the tailored intervention arm compared to the attention control arm of FRESH START.
Methods
Secondary data analyses were conducted using baseline and 1-year follow-up data from participants who enrolled and completed FRESH START, a home-based randomized controlled trial to promote physical activity and a healthy diet among cancer survivors. This trial had two arms: a tailored intervention, which distributed sequentially-tailored diet and exercise materials based on participant’s characteristics, and an attention control, which distributed (non-tailored) standardized diet and exercise brochures. Participants in the tailored intervention arm received two of the following three intervention modules: increasing fruit and vegetable consumption, restricting total and saturated fats, and/or increasing physical activity. Within the exercise intervention module, participants received mailed newsletters that provided information on how to overcome their specific exercise barriers. At one year follow-up, significantly more participants in the tailored intervention arm reported practicing two or more goal behaviors (≥150 minutes/week of exercise, consumption of ≥5 daily servings of fruits and vegetables, or consumption of a low fat diet) compared to the attention control arm [16]. Other details of the original study and main outcomes have been described previously [16,17]. Duke University Medical Center’s Institutional Review Board approved this trial conducted from July 2002 through October 2005.
Sample
FRESH START recruited cancer survivors from several cancer registries and oncologic practices throughout North America. Participants provided written consent and were screened for eligibility; criteria included: 1) diagnosis of loco-regionally staged prostate or female breast cancer within the past nine months, 2) free of conditions limiting adherence to physical activity (congestive heart failure, recent myocardial infarction, severe breathing difficulties, mobility problems, kidney failure, and progressive cancer), 3) English-speaking/writing, and 4) not engaging in 2-of-3 goal behaviors (i.e., ≥150 minutes/week of exercise, consumption of ≥5 daily servings of fruits and vegetables, or consumption of a low fat diet). The study sample resided in 39 U.S. states and 2 Canadian provinces. The current analyses included participants who 1) received standardized (attention control arm) or exercise intervention materials (72 excluded) and 2) completed baseline and 1-year measures (an additional 19 excluded) (N = 452).
Exercise barriers
Exercise barriers were assessed by telephone interview using a checklist which included 14 personal, social, and environmental barriers previously reported in the literature to be associated with physical inactivity in survivors or the general population [12–14]. Individuals were asked to respond affirmatively if the barrier applied to them or negatively if it did not (yes/no). Barrier change was calculated as either those who decreased barriers from baseline to 1 year vs. those who increased or reported the same number of barriers.
Minutes of physical activity
Minutes of physical activity were assessed by telephone interview using the 7-Day Physical Activity Recall (PAR), a validated instrument with proven reliability [18]. Participants reported their occupational and leisure activity (in minutes) during the previous week. Previous analyses in a subsample of FRESH START participants observed a moderate association between the subjective 7-Day PAR and an objective measure of the RT3 Triaxial Accelerometer [19].
Covariates
Information was collected on participants’ age, race, education, body mass index (BMI), number of comorbidities (Older Americans Resources & Services scale) [20], cancer stage, treatment modality, cancer coping style (mini-Mental Adjustment to Cancer) [21], and stage of readiness to exercise (Transtheoretical Model outlined by Prochaska et al) [22]. These variables were obtained at baseline through cancer registries and self-reported responses from telephone surveys.
Statistical analysis
Because cancer site was confounded by gender, and several differences in reported barriers were observed between cancer sites, the analyses were stratified by site (breast and prostate). When appropriate, demographic and clinical characteristics among breast and prostate cancer survivors were compared using t-tests and Chi-square tests. Chi-square tests were used to compare barriers between survivor groups. T-tests were used to compare minutes of physical activity among those reporting and not reporting specific exercise barriers at baseline. The number of participants who reported change in barriers from baseline to 1-year (decrease vs. increase or same) was calculated. Intervention assignment and barrier change was assessed using a chi-sqaure test. A t-test was used to evaluate the association between barrier change and change in minutes of physical activity from baseline to 1 year. Given the exploratory nature of this study, no modifications were made for multiple comparisons; thus, all significance levels were set at p < 0.05 (two-sided tests).
Results
From the original FRESH START sample of 543, the current sample of 452 participants was composed of 259 breast cancer survivors and 193 prostate cancer survivors. The current sample did not differ statistically from those excluded with respect to age, BMI, number of comorbidities, race, cancer stage, education, cancer coping style, treatment, or stage of readiness for exercise.
Participant characteristics
Table 1 presents characteristics of this study sample. At baseline, prostate cancer survivors reported more minutes of physical activity, were significantly older, included fewer racial-ethnic minorities, and had higher proportions reporting a fatalist coping style and precontemplative exercise stages of readiness than breast cancer participants.
Table 1.
Baseline participant characteristics
| Characteristic | Cancer survivor subgroup | P-valuea | ||
|---|---|---|---|---|
| Breast | Prostate | |||
| n (%) | n (%) | |||
| Minutes of physical activity | mean (SD) | 23.5 (50.6) | 52.2 (94.7) | <0.01 |
| Age, years | mean (SD) | 53.5 (11.4) | 62.0 (8.3) | <0.01 |
| BMI | mean (SD) | 27.5 (6.1) | 27.7 (4.1) | 0.74 |
| Comorbidities | mean (SD) | 2.2 (1.7) | 2.1 (1.6) | 0.57 |
| Race | <0.01 | |||
| Non-White | 58 (22) | 21 (11) | ||
| White | 201 (78) | 172 (89) | ||
| Cancer stageb | - | |||
| 0 | 33 (13) | 0 (0) | ||
| 1 | 132 (51) | 77 (40) | ||
| 2 | 78 (30) | 101 (52) | ||
| 3 | 16 (6) | 0 (0) | ||
| unknown | 0 (0) | 15 (8) | ||
| Treatmentb,c | - | |||
| Radiation | 153 (59) | 35 (18) | ||
| Hormonal therapy | 147 (57) | 27 (14) | ||
| Chemotherapy | 122 (47) | 0 (0) | ||
| Surgery | 257 (99) | 125 (65) | ||
| Education | 0.20 | |||
| High school graduate or less | 30 (12) | 21 (11) | ||
| Some college or associate | 89 (34) | 52 (27) | ||
| College graduate/postgraduate | 140 (54) | 120 (62) | ||
| Cancer coping style | <0.01 | |||
| Fighting spirit | 105 (40) | 61 (32) | ||
| Fatalist | 129 (50) | 122 (63) | ||
| Other | 25 (10) | 10 (5) | ||
| Stage of readiness for exercise | <0.01 | |||
| Precontemplator | 13 (5) | 25 (13) | ||
| Contemplator | 27 (10) | 20 (10) | ||
| Preparation/Action | 219 (85) | 148 (77) | ||
t-test or Chi-square
not a logical comparison between groups
Column percents don’t add to 100. Treatments are not mutually exclusive, participants may be counted in more than one category
Baseline exercise barriers among breast and prostate cancer survivors
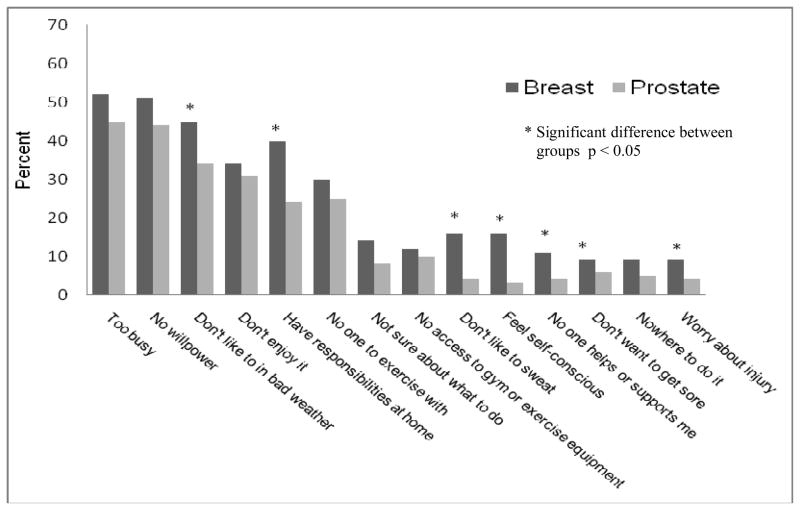
The top three exercise barriers reported among both groups of survivors were “too busy,” “no willpower,” and “don’t like to exercise in bad weather” (Figure 1). “Too busy” was the leading barrier reported among both breast and prostate cancer survivors (breast 52%, prostate 45%). Barriers reported less frequently included “nowhere to exercise” and “worry about injury.”
Figure 1.
Percent of participants reporting exercise barriers by cancer survivor group
Overall, more breast cancer survivors endorsed each exercise barrier compared to prostate cancer survivors, and also reported more total barriers (3.5 vs. 2.4, p < 0.01). There were significant differences between survivor groups for seven individual barriers (Figure 1).
Associations between baseline barriers and minutes of physical activity
Table 2 compares the average minutes of physical activity for participants who did and did not report specific exercise barriers at baseline. Among breast cancer survivors, participants who reported the barriers of “no one to exercise with,” “nowhere to do it,” “not sure about what to do,” “don’t want to get sore,” “don’t enjoy it,” “don’t like to sweat,” and “no willpower” reported significantly fewer minutes of physical activity than those who did not report these barriers. Among prostate cancer survivors, differences in physical activity were observed in the barriers “no access to gym or exercise equipment,” “not sure about what to do,” “worry about injury,” “don’t like to sweat,” and “no willpower.”
Table 2.
Baseline self reported minutes of activity at baseline, by barrier status
| Exercise barrier | Cancer survivor subgroup | |||
|---|---|---|---|---|
| Breast | Prostate | |||
| Not reported at baseline | Reported at baseline | Not reported at baseline | Reported at baseline | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Too busy | 25.3 (56.1) | 21.8 (45.0) | 54.7 (104.9) | 49.2 (81.1) |
| Have responsibilities at home | 22.6 (48.8) | 24.7 (53.3) | 50.6 (94.2) | 57.2 (97.3) |
| No one to exercise with | 28.9 (56.9) | 10.8 (27.8)* | 58.0 (96.2) | 34.7 (89.0) |
| No one helps or supports me | 24.9 (52.6) | 12.2 (28.1) | 52.8 (95.8) | 35.7 (60.5) |
| Nowhere to do it | 25.8 (52.4) | 0 (0)* | 52.9 (96.3) | 38.6 (54.2) |
| No access to gym or exercise equipment | 23.6 (49.1) | 22.8 (61.5) | 55.3 (98.4) | 24.3 (42.7)* |
| Don’t like to in bad weather | 28.4 (56.6) | 17.4 (41.3) | 56.0 (108.2) | 44.8 (60.1) |
| Not sure about what to do | 26.6 (53.6) | 4.5 (15.4)* | 56.0 (97.6) | 6.7 (18.0)* |
| Worry about injury | 23.4 (51.1) | 24.6 (45.5) | 53.7 (96.1) | 11.4 (22.7)* |
| Don’t want to get sore | 25.2 (52.5) | 5.9 (14.9)* | 53.3 (96.8) | 34.5 (46.8) |
| Don’t enjoy it | 32.4 (58.7) | 5.8 (18.5)* | 58.2 (101.6) | 38.9 (76.4) |
| Don’t like to sweat | 26.2 (53.4) | 9.2 (28.8)* | 53.7 (96.1) | 11.4 (20.4)* |
| Feel self-conscious | 24.2 (52.5) | 19.5 (38.9) | 53.6 (95.6) | 0 (0)* |
| No willpower | 33.0 (62.6) | 14.2 (32.6)* | 69.6 (117.0) | 30.1 (46.7)* |
t-test: p < 0.05
Changes in exercise barriers and physical activity throughout the intervention period
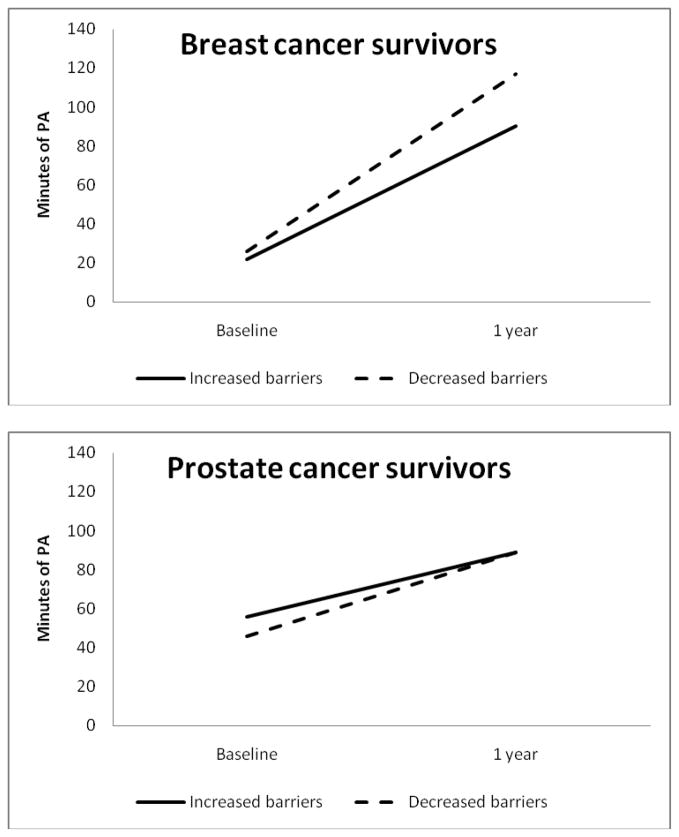
Overall, 37% of breast and 36% of prostate cancer survivors reduced barriers from baseline to 1-year follow-up. Intervention assignment was associated with barrier change among prostate cancer survivors, with 42% in the attention control arm reducing barriers, compared to 25% in the tailored intervention arm (p = 0.02). Among breast cancer survivors, those who reduced barriers to exercise (regardless of what intervention arm they were assigned to) reported an increase of 91 minutes of activity from baseline to 1 year, compared to an increase of 68 minutes among those who reported the same number or increased barriers (this was a non-significant increase between barrier change groups, p = 0.19) (Figure 2). Among prostate cancer survivors, those who reduced barriers from baseline to 1 year increased minutes by 43, compared to an increase of 33 minutes among those who reported the same number or increased number of barriers (p = 0.64).
Figure 2.
Change in exericse barriers and physical activity from baseline to 1-year follow-up, by cancer survivor group
Discussion
This study is the first to characterize and compare exercise barriers in both breast and prostate cancer survivors who participated in a home-based physical activity intervention. It provides much needed information about the type of barriers reported by these two prevalent survivor groups and their association with levels of physical activity, as well as changes in physical activity that occur longitudinally in response to a home-based intervention.
Compared to prostate cancer survivors, breast cancer survivors reported more barriers, and consistently greater endorsement of each individual barrier. However, both breast and prostate cancer survivors had similar rankings of their top three barriers (too busy; no willpower; and don’t like to exercise in bad weather). “Too busy” has been reported as a major exercise barrier among samples of head and neck (30%), breast cancer (73%), and colorectal cancer patients (32%) [12, 14, 23]. Lack of willpower and issues with weather also have been reported as substantial exercise barriers in both cancer and non-cancer samples [24–27].
More breast as compared to prostate cancer survivors responded affirmatively to each barrier. Given that breast cancer survivors tended to be middle-aged or older women, this finding could be attributed to social norms against exercise and perceptions that it is unfeminine. Also, women may cite lack of time as a reason for limiting activity based on family and care giving responsibilities [28,29]. Another possibility is that women may more readily divulge barriers and health-related information than men.
Several different exercise barriers were associated with baseline levels of physical activity in the two survivors groups, three of which were significant (not sure about what to do; don’t like to sweat; and no willpower). No willpower also was one of the most commonly reported exercise barriers among both survivor groups, suggesting this is an important barrier to address in future interventions. This may be difficult though, as “no willpower” is a fairly abstract internal construct and it may be difficult to measurably increase willpower in individuals. Instead, a successful intervention strategy may be to intervene on barriers associated with activity such as “not sure what to do,” as this is more tangible and participants can be educated on a variety of exercises and activities, and how to perform them. The most commonly reported barrier, “too busy,” was not associated with minutes of baseline physical activity, suggesting that time constraints are an acknowledged barrier among those who are sedentary, but that lack of time is also perceived and felt to influence exercise behavior even among those who exercise regularly.
Approximately 36–37% percent of breast and prostate cancer survivors, respectively, reduced their exercise barriers from baseline to 1 year. Interestingly, intervention assignment was associated with barrier change among prostate cancer survivors, but in the opposite direction of our hypothesis. Fewer individuals in the tailored intervention arm, as compared to the attention control arm reduced exercise barriers over time. Previous studies also have also reported this counter-intuitive finding, and posit that those who continue to be the most active over time have increased awareness of symptoms and side effects [30, 31].
Gains in reported physical activity were observed in both survivor groups regardless of barrier change direction. Thus, this suggests a possible consequence of physical activity interventions is an actual increase in exercise barriers. Physical activity interventions are likely to have a mix of participants, including those who, after reducing barriers will increase activity, as well as those who after increasing activity, will report more exercise barriers. The important message from this analysis is that at any snapshot in time, exercise barriers appear to influence activity levels, and they are different according to cancer type and, or gender. Those who report certain barriers typically report less activity. However, in the setting of a physical activity intervention, increasing activity may actually lead to higher reporting of exercise barriers.
Strengths and Limitations
The primary limitations of this study are as follows: 1) participants in FRESH START may have had greater motivation to adopt healthy behaviors compared to the overall survivor population; 2) the majority of measures were based on self report, and the psychometric properties of the barriers checklist are unknown; and 3) this trial was not powered for this secondary analysis. These limitations are mitigated by the fact that the response and retention rates of FRESH START were excellent (42% and 8%, respectively), the barriers checklist was composed of a compendium of items that have been validated in other studies, and self-report of exercise was validated via accelerometry [19]. In addition, given the fact that FRESH START was one of the largest lifestyle interventions ever conducted among a diverse sample of cancer survivors, our power to detect differences and associations is better than in most studies conducted to date. Furthermore, given the wide geographic distribution of FRESH START, these findings have the potential to generalize to a broad population and evaluates barriers at the time of participation, thereby reducing recall bias, and assesses changes in exercise barriers prospectively which few studies have done. Thus, this study provides important data where a current void exists.
Conclusion
Breast cancer survivors consistently reported a greater number of exercise barriers compared to prostate cancer survivors. Several exercise barriers were significantly inversely associated with reported physical activity at baseline, however the overall change in barriers was not associated with physical activity change throughout the FRESH START trial. In addition to knowledge gained from this study, future longitudinal research is still needed to investigate these ideas: 1) further explore differences between cancer types, as well as gender differences in regards to exercise barriers, and 2) explore the combination of exercise barriers that may act synergistically to impact physical activity.
Acknowledgments
The original FRESH START trial was supported by grants R01 CA81191, CA74000, CA63782, and M01-RR-30 from the National Institutes of Health and also by the American Institute of Cancer Research and the Susan G. Komen Foundation.
This material is the result of work supported with resources and the use of facilities at the Durham VA medical center.
Footnotes
No financial disclosures from any authors.
References
- 1.American Cancer Society. Cancer Facts & Figures 2010. Atlanta: American Cancer Society; 2010. http://www.cancer.org/acs/groups/content/@nho/documents/document/acspc-024113.pdf. [Google Scholar]
- 2.Age-adjusted SEER incidence and U.S. death rates and 5-year relative survival rates by primary cancer site, sex and time period (Table 1.4) SEER Cancer Statistics. http://seer.cancer.gov/csr/1975_2006/results_merged/topic_survival.pdf.
- 3.Alfano CM, Rowland JH. Recovery issues in cancer survivorship: A new challenge for supportive care. Cancer J. 2006;12(5):432–43. doi: 10.1097/00130404-200609000-00012. [DOI] [PubMed] [Google Scholar]
- 4.McNeely ML, Campbell KL, Rowe BH, et al. Effects of exercise on breast cancer patients and survivors: A systematic review and meta-analysis. CMAJ. 2006;175(1):34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinto BM, Frierson GM, Rabin C, et al. Home-based physical activity intervention for breast cancer patients. J Clin Oncol. 2005;23(15):3577–87. doi: 10.1200/JCO.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 6.Thorsen L, Courneya KS, Stevinson C, et al. A systematic review of physical activity in prostate cancer survivors: Outcomes, prevalence, and determinants. Support Care Cancer. 2008;16(9):987–97. doi: 10.1007/s00520-008-0411-7. [DOI] [PubMed] [Google Scholar]
- 7.Segal RJ, Reid RD, Courneya KS, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21(9):1653–9. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- 8.Doyle C, Kushi LH, Byers T, et al. Nutrition and physical activity during and after cancer treatment: An American cancer society guide for informed choices. CA Cancer J Clin. 2006;56:323–53. doi: 10.3322/canjclin.56.6.323. [DOI] [PubMed] [Google Scholar]
- 9.Bellizzi KM, Rowland JH, Jeffery DD, et al. Health behaviors of cancer survivors: examining opportunities for cancer control intervention. J Clin Oncol. 2005;23(34):8884– 8893. doi: 10.1200/JCO.2005.02.2343. [DOI] [PubMed] [Google Scholar]
- 10.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 11.Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Thomas J, Nelson H, Whittom R, Hantel A, Schilsky RL, Fuchs CS. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 24(22):3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 12.Rogers LQ, Courneya KS, Robbins KT, et al. Physical activity correlates and barriers in head and neck cancer patients. Support Care Cancer. 2008;16(1):19–27. doi: 10.1007/s00520-007-0293-0. [DOI] [PubMed] [Google Scholar]
- 13.Satia JA, Walsh JF, Pruthi RS. Health behavior changes in white and African American prostate cancer survivors. Cancer Nurs. 2009;32(2):107–17. doi: 10.1097/NCC.0b013e3181982d4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leddy SK. Incentives and barriers to exercise in women with a history of breast cancer. Oncol Nurs Forum. 1997;24:885–90. [PubMed] [Google Scholar]
- 15.Trost SG, Owen N, Bauman AE, Sallis JF, Brown W. Correlates of adults’ participation in physical activity: review and update. Med Sci Sports Exerc. 2002;34(12):1996–2001. doi: 10.1097/00005768-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Demark-Wahnefried W, Clipp EC, Lipkus IM, et al. Main outcomes of the FRESH START trial: A sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. J Clin Oncol. 2007;25(19):2709–18. doi: 10.1200/JCO.2007.10.7094. [DOI] [PubMed] [Google Scholar]
- 17.Demark-Wahnefried W, Clipp EC, McBride C, et al. Design of FRESH START: A randomized trial of exercise and diet among cancer survivors. Med Sci Sports Exerc. 2003;35(3):415–24. doi: 10.1249/01.MSS.0000053704.28156.0F. [DOI] [PubMed] [Google Scholar]
- 18.Blair SN, Haskell WL, Ho P, Paffenbarger RS, Jr, Vranizan KM, Farquhar JW, Wood PD. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122(5):794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- 19.Sloane R, Snyder DC, Demark-Wahnefried W, et al. Comparing the 7-Day physical activity recall with a triaxial accelerometer for measuring time in exercise. Med Sci Sports Exerc. 2009;41(6):1334–1340. doi: 10.1249/MSS.0b013e3181984fa8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fillenbaum GG. Multidimensional Functional Assessment of Older Adults. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 21.Watson MM, Law M, dos Santos M, et al. The Mini-MAC further development of the Mental Adjustment to Cancer scale. J Psychosoc Oncol. 1994;12(3):33–45. [Google Scholar]
- 22.Prochaska JO, Velicer WF, Rossi JS, et al. Stages of change and decisional balance for 12 problem behaviors. Health Psychol. 1994;13(1):39–46. doi: 10.1037//0278-6133.13.1.39. [DOI] [PubMed] [Google Scholar]
- 23.Courneya KS, Friedenreich CM, Quinney HA, Fields AL, Jones Lee W, Vallance JK, Fairey AS. A longitudinal study of exercise barriers in colorectal cancer survivors participating in a randomized controlled trial. Annals of Behavioral Medicine. 2005;29:147–53. doi: 10.1207/s15324796abm2902_9. [DOI] [PubMed] [Google Scholar]
- 24.Clark DO. Identifying psychological, physiological, and environmental barriers and facilitators to exercise among older low income adults. Journal of Clinical Geropsychology. 1999;5(1):51–62. [Google Scholar]
- 25.Mancuso CA, Sayles W, Robbins L, et al. Barriers and facilitators to healthy physical activity in asthma patients. Journal of Asthma. 2006;43(2):137–43. doi: 10.1080/02770900500498584. [DOI] [PubMed] [Google Scholar]
- 26.Sánchez ZV, Cashion AK, Cowan PA, et al. Perceived barriers and facilitators to physical activity in kidney transplant recipients. Progress in Transplantation. 2007;17(4):324–31. doi: 10.1177/152692480701700411. [DOI] [PubMed] [Google Scholar]
- 27.Rye JA, Rye SL, Tessaro I, et al. Perceived barriers to physical activity according to stage of change and body mass index in the west virginia wisewomen population. Women’s Health Issues. 2009;19(2):126–134. doi: 10.1016/j.whi.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Artazcoz L, Borrell C, Benach J. Gender inequalities in health among workers: the relation with family demands. J Epidemiol Community Health. 2001;55(9):639–647. doi: 10.1136/jech.55.9.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verhoef MJ, Love EJ, Rose MS. Women’s social roles and their exercise participation. Women and Health. 1992;19(4):15–29. doi: 10.1300/j013v19n04_02. [DOI] [PubMed] [Google Scholar]
- 30.Lynch B, Owen N, Hawkes A, et al. Perceived barriers to physical activity for colorectal cancer survivors. Support Care Cancer. 2010;18(6):729–734. doi: 10.1007/s00520-009-0705-4. [DOI] [PubMed] [Google Scholar]
- 31.Dawson J, Hillsdon M, Boller I, Foster C. Perceived barriers to walking in the neighbourhood environment and change in physical activity levels over 12 months. Br J Sports Med. 2007;41(9):562–568. doi: 10.1136/bjsm.2006.033340. [DOI] [PMC free article] [PubMed] [Google Scholar]