Abstract
Macrophages are an important component of muscle where they are involved in complex processes such as repair, regeneration, and hypertrophy. We recently reported that macrophage numbers increase in the muscle of obese patients, suggesting that muscle resident macrophages could be involved in the development of muscle insulin resistance that is associated with obesity. Co-culture of activated macrophages with human muscle cells impairs insulin signaling and induces atrophy signaling pathways in the human muscle cells; this is exacerbated by the addition of palmitic acid. In this study, we tested the hypothesis that docosahexaenoic acid (DHA), a polyunsaturated fatty acid that has anti-inflammatory properties, would have the opposite effect of palmitic acid on muscle-macrophage co-cultures. Surprisingly, DHA did not stimulate insulin signaling in human muscle myotubes that were co-cultured with fibroblasts or macrophages. However, DHA inhibited Fn14, the TWEAK receptor that increases the expression of the muscle specific ubiquitin ligase MuRF-1. DHA treatment also increased the apparent molecular mass of MuRF-1 on SDS-PAGE gels, suggesting that DHA causes MuRF-1 to be post-translationally modified. In conclusion, these results suggest that DHA may have a beneficial effect on muscle mass in humans by inhibiting the induction of Fn14 by infiltrating macrophages.
Keywords: docosahexaenoic acid, palmitic acid, muscle atrophy, Fn14, MuRF-1, MAFbx
1. Introduction
Obesity increases the macrophage content of insulin responsive tissues [1]. It is well accepted that adipose tissue from obese individuals has more macrophages than the adipose tissue of lean individuals. Moreover, adipose resident macrophages increase the concentration of circulating inflammatory cytokines [2–4]. These proinflammatory cytokines in conjunction with elevated free fatty acids cause insulin resistance in muscle [5]. However, obesity also increases the number of tissue resident macrophages in muscle [6]; these macrophages, in close proximity to myoblasts and myofibers, are in a position to directly affect muscle by secreting various cytokines. Macrophage-muscle interactions can be studied with co-culture systems. We have showed that activated macrophages alter insulin signaling pathways in muscle and that this effect is enhanced by the saturated free fatty acid (FFA) palmitic acid [6]. Furthermore, co-culture with macrophages induced atrophy signaling in human muscle, and this was enhanced by palmitic acid [6]. The effects of unsaturated FFAs on macrophage-myotube interactions are unknown, but are likely to be different than palmitic acid. Therefore, we evaluated the effect of oleic acid, which is monounsaturated, and DHA, which is polyunsaturated, in this co-culture study.
Free fatty acids are an important fuel source for muscle and may become incorporated into phospholipids subsequently influencing both the properties of muscle cell membranes and signal transduction pathways [7]. However, high concentrations of FFA, which occur with obesity, cause detrimental effects in muscle. Palmitic acid increases inflammation by stimulating toll receptors [8], and palmitic acid induces the de novo synthesis of ceramide and diacylglycerol (DAG) [9, 10], both of which inhibit insulin signaling [8, 11, 12]. The addition of palmitic acid to muscle-macrophage co-cultures decreases the phosphorylation of the insulin signaling target Akt in myotubes [6, 13]. In addition to regulating GLUT4 translocation to the plasma membrane in response to insulin, Akt also regulates muscle mass, and we found that palmitic acid treatment increases atrophy signaling in myotubes [6].
In contrast to saturated fatty acids, n-3 fatty acids, which include docosahexaenoic acid (DHA; 22:6n-3) and eicosapentaenoic acid (EPA; 20:5n-3), are anti-inflammatory [14]. The anti-inflammation mechanism is complex and involves reducing the n-6 to n-3 ratio, altering transcription, producing resolvins, and stimulating the hetero-trimeric G-protein coupled receptor GPR120 [14, 15]. Because DHA is anti-inflammatory, we hypothesized that the addition of DHA to muscle-macrophage co-cultures would enhance myotube insulin signaling and decrease myotube atrophy signaling. DHA enhances insulin signaling in adipocytes [15], improves insulin sensitivity in cattle [16], and abrogates the effect of high fat feeding on insulin sensitivity in rats and mice [15, 17]. Finally, there is limited evidence that n-3 fatty acids are protective against muscle atrophy since they protect muscle from cytokines in cell culture studies [18] and preserve muscle mass in rats [19].
In this study, we examined the effects of n-3 fatty acids on myotube insulin and atrophy signaling in macrophage-myotube co-cultures. We found that DHA did not increase stimulation of Akt phosphorylation by insulin or protect against the dramatic reduction in pAkt levels that occurs when myotubes are co-cultured with macrophages. Interestingly, Fn14, which is a newly discovered regulator of the muscle specific ubiquitin ligase MuRF-1, was induced in myotubes by macrophage co-culture, and DHA treatment reduced Fn14 protein levels. Finally, MuRF-1 is likely post-translationally modified in response to DHA treatment. Thus, DHA supplementation may have a beneficial effect on human muscle mass by inhibiting Fn14 induction by macrophages.
2. Materials and Methods
2.1. Human Subjects and Muscle Biopsies
Healthy, sedentary, non-diabetic human subjects were recruited by local advertisement. All subjects recruited signed informed consent forms under protocols that were approved by the institutional review board at the University of Arkansas for Medical Sciences. Muscle biopsy specimens from 6 subjects were included in this study. Muscle biopsies were obtained from vastus lateralis muscle under local anesthesia. Subjects in this group were between 22 and 52 years old; their BMI was between 23 and 40; none of the subjects were taking anti-inflammatory medications.
2.2. Isolation and culture of myoblasts from human muscle biopsies
Myoblasts were isolated from the biopsied tissue as described previously [6]. Myoblasts that were 90% or greater MyoD positive and that were at passages between 4 and 5 were used in the experiments. When the cells were 90% confluent, they were induced to differentiate into myotubes by culturing in alpha MEM medium (Invitrogen, Carlsbad, CA) containing 5 mM glutamine, 1× penicillin-streptomycin and 2% fetal bovine serum (FBS) for 48 to 72 hours.
2.3. Myotube–fibroblast and myotube–macrophage co-cultures
THP-1 cells, a human monocyte cell line, were maintained in RPMI medium with 10% FBS and 1% penicillin/streptomycin. Differentiated macrophages were obtained by plating THP-1 monocytes at 1.4 × 107 cells/100 mm culture dish in Macrophage-SFM (Invitrogen) with 1% penicillin/streptomycin and 250 nM phorbol ester (12-O tetradecanoylphorbol-13-acetate (Sigma-Aldrich, St. Louis, MO)) for 72h. MRC-5 cells, a human fibroblast cell line, were grown in Dulbecco's Modified Eagle's Medium (DMEM), 1g/L glucose (Mediatech, Manassas, VA) containing 10% FBS and 1× penicillin- streptomycin. Differentiated myotubes were cultured alone, co-cultured with THP-1 macrophages, or as a control, co-cultured with MRC-5 fibroblasts. For co-cultures, the TPA differentiated THP-1 macrophages were gently scraped, counted using trypan blue, plated at 1.8–2.0 × 105 cells on inserts, and allowed to settle and adhere in 6-well plates for 12h prior to starting the co-culture. Thus, the macrophages were plated at 20 to 25% of the confluent numbers of myoblasts (7–9 × 105); this is similar to the macrophage to myotube ratio observed in the muscle from obese subjects [6]. Differentiated myotubes were co-cultured with inserts containing either THP-1 macrophages, MRC fibroblasts, or no cells. The co-culture medium contained either 0.2 mM palmitic acid, 0.2 mM oleic acid, 0.2 mM DHA, or ethanol (vehicle control). Palmitic acid, oleic acid, or DHA (Sigma-Aldrich) were first conjugated with fatty acid free bovine serum albumin (BSA) (MP Biochemicals, Irvine, California) in a ratio of 2.5:1 as follows. Each fatty acid was dissolved in ethanol and mixed with 20% fatty acid free BSA (MP Biomedicals, Solon, OH), which was dissolved in alpha MEM medium. The fatty acid was then conjugated to BSA at 45-°C for 20 minutes. Ethanol was used as a vehicle control. The final concentration of ethanol was less than 0.5%. The co-culture experiments were performed for 24h in duplicate. Following co-culture, the cells from the inserts and plate wells were collected with either RNA lysis buffer or cell lysis buffer.
2.4. Total RNA isolation and real time RT-PCR
Total RNA from differentiated myotubes, THP-1 macrophages, and MRC-5 fibroblasts was obtained using the RNAqueous kit (Ambion Inc., Austin, TX) according to the manufacturer’s instructions. The quantity and quality of the isolated RNA was determined with an Agilent 2100 Bioanalyzer (Palo Alto, CA). cDNA was prepared and levels of MafBX and MyoD were determined using real-time RT-PCR as described earlier [6].
2.5. Immunoblot analysis
Cell lysates were prepared by adding M-PER (Thermo Fisher Scientific, Waltham, MA) containing both protease and phosphatase inhibitors to the wells, scraping the cells and then centrifuging the total lysate to remove cellular debris. Thirty µg of lysate was resolved on a 10% gel, transferred to nitrocellulose, and immunoblotted as follows. The membranes were blocked in TBST-C (20 mM Tris pH 8.0, 150 mM NaCl, 0.1% Tw-20, 1% casein (technical grade, Sigma)). Membranes were incubated with primary antibody for 1 h, washed three times with TBST, incubated with IRDye 800CW secondary antibodies (LI-COR Biosciences, Lincoln, NE), washed three times with TBST, and then quantified using an Odyssey imaging system (LI-COR Biosciences, Lincoln, NE). Actin was used as a loading control. When necessary, membranes were stripped with 0.2 M glycine pH 2.5, 0.05% Tween-20 at 80-°C for 30 min. The membranes were then blocked and probed with the appropriate secondary antibody to confirm stripping. Integrated intensities of the bands were determined with Odyssey Software (LI-COR Biosciences). The data were normalized by dividing by the integrated intensity of the load control (actin or Akt). Antibodies against Fn14 (4403), pAkt Ser473 (4051), and Akt (9272) were from Cell Signaling (Danvers, MA); against MuRF-1 (MP3401) was from ECM biosciences (Versailles, KY), and against actin (A1978) was from Sigma.
2.6. Phosphatase treatment
Lysates from either control or DHA treated myotubes were dialyzed for 1 in ice-cold phosphatase buffer (50 mM Hepes pH 7.4, 100 mM NaCl, 0.5% Triton X-100, protease inhibitors (EMD, Gibbstown, NJ), and 2 mM DTT). Thirty µg lysate was then dephosphorylated in a 20 µL reaction containing 2 µL (800 units) lambda phosphatase (NEB, Danvers, MA) and 1 mM MnCl2. The reaction was incubated at 30-°C for 30 min, resolved on a 10% SDS-PAGE gel, transferred to nitrocellulose, and analyzed by immunoblotting as described above.
2.7 Measurement of myotube DAG and ceramide
Quantification of DAG and ceramide content in lipid extracts from myotubes was performed as previously reported [20] with some modifications. Briefly, lipids were extracted from myotubes and DAG and ceramide phosphorylated in the lipid extract by the addition of diacylglycerol-kinase and 5 µCi [32P] ATP. DAG and ceramide were separated by thin-layer chromatography. The silica plates were then exposed to autoradiograph film, DAG and ceramide located and scraped into separate scintillation vials. DAG and ceramide concentrations were calculated as nanomoles/mg protein based on known standards run on the same plate.
2.8 Statistical Analysis
Ratios were treated as continuous variables and described with means and standard errors for FFA treatment and co-culture cell type. Analysis of the means plots indicated that the no cell co-culture control behaved similarly to co-culture with fibroblasts. Therefore, for all analyses, the no cell co-culture was removed as a treatment. Two-way ANOVAs were used to investigate the main effects of co-culture type, lipid type, and the interaction of cell co-culture type and lipid type on means ratios. When the data suggested potential interactions between lipid and cell co-culture type (p≤ 0.25), comparisons were made using post-hoc tests to investigate the effect of lipid on the fibroblast and macrophage co-culture types. SAS v9.2 was used for all statistical tests and p-values less than 0.05 were considered statistically significant.
3. Results
3.1. Effect of macrophage co-culture and free fatty acids (FFAs) on human muscle insulin signaling pathways
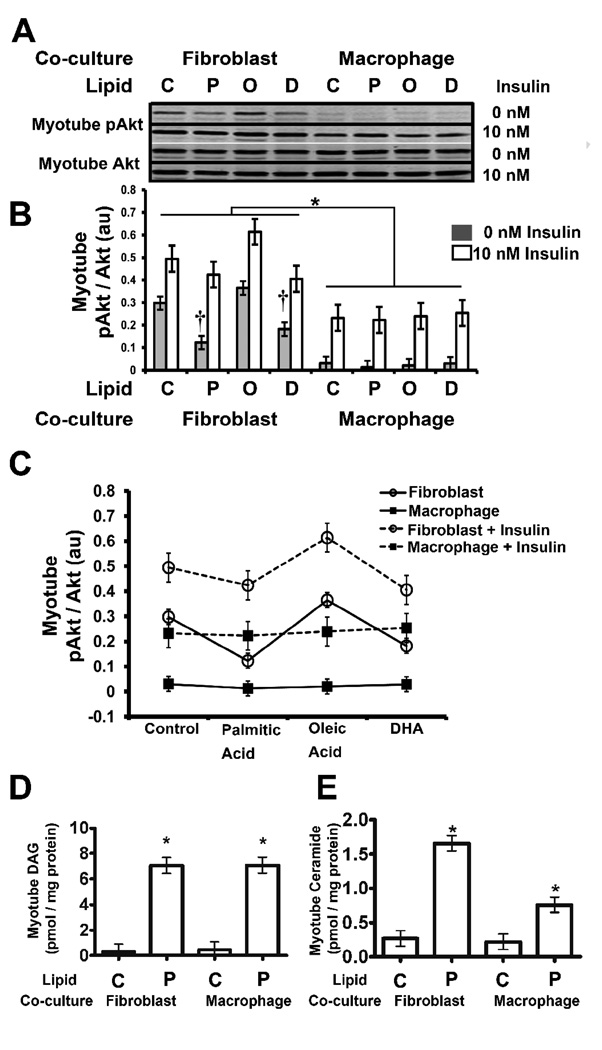
We showed previously that palmitic acid and macrophages act synergistically to blunt insulin signaling in muscle cells [6]. To explore this further, the effects of palmitic acid were compared to oleic acid and DHA for their ability to alter insulin signaling pathways in human myotubes. DHA has anti-inflammatory effects on many immune cell types including macrophages; therefore, it was hypothesized that DHA would have the opposite effect of palmitic acid in the co-culture system. Myotubes from six different subjects were cultured alone, with human fibroblasts (on inserts), or with differentiated (prior to co-culture) THP-1 human macrophages (on inserts) in medium containing either ethanol (vehicle control), palmitic acid, oleic acid, or DHA as indicated (Fig. 1A). After 24 hours, the cultures were stimulated with 0 or 10 nM insulin for 20 minutes, and then one set of myotubes was analyzed by immunoblotting and another set was analyzed by real time RT-PCR (see below). There were no significant differences between myotubes co-cultured with fibroblasts or cultured alone; therefore, culture alone data are not shown.
Fig. 1. Effect of macrophage co-culture and free fatty acids (FFA) on human muscle insulin signaling pathways.
A) Human myotubes were co-cultured for 24 hours with the indicated cells (on inserts) and either 0.2 mM palmitic acid (P), oleic acid (O), DHA (D) or ethanol vehicle control (C); the FFAs were added as BSA conjugates. The co-cultures were then stimulated with 0 nM or 10 nM insulin for 20 minutes; then the inserts were removed, and the myotubes were harvested and immunoblotted for phospho-S473 Akt (pAkt), Akt, or actin as described under “Materials and Methods.” A representative cell line is shown. B) The pAkt to Akt ratio (au: arbitrary units) of all six muscle cell lines was quantified, and statistical analysis of the data was performed using two-way ANOVA as described under “Materials and Methods.” Shaded boxes represent cells treated with 0 nM insulin and open boxes represent cells treated with 10 nM insulin. The data are represented as means ± SE (n=6); †: compare baseline (0 nM insulin) control to either palmitic acid or DHA treatment (p<0.05); *: compare baseline macrophage co-culture to fibroblast co-culture (p< 0.05). C) Means plots of the myotube pAkt to Akt ratio (au: arbitrary units) by lipid treatment, cell type (fibroblasts: open circles; macrophages: closed squares), and insulin treatment (0 nM insulin: solid line; 10 nM insulin: dashed line) are shown. Insulin treatment significantly increased the myotube pAkt to Akt ratio in all co-culture conditions (p<0.0001). D, E) DAG and ceramide levels were determined as described under “Materials and Methods.”
Insulin signaling was measured by immunoblotting phospho-serine 473 Akt (pAkt) and total Akt, and then determining the pAkt to Akt ratio. The results of a representative cell line are shown in Fig. 1A, and the average pAkt to Akt ratio of the six human myotube lines is shown in Fig. 1B. Macrophage co-culture reduced baseline (non-stimulated) pAkt levels in myotubes (p<0.0001) as previously observed [6], and this was not reversed by DHA (Fig. 1). Palmitic acid reduced baseline pAkt levels in myotubes co-cultured with fibroblasts (p=0.002), and surprisingly, DHA had the same effect (p=0.0095) whereas oleic acid had no effect. Means plots were generated to analyze the pAkt to Akt ratio as a function of FFA treatment, insulin treatment, and co-cultured cell type (Fig. 1C). Insulin treatment caused an increase in the pAkt to Akt ratio in all co-culture conditions (p<0.0001). Furthermore, this analysis revealed that insulin uniformly increased the ratio from the baseline level (Fig. 1C), indicating that the magnitude of the stimulation of Akt phosphorylation was unaffected by either FFA treatment or co-cultured cell type. Finally, the FFA treatments of the myotube-macrophage co-cultures did not significantly change the pAkt to Akt ratio. Thus, the major effect of either macrophage co-culture or the FFAs palmitic acid and DHA is to reduce baseline pAkt levels in human myotubes. Furthermore, DHA does not protect myotubes from the adverse effect that macrophage co-culture has on pAkt levels, nor does DHA enhance myotube Akt phosphorylation in response to insulin.
Palmitic acid has been reported to inhibit insulin signaling; therefore, the observation that palmitic acid did not have an inhibitory effect on myotube insulin signaling was surprising. In order to ensure that we had induced metabolic dysfunction, we measured DAG and ceramide in the mytotubes. The addition of palmitic acid to the co-cultures resulted in statistically significant increases in the levels of DAG in the myotubes that were co-cultured with either fibroblasts or macrophages (p<0.0001) (Fig. 1 D). The addition of palmitic acid to the co-cultures also resulted in statistically significant increases in the levels of ceramide in the myotubes that were co-cultured with either fibroblasts (p<0.0001) or macrophages (p=0.0259) (Fig. 1E). Thus, the myotube lines used in this study were resistant to the effects of these lipids with respect to insulin signaling (Fig. 1C). We also examined the effect of DHA on cytokine signaling in order to gain insight as to why DHA did not reverse the effect of macrophages on pAkt levels. As previously reported, macrophage co-culture significantly increased the level of IL-6 in the culture medium (p<0.0001); however, this was not inhibited by DHA (data not shown). The lack of effect of DHA on insulin signaling and IL-6 levels, and the reduction in baseline pAkt, led us to explore atrophy signaling, which we previously demonstrated to be affected by macrophage co-culture and palmitic acid [6].
3.2. Effect of macrophage co-culture on human muscle atrophy-inducing signaling
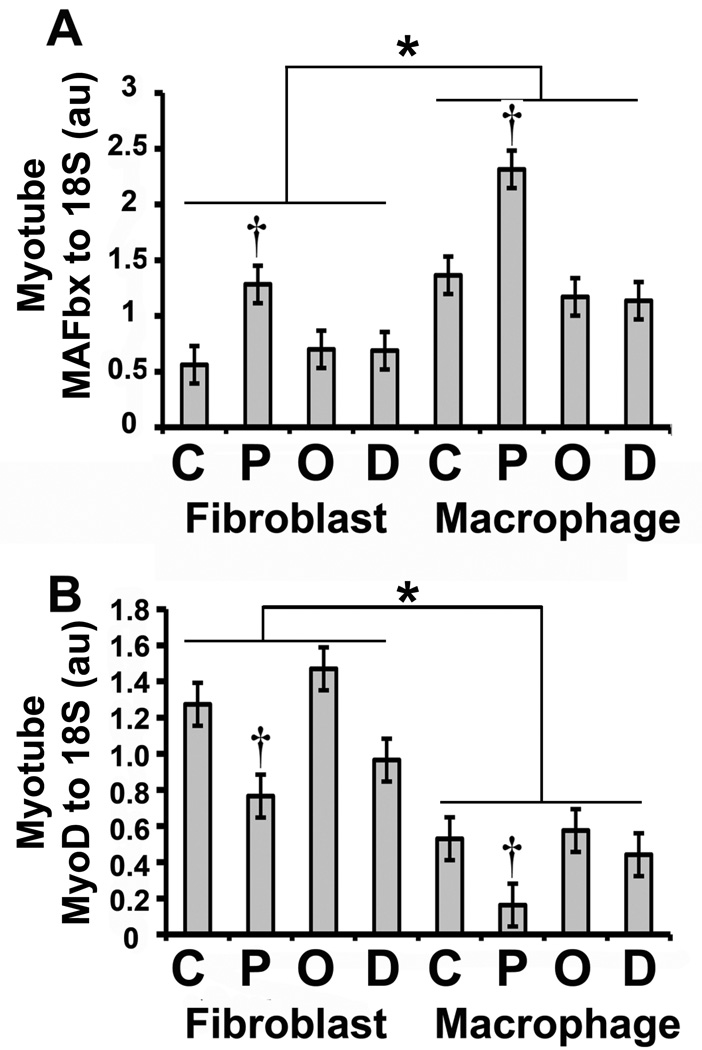
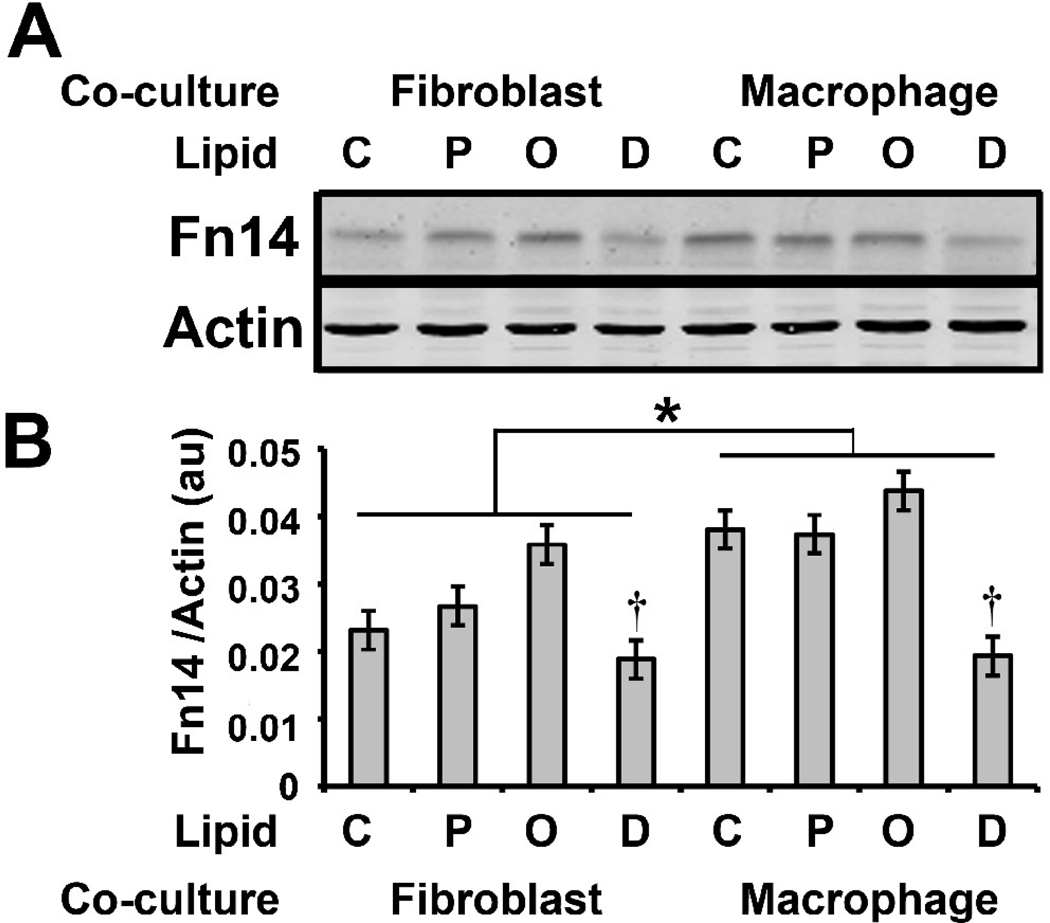
In addition to insulin signaling, Akt integrates numerous signals that regulate muscle mass. When active, Akt phosphorylates FOXO transcription factors, preventing their translocation to the nucleus where they up-regulate the expression of the ubiquitin ligases MuRF-1 and MAFbx [21, 22]. Macrophage co-culture increased myotube MAFbx mRNA levels (p<0.0001) (Fig. 2A), and this was consistent with the reduction in myotube pAkt levels caused by macrophage co-culture (Fig. 1); however, MuRF-1 mRNA was not increased (data not shown). Interestingly, the mRNA levels of MyoD, a transcription factor that controls muscle-specific genes, correlated with myotube pAkt levels, decreasing as a result of macrophage co-culture (p<0.0001) (Fig. 2B). Other pathways in addition to the Akt pathway regulate ubiquitin ligase expression, including the Tweak-Fn14 system that regulates MuRF-1 [23]. Fn14 protein expression was increased by macrophage co-culture (p=0.005) (Fig. 3B); however, this did not result in increased MuRF-1 protein after 24 hours of co-culture (Fig. 4B).
Fig. 2. Effect of macrophage co-culture and FFA on human muscle atrophy signaling.
Human myotubes were co-cultured as in Fig. 1. Total RNA was prepared and analyzed by real-time RT PCR as described under “Materials and Methods.” The mRNA level of the indicated gene was normalized to the level of 18S RNA and the ratio is represented in arbitrary units (au). Statistical analysis of the data was performed using two-way ANOVA as described under “Materials and Methods.” A) MAFbx to 18S ratio: the data are represented as means ± SE (n=6); †: compare control to palmitic acid treatment (p<0.05); *: compare macrophage co-culture to fibroblast co-culture (p< 0.05). B) MyoD to 18S ratio: the data are represented as means ± SE (n=6); †: compare control to palmitic acid treatment (p<0.05); *: compare macrophage co-culture to fibroblast co-culture (p< 0.05).
Fig. 3. Effect of macrophage co-culture and FFA on Fn14.
A) Human myotubes were co-cultured as in Fig. 1. The protein lysates were analyzed by immunoblotting with antibodies against Fn14 and actin. B) The Fn14 and actin levels were quantified as described under “Materials and Methods” and the Fn14 to actin ratio is represented in arbitrary units (au). Statistical analysis of the data was performed using two-way ANOVA as described under “Materials and Methods.” The data are represented as means ± SE (n=6); †: compare control to DHA treatment (p<0.05); *: compare macrophage co-culture to fibroblast co-culture (p< 0.05).
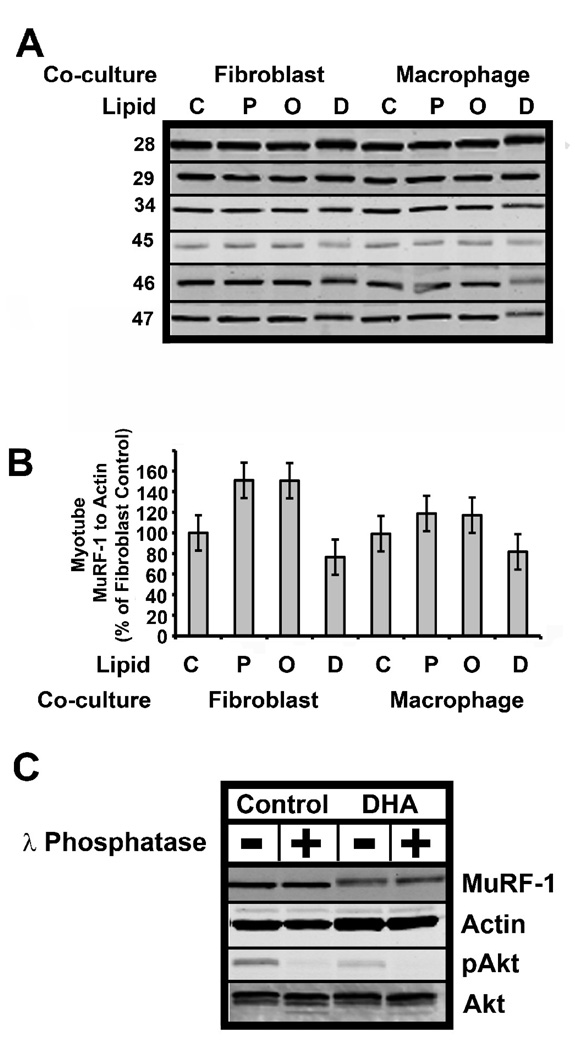
Fig. 4. Effect of DHA treatment on MuRF-1.
A) Human myotubes were co-cultured as in Fig. 1. The protein lysates were analyzed by immunoblotting for MuRF-1 or actin (not shown) as a load control. B) The MuRF-1 and actin levels were quantified as described under “Materials and Methods.” The MuRF-1 to actin ratio was normalized to the control-fibroblast level, which was set to 100%. C) Phosphatase treatment was used to determine whether the apparent increase in the mass of MuRF-1 was due to phosphorylation of MuRF-1. Thirty µg protein lysate from control or DHA treated muscle was treated with λ phosphatase as indicated. The lysate was then run on 10% SDS-PAGE gels, the gels were transferred to nitrocellulose and immunoblotted with MuRF-1, actin, phospho-S473 Akt or Akt as indicated. The data are representative of an experiment repeated three times.
3.3. Effect of FFAs on human muscle atrophy-inducing signaling
FFAs influenced both Fn14 and ubiquitin ligase expression in myotubes. Palmitic acid increased MAFbx mRNA in myotubes co-cultured cell with fibroblasts (p=0.0042) or macrophages (p=0.0003), but neither oleic acid nor DHA had an effect (Fig. 2A). DHA treatment reduced myotube Fn14 protein levels (p=0.007) in both fibroblast and macrophage co-cultures; furthermore, Fn14 levels did not become elevated in myotubes co-cultured with macrophages and treated with DHA (Fig. 3, compare macrophage co-culture lanes C and D). MuRF-1 protein levels were also affected by DHA treatment; however, there was a large variance in the baseline expression of MuRF-1 in the six cell lines (Fig. 4A). Therefore, we normalized MuRf-1 expression to the level in control (no FFA treatment) myotubes co-cultured with fibroblasts. Four of the six myotube lines treated with DHA (Fig. 4A; lines 45, 47, 34, and 46) had reduced MuRF-1 protein levels independent of co-cultured cell (Fig. 4). Although there was a trend towards reduction of MuRF-1 by DHA, it did not reach statistical significance. These results suggest that DHA may have a protective effect on muscle mass by reducing both Fn14 signaling and the ubiquitin ligase MuRF-1 in some individuals.
3.4. Post-translational modification of MuRF-1
Interestingly, the electrophoretic mobility of MuRF-1 was reduced in all six of the muscle lines when DHA was added (Fig. 4A). This observation suggests the possibility that DHA treatment causes post-translational modification of MuRF-1 that may make it more susceptible to degradation and explain the reduction in MuRF-1 protein levels by DHA treatment. Since it is known that phosphorylation may target proteins for proteasomal degradation [24], we determined whether phosphorylation of MuRF-1 caused the reduced mobility of MuRF-1. The addition of lambda protein phosphatase, which can dephosphorylate phospho- serine, threonine, and tyrosine, did not increase the migration of MuRF-1 from DHA treated myotubes. However, this treatment reduced the phosphorylation of Akt serine 473 (pAkt), and slightly increased the mobility of Akt, suggesting that dephosphorylation of the lysate was effective (Fig. 4C). Thus, the reduced mobility of MuRF-1 induced by DHA treatment is likely not due to phosphorylation. Identifying this post-translational modification of MuRF-1 will be the subject of future studies.
4. Discussion
Obesity causes an increase in macrophage infiltration into tissues that are the targets of insulin signaling including adipose, liver and muscle (reviewed in [1]). We reported that macrophage co-culture with human myotubes blunts insulin signaling and augments atrophy signaling pathways, and these effects are exacerbated by palmitic acid, a saturated FFA that is inflammatory [6]. In this study, the same co-culture system was used to compare the effect of unsaturated FFAs, oleic acid and DHA, to that of palmitic acid on insulin and atrophy signaling pathways in myotubes. Macrophage co-culture dramatically decreased myotube pAkt levels, and DHA did not protect against this. The major effects of either palmitic acid or DHA were to reduce baseline pAkt levels, and there was no effect of either macrophages or FFAs on the relative increase in pAkt due to insulin stimulation. This is surprising, given that ceramide and DAG levels were increased. Atrophy signaling pathways were induced by both macrophage co-culture and palmitic acid which increased MAFbx mRNA levels and Fn14 protein levels. The addition of DHA reduced Fn14 protein levels in a statistically significant manner and there was a trend to reduce MuRF-1 protein levels. Finally, DHA treatment likely induced a post-translational modification of MuRF-1 suggesting a possible mechanism to target MuRF-1 for degradation. Thus, DHA may have a beneficial effect on human muscle mass by reducing MuRF-1 ubiquitin ligase levels.
Suppression of baseline pAkt levels by DHA was unexpected, but its mechanism is likely to be different than palmitic acid. DHA has been shown to increase PTEN levels [25] and inhibit PI3K [26], both of which could reduce pAkt levels. Alternatively, in adipocytes, DHA increases pAkt levels by a pathway that involves the heterotrimeric G-protein coupled receptor GPR120 [15]. However, in mice this receptor is specifically expressed in cell types that include adipocytes and macrophages, but not muscle [15]. Thus, DHA treatment either increases or decreases pAkt depending upon the cell type. The precise mechanism that is induced by DHA treatment to regulate pAkt levels in human myotubes remains to be elucidated.
Although reducing baseline pAkt, palmitic acid treatment did not reduce the magnitude of the stimulation of pAkt levels in response to insulin. Whereas FFAs are known to induce insulin resistance in humans [5], there have been reports that cultured myotubes can be resistant to palmitic acid treatment. Bikman and colleagues reported that pooled myotubes from obese individuals were not inhibited by a 16 h pretreatment with 0.45 mM palmitic acid, a higher concentration than used in this study [27]. Alternatively, Thrush and colleagues, using isolated muscle preparations, found that muscle from lean but not obese humans was resistant to the effects of 2 mM palmitic acid [28]. We confirmed that palmitic acid increased DAG and ceramide levels. However, further investigation will be necessary to determine the mechanism for resistance to palmitic acid treatment.
Because of the remarkable reduction in pAkt in myotubes co-cultured with macrophages and our previous work [6], we also evaluated atrophy signaling. Macrophages are required for many aspects of muscle biology such as repair [29] and hypertrophy responses [30]. Depending on their activation state, macrophages have dramatically different effects on muscle including damage [31], induction of atrophy signaling [6], modulation of insulin signaling [6, 13], and supporting satellite cell proliferation and repair [29, 32, 33]. Both M1 and M2 macrophages are found in human muscle, with M2 primarily responding to hypertrophic stimuli from resistance training [34]. We hypothesize that obesity results in increased abundance of classically activated macrophages in muscle, and that this contributes to insulin resistance. The THP-1 monocytes used in this study were activated by PMA treatment which induces a classical activation state that is inflammatory and induces atrophy signaling [6]. Co-culture with these macrophages results in a dramatic reduction of pAkt which would be predicted to induce both MAFbx and MuRF-1 since activation of Akt can dominantly repress both of these ubiquitin ligases [35]. Furthermore, macrophage co-culture also increased Fn14. Fn14 is a receptor for TWEAK (TNF-like weak inducer of apoptosis), and Fn14-TWEAK signaling is thought to play a role in tissue repair [36]. In muscle, Fn14 is induced after denervation; whereas, TWEAK remains constant, indicating an “inside-out” signaling mechanism in which receptor levels are critical [23]. Furthermore, the analysis of TWEAK-Tg and TWEAK-null mice suggests that TWEAK-Fn14 signaling induces atrophy in mice by inducing MuRF-1 and that disruption of TWEAK-Fn14 signaling could have beneficial effects on muscle mass [23, 37]. The observation in this study that macrophage co-culture induces Fn14 suggests that MuRF-1 should increase. Whereas MAFbx mRNA was induced by macrophage co-culture at 24 hours, MuRF-1 mRNA was not induced. This suggests that the kinetics of induction of MAFbx and MuRF-1 in myotubes in response to macrophage co-culture are different. This could be due to a differential response to the pAkt-FOXO pathway and the Fn14-TWEAK pathway, both of which are induced by macrophage co-culture.
Omega-3 fatty acids such as DHA are anti-inflammatory and use multiple biochemical mechanisms to down-regulate inflammatory signaling pathways in cells [15, 38]. Although the macrophages used in this study were activated towards an inflammatory state, the effect that they had on human muscle pAkt could not be disrupted by DHA treatment. However, the atrophy-inducing signaling by macrophage co-culture could be disrupted by DHA since Fn14 was not up-regulated by macrophage co-culture in the presence of DHA. Furthermore, DHA treatment reduced MuRF-1 protein levels in the majority of the cell lines tested. Atrophy occurs with aging and certain diseases such as cancer and diabetes [39]. There have been reports that fish oil supplementation protects against weight loss in cancer [40, 41], and that reduced plasma omega-3 fatty acid levels are associated with muscle loss [42]. Finally, omega-3 fatty acid supplementation was shown to increase grip strength in the elderly, suggesting that fish oil supplementation preserves muscle mass in humans [43]. Thus, the ability of DHA to inhibit Fn14 signaling by reducing Fn14 protein levels and the ability of DHA to reduce MuRF-1 protein levels suggest that DHA treatment induces multiple mechanisms of lowering MuRF-1 protein levels and that DHA dietary supplementation may preserve muscle mass in humans.
Finally, DHA treatment caused a decrease in the mobility of MuRF-1, suggesting that MuRF-1 is post-translationally modified in myotubes. Since MuRF-1 protein levels tended to be reduced by DHA treatment, and phosphorylation is the first step in directing a number of proteins to the ubiquitin-proteasome degradation pathway, we determined whether phosphorylation caused the decrease in the mobility of Murf-1. Dephosphorylation of the protein extract with lambda phosphatase did not increase the electrophoretic mobility of MuRF-1, but was effective since pAkt was dephosphorylated (Fig. 4). Thus, phosphorylation is not responsible for the reduced electrophoretic mobility of MuRF-1 observed in response to DHA treatment. The modification may be acylation, perhaps by DHA itself, although palmitic acid and oleic acid treatment did not affect MuRF-1electrophoretic mobility. Thus, the identity of the post-translational modification and whether it causes MuRF-1 to be a substrate for ubiquitin ligases remain to be determined.
Acknowledgements
We would like to acknowledge Robert E. McGehee, Jr (Department of Pediatrics, University of Arkansas for Medical Sciences) for helpful discussions during the design of the study. We would also like to acknowledge Heather Bush (College of Public Health, University of Kentucky) for help with the statistical analysis of the data.
This work was supported by the following grants: DK 80327 (P.A.K.), DK 71349 (C.A.P. and P.A.K.), AG20941 (C.A.P.), and a merit grant from the Veterans Administration (N.R).
Abbreviations
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- DAG
diacylglycerol
- FFA
free fatty acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 2.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Gregorio GB, Yao-Borengasser A, Rasouli N, Varma V, Lu T, Miles LM, et al. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes. 2005;54:2305–2313. doi: 10.2337/diabetes.54.8.2305. [DOI] [PubMed] [Google Scholar]
- 4.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011 doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varma V, Yao-Borengasser A, Rasouli N, Nolen GT, Phanavanh B, Starks T, et al. Muscle inflammatory response and insulin resistance: synergistic interaction between macrophages and fatty acids leads to impaired insulin action. Am J Physiol Endocrinol Metab. 2009;296:E1300–E1310. doi: 10.1152/ajpendo.90885.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glatz JF, Luiken JJ, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev. 2010;90:367–417. doi: 10.1152/physrev.00003.2009. [DOI] [PubMed] [Google Scholar]
- 8.Senn JJ. Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J Biol Chem. 2006;281:26865–26875. doi: 10.1074/jbc.M513304200. [DOI] [PubMed] [Google Scholar]
- 9.Summers SA. Sphingolipids and insulin resistance: the five Ws. Curr Opin Lipidol. 2010;21:128–135. doi: 10.1097/MOL.0b013e3283373b66. [DOI] [PubMed] [Google Scholar]
- 10.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375:2267–2277. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J Biol Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 12.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samokhvalov V, Bilan PJ, Schertzer JD, Antonescu CN, Klip A. Palmitate- and lipopolysaccharide-activated macrophages evoke contrasting insulin responses in muscle cells. Am J Physiol Endocrinol Metab. 2009;296:E37–E46. doi: 10.1152/ajpendo.90667.2008. [DOI] [PubMed] [Google Scholar]
- 14.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 15.Oh da Y, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gingras AA, White PJ, Chouinard PY, Julien P, Davis TA, Dombrowski L, et al. Long-chain omega-3 fatty acids regulate bovine whole-body protein metabolism by promoting muscle insulin signalling to the Akt-mTOR-S6K1 pathway and insulin sensitivity. J Physiol. 2007;579:269–284. doi: 10.1113/jphysiol.2006.121079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buettner R, Parhofer KG, Woenckhaus M, Wrede CE, Kunz-Schughart LA, Scholmerich J, et al. Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J Mol Endocrinol. 2006;36:485–501. doi: 10.1677/jme.1.01909. [DOI] [PubMed] [Google Scholar]
- 18.Magee P, Pearson S, Allen J. The omega-3 fatty acid, eicosapentaenoic acid (EPA), prevents the damaging effects of tumour necrosis factor (TNF)-alpha during murine skeletal muscle cell differentiation. Lipids Health Dis. 2008;7:24. doi: 10.1186/1476-511X-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jho DH, Babcock TA, Tevar R, Helton WS, Espat NJ. Eicosapentaenoic acid supplementation reduces tumor volume and attenuates cachexia in a rat model of progressive non-metastasizing malignancy. JPEN J Parenter Enteral Nutr. 2002;26:291–297. doi: 10.1177/0148607102026005291. [DOI] [PubMed] [Google Scholar]
- 20.Dube JJ, Bhatt BA, Dedousis N, Bonen A, O'Doherty RM. Leptin, skeletal muscle lipids, and lipid-induced insulin resistance. Am J Physiol Regul Integr Comp Physiol. 2007;293:R642–R650. doi: 10.1152/ajpregu.00133.2007. [DOI] [PubMed] [Google Scholar]
- 21.Glass DJ. Signaling pathways perturbing muscle mass. Curr Opin Clin Nutr Metab Care. 2010;13:225–229. doi: 10.1097/mco.0b013e32833862df. [DOI] [PubMed] [Google Scholar]
- 22.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mittal A, Bhatnagar S, Kumar A, Lach-Trifilieff E, Wauters S, Li H, et al. The TWEAK-Fn14 system is a critical regulator of denervation-induced skeletal muscle atrophy in mice. J Cell Biol. 2010;188:833–849. doi: 10.1083/jcb.200909117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunter T. The age of crosstalk: phosphorylation, ubiquitination, and beyond. Mol Cell. 2007;28:730–738. doi: 10.1016/j.molcel.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh-Choudhury T, Mandal CC, Woodruff K, St Clair P, Fernandes G, Choudhury GG, et al. Fish oil targets PTEN to regulate NFkappaB for downregulation of anti-apoptotic genes in breast tumor growth. Breast Cancer Res Treat. 2009;118:213–228. doi: 10.1007/s10549-008-0227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Couplan E, Le Cann M, Le Foll C, Corporeau C, Blondel M, Delarue J. Polyunsaturated fatty acids inhibit PI3K activity in a yeast-based model system. Biotechnol J. 2009;4:1190–1197. doi: 10.1002/biot.200800229. [DOI] [PubMed] [Google Scholar]
- 27.Bikman BT, Zheng D, Reed MA, Hickner RC, Houmard JA, Dohm GL. Lipid-induced insulin resistance is prevented in lean and obese myotubes by AICAR treatment. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1692–R1699. doi: 10.1152/ajpregu.00190.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thrush AB, Heigenhauser GJ, Mullen KL, Wright DC, Dyck DJ. Palmitate acutely induces insulin resistance in isolated muscle from obese but not lean humans. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1205–R1212. doi: 10.1152/ajpregu.00909.2007. [DOI] [PubMed] [Google Scholar]
- 29.Tidball JG, Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J Physiol. 2007;578:327–336. doi: 10.1113/jphysiol.2006.118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiPasquale DM, Cheng M, Billich W, Huang SA, van Rooijen N, Hornberger TA, et al. Urokinase-type plasminogen activator and macrophages are required for skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol. 2007;293:C1278–C1285. doi: 10.1152/ajpcell.00201.2007. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen HX, Tidball JG. Interactions between neutrophils and macrophages promote macrophage killing of rat muscle cells in vitro. J Physiol. 2003;547:125–132. doi: 10.1113/jphysiol.2002.031450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chazaud B, Sonnet C, Lafuste P, Bassez G, Rimaniol AC, Poron F, et al. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol. 2003;163:1133–1143. doi: 10.1083/jcb.200212046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massimino ML, Rapizzi E, Cantini M, Libera LD, Mazzoleni F, Arslan P, et al. ED2+ macrophages increase selectively myoblast proliferation in muscle cultures. Biochem Biophys Res Commun. 1997;235:754–759. doi: 10.1006/bbrc.1997.6823. [DOI] [PubMed] [Google Scholar]
- 34.Przybyla B, Gurley C, Harvey JF, Bearden E, Kortebein P, Evans WJ, et al. Aging alters macrophage properties in human skeletal muscle both at rest and in response to acute resistance exercise. Exp Gerontol. 2006;41:320–327. doi: 10.1016/j.exger.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 36.Winkles JA. The TWEAK-Fn14 cytokine-receptor axis: discovery, biology and therapeutic targeting. Nat Rev Drug Discov. 2008;7:411–425. doi: 10.1038/nrd2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mittal A, Bhatnagar S, Kumar A, Paul PK, Kuang S, Kumar A. Genetic ablation of TWEAK augments regeneration and post-injury growth of skeletal muscle in mice. Am J Pathol. 2010;177:1732–1742. doi: 10.2353/ajpath.2010.100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jump DB. The biochemistry of n-3 polyunsaturated fatty acids. J Biol Chem. 2002;277:8755–8758. doi: 10.1074/jbc.R100062200. [DOI] [PubMed] [Google Scholar]
- 39.Park SW, Goodpaster BH, Lee JS, Kuller LH, Boudreau R, de Rekeneire N, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32:1993–1997. doi: 10.2337/dc09-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barber MD, Ross JA, Voss AC, Tisdale MJ, Fearon KC. The effect of an oral nutritional supplement enriched with fish oil on weight-loss in patients with pancreatic cancer. Br J Cancer. 1999;81:80–86. doi: 10.1038/sj.bjc.6690654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colomer R, Moreno-Nogueira JM, Garcia-Luna PP, Garcia-Peris P, Garcia-de-Lorenzo A, Zarazaga A, et al. N-3 fatty acids, cancer and cachexia: a systematic review of the literature. Br J Nutr. 2007;97:823–831. doi: 10.1017/S000711450765795X. [DOI] [PubMed] [Google Scholar]
- 42.Murphy RA, Mourtzakis M, Chu QS, Reiman T, Mazurak VC. Skeletal muscle depletion is associated with reduced plasma (n-3) fatty acids in non-small cell lung cancer patients. J Nutr. 2010;140:1602–1606. doi: 10.3945/jn.110.123521. [DOI] [PubMed] [Google Scholar]
- 43.Robinson SM, Jameson KA, Batelaan SF, Martin HJ, Syddall HE, Dennison EM, et al. Diet and its relationship with grip strength in community-dwelling older men and women: the Hertfordshire cohort study. J Am Geriatr Soc. 2008;56:84–90. doi: 10.1111/j.1532-5415.2007.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]