Abstract
A fundamental but unsolved problem in neuroscience is how connections between neurons might underlie information processing in central circuits. Building wiring diagrams of neural networks may accelerate our understanding of how they compute. But even if we had wiring diagrams, it is critical to know what neurons in a circuit are doing: their physiology. In both the retina and cerebral cortex, a great deal is known about topographic specificity, such as lamination, and cell-type specificity of connections. Little, however, is known about connections as they relate to function. Here, we review how advances in functional imaging and electron microscopy have recently allowed the examination of relationships between sensory physiology and synaptic connections in cortical and retinal circuits.
Introduction
One of the major challenges in neuroscience is to make and test realistic models of information processing in neuronal networks. The problem remains largely unsolved, even for networks such as the retina, arguably the best understood part of the central nervous system, or the visual cortex, perhaps the most studied cortical area. The biggest impediment to our understanding is that we have little idea how connections between neurons in a local circuit carry out specific computations. Examining the relationship between cellular function and network structure - between sensory physiology and specific synaptic connectivity - has the potential to address this problem.
What we discuss as ‘specificity’, however, should be clear. Most neural circuits have topographic specificity, for instance when axons selectively target brain regions or neuronal lamina. Specific connectivity can alternatively be interpreted as the selective targeting of synapses onto particular cell types or sub-cellular compartments: examples of cell-type specificity. Here, we concentrate on functional specificity, as it relates to information processing in neuronal circuits.
For example, do neurons specifically connect to others respecting functional properties, or is the network haphazardly connected with respect to function? In the visual cortex, the question might be whether neurons with similar orientation, direction, or spatial preferences preferentially synapse with one another. In the retina, it may be how the function of output neurons - retinal ganglion cells - is produced by connections from bipolar or amacrine cells, specifically selected from other neurons of the same class with overlapping arbors. Here, we review recent reports examining structure-function relationships in neuronal networks. We focus mainly on the mammalian visual system where sensory physiology has been combined with techniques for assessing connectivity: in vitro electrophysiology and large-scale electron microscopy (EM).
Connectivity and function in cortical circuits
Although some have argued that the local cortical network is for the most part randomly connected, other than topographic specificity [1], detailed examination of cortical connectivity supports the idea that synaptic connectivity is specific at several different levels. Although at least one axonal and dendritic apposition is observed for every pair of pyramidal neurons sharing the same cortical column in somatosensory cortex, only a fraction of appositions have synaptic boutons reflecting synaptic connectivity [2,3]. Similarly, neurons are synaptically connected to only a small fraction of their neighbors, and such synaptic connections are specific to both cell types and sub-cellular compartments, and thus have cell-type specificity [4–8]. Therefore, whereas the potential connectivity between neurons in the cortex is dense, the actual synaptic connections are sparse and likely may be functionally specific. Finally, statistical analysis of simultaneous recordings between a seemingly homogeneous population of layer 5 (L5) pyramidal cells has shown that connections are far more clustered than expected from a random network [9,10] suggesting the presence of highly interconnected sub-networks embedded in local circuits. Indeed, lateral connections in L2/3 are frequently observed only between cells that received common inputs from L4 [11] and connections between L5 pyramidal cells are dependent on their long-range targets [12]. Unfortunately, we still do not understand how the specificity of these connections underlies information processing in vivo.
Until recently, study of the relationship between identified neurons and their in vivo function has only been possible one cell at a time. Most commonly, the physiology of individual cortical neurons was recorded, and neurons were labeled to determine their axonal and dendritic morphology. With this approach, the general features of cortical microcircuits were described [13–19]. A new combination of technologies is poised to build upon this foundation.
The advent of optical methods to record the activity from populations of neurons at cellular resolution in vivo, allows investigators to simultaneously interrogate the functional properties of hundreds or thousands of cells [20–22]. Much of this recent work has focused on functional organization of neurons at cellular resolution [22–31], accompanied by more recent attempts at examining the functional properties of different cell types [32,33*,34*,35*]. A logical next step is to examine the fine-scale connectivity underlying these local circuits. In vivo multi-photon calcium imaging is ideal for studying functional organization and cell type-specific physiology because its inherent spatial information provides the ability to precisely locate and identify cells with known physiological properties. This also makes the technique well suited to being combined with several different approaches to measure network connectivity.
Excitatory functional connectivity
While the study of cortical connectivity may at the surface seem an anatomical endeavor, in vitro physiology has been the most effective approach in recent years. Due to the relative ease of the experiments and the strong signal-to-noise ratio of the data, dual-cell recordings have become a fast and reliable method of assessing synaptic connectivity [36]. Indeed, most of what we know about the probabilities of connectivity - the likelihood that any pair of neurons exhibit the electrophysiological signature of a synapse - within and between cortical layers was determined by paired recordings between known cell types [4–8,12,37,38].
Recently, Ko and colleagues combined in vivo calcium imaging with subsequent recordings in vitro to ask if neurons with similar functional properties were preferentially connected [39**]. They used simultaneous whole-cell patch-clamp recordings in acute slice preparations to test for monosynaptic connections between pairs of neighboring visual cortical neurons whose receptive fields had been mapped prior to tissue sectioning. Nearby neurons with similar orientation selectivities exhibited a connection probability of 0.38, whereas neurons whose orientation preference were dissimilar (i.e., nearly orthogonal) had a connection probability of 0.17. Moreover, neurons with highly correlated stimulus-driven activity were connected 50% of the time. This is in contrast to conventional slice recordings, blind to in vivo function, that typically find 1 in 10 nearby pairs of pyramidal cell connected in the superficial layers of the cortex [4,37]. Taken together, these results suggest that subnetworks are embedded in the visual cortex and that some of their connectivity is related to processing similar features, or to correlated activity. Although very powerful, slice recordings suffer from a few shortcomings. First, connectivity testing is limited to the volume of the slice. More importantly, the number of cells from which one can record is limited by the health of the slice over time and the technical difficulties of simultaneous electrophysiological recordings. In comparison, several emerging anatomical circuit tracing methods may offer some complementary advantages.
Circuit connectivity by large-scale electron microscopy
Since its origins in the 1950s, serial-section EM [40] has proven the only way to image all the dense neural tissue of the central nervous system. With increases in computing power, high-speed data acquisition, and laboratory automation, there has been a resurgence of interest in the method [41–47]. It is now possible to extend this fine-scale analysis of neural tissue—in which virtually every single axon, dendrite and synapse can be visualized—to large volumes: circuit-scale ultrastructural anatomy. Previous reviews have previewed technology and methods for large-scale EM [42,43,45] and championed development of the methodology and what might be gleaned [46,47]. For the remainder of this review, we focus on recent applications of such techniques and their results in the mammalian retina and cerebral cortex.
The retinal network anatomy of direction selectivity
In the retina, the brain’s window on the world, a wealth of information processing occurs. These are reviewed in this issue by Azeredo da Silveira and Roska [48] and Taylor and Smith [49], so we focus on one aspect - direction selectivity - that has received renewed attention with newer network mapping methods. Direction selectivity in some ganglion cells is one of the clearest examples of complex information processing that occurs in the retina. Direction selective ganglion cells in the retina exhibit preferential activity for motion of bars in a particular direction [50]. Mechanisms underlying this phenomenon have long been investigated and point toward inhibition of direction selective ganglion cells by starburst amacrine cells as a key component [51–53]. Starburst amacrine cells are retinal interneurons with radially symmetric stellate morphology that have varicosities on their distal processes contacting direction selective ganglion cells. Centrifugal motion along their processes activates these dendrites [54] suggesting that starburst cells can inhibit ganglion cells when the direction of motion is away from the starburst’s cell body. This inhibition, opposite the preferred direction of the ganglion cell’s, is thought to be a major mechanism underlying driving direction selectivity in the retina.
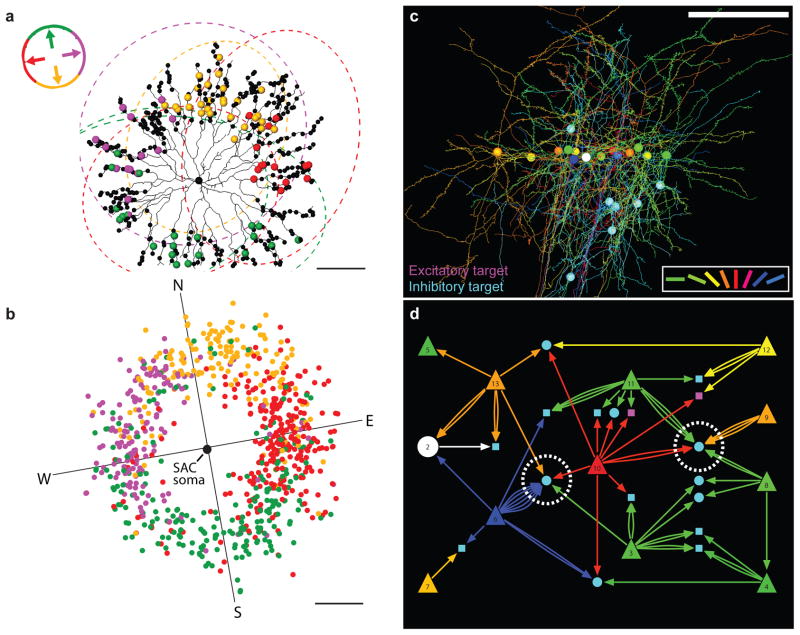
Two great advantages of the retina are that its visual physiology can be studied thoroughly in vitro [55] and it has a relatively orderly, laminated anatomy with well-defined cell classes [56]. Historically, many have exploited this accessibility and anatomical simplicity, including two groups who have recently used these advantages to investigate its fine-scale network anatomy [57,58*,59**]. Briggman and colleagues recently provided circuit-scale ultrastructural evidence that direction selectivity can be derived from wiring between these cell-types with functional specificity [59**]. By combining two-photon calcium imaging and serial block-face scanning EM they show that starburst amacrine cells selectively target most of their putative inhibitory synapses onto direction selective ganglion cells of the appropriate preferred direction. That is to say that, despite their morphological symmetry, they have specific asymmetrical functional connectivity whereby their distal inhibitory synapses target ganglion cells with the opposite the preferred direction of motion of the starburst neurite (Figure 1a,b). This demonstrates that inhibitory afferents can have exquisite synaptic specificity with a critical spatial component to shape cell physiology.
Figure 1.
Inhibitory specificity and randomness in visual circuits, as demonstrated with functional network anatomy. a, An example of an inhibitory starburst amacrine cell (black skeleton), with presynaptic varicosities (small black dots). Synapses onto dendrites of direction-selective ganglion cells (outlined with dashes) are indicated by large dots, colour-coded by the preferred direction (key, top left). b, The locations of synapses onto direction-selective ganglion cells (colour-coded as in a), plotted relative to the somatas of 24 starburst amacrine cells (SAC). Scale bars, 50 μm. Ganglion cells receive input from SAC dendrites that radiate from the SAC in the direction opposite to the ganglion cell’s preferred direction. c, Three-dimensional rendering of the dendrites, axons and cell bodies of functionally characterized visual cortical neurons (coloured according to their orientation preference; key, lower right), along with postsynaptic inhibitory targets (cyan) that receive convergent input from multiple functionally characterized neurons. Scale bar, 100 μm. d, A subset of the network diagram showing the connections in c. Presynaptic pyramidal cells are triangles colour-coded by preferred orientation (as in c). Multiple arrows indicate several synapses from the same pyramidal cell. Postsynaptic targets that could be traced to cell bodies are drawn as circles; post-synaptic dendritic fragments are drawn as squares. Dashed white circles highlight interneuron targets that received multiple excitatory inputs with a broad range of preferred orientations (a, b reproduced with permission from [59**] and c, d from [68**]).
Recently, the early postnatal development of directionally-selective ganglion cells was closely examined in two studies that demonstrated a rapid and activity-independent development of asymmetric inhibition from starburst amacrine cells [60*,61*]. In addition to electrophysiological recording, one group mapped connectivity with two promising techniques: photostimulation and virally mediated trans-synaptic tracing to demonstrate direct connectivity between direction selective ganglion cells and starburst amacrine cells [61*]. This viral technique uses a replication-deficient virus to specifically label only neurons that provide synaptic input to an isolated target cell in which the deficient viral components are expressed [62–64]. The anatomical approach in particular will likely prove powerful in cortical experiments in which the in vivo physiology of a single neuron and all its presynaptic inputs could be compared.
Cortical network anatomy and the functional role of interneurons
The adult visual cortex is comprised of two broad classes of neurons 80% of which are excitatory pyramidal cells with the remainder inhibitory interneurons. Visually responsive pyramidal cells are generally highly selective for specific stimulus properties. Currently, there is debate in the field over the tuning properties of inhibitory interneurons. Recent studies have found inhibitory interneurons generally appear much more broadly tuned than pyramidal cells [32,33*,65,66]. Several others, however, report different interneuron subtypes with quite selective tuning [34*,67], while still others described examples of intermediate selectivity [35*].
Is it possible that the underlying cortical network’s anatomy might explain interneuron receptive field properties? Bock and colleagues addressed this question, while providing a framework for untangling cortical wiring by combining in vivo two-photon calcium imaging and large-scale serial-section transmission EM [68**]. This study found that synapses from pyramidal neurons converged onto interneurons in their general vicinity irrespective of presynaptic function (Figure 1c,d). Pairs of pyramidal neurons with different preferred orientations were found to converge input onto inhibitory targets with the same probability as pairs of inputs with the same preferred orientation. This result is consistent with haphazard inhibitory interneuron connectivity in light of the recent demonstration that Martinotti cells (an interneuron subtype) appear to provide synaptic input to nearly every local pyramidal cell in the superficial layers of the cortex [69*] and with the convergence of pyramidal output onto fast-spiking putative interneurons in other regions of the neocortex [37,70]. Thus, evidence from studies of connectivity are consistent with most interneurons receiving a broad array of differently tuned input, which would combine non-specifically to control the gain of excitation in a local circuit [33*].
Conclusions
The functional connectivity of mammalian neural circuits appears to span from specific to haphazard. Excitatory pyramidal cells in the visual cortex and inhibitory interneurons in the retina exhibit functionally specific connectivity, whereas inhibitory wiring in the cortex appears less so. What does this connectivity tell us about the function of circuits and their components? Broadly speaking, a circuit’s structure develops to suit its purpose. Unlike the cortex, the retina, with its orderly tiling of cell types, appears more like a mosaic of individual processing units with minimal cross-talk between the same cell types, so that computations are localized to discrete retinotopic locations. A lattice of parallel processing pathways may demand specific connections within processing units to maintain order and efficiency. In the cerebral cortex, however, information being processed becomes more complex and integrated. It is therefore reasonable that functionally specific connections exists between excitatory neurons - the main thoroughfare for information processing - but that inhibitory interneurons, which largely appear to regulate local gain of a circuit and network oscillations, are promiscuous in their local wiring.
One benchmark of our understanding of network information processing is the extent to which we can predict neuronal function from connectivity. Attempts to do so with the wiring diagram of C. elegans [71] have not seemingly borne this out, but critically missing was a rich literature of single-cell physiology. Recent work using large-scale EM in mammalian systems has demonstrated that structure appears to confirm predictions from function in systems with well-understood physiology. This is reassuring, as it suggests that we may soon have sufficient understanding of how information flows and is transformed through some networks to make accurate predictions of their function from circuit structure.
Ultimately, a variety of approaches - and likely a synergy of methods - will be employed to reach a satisfying understanding of how neural circuits process information. Circuit-scale EM is now amongst the tools available [41,57,58*,59**,68**,72]. EM datasets and their analysis will get ever larger and richer. This richness can be in the form of more function [59**,68**,73–75], correlated labeling for cell identity or biochemical analysis [57,58*,76,77], EM dense labeling [78–80] for information on long-range connections, and more complete 3D segmentation [45,81,82] for volumetric properties like synaptic strength as correlated to synapse size [83–85].
Large-scale EM datasets should also serve as resources that can be mined by multiple investigators. The EM dataset from Bock and colleagues is publicly available online from the UCSD Cell Centered Database [86] (http://ccdb.ucsd.edu/, accession number 8448) and browsable [87] from Open Connectome Project at Johns Hopkins University (http://openconnectomeproject.org/). A potentially useful analogy is with that of a perpetual slice experiment where investigators can test for synaptic connectivity between any cells in the volume. Higher-order patterns of connectivity can be found in such datasets as the reconstruction becomes increasingly dense, generating many-to-many graphs of network connectivity, currently unachievable with other methods. Finally, recently acquired EM datasets demonstrate our coming ability to routinely image at synaptic resolution the entire nervous systems of model organisms such as C. elegans, Drosophila, and zebrafish. Thus, with recent advances in imaging techniques—in vivo two-photon calcium imaging and large-scale serial-section EM—as well as traditional slice physiology and sophisticated transynaptic tracing methods the stage is set to get a functional and structural understanding of neuronal networks. Very recent reports show additional examples of broadly tuned interneurons in the adult visual cortex [88,89] and describe a new suite of annotation tools that will likely accelerate manual reconstruction of large-scale EM datasets with high-fidelity [90].
Highlights.
Reviews recent reports on structure-function relationships in neural networks
Combinations of two-photon calcium imaging with electrophysiological mapping or large-scale EM
Functionally specific excitatory connections in the cortex
Functionally specific inhibitory connections in the retina
Functionally haphazard inhibitory connections in the cortex
Acknowledgments
We are extremely grateful to Davi Bock, for valuable discussions, insight, and for our longstanding collaboration on network anatomy. We thank Mark Andermann, Vincent Bonin, Lindsey Glickfeld, David Hildebrand, and Aaron Kerlin for comments on the manuscript. This work was supported by the Center for Brain Science at Harvard University, Microsoft Research, and the National Institutes of Health through the National Eye Institute to R.C.R. (EY10115 and EY18742) and W.-C.A.L. (EY18532).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Wei-Chung Allen Lee, Email: wei-chung_lee@hms.harvard.edu.
R. Clay Reid, Email: clay_reid@hms.harvard.edu.
References and recommended reading
- 1.Braitenberg V, Schüz A. Cortex: statistics and geometry of neuronal connectivity. 2. Berlin; New York: Springer; 1998. thoroughly rev. [Google Scholar]
- 2.Kalisman N, Silberberg G, Markram H. The neocortical microcircuit as a tabula rasa. Proc Natl Acad Sci U S A. 2005;102:880–885. doi: 10.1073/pnas.0407088102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stepanyants A, Chklovskii DB. Neurogeometry and potential synaptic connectivity. Trends Neurosci. 2005;28:387–394. doi: 10.1016/j.tins.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Mason A, Nicoll A, Stratford K. Synaptic transmission between individual pyramidal neurons of the rat visual cortex in vitro. J Neurosci. 1991;11:72–84. doi: 10.1523/JNEUROSCI.11-01-00072.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markram H, Lubke J, Frotscher M, Roth A, Sakmann B. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J Physiol. 1997;500 ( Pt 2):409–440. doi: 10.1113/jphysiol.1997.sp022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomson AM, Deuchars J. Synaptic interactions in neocortical local circuits: dual intracellular recordings in vitro. Cereb Cortex. 1997;7:510–522. doi: 10.1093/cercor/7.6.510. [DOI] [PubMed] [Google Scholar]
- 7.Feldmeyer D, Lubke J, Silver RA, Sakmann B. Synaptic connections between layer 4 spiny neurone-layer 2/3 pyramidal cell pairs in juvenile rat barrel cortex: physiology and anatomy of interlaminar signalling within a cortical column. The Journal of physiology. 2002;538:803–822. doi: 10.1113/jphysiol.2001.012959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomson AM, Bannister AP. Interlaminar connections in the neocortex. Cereb Cortex. 2003;13:5–14. doi: 10.1093/cercor/13.1.5. [DOI] [PubMed] [Google Scholar]
- 9.Song S, Sjostrom PJ, Reigl M, Nelson S, Chklovskii DB. Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS Biol. 2005;3:e68. doi: 10.1371/journal.pbio.0030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perin R, Berger TK, Markram H. A synaptic organizing principle for cortical neuronal groups. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5419–5424. doi: 10.1073/pnas.1016051108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshimura Y, Dantzker JL, Callaway EM. Excitatory cortical neurons form fine-scale functional networks. Nature. 2005;433:868–873. doi: 10.1038/nature03252. [DOI] [PubMed] [Google Scholar]
- 12.Brown SP, Hestrin S. Intracortical circuits of pyramidal neurons reflect their long-range axonal targets. Nature. 2009;457:1133–1136. doi: 10.1038/nature07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly JP, Van Essen DC. Cell structure and function in the visual cortex of the cat. The Journal of physiology. 1974;238:515–547. doi: 10.1113/jphysiol.1974.sp010541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert CD, Wiesel TN. Morphology and intracortical projections of functionally characterized neurons in the cat visual cortex. Nature. 1979;280:120–125. doi: 10.1038/280120a0. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert CD, Wiesel TN. Intrinsic connectivity and receptive field properties in visual cortex. Vision research. 1985;25:365–374. doi: 10.1016/0042-6989(85)90061-6. [DOI] [PubMed] [Google Scholar]
- 16.Douglas RJ, Martin KA. A functional microcircuit for cat visual cortex. J Physiol. 1991;440:735–769. doi: 10.1113/jphysiol.1991.sp018733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Binzegger T, Douglas RJ, Martin KA. A quantitative map of the circuit of cat primary visual cortex. J Neurosci. 2004;24:8441–8453. doi: 10.1523/JNEUROSCI.1400-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin KA. Microcircuits in visual cortex. Curr Opin Neurobiol. 2002;12:418–425. doi: 10.1016/s0959-4388(02)00343-4. [DOI] [PubMed] [Google Scholar]
- 19.Douglas RJ, Martin KA. Neuronal circuits of the neocortex. Annu Rev Neurosci. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- 20.Stosiek C, Garaschuk O, Holthoff K, Konnerth A. In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci U S A. 2003;100:7319–7324. doi: 10.1073/pnas.1232232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerr JN, Greenberg D, Helmchen F. Imaging input and output of neocortical networks in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14063–14068. doi: 10.1073/pnas.0506029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohki K, Chung S, Ch’ng YH, Kara P, Reid RC. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature. 2005;433:597–603. doi: 10.1038/nature03274. [DOI] [PubMed] [Google Scholar]
- 23.Ohki K, Chung S, Kara P, Hubener M, Bonhoeffer T, Reid RC. Highly ordered arrangement of single neurons in orientation pinwheels. Nature. 2006;442:925–928. doi: 10.1038/nature05019. [DOI] [PubMed] [Google Scholar]
- 24.Kerr JN, de Kock CP, Greenberg DS, Bruno RM, Sakmann B, Helmchen F. Spatial organization of neuronal population responses in layer 2/3 of rat barrel cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:13316–13328. doi: 10.1523/JNEUROSCI.2210-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato TR, Gray NW, Mainen ZF, Svoboda K. The functional microarchitecture of the mouse barrel cortex. PLoS biology. 2007;5:e189. doi: 10.1371/journal.pbio.0050189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stettler DD, Axel R. Representations of odor in the piriform cortex. Neuron. 2009;63:854–864. doi: 10.1016/j.neuron.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Dombeck DA, Graziano MS, Tank DW. Functional clustering of neurons in motor cortex determined by cellular resolution imaging in awake behaving mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:13751–13760. doi: 10.1523/JNEUROSCI.2985-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bandyopadhyay S, Shamma SA, Kanold PO. Dichotomy of functional organization in the mouse auditory cortex. Nature neuroscience. 2010;13:361–368. doi: 10.1038/nn.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dombeck DA, Harvey CD, Tian L, Looger LL, Tank DW. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nature neuroscience. 2010;13:1433–1440. doi: 10.1038/nn.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothschild G, Nelken I, Mizrahi A. Functional organization and population dynamics in the mouse primary auditory cortex. Nature neuroscience. 2010;13:353–360. doi: 10.1038/nn.2484. [DOI] [PubMed] [Google Scholar]
- 31.Komiyama T, Sato TR, O’Connor DH, Zhang YX, Huber D, Hooks BM, Gabitto M, Svoboda K. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature. 2010;464:1182–1186. doi: 10.1038/nature08897. [DOI] [PubMed] [Google Scholar]
- 32.Sohya K, Kameyama K, Yanagawa Y, Obata K, Tsumoto T. GABAergic neurons are less selective to stimulus orientation than excitatory neurons in layer II/III of visual cortex, as revealed by in vivo functional Ca2+ imaging in transgenic mice. J Neurosci. 2007;27:2145–2149. doi: 10.1523/JNEUROSCI.4641-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.Kerlin AM, Andermann ML, Berezovskii VK, Reid RC. Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron. 2010;67:858–871. doi: 10.1016/j.neuron.2010.08.002. Using in vivo two-photon microscopy and post-mortem immunohistochemistry, these authors found the majority of inhibitory neurons in visual cortex—of several different subclasses—were far less selective for stimulus orientation than were pyramidal cells. Some interneurons had a small orientation bias, but tended to be embedded in a neighborhood of cells with an average similar orientation bias, further suggesting that inhibitory neurons receive excitation haphazardly from the local pool of pyramidal cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34*.Runyan CA, Schummers J, Van Wart A, Kuhlman SJ, Wilson NR, Huang ZJ, Sur M. Response features of parvalbumin-expressing interneurons suggest precise roles for subtypes of inhibition in visual cortex. Neuron. 2010;67:847–857. doi: 10.1016/j.neuron.2010.08.006. Using in vivo two-photon calcium imaging and mice with parvalbumin-positive fast-spiking cells that were transgenically labeled, these authors conclude that this interneuron subtype is nearly as stimulus-selective as pyramidal cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Zariwala HA, Madisen L, Ahrens KF, Bernard A, Lein ES, Jones AR, Zeng H. Visual tuning properties of genetically identified layer 2/3 neuronal types in the primary visual cortex of cre-transgenic mice. Frontiers in systems neuroscience. 2011;4:162. doi: 10.3389/fnsys.2010.00162. These authors show examples of genetically labeled interneuron subtypes with intermediate selectivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Debanne D, Boudkkazi S, Campanac E, Cudmore RH, Giraud P, Fronzaroli-Molinieres L, Carlier E, Caillard O. Paired-recordings from synaptically coupled cortical and hippocampal neurons in acute and cultured brain slices. Nature protocols. 2008;3:1559–1568. doi: 10.1038/nprot.2008.147. [DOI] [PubMed] [Google Scholar]
- 37.Holmgren C, Harkany T, Svennenfors B, Zilberter Y. Pyramidal cell communication within local networks in layer 2/3 of rat neocortex. J Physiol. 2003;551:139–153. doi: 10.1113/jphysiol.2003.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lefort S, Tomm C, Floyd Sarria JC, Petersen CC. The excitatory neuronal network of the C2 barrel column in mouse primary somatosensory cortex. Neuron. 2009;61:301–316. doi: 10.1016/j.neuron.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 39**.Ko H, Hofer SB, Pichler B, Buchanan KA, Sjostrom PJ, Mrsic-Flogel TD. Functional specificity of local synaptic connections in neocortical networks. Nature. 2011 doi: 10.1038/nature09880. The authors examined the relationship between function and connectivity in the visual cortex, using multiple whole-cell patch-clamp recording from pyramidal cells that had been functionally characterized in vivo with two-photon calcium imaging. To our knowledge, this is the first demonstration that nearby excitatory neurons in the cortex are more likely to be connected if they have similar sensory physiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sjostrand FS. Ultrastructure of retinal rod synapses of the guinea pig eye as revealed by three-dimensional reconstructions from serial sections. Journal of ultrastructure research. 1958;2:122–170. doi: 10.1016/s0022-5320(58)90050-9. [DOI] [PubMed] [Google Scholar]
- 41.Denk W, Horstmann H. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol. 2004;2:e329. doi: 10.1371/journal.pbio.0020329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Briggman KL, Denk W. Towards neural circuit reconstruction with volume electron microscopy techniques. Curr Opin Neurobiol. 2006;16:562–570. doi: 10.1016/j.conb.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 43.Smith SJ. Circuit reconstruction tools today. Current opinion in neurobiology. 2007;17:601–608. doi: 10.1016/j.conb.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knott G, Marchman H, Wall D, Lich B. Serial section scanning electron microscopy of adult brain tissue using focused ion beam milling. J Neurosci. 2008;28:2959–2964. doi: 10.1523/JNEUROSCI.3189-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Helmstaedter M, Briggman KL, Denk W. 3D structural imaging of the brain with photons and electrons. Curr Opin Neurobiol. 2008;18:633–641. doi: 10.1016/j.conb.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 46.Lichtman JW, Sanes JR. Ome sweet ome: what can the genome tell us about the connectome? Curr Opin Neurobiol. 2008;18:346–353. doi: 10.1016/j.conb.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seung HS. Reading the book of memory: sparse sampling versus dense mapping of connectomes. Neuron. 2009;62:17–29. doi: 10.1016/j.neuron.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 48.Azeredo da Silveira R, Roska B. Cell Types, Circuits, Computation. Current opinion in neurobiology. 2011 doi: 10.1016/j.conb.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 49.Taylor WR, Smith RG. Trigger Features and Excitation in the Retina. Current opinion in neurobiology. 2011 doi: 10.1016/j.conb.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barlow HB, Hill RM. Selective sensitivity to direction of movement in ganglion cells of the rabbit retina. Science. 1963;139:412–414. doi: 10.1126/science.139.3553.412. [DOI] [PubMed] [Google Scholar]
- 51.Barlow HB, Levick WR. The mechanism of directionally selective units in rabbit’s retina. The Journal of physiology. 1965;178:477–504. doi: 10.1113/jphysiol.1965.sp007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fried SI, Munch TA, Werblin FS. Mechanisms and circuitry underlying directional selectivity in the retina. Nature. 2002;420:411–414. doi: 10.1038/nature01179. [DOI] [PubMed] [Google Scholar]
- 53.Lee S, Kim K, Zhou ZJ. Role of ACh-GABA cotransmission in detecting image motion and motion direction. Neuron. 2010;68:1159–1172. doi: 10.1016/j.neuron.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Euler T, Detwiler PB, Denk W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature. 2002;418:845–852. doi: 10.1038/nature00931. [DOI] [PubMed] [Google Scholar]
- 55.Field GD, Gauthier JL, Sher A, Greschner M, Machado TA, Jepson LH, Shlens J, Gunning DE, Mathieson K, Dabrowski W, et al. Functional connectivity in the retina at the resolution of photoreceptors. Nature. 2010;467:673–677. doi: 10.1038/nature09424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sterling P. Microcircuitry of the cat retina. Annu Rev Neurosci. 1983;6:149–185. doi: 10.1146/annurev.ne.06.030183.001053. [DOI] [PubMed] [Google Scholar]
- 57.Anderson JR, Jones BW, Yang JH, Shaw MV, Watt CB, Koshevoy P, Spaltenstein J, Jurrus E, UVK, Whitaker RT, et al. A computational framework for ultrastructural mapping of neural circuitry. PLoS Biol. 2009;7:e1000074. doi: 10.1371/journal.pbio.1000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58*.Anderson JR, Jones BW, Watt CB, Shaw MV, Yang JH, Demill D, Lauritzen JS, Lin Y, Rapp KD, Mastronarde D, et al. Exploring the retinal connectome. Molecular vision. 2011;17:355–379. Transmission EM acquisition and 3D volume generation were automated to produce a large, high-resolution EM dataset through rabbit retina with cell-type and activity markers. The authors focus on reconstructing network motifs involving amacrine cells. [PMC free article] [PubMed] [Google Scholar]
- 59**.Briggman KL, Helmstaedter M, Denk W. Wiring specificity in the direction-selectivity circuit of the retina. Nature. 2011;471:183–188. doi: 10.1038/nature09818. Combining two-photon calcium imaging and serial block face scanning EM, these authors demonstrate the functional specificity of starburst amacrine cell to direction selective ganglion cell synapses. This is arguably the strongest evidence to date for this mechanism of specific inhibitory connectivity underlying of direction selectivity in the retina. [DOI] [PubMed] [Google Scholar]
- 60*.Wei W, Hamby AM, Zhou K, Feller MB. Development of asymmetric inhibition underlying direction selectivity in the retina. Nature. 2011;469:402–406. doi: 10.1038/nature09600. These authors show that the strength of inhibitory inputs from mouse starburst amacrine cells onto direction selective ganglion cells selectively increases between the first and second postnatal weeks, and that the development of this inhibitory asymmetry emerges even in the presence of activity blockers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61*.Yonehara K, Balint K, Noda M, Nagel G, Bamberg E, Roska B. Spatially asymmetric reorganization of inhibition establishes a motion-sensitive circuit. Nature. 2011;469:407–410. doi: 10.1038/nature09711. These authors combine electrophysiological recordings, photostimulation, and monosynaptic viral tracing to show that the inhibitory wiring between mouse starburst amacrine cells and direction selective ganglion cells is symmetrical up to postnatal day 6, but is subsequently rewired asymmetrically within just two days. [DOI] [PubMed] [Google Scholar]
- 62.Wickersham IR, Lyon DC, Barnard RJ, Mori T, Finke S, Conzelmann KK, Young JA, Callaway EM. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007;53:639–647. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marshel JH, Mori T, Nielsen KJ, Callaway EM. Targeting single neuronal networks for gene expression and cell labeling in vivo. Neuron. 2010;67:562–574. doi: 10.1016/j.neuron.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rancz EA, Franks KM, Schwarz MK, Pichler B, Schaefer AT, Margrie TW. Transfection via whole-cell recording in vivo: bridging single-cell physiology, genetics and connectomics. Nature neuroscience. 2011;14:527–532. doi: 10.1038/nn.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Niell CM, Stryker MP. Highly selective receptive fields in mouse visual cortex. J Neurosci. 2008;28:7520–7536. doi: 10.1523/JNEUROSCI.0623-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu BH, Li P, Li YT, Sun YJ, Yanagawa Y, Obata K, Zhang LI, Tao HW. Visual receptive field structure of cortical inhibitory neurons revealed by two-photon imaging guided recording. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:10520–10532. doi: 10.1523/JNEUROSCI.1915-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma WP, Liu BH, Li YT, Huang ZJ, Zhang LI, Tao HW. Visual representations by cortical somatostatin inhibitory neurons--selective but with weak and delayed responses. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:14371–14379. doi: 10.1523/JNEUROSCI.3248-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68**.Bock DD, Lee WC, Kerlin AM, Andermann ML, Hood G, Wetzel AW, Yurgenson S, Soucy ER, Kim HS, Reid RC. Network anatomy and in vivo physiology of visual cortical neurons. Nature. 2011;471:177–182. doi: 10.1038/nature09802. Combining in vivo two-photon calcium imaging and high-throughput serial-section transmission electron microscopy in the visual cortex, the authors show that when pyramidal cells converge onto a common inhibitory target, the inputs can have a broad range of different stimulus-selective responses. The result is consistent with a model of functionally non-specific inhibition in rodent visual cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69*.Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron. 2011;69:1188–1203. doi: 10.1016/j.neuron.2011.02.025. The authors used two-photon uncaging of glutamate in cortical brain slices to demonstrate that somatostatin-positive Martinotti cells have synaptic connections to and from virtually all local pyramidal cells. Therefore, this class of interneurons likely provides functionally non-specific inhibition to the local circuit. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fujisawa S, Amarasingham A, Harrison MT, Buzsaki G. Behavior-dependent short-term assembly dynamics in the medial prefrontal cortex. Nature neuroscience. 2008;11:823–833. doi: 10.1038/nn.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil Trans Royal Soc London B. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 72.Hayworth K, Kasthuri N, Schalek R, Lichtman J. Automating the Collection of Ultrathin Serial Sections for Large Volume TEM Reconstructions. Microsc and Microanal. 2006:86–87. [Google Scholar]
- 73.Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat Methods. 2005;2:932–940. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- 74.Grewe BF, Helmchen F. Optical probing of neuronal ensemble activity. Curr Opin Neurobiol. 2009;19:520–529. doi: 10.1016/j.conb.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 75.Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Micheva KD, Smith SJ. Array tomography: a new tool for imaging the molecular architecture and ultrastructure of neural circuits. Neuron. 2007;55:25–36. doi: 10.1016/j.neuron.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Micheva KD, Busse B, Weiler NC, O’Rourke N, Smith SJ. Single-synapse analysis of a diverse synapse population: proteomic imaging methods and markers. Neuron. 2010;68:639–653. doi: 10.1016/j.neuron.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagiel A, Andor-Ardo D, Hudspeth AJ. Specificity of afferent synapses onto plane-polarized hair cells in the posterior lateral line of the zebrafish. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:8442–8453. doi: 10.1523/JNEUROSCI.2425-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li J, Erisir A, Cline H. In vivo time-lapse imaging and serial section electron microscopy reveal developmental synaptic rearrangements. Neuron. 2011;69:273–286. doi: 10.1016/j.neuron.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shu X, Lev-Ram V, Deerinck TJ, Qi Y, Ramko EB, Davidson MW, Jin Y, Ellisman MH, Tsien RY. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS biology. 2011;9:e1001041. doi: 10.1371/journal.pbio.1001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chklovskii DB, Vitaladevuni S, Scheffer LK. Semi-automated reconstruction of neural circuits using electron microscopy. Current opinion in neurobiology. 2010;20:667–675. doi: 10.1016/j.conb.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 82.Jain V, Seung HS, Turaga SC. Machines that learn to segment images: a crucial technology for connectomics. Curr Opin Neurobiol. 2010;20:653–666. doi: 10.1016/j.conb.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harris KM, Stevens JK. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1989;9:2982–2997. doi: 10.1523/JNEUROSCI.09-08-02982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nusser Z, Lujan R, Laube G, Roberts JD, Molnar E, Somogyi P. Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron. 1998;21:545–559. doi: 10.1016/s0896-6273(00)80565-6. [DOI] [PubMed] [Google Scholar]
- 85.Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nature neuroscience. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martone ME, Gupta A, Wong M, Qian X, Sosinsky G, Ludascher B, Ellisman MH. A cell-centered database for electron tomographic data. Journal of structural biology. 2002;138:145–155. doi: 10.1016/s1047-8477(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 87.Saalfeld S, Cardona A, Hartenstein V, Tomancak P. CATMAID: collaborative annotation toolkit for massive amounts of image data. Bioinformatics. 2009;25:1984–1986. doi: 10.1093/bioinformatics/btp266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hofer SB, Ko H, Pichler B, Vogelstein J, Ros H, Zeng H, Lein E, Lesica NA, Mrsic-Flogel TD. Differential connectivity and response dynamics of excitatory and inhibitory neurons in visual cortex. Nat Neurosci. 2011;14:1045–1052. doi: 10.1038/nn.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuhlman SJ, Tring E, Trachtenberg JT. Fast-spiking interneurons have an initial orientation bias that is lost with vision. Nat Neurosci. 2011 doi: 10.1038/nn.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Helmstaedter M, Briggman KL, Denk W. High-accuracy neurite reconstruction for high-throughput neuroanatomy. Nat Neurosci. 2011;14:1081–1088. doi: 10.1038/nn.2868. [DOI] [PubMed] [Google Scholar]