Abstract
OBJECTIVES
To investigate whether sustained long-term separate treatments of diabetic inducible nitric oxide synthase knockout (iNOSKo) mice with allopurinol, an antioxidant inhibiting xanthine oxidoreductase, decorin, a transforming growth factor-β1 (TGFβ1) -binding antagonist, and molsidomine, a long-life nitric oxide donor, prevent the processes of diabetes-induced cavernosal fibrosis.
METHODS
iNOSKo mice were divided into groups and treated
Blood chemistry and histopathology were investigated.
RESULTS
Eight-week treatment with either allopurinol or decorin counteracted the decrease in smooth muscle cells and the increase in apoptosis and local oxidative stress within the corpora tissue.
Decorin but not allopurinol increased the smooth muscle cell/collagen ratio, whereas allopurinol but not decorin inhibited systemic oxidative stress.
Molsidomine was effective in reducing both local and systemic oxidative stress, but did not prevent corporal fibrosis.
CONCLUSION
Both allopurinol and decorin appear as promising approaches either as a single or a combined pharmacological modality for protecting the diabetic corpora from undergoing apoptosis and fibrosis although their functional effects still need to be defined.
Keywords: erectile dysfunction, smooth muscle, penis, collagen, nitric oxide, decorin, allopurinol, molsidomine, apoptosis
INTRODUCTION
It is well established both in human tissue and in experimental animal models that the combination of fibrosis and oxidative stress, either localized or diffuse, is the common pathophysiological denominator of the two major disorders affecting the penis, namely Peyronie’s disease [1,2]and the most common form of erectile dysfunction: corporal veno-occlusive dysfunction (CVOD) [2]. In the case of CVOD this occurs in conditions as varied as aging [3,4], types 1 and 2 diabetes mellitus [5,6], cavernosal nerve damage [7-10] and certain animal models of systemic hypertension [11]. The combined production of active TGF-β1, reactive oxygen species (ROS) and other profibrotic factors stimulates the excessive deposition of collagen and extracellular matrix by fibroblasts and myofibroblasts in the tunica albuginea and corpora cavernosa in Peyronie’s disease and CVOD, respectively. In CVOD, the corporal smooth muscle cells (SMC) also undergo a switch from the contractile phenotype to the synthetic phenotype, leading to deposition of extracellular matrix components. This is compounded by a loss of SMC, which leads to an impairment in the ability of the corporal tissue to undergo relaxation by the nitric oxide/cGMP pathway and the resulting passive occlusion of the subtunical veins egressing the corpora [12].
Another common denominator of both Peyronie’s disease and CVOD is the steady expression of inducible nitric oxide synthase (iNOS) by different cell types leading to the sustained generation of nitric oxide and cGMP that inhibit myofibroblast generation or SMC activation and collagen synthesis [12,13]. Nitric oxide also reduces the profibrotic effects of oxidative stress by quenching ROS, stimulates collagen degradation and protects the SMC, so in this scenario iNOS induction is considered to act as an antifibrotic mechanism. This role is supported by the inhibition of oxidative stress and fibrosis by iNOS gene transfer or long-term continuous administration of nitric oxide generators and phosphodiesterase 5 inhibitors [3,8-10,14,15] or by the exacerbation of these processes by chronic inhibition of iNOS activity by N-iminoethyl l-lysine [9,16,17]. Moreover, the genetic inactivation of iNOS expression in the iNOS knockout (iNOSKo) mouse leads per se to an increase in fibrosis and oxidative stress within the corporal tissue and both of these are further exacerbated in the presence of diabetes [18].
Experimental approaches to ameliorate this underlying fibrotic corporal histopathology induced by either diabetes or after a cavernosal nerve injury with phosphodiesterase 5 inhibitors [19-21] still require clinical validation. To increase the efficacy of such agents, it may be necessary to combine those that target different fibrotic pathways. One of the most obvious is the use of long half-life nitric oxide generators to mimic the effects of iNOS induction, e.g. molsidomine or SIN-10, an agent currently studied clinically as a vasodilator for the treatment of coronary artery disease and angina pectoris [22-24], and experimentally for its antifibrotic effects in the kidney and liver [25-27]. These have not been investigated so far for counteracting corporal fibrosis.
A second type of agent is an antioxidant that targets xanthine oxidoreductase (XOR), a critical enzyme involved in oxidative stress in the penis. One such example is allopurinol, widely used clinically [28,29], and having a potent experimental antifibrotic action not yet explored for erectile dysfunction [30-32]. Finally, agents that aim to inactivate TGF-β signalling, such as decorin, a proteoglycan endogenously expressed in many organs, that binds several members of the TGF-β super-family is being preclinically investigated as an antifibrotic agent in wound healing and kidney, heart and skeletal muscle fibrosis [33-38] but has not yet been used for the treatment of corporal fibrosis.
The streptozotocin-induced diabetic iNOSKo mouse model, which shows the impact of diabetes on the corpora cavernosa under conditions of iNOS deprivation, i.e. an exacerbation of corporal fibrosis [18], lends itself for the investigation of antifibrotic and antioxidant compounds with the potential for the prevention or reversal of this process. In the current study, we have tested in the diabetic iNOSKo mouse the effects of the continuous long-term separate administration of allopurinol, decorin and molsidomine on corporal fibrosis, oxidative stress and SMC turnover.
MATERIALS AND METHODS
All the experiments were approved by the Institutional Animal Care and Use Committee at our institution, and according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Four-month-old iNOSKo B6.129P2-Nos2tm1Lau/J (iNOSKo) mice were divided into the following groups and maintained for 8 weeks before being killed (n = 8 mice/group): (1) iNOSKo injected once i.p. with 150 mg/kg body weight streptozotocin (iNOSKo+STZ); (2) as #1 treated with 40 mg/kg/day allopurinol in the drinking water (iNOSKo +STZ+ALLO); (3) as #1 treated with 50 μg decorin per animal; i.p. twice a day (4 mg/kg/day) (iNOSKo +STZ+DECO); (4) as #1 treated with 5 mg/kg body weight molsidomine i.p. daily (iNOSKo +STZ+MOL).
Body weights were recorded weekly. Blood for glycaemia determination was withdrawn at baseline and then weekly under 3% isofluorane anaesthesia. Urine was collected from the urinary bladder under anaesthesia before killing. Mice were killed by a bolus administration of sodium pentobarbital. Blood for the determination of the ratio of reduced to oxidized glutathione (GSH/GGSG) was collected from the heart. Penises were rapidly excised, weighed and the shaft was denuded of skin, a mid-region was fixed in 10% formalin for tissue sectioning and the rest was frozen on dry ice and stored at − 80°C for further use.
Glycaemia was determined in serum by an Accu-Chek Active blood glucose meter (Roche, Dublin, Ireland), and urinary glucose, ketone bodies, specific gravity, pH, and protein were determined using a Multistix Dip Stick (Bayer, Leverkusen, Germany).
For the measurement of GSH/GSSG ratio [16], blood was collected with or without 1-methyl-2 vinylpyridinium trifluoromethane sulphonate (M2VP) scavenger of reduced glutathione, described in the commercial kit protocol (‘Bioxytech GSH/GSSG-412 kit’ from Oxis Health Products). The omission or addition of M2VP allows the measurement of reduced (GSH) and oxidized (GSSG) glutathione, respectively. The spectrophotometric detection was recorded at 412 nm for 3 min after the addition of 3.8 μmol NADPH. The GSH/GSSG ratio is inversely related to ROS levels.
Histochemistry and immunohistochemistry investigations used paraffin-embedded tissue sections (5 μm) for the following procedures [3,4,7-10]. (a) Masson trichrome staining for collagen (blue) and SMC (red); (b) immunodetection with: monoclonal antibody against α-smooth muscle actin (ASMA) as an SMC marker (Sigma kit, Sigma Diagnostics, St Louis, MO, USA); polyclonal antibody against TGF-β1 (1:200) (Promega, Madison, WI, USA), as profibrotic factor; monoclonal antibody against proliferating cell nuclear antigen (PCNA) as a marker of cell proliferation (1:400) (Chemicon, Temecula, CA, USA); and polyclonal antibody against XOR (1:5000; Abcam, Cambridge, UK), as a marker of oxidative stress. The specificity of the antibodies was validated by Western blot.
Briefly, tissue sections were treated with proteinase K (20 μg/mL), followed by quenching in 0.3% H2O2-PBS, blocked with goat serum (Vector Laboratories, Burlingame, CA, USA), and incubated overnight at 4°C with the primary antibody. In the case of PCNA and XOR, antigen retrieval was performed by boiling the slides for 3 min in an antigen unmasking solution (Vector Laboratories). After the overnight incubation with the first antibodies, sections were then incubated with biotinylated anti-mouse IgG (ASMA, PCNA), or biotinylated anti-rabbit IgG (TGF-β1, XOR), respectively, followed by ABC complex (Vector Laboratories) and 3,3’-diaminobenzidine (Sigma) (PCNA and iNOS), or with the ASMA Sigma kit (ASMA) and 3-amino-9-ethylcarbazole.
Terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay was performed as described previously [3,4,7-10] by applying the Apoptag peroxidase detection assay (Chemicon), with TdT enzyme and anti-digoxigenin-conjugated peroxidase, and 3,3’-diaminobenzidine/H2O2. Sections were counterstained with haematoxylin QS (Vector Laboratories). Negative controls in the immunohistochemical detections were performed by replacing the first antibody with IgG isotype. The negative control for TUNEL was made by substituting buffer for the TdT enzyme. Testicular tissue sections were used as positive controls for TUNEL.
Quantitative image analysis was performed by computerized densitometry using the ImagePro 4.01 program (Media Cybernetics, Silver Spring, MD, USA), coupled to a Leica B microscope equipped with a Spot RT digital camera (Diagnostic Instruments, Portland OR, USA) [1-7]. For Masson staining, 40 x magnification pictures of the whole penis were analysed for SMC (stained in red) and collagen (stained in blue), and expressed as SMC/collagen ratio. For ASMA and XOR staining, only the corpora cavernosa were analysed in a computerized grid and expressed as % of positive area vs total area of the corpora cavernosa. For PCNA and TUNEL determinations, the number of positive cells at 400 x was counted and results were expressed as % of positive cells/total cells in the corpora cavernosa. In all cases, four penile anatomically matched tissue sections were examined per animal at 40 x, with enough fields to cover the whole corpora cavernosa, and in certain cases at 400 x with eight fields per section, with eight animals per group.
Values were expressed as mean ± SEM. The normality distribution of the data was established using the Wilks–Shapiro test, followed by one-way anova and post-hoc comparisons with the Bonferroni test, according to the GraphPad Prism V 4.1. Differences were considered significant at P < 0.05.
RESULTS
The effects of iNOS deletion and of diabetes induction in the streptozotocin-injected iNOSKo mice have been described previously [18]. Briefly, iNOS deletion alone causes a reduction in the corporal SMC/collagen ratio and the SMC content when compared with the normal animals (wild-type; WT). In the iNOSKo mouse that then undergoes STZ-induced diabetes, there is a further reduction in the SMC/collagen ratio which is then completely prevented by insulin in the WT mice but only partially so in the iNOSKo mice.
In the current work, as expected, 8-week treatments of this STZ-induced diabetic iNOSKo model with allopurinol, decorin and molsidomine, did not significantly affect body weight. Similarly, allopurinol and decorin did not significantly affect the streptozotocin-induced hyperglycaemia, but surprisingly in the untreated controls, molsidomine increased it to 431 ± 32 mg/dL from 354 ± 17 mg/dL (P < 0.001), and induced a considerable glucosuria from a basal level in the control (not shown). No ketonuria was found in any case, but the considerable proteinuria in the control (72.5 ± 33.6 mg/dL) was significantly reduced by allopurinol to 15.4 ± 5.5 mg/dL, suggesting a protective effect directly on the kidney tissue independent from glycaemic control. Decorin and molsidomine did not affect proteinuria. As expected, nitrites in the urine were present in all molsidomine-treated animals
The effects of these treatments in preventing the underlying histopathology caused by diabetes and iNOS deletion in the corpora cavernosa of the iNOSKo mice were determined in paraffin-embedded corporal tissue sections. Figure 1, like the other figures, shows representative pictures for each group and bar graphs for the quantitative image analysis. Despite our initial assumption, neither allopurinol nor molsidomine affected the SMC/collagen ratio as estimated by Masson trichrome. However, decorin did increase it considerably, by 2.5-fold. In contrast, the SMC content (Fig. 2), as estimated by ASMA, was more sensitive because not only did decorin increase it by 2.0-fold but allopurinol also increased it by 2.3-fold. Although molsidomine exerted a smaller stimulation, it did not achieve statistical significance.
FIG. 1.
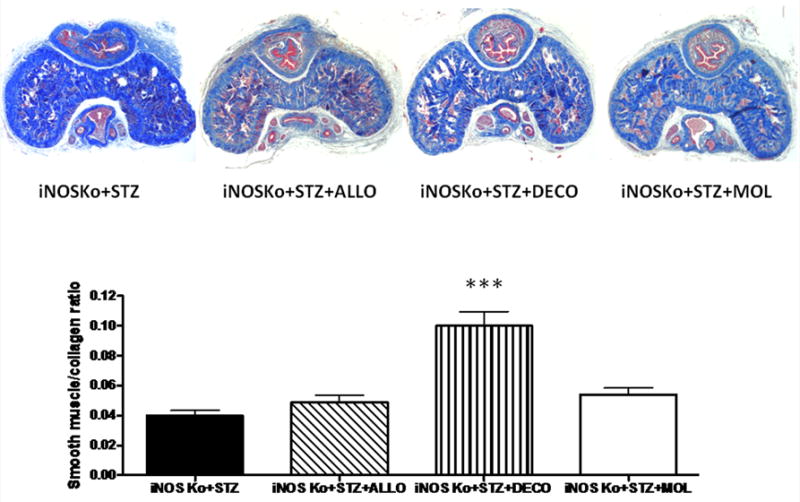
Long-term treatment of the diabetic inducible nitric oxide synthase knockout (iNOSKo) mice with decorin (DECO) increases the corporal smooth muscle cell (SMC)/collagen ratio. Top panel: representative pictures of Masson trichrome staining. iNOSKo+STZ; streptozotocin-injected iNOSKo mouse, untreated; iNOSKo+STZ+ALLO: iNOSKo+STZ treated with allopurinol. iNOSKo+STZ+DECO: iNOSKo+STZ treated with decorin. iNOSKo+STZ+MOL: iNOSKo+STZ treated with molsidomine. Bottom panel: quantitative image analysis for the SMC/collagen ratio expressed as means ± SEM; ***P < 0.001.
FIG. 2.
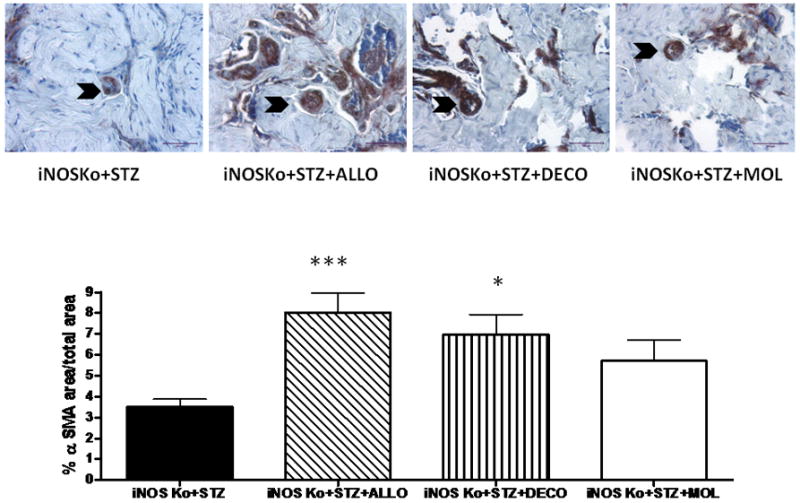
Long-term treatment of the diabetic inducible nitric oxide synthase knockout (iNOSKo) mice with decorin or allopurinol, increases the corporal smooth muscle cell (SMC) content. Top panel: representative pictures of α-smooth muscle actin (ASMA) immunostaining; symbols as for Fig. 1. Bottom panel: quantitative image analysis for the SMC content expressed as means ± SEM; ***P < 0.001; *P < 0.05.
The protective effects of both decorin and allopurinol on the corporal SMC were reflected by a significant reduction in the apoptotic index (Fig. 3). Molsidomine did not reduce cell death. (Fig. 4A). Allopurinol increased cell replication 1.5-fold whereas decorin was ineffective (Fig. 4A), but the positive cell turnovers (proliferation predominating over cell death) were increased in both cases (Fig. 4B).
FIG. 3.
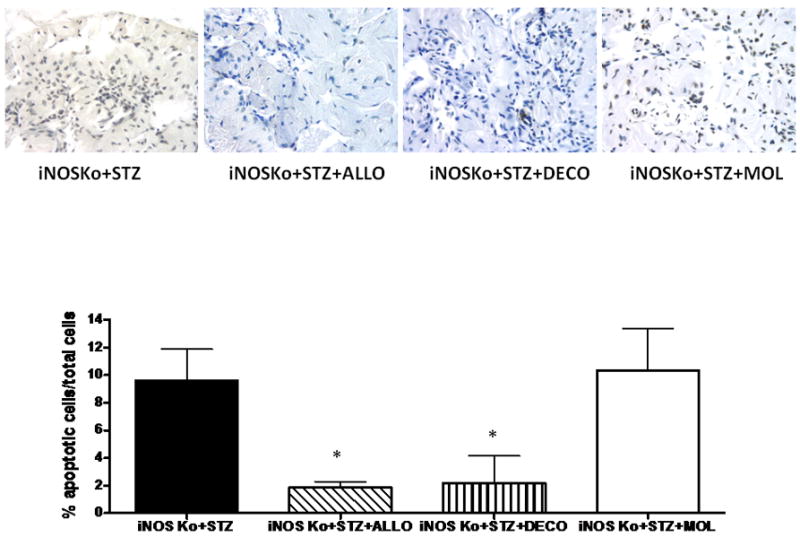
Long-term treatment of the diabetic inducible nitric oxide synthase knockout (iNOSKo) mice with decorin or allopurinol, reduces the corporal apoptotic index. Top panel: representative pictures of apoptosis by TUNEL immunostaining; symbols as for Fig. 1. Bottom panel: quantitative image analysis for the apoptotic index expressed as means ± SEM; *P < 0.05.
FIG. 4.
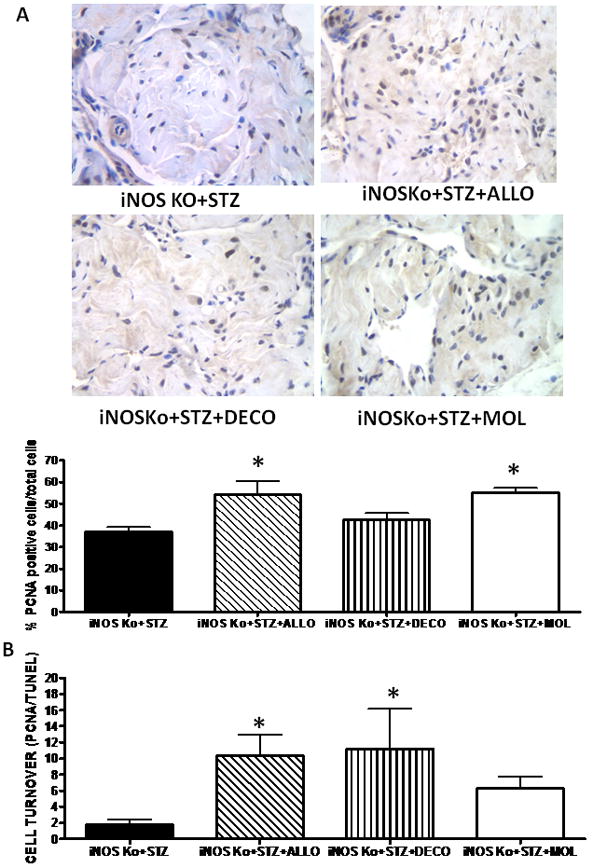
Long-term treatment of inducible nitric oxide synthase knockout (iNOSKo) mice with allopurinol increases corporal cell replication and induces a positive cell turnover. (A; top panel) Representative pictures of proliferating cell nuclear antigen (PCNA) immunostaining; symbols as for Fig. 1. (A; bottom panel) Quantitative image analysis for the number of PCNA-positive cells. (B) Cell turnover determined as ratio of cell proliferation and cell death expressed as means ± SEM; *P < 0.05.
The three types of treatments were uniformly effective in reducing oxidative stress in the corpora cavernosa by 46–60% as estimated by XOR (Fig. 5A). This is reflected in the expected decrease of systemic oxidative stress by allopurinol and molsidomine, represented by the nearly three-fold decrease of ROS in the blood as measured by the GSH/GSSG ratio (the higher the ratio, the lower the oxidative stress) (Fig. 5B). As expected, decorin which acts by a mechanism different from an antioxidant or a nitric oxide donor, did not reduce systemic oxidative stress.
FIG. 5.
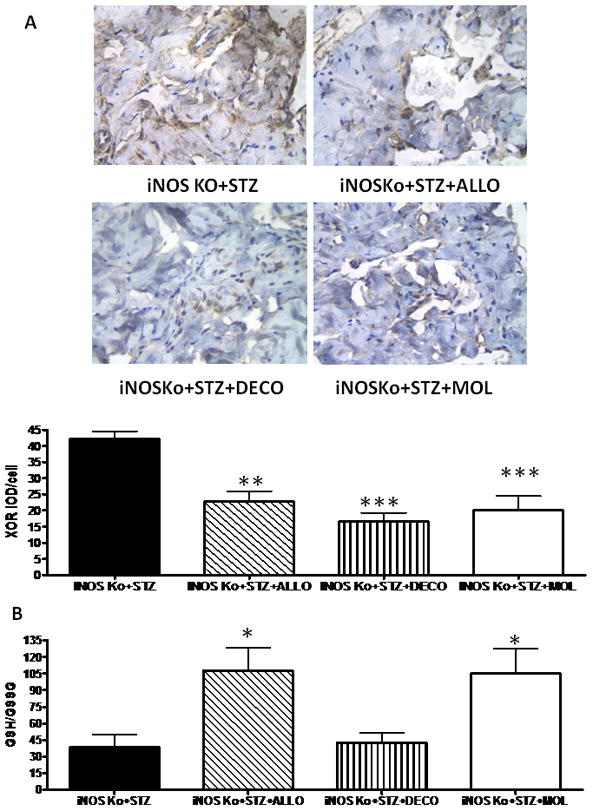
Long-term treatment of inducible nitric oxide synthase knockout (iNOSKo) mice with allopurinol, decorin and molsidomine reduces corporal oxidative stress and, except for decorin, decreases systemic oxidative stress. (A; top panel) Representative pictures of xanthine oxidoreductase (XOR) immunostaining; symbols as for Fig. 1. (A; bottom panel) Quantitative image analysis for the area of XOR+ staining. (B) Reduced to oxidized glutathione (GSH/GSSG) ratio in blood expressed as means ± SEM; ***P < 0.001; **P < 0.01; *P < 0.05.
Finally, none of the protective effects of these agents on the corporal histology seemed to be the result of a reduction in the expression of the key profibrotic factor, TGF-β1. In the case of allopurinol, TGFB1 expression was actually stimulated 2.0-fold (not shown). The fact that decorin did not induce any significant change in TGFB1 is as expected based on its mechanism of action of its binding to TGF-β1, and so neutralizing it, without affecting its expression.
DISCUSSION
This report is the first to compare concurrently in a mouse model of exacerbated fibrosis, the diabetic iNOSKo mouse, the potential antifibrotic, antioxidant and SMC-protective action of three pharmacological agents. They act by different mechanisms on the penile corpora cavernosa, although each one reduces the levels of some but not all of the key profibrotic factors. Allopurinol reduces ROS and the subsequent oxidative stress through direct antioxidant activity whereas molsidomine quenches it. Decorin binds to, TGF-β1 and therefore blocks the signalling triggered by its receptor. However, although decorin and allopurinol are effective in protecting the SMC through inhibition of apoptosis, molsidomine (which theoretically should replace the effects of the absent iNOS) did not.
The critical profibrotic role of TGF-β1 expression in the diabetic iNOSKo was confirmed by the results of a long-term treatment with decorin, a small leucine-rich proteoglycan that counteracts TGF-β1 binding to its receptor and so acts as an antifibrotic agent [33-38]. The increase in the corporal SMC/collagen ratio and in SMC content while decreasing apoptosis, and the local tissue protection against oxidative stress without affecting systemic ROS, is in agreement with this mechanism. The effects of decorin have not been reported for penile tissues, other than in terms of the potentially compensatory expression of decorin observed in the human Peyronie’s disease fibrotic plaque [39]. However, our results do not predict how decorin would act in a setting of normal iNOS induction, because TGF-β1 overexpression was not observed in the non-diabetic iNOSKo or in the diabetic wild-type mice in comparison with the non-diabetic wild-type animals [18]. So far, the role of TGF-β1 in corporal fibrosis induced by aging, diabetes or cavernosal damage remains elusive, in contrast to its very clear significance for Peyronie’s disease [1,2], and fibrosis of other organs such as the kidney, liver and heart [33-38].
Allopurinol is perhaps the most promising agent because it was very effective in preventing corporal SMC loss in the diabetic iNOSKo mice by reducing apoptosis and oxidative stress, both systemic and local, and stimulating cell proliferation, so confirming the beneficial effects of antioxidant therapy on corporal fibrosis and erectile dysfunction in diabetes and on tissue fibrosis in general [40-42]. The lack of allopurinol effects on the corporal SMC/collagen ratio, which is in contrast to its well known effects in reducing collagen deposition in tissues such as the heart and liver [30-32], is probably related to the also unexpected increase in TGF-β1 expression. The systemic effects of allopurinol on ROS agree with the decrease in proteinuria that reflects an antifibrotic effect on diabetic nephropathy, previously reported for this agent, since allopurinol has been proved to be an effective antifibrotic and antioxidant in the heart and kidney [30-32]. This also confirms the pivotal role of XOR in corporal oxidative stress that has been essentially ignored in favour of NADPH oxidase [43,44], which is reflected on the absence of published reports on the effects of allopurinol on erectile dysfunction or the penis. The single exception is a study that showed acute effects of allopurinol in the attenuation of ischaemia-induced and reperfusion-induced corporal injury in a rat model of veno-occlusive priapism, presumably based on the reduction of corporal lipid peroxidation [45].
Perhaps the most surprising result was the inability of the long-term administration of the long half-life exogenous nitric oxide generator, molsidomine, to affect the SMC/collagen ratio, SMC content or apoptosis, that was expected from the antifibrotic effects reported for the kidney and liver [25-27]. Linked to the vasodilating activity, but still poor efficacy of the acute administration of molsidomine or its derivative SIN-1 to induce corporal relaxation in comparison with prostaglandin E1 [46,47], this may rule out further consideration of this drug for erectile dysfunction. However, its antioxidant activity, both local and systemic, supports the view that iNOS is in fact antioxidant through a sustained production of nitric oxide in the corpora cavernosa. Nitric oxide leads to XOR downregulation in addition to ROS quenching, and is not necessarily a cause of oxidative stress because it may occur in other tissue settings. In turn, the stimulation of cell proliferation by molsidomine in the corporal tissue by nitric oxide/cGMP agrees with our previous studies [7,9,16], and again establishes an interesting divergence with the effects in general seen in the arterial wall SMC [48]. Therefore further studies may be needed to rule out any use for molsidomine for erectile dysfunction.
In summary, under conditions where the inactivation of the iNOS gene exacerbates corporal fibrosis in diabetes this histopathology is ameliorated by long-term pharmacological reduction of oxidative stress or counteracting TGF-β1, but not by simply producing nitric oxide from exogenous sources. This indicates that the use of certain antioxidant or antifibrotic agents would be effective to ameliorate corporal fibrosis and improve erectile dysfunction in diabetes, suggesting that combination therapy with some of these types of compounds, perhaps together with long-term continuous treatment with phosphodiesterase 5 inhibitors, may be beneficial by targeting different sites in the fibrosis pathways. Allopurinol, because of its long clinical use and its oral route of administration, is an interesting candidate in this respect, despite the negligible effect on corporal collagen deposition observed here. Decorin was more uniformly effective, but we are not aware of any clinical use. Further investigations in animal models are required to confirm preclinically these assumptions, particularly by measuring the penile erection response, which was not studied in the current report.
Acknowledgments
This work was funded by grants NIH 5RO1DK53069 (NGC), NIH R21DK-070003 (NGC); and DOD W81XWH-07-1-0129 (NGC).
Abbreviations
- ALLO
allopurinol
- ASMA
α-smooth muscle actin
- CVOD
corporal veno-occlusive dysfunction
- DECO
decorin
- GSH/GSSG
reactive glutathione/oxidized glutathione
- iNOS
NOS II, inducible nitric oxide synthase
- iNOSKo
iNOS knockout mouse
- PCNA
proliferating cell nuclear antigen
- ROS
reactive oxygen species
- SMC
smooth muscle cells
- STZ
streptozotocin
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labelling
- XOR
xanthine oxidoreductase
References
- 1.Gonzalez-Cadavid NF, Rajfer J. Experimental models for Peyronie’s disease. Implications for therapy. J Sex Medic. 2009;6:303–13. doi: 10.1111/j.1743-6109.2008.01104.x. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Cadavid NF. Mechanisms of penile fibrosis. J Sex Med. 2009;6(Suppl 3):353–62. doi: 10.1111/j.1743-6109.2008.01195.x. [DOI] [PubMed] [Google Scholar]
- 3.Ferrini MG, Kovanecz I, Sanchez S, et al. Long-term continuous treatment with sildenafil ameliorates aging-related erectile dysfunction and the underlying corporal fibrosis. Biol Reprod. 2007;76:915–23. doi: 10.1095/biolreprod.106.059642. [DOI] [PubMed] [Google Scholar]
- 4.Kovanecz I, Ferrini MG, Davila HH, Rajfer J, Gonzalez-Cadavid NF. Pioglitazone ameliorates penile corpora veno-occlusive dysfunction (CVOD) in the aged rat. BJU Int. 2007;100:867–74. doi: 10.1111/j.1464-410X.2007.07070.x. [DOI] [PubMed] [Google Scholar]
- 5.Ahn GJ, Sohn YS, Kang KK, et al. The effect of PDE5 inhibition on the erectile function in streptozotocin-induced diabetic rats. Int J Impot Res. 2005;17:134–41. doi: 10.1038/sj.ijir.3901295. Erratum in: Int J Impot Res 2006; 18: 576. [DOI] [PubMed] [Google Scholar]
- 6.Kovanecz I, Ferrini MG, Vernet D, Nolazco G, Rajfer J, Gonzalez-Cadavid NF. Pioglitazone prevents corporal veno-occlusive dysfunction (CVOD) in a rat model of type 2 diabetes melittus. BJU Int. 2006;98:116–24. doi: 10.1111/j.1464-410X.2006.06268.x. [DOI] [PubMed] [Google Scholar]
- 7.Ferrini MG, Kovanecz I, Sanchez S, et al. Fibrosis and loss of smooth muscle in the corpora cavernosa precede corporal veno-occlusive dysfunction (CVOD) induced by experimental cavernosal nerve damage in the rat. J Sex Medic. 2009;6:415–28. doi: 10.1111/j.1743-6109.2008.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovanecz I, Rambhatla A, Ferrini MG, et al. Chronic daily tadalafil prevents the corporal fibrosis and veno-occlusive dysfunction (CVOD) that occurs following cavernosal nerve resection in the rat. BJU Int. 2008;101:203–10. doi: 10.1111/j.1464-410X.2007.07223.x. [DOI] [PubMed] [Google Scholar]
- 9.Kovanecz I, Rambhatla A, Ferrini MG, et al. Long term sildenafil treatment ameliorates corporal veno-occlusive dysfunction (CVOD) induced by cavernosal nerve resection in rats. Int J Impot Res. 2007;100:867–74. doi: 10.1038/sj.ijir.3901612. [DOI] [PubMed] [Google Scholar]
- 10.Ferrini MG, Davila H, Kovanecz I, Sanchez S, Gonzalez-Cadavid NF, Rajfer J. Long-term continuous treatment with vardenafil prevents fibrosis and preserves smooth muscle content in the rat corpora cavernosa after bilateral cavernosal nerve transection. Urology. 2006;68:429–35. doi: 10.1016/j.urology.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Jiang R, Chen JH, Jin J, Shen W, Li QM. Ultrastructural comparison of penile cavernous tissue between hypertensive and normotensive rats. Int J Impot Res. 2005;17:417–23. doi: 10.1038/sj.ijir.3901329. [DOI] [PubMed] [Google Scholar]
- 12.Davila HH, Rajfer J, Gonzalez-Cadavid NF. Corporal veno-occlusive dysfunction in aging rats: evaluation by cavernosometry and cavernosography. Urology. 2004;64:1261–6. doi: 10.1016/j.urology.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Cadavid NF, Rajfer J. The pleiotropic effects of inducible nitric oxide synthase on the physiology and pathology of penile erection. Curr Pharm Des. 2005;11:4041–6. doi: 10.2174/138161205774913372. [DOI] [PubMed] [Google Scholar]
- 14.Davila HH, Magee TR, Rajfer J, Gonzalez-Cadavid NF. Gene therapy with the inducible nitric oxide synthase (iNOS) cDNA regresses the fibrotic plaque in an animal model of Peyronie’s disease. Biol Reprod. 2004;71:1568–77. doi: 10.1095/biolreprod.104.030833. [DOI] [PubMed] [Google Scholar]
- 15.Vernet D, Ferrini MG, Valente E, et al. Effect of nitric oxide on the differentiation of fibroblasts into myofibroblasts in the Peyronie’s fibrotic plaque and in its rat model. Nitric Oxide. 2002;7:262–76. doi: 10.1016/s1089-8603(02)00124-6. [DOI] [PubMed] [Google Scholar]
- 16.Ferrini MG, Davila H, Valente EG. Aging-related induction of inducible nitric oxide synthase (iNOS) is vasculo-protective in the arterial media. Cardiovascular Res. 2004;61:796–805. doi: 10.1016/j.cardiores.2003.12.006. Gonzalez-Cadavid NF*, Rajfer J*, *equal contributors. [DOI] [PubMed] [Google Scholar]
- 17.Ferrini MG, Vernet D, Magee TR, et al. Antifibrotic role of inducible nitric oxide synthase (iNOS) Nitric Oxide. 2002;6:283–94. doi: 10.1006/niox.2001.0421. [DOI] [PubMed] [Google Scholar]
- 18.Ferrini MG, Rivera S, Moon J, Vernet D, Rajfer J, Gonzalez-Cadavid NF. The Genetic Inactivation of Inducible Nitric Oxide Synthase (iNOS) Intensifies Fibrosis and Oxidative Stress in the Penile Corpora Cavernosa in Type 1 Diabetes. J Sex Med. 2010;7:3033–44. doi: 10.1111/j.1743-6109.2010.01884.x. [DOI] [PubMed] [Google Scholar]
- 19.Rambhatla A, Kovanecz I, Ferrini M, Gonzalez-Cadavid NF, Rajfer J. Rationale for phosphodiesterase 5 inhibitor use post-radical prostatectomy: experimental and clinical review. Int J Impot Res. 2008;20:30–4. doi: 10.1038/sj.ijir.3901588. [DOI] [PubMed] [Google Scholar]
- 20.Magheli A, Burnett AL. Erectile dysfunction following prostatectomy: prevention and treatment. Nat Rev Urol. 2009;6:415–27. doi: 10.1038/nrurol.2009.126. [DOI] [PubMed] [Google Scholar]
- 21.Mulhall JP. Penile rehabilitation following radical prostatectomy. Curr Opin Urol. 2008;18:613–20. doi: 10.1097/MOU.0b013e3283136462. [DOI] [PubMed] [Google Scholar]
- 22.Messin R, Dubois C, Famaey JP. Comparative effects of once-daily molsidomine in coronary patients from two distinct European ethnicities. Adv Ther. 2008;25:1200–14. doi: 10.1007/s12325-008-0117-8. [DOI] [PubMed] [Google Scholar]
- 23.Banasiak W, Wilkins A, Pociupany R, Ponikowski P. Pharmacotherapy in patients with stable coronary artery disease treated on an outpatient basis in Poland. Results of the multicentre RECENT study. Kardiol Pol. 2008;66:642–9. [PubMed] [Google Scholar]
- 24.Messin R, Cerreer-Bruhwyler F, Dubois C, Famaey JP, Géczy J. Efficacy and safety of once- and twice-daily formulations of molsidomine in patients with stable angina pectoris: double-blind and open-label studies. Adv Ther. 2006;23:107–30. doi: 10.1007/BF02850352. [DOI] [PubMed] [Google Scholar]
- 25.Oztürk H, Yağmur Y, Buyukbayram H, Dokucu AI, Gurel A. Effects of the nitric oxide donor molsidomine on the early stages of liver damage in rats with bile duct ligation: a biochemical and immunohistochemical approach. Eur Surg Res. 2002;34:285–90. doi: 10.1159/000063069. [DOI] [PubMed] [Google Scholar]
- 26.Benigni A, Zoja C, Noris M, et al. Renoprotection by nitric oxide donor and lisinopril in the remnant kidney model. Am J Kidney Dis. 1999;33:746–53. doi: 10.1016/s0272-6386(99)70229-5. [DOI] [PubMed] [Google Scholar]
- 27.Peters H, Daig U, Martini S, et al. NO mediates antifibrotic actions of L-arginine supplementation following induction of anti-thy1 glomerulonephritis. Kidney Int. 2003;64:509–18. doi: 10.1046/j.1523-1755.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- 28.McGill NW. Management of gout: beyond allopurinol. Intern Med J. 2010;40:545–53. doi: 10.1111/j.1445-5994.2010.02293.x. [DOI] [PubMed] [Google Scholar]
- 29.Tsai TF, Yeh TY. Allopurinol in dermatology. Am J Clin Dermatol. 2010;11:225–32. doi: 10.2165/11533190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Jia N, Dong P, Ye Y, Qian C, Dai Q. Allopurinol Attenuates Oxidative Stress and Cardiac Fibrosis in Angiotensin II-Induced Cardiac Diastolic Dysfunction. Cardiovasc Ther. 2010 doi: 10.1111/j.1755-5922.2010.00243.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.Rajesh M, Mukhopadhyay P, Bátkai S, et al. Xanthine oxidase inhibitor allopurinol attenuates the development of diabetic cardiomyopathy. J Cell Mol Med. 2009;13:2330–41. doi: 10.1111/j.1582-4934.2008.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayashi K, Kimata H, Obata K, et al. Xanthine oxidase inhibition improves left ventricular dysfunction in dilated cardiomyopathic hamsters. J Card Fail. 2008;14:238–44. doi: 10.1016/j.cardfail.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Järvinen TA, Ruoslahti E. Target-seeking antifibrotic compound enhances wound healing and suppresses scar formation in mice. Proc Natl Acad Sci USA. 2010;107:21671–6. doi: 10.1073/pnas.1016233107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohan RR, Gupta R, Mehan MK, Cowden JW, Sinha S. Decorin transfection suppresses profibrogenic genes and myofibroblast formation in human corneal fibroblasts. Exp Eye Res. 2010;91:238–45. doi: 10.1016/j.exer.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Z, Wu F, Zheng F, Li H. Adenovirus-mediated decorin gene transfection has therapeutic effects in a streptozocin-induced diabetic rat model. Nephron Exp Nephrol. 2010;116(1):e11–21. doi: 10.1159/000314669. [DOI] [PubMed] [Google Scholar]
- 36.Faust SM, Lu G, Wood SC, Bishop DK. TGFbeta neutralization within cardiac allografts by decorin gene transfer attenuates chronic rejection. J Immunol. 2009;183:7307–13. doi: 10.4049/jimmunol.0902736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan W, Wang P, Zhao CX, Tang J, Xiao X, Wang DW. Decorin gene delivery inhibits cardiac fibrosis in spontaneously hypertensive rats by modulation of transforming growth factor-beta/Smad and p38 mitogen-activated protein kinase signaling pathways. Hum Gene Ther. 2009;20:1190–200. doi: 10.1089/hum.2008.204. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Li J, Zhu J, et al. Decorin gene transfer promotes muscle cell differentiation and muscle regeneration. Mol Ther. 2007;15:1616–22. doi: 10.1038/sj.mt.6300250. [DOI] [PubMed] [Google Scholar]
- 39.Qian A, Meals R, Rajfer J, Gonzalez-Cadavid NF. Comparison of gene expression profiles between Peyronie’s disease and Dupuytren’s contracture. Urology. 2004;64:399–404. doi: 10.1016/j.urology.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Vicari E, La Vignera S, Condorelli R, Calogero AE. Endothelial Antioxidant Administration Ameliorates the Erectile Response to PDE5 Regardless of the Extension of the Atherosclerotic Process. J Sex Med. 2010;7:1247–53. doi: 10.1111/j.1743-6109.2009.01420.x. [DOI] [PubMed] [Google Scholar]
- 41.Villalba N, Martínez P, Bríones AM, et al. Differential structural and functional changes in penile and coronary arteries from obese Zucker rats. Am J Physiol Heart Circ Physiol. 2009;297:H696–707. doi: 10.1152/ajpheart.01308.2008. [DOI] [PubMed] [Google Scholar]
- 42.Shukla N, Hotston M, Persad R, Angelini GD, Jeremy JY. The administration of folic acid improves erectile function and reduces intracavernosal oxidative stress in the diabetic rabbit. BJU Int. 2009;103:98–103. doi: 10.1111/j.1464-410X.2008.07911.x. [DOI] [PubMed] [Google Scholar]
- 43.Tziomalos K, Hare JM. Role of xanthine oxidoreductase in cardiac nitroso-redox imbalance. Front Biosci. 2009;14:237–62. doi: 10.2741/3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeremy JY, Jones RA, Koupparis AJ, et al. Reactive oxygen species and erectile dysfunction: possible role of NADPH oxidase. Int J Impot Res. 2007;19:265–80. doi: 10.1038/sj.ijir.3901523. [DOI] [PubMed] [Google Scholar]
- 45.Evliyaoglu Y, Kayrin L, Kaya B. Effect of allopurinol on lipid peroxidation induced in corporeal tissue by veno-occlusive priapism in a rat model. Br J Urol. 1997;80:476–9. doi: 10.1046/j.1464-410x.1997.00371.x. [DOI] [PubMed] [Google Scholar]
- 46.Sazova O, Kadioğlu A, Gürkan L, et al. Intracavernous administration of SIN-1+VIP in an in vivo rabbit model for erectile function. Int J Impot Res. 2002;14:44–9. doi: 10.1038/sj.ijir.3900813. [DOI] [PubMed] [Google Scholar]
- 47.von Heyden B, Brock GB, Martinez-Piñeiro L, Lue TF. Intracavernous injection of linsidomine chlorhydrate in monkeys: lack of toxic effect with long-term use. Eur Urol. 1996;30:502–5. doi: 10.1159/000474224. [DOI] [PubMed] [Google Scholar]
- 48.Ahanchi SS, Tsihlis ND, Kibbe MR. The role of nitric oxide in the pathophysiology of intimal hyperplasia. J Vasc Surg. 2007;45(Suppl. A):A64–73. doi: 10.1016/j.jvs.2007.02.027. [DOI] [PubMed] [Google Scholar]


