Abstract
Wnt signaling has been implicated in many developmental processes, but its role in early endoderm development is not well understood. Wnt signaling is active in posterior endoderm as early as E7.5. Genetic and chemical activation show that the Wnt pathway acts directly on endoderm to induce the intestinal master regulator Cdx2, shifting global gene away from anterior endoderm and toward a posterior, intestinal program. In a mouse embryonic stem cell differentiation platform that yields pure populations of definitive endoderm, Wnt signaling induces intestinal gene expression in all cells. We have identified a set of genes specific to the anterior small intestine, posterior small intestine, and large intestine during early development, and show that Wnt, through Cdx2, activates large intestinal gene expression at high doses and small intestinal gene expression at lower doses. These findings shed light on the mechanism of embryonic intestinal induction and provide a method to manipulate intestinal development from embryonic stem cells.
Keywords: Endoderm, Embryonic stem cell differentiation, Intestinal development
Introduction
There are two main reasons for studying the development of the intestine. First, the intestine is responsible for digestion and absorption of food. Second, colorectal cancers are the second leading cause of cancer-related mortality, and Inflammatory Bowel Syndromes afflict hundreds of thousands of people. Despite its essential functions and medical problems, rather little is known about how different cell types within the intestine arise during embryogenesis.
During mouse development, the endoderm forms between E6.5–E7.5 as a flat sheet on the outside of the cup-shaped embryo. Endoderm begins to fold ventrally at the caudal end of the endoderm, at E8.25, forming a tube known as the caudal intestinal portal (CIP). This nascent tube zips progressively anteriorly until it joins the anterior intestinal portal (AIP), forming an internal single-cell-thick gut tube by E8.75. The single-layered intestinal epithelial tube proliferates and begins to become stratified between E8.75 and E14.5, at which point the intestine begins to deform in the radial plan, eventually forming fingerlike protrusions into the lumen. The adult intestine is composed of these protrusions, known as villi, which contain mature intestinal absorptive, secretive, and endocrine cells, as well as crypts that contain stem cells that replenish the intestinal epithelium throughout adult life (van der Flier and Clevers, 2009).
While considerable insight has been gained about adult intestinal stem cell self-renewal, differentiation, and dysfunction in disease (Barker et al., 2008), relatively little attention has been given to the initial specification and morphogenesis of the intestine. Protohox genes of the Cdx family, the mammalian homologues of the Drosophila posterior determinant Caudal (Mlodzik et al 1985), are required for posterior development in all germ layers (Chawengsaksophak et al., 2004; Subramanian et al., 1995; van den Akker et al., 2002). Cdx2 is the first known molecular marker for the future intestinal epithelium, first expressed in the posterior endoderm at embryonic day 7.5 (E7.5, (Beck et al., 1995; Sherwood et al., 2009)). Cdx2 has been shown to be necessary for intestinal specification in the endoderm (Gao et al., 2009), controlling a network of intestinal genes.
Tcf1/Tcf4 mutants have embryonic intestinal defects, implicating the Wnt pathway in intestinal development (Gregorieff et al., 2004) and inhibition of Wnt signaling appears necessary for proper differentiation of foregut endoderm derivatives (Heller et al., 2002; Kim et al., 2005; McLin et al., 2007; Okubo and Hogan, 2004). Numerous Wnt ligands are present in the early node and primitive streak, including Wnt3a (Takada et al., 1994), Wnt5a (Yamaguchi et al., 1999), and Wnt8a (Bouillet et al., 1996). The developing gut also expresses a multitude of secreted Wnt repressors including those of the Sfrp and Dkk families (Theodosiou and Tabin, 2003), so expression alone is insufficient to predict where Wnt signaling is occurring. Other signaling pathways including the Hedgehog and Bmp families (Roberts et al., 1998) and Notch (Stanger et al., 2005) have been implicated in intestinal development as well.
The small intestine is traditionally divided into three segments, the duodenum, jejunum and ileum, by morphological criteria, and the large intestine is similarly divided into the cecum, ascending, transverse, and descending colon and rectum. Villous architecture and Paneth cells at the base of crypts are present only in small intestine, while the large intestine is characterized by a flat epithelium with deep crypts. Many digestive enzymes are exclusive to the duodenum and pancreas (Whitcomb and Lowe, 2007). Enteroendocrine cells display segment-specific expression, with CCK primarily produced in anterior small intestine and PYY and GLP1 primarily in distal small intestine and large intestine (Ratineau et al., 2003). To what extent molecular distinctions exist among the small and large intestinal segments is unknown and no mechanism has been proposed for the acquisition of these differences.
Here we describe the role of Wnt signaling in intestinal specification and regionalization. We find that Wnt signaling activates Cdx2 in the early endoderm and is capable of inducing an intestinal gene program in anterior endoderm cells. Wnt signaling not only specifies intestinal fate from undifferentiated endoderm, it appears to have concentration-dependent effects on anterior-posterior intestinal segments. Consequently, a molecular heterogeneity appears before any morphological distinctions are apparent. Finally, we have transferred these findings to the in vitro differentiation of embryonic stem cells and show that Wnt signaling efficiently specifies intestinal fate in endoderm derived from mouse and human ES cells.
Materials and Methods
Tissue manipulation and bead implantation
For all experiments, outbred ICR mice (Taconic, Germantown, NY) and transgenic strains were bred and maintained at the Harvard Biomedical Research Infrastructure. Embryos were considered to be E0.5 at noon of the day the plug was detected. Sox17-CreER mice were generated using a similar cloning strategy to that of Borowiak et al (Borowiak et al., 2009) and will be described in a manuscript in preparation.
Tissues were dissected in HBSS (Sigma, St. Louis, MO). AG 1-X8 beads, formate form, 200–400 µm diameter (Bio-Rad, Hercules, CA) were soaked in either DMSO (Sigma) or GSK3iXV (EMD Biosciences, Gibbstown, NJ) and implanted into the foregut cavity with forceps. Dissected tissues were cultured in DMEM:F12 (Invitrogen, Carlsbad, CA) with 10% FBS (Hyclone, Logan, UT) and penicillin/streptinomycin (Invitrogen).
Electroporation experiments were performed according to published protocols (Pierreux et al., 2005). Embryos were bathed in 1 µg/µL plasmid solution and electroporated using a T820 Electro Square Porator (BTX, Holliston, MA) using three 12-Volt pulses with 50 µs interval. Embryos were cultured in static culture for 24 hours in 1:1 DMEM:F12 (Invitrogen): rat serum (Valley Biomedical, Winchester, VA) or dissected and cultured as dissected tissues as above.
Flow cytometry and microarray profiling
Tissues were dissociated in 0.25% Trypsin (Invitrogen) for 2–5 minutes at 37 degrees and, after neutralization and centrifugation, were stained on ice for 15 minutes in DMEM:F12 (Invitrogen) with 2% FBS (Hyclone) and 2 mM EDTA. Before flow cytometric sorting, cells were resuspended in staining buffer with calcein blue AM (Invitrogen) and sorted using a FACSAria (Becton Dickinson, San Jose, CA).
Sorted cell populations were collected in Trizol (Invitrogen), and RNA was isolated according to the manufacturer’s instructions. RNA was amplified and biotinylated cRNA probe generated using the Ambion Illumina TotalPrep RNA Amplification Kit (Applied Biosystems/Ambion, Austin, TX). Biotinylated cRNA was hybridized onto Illumina MouseRef-8 v2 microarrays (Illumina, San Diego, CA) according to the manufacturer’s instructions. Microarrays were scanned using Beadstation (Illumina), and data was analyzed using BeadStudio (Illumina). Two to three biological replicates were performed for each tissue analyzed.
Antibodies and immunofluorescence
For immunofluorescence analysis, wholemount tissues were fixed for 15 minutes in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) and washed in PBS with 1% Tween-20 (Sigma). Tissues were blocked by 1 hour incubation at 4 degrees in PBS with 20% donkey serum (Jackson Immunoresearch, West Grove, PA) and 1% Tween-20. Primary and secondary antibody staining were performed by rocking overnight at 4 degrees in PBS with 5% donkey serum, and after primary and secondary antibody staining, washing was performed for >5 hours in PBS with 1% Tween-20.
After staining, wholemount embryos were transferred to 1:1 glycerol:PBS with 1% Tween and placed between two coverslips. Confocal imaging was performed using an LSM 510Meta confocal microscope (Carl Zeiss Inc., Germany).
The following primary antibodies were used: rabbit anti- β-galactosidase, chicken anti-GFP (Millipore, Billerica, MA); G8.8 (anti-EpCAM; Developmental Studies Hybridoma Bank, Iowa City, IA); goat anti-Foxa2 (M-20), goat anti-Sox2 (Y-17; Santa Cruz Biotechnology, Santa Cruz, CA); biotinylated DBA (Vector Labs, Burlingame, CA); mouse anti-Cdx2 (Biogenex, San Ramon, CA); biotin anti-Ly-6A (D7; ebioscience, San Diego, CA) and FITC-conjugated rat anti-CD26 (BD Biosciences, San Jose, CA). Alexa Fluor 488, 594, 647 and Pacific Blue secondary antibody conjugates (Invitrogen) as well as PE and APC conjugates (Jackson Immunoresearch) were used for secondary detection.
In situ hybridization
In situ hybridization was performed according to (Wilkinson and Nieto, 1993). cDNA was obtained from commercial clone libraries (Open Biosystems, Huntsville, AL) and digoxigenin-labeled probes were generated. Tissues were fixed overnight in 4% paraformaldehyde, dehydrated in methanol, bleached in hydrogen peroxide and treated with proteinase K, followed by re-fixation. Probe was added at 1 µg/mL overnight at 70 degrees. Embryos were washed and labeled overnight with anti-digoxigenin-AP antibody (Roche Applied Science, Indianapolis, IN). Embryos were developed in BM Purple (Roche Applied Science).
Embryonic stem cell culture and differentiation
Undifferentiated 129P2/OlaHsd mouse ES cells were maintained on gelatin-coated plates with mouse embryonic fibroblast (MEF) feeders in DMEM (Invitrogen) supplemented with 15% defined fetal bovine serum (FBS) (HyClone), 0.1mM nonessential amino acids (Invitrogen), Glutamax (Invitrogen), penicillin/streptomycin (Invitrogen), 0.55mM 2-mercaptoethanol (Sigma), and 1X ESGRO LIF (Chemicon, Temecula, CA).
Prior to differentiation, ES cells were passaged onto gelatin-coated plates for 30 minutes to deplete MEFs. MEF-depleted ES cells were then seeded at 1 *10^4 cells/cm2 onto gelatin-coated dishes in Advanced DMEM (Invitrogen) supplemented with N-2 (Invitrogen), B27 Supplement without vitamin A (Invitrogen), 0.05% Albumax II (Invitrogen), Glutamax, and 2.5 µM Y-27632 (Tocris Bioscience, Ellisville, MO). After 40–44 hours, media was changed to Advanced DMEM with 2% FBS, Glutamax, 5 nM GSK-3 inhibitor XV and 50 ng/mL E. coli-derived Activin A (Peprotech, Rocky Hill, NJ). After 1 day, cells were fed with Advanced DMEM with 2% FBS, Glutamax, 50 ng/mL Activin A and 1 µM Dorsomorphin (Sigma). After 5 days of differentiation, cells were either stained for endodermal genes or passaged using 0.25% Trypsin onto plates coated with 804G cell conditioned media and treated with DMSO or GSK3iXV at the concentrations indicated in Advanced DMEM with B-27 supplement without vitamin A and Glutamax.
ES cells with a doxycycline-inducible Cdx2 allele in the HPRT locus were created as described (Iacovino et al., 2009) and maintained and differentiated as above.
HUES8 human embryonic stem cells were maintained and differentiated toward endoderm as described (Chen et al., 2009). After endoderm induction, cells were treated with 0.05% Trypsin and re-plated onto plates coated with conditioned media from the 804G cell line in RPMI (Invitrogen) with 2% FBS, Glutamax and peniciliin/streptomycin (Invitrogen). Cells were cultured for 48 hours in the same media with 125 nM GSK3iXV when indicated prior to fixation and immunostaining.
Results
Wnt signaling is active in posterior endoderm between E7.5–E8.5
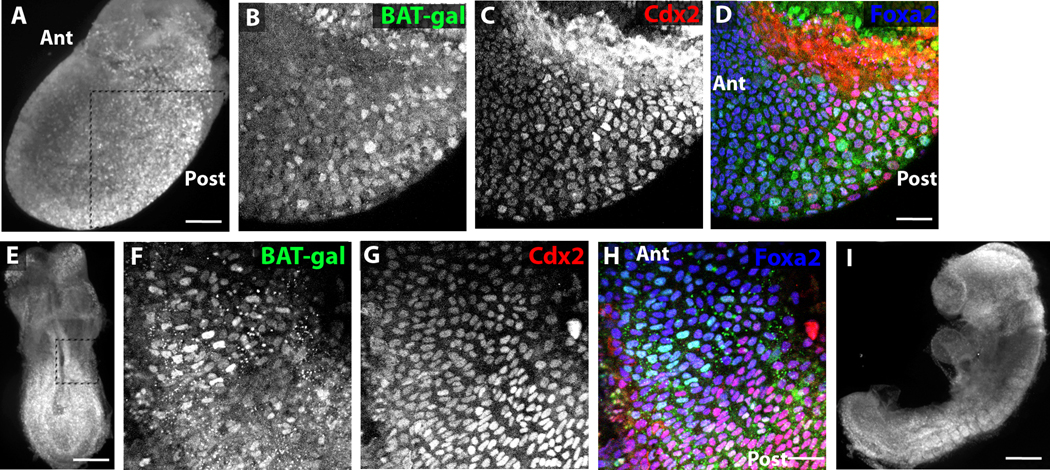
To determine whether Wnt signaling is active in early definitive endoderm, wholemount confocal analysis was performed on E7.5–E9.5 BAT-gal mouse embryos. This mouse strain reports canonical Wnt activity through expression of nuclear β-galactosidase (Maretto et al., 2003), and Wnt is known to be expressed during gastrulation and neurulation in the posterior region of the embryo. Cells co-expressing the pan-endodermal transcription Foxa2 and β -galactosidase can be seen in the posterior of the embryo starting at E7.5 (Figure 1A–D). Many of these cells also express the posterior endodermal and future intestinal transcriptional regulator Cdx2. β-gal-expressing cells can also be found more anteriorly in the endoderm than Cdx2+ cells (Figure 1D). At E8.25, β -gal+Foxa2+ cells occur as a swath of cells spanning the anterior border of Cdx2 expression in the midgut with the anterior border of β-gal expression occurring a few cell diameters more anteriorly than the border of Cdx2 expression (Figure 1E–H). By E8.75, β -gal expression in the posterior endoderm can no longer be detected (Figure 1I). Additionally, the Wnt target gene Axin2 (Jho et al., 2002) is expressed highly in E7.5–E8.5 endoderm but is downregulated by E9.5, and its expression is significantly weaker in anterior endoderm than in posterior endoderm at E8.5 (Supplemental Figure 1). Thus, in the E7.5–E8.5 endoderm, a correlation between the anterior border of Cdx2 expression and Wnt activity is apparent.
Figure 1. Wnt signaling is active at the anterior border of Cdx2+ endoderm between E7.5–E8.5.
(A) Wholemount immunofluorescence image of E7.5 BAT-Gal embryo stained for β-gal. Boxed area represents embryonic region imaged in higher detail in B–D. (B–D) Wholemount confocal immunofluorescence images of E7.5 BAT-Gal embryo stained for β-gal (B, green in D), Cdx2 (C, red in D), and Foxa2 (blue in D). (E) Wholemount immunofluorescence image of E8.25 BAT-Gal embryo stained for β-gal. Boxed area represents embryonic region imaged in higher detail in F–H. (F–H) Wholemount confocal immunofluorescence images of E8.5 BAT-Gal embryo stained for β-gal (F, green in H), Cdx2 (G, red in H), and Foxa2 (blue in H). (I) Wholemount immunofluorescence image of E8.75 BAT-Gal embryo stained for β-gal. Scale bar equals 500 µm in A, E, and I and 50 µm in D and H.
Activation of Wnt signaling induces Cdx2 expression and posterior migration of definitive endoderm
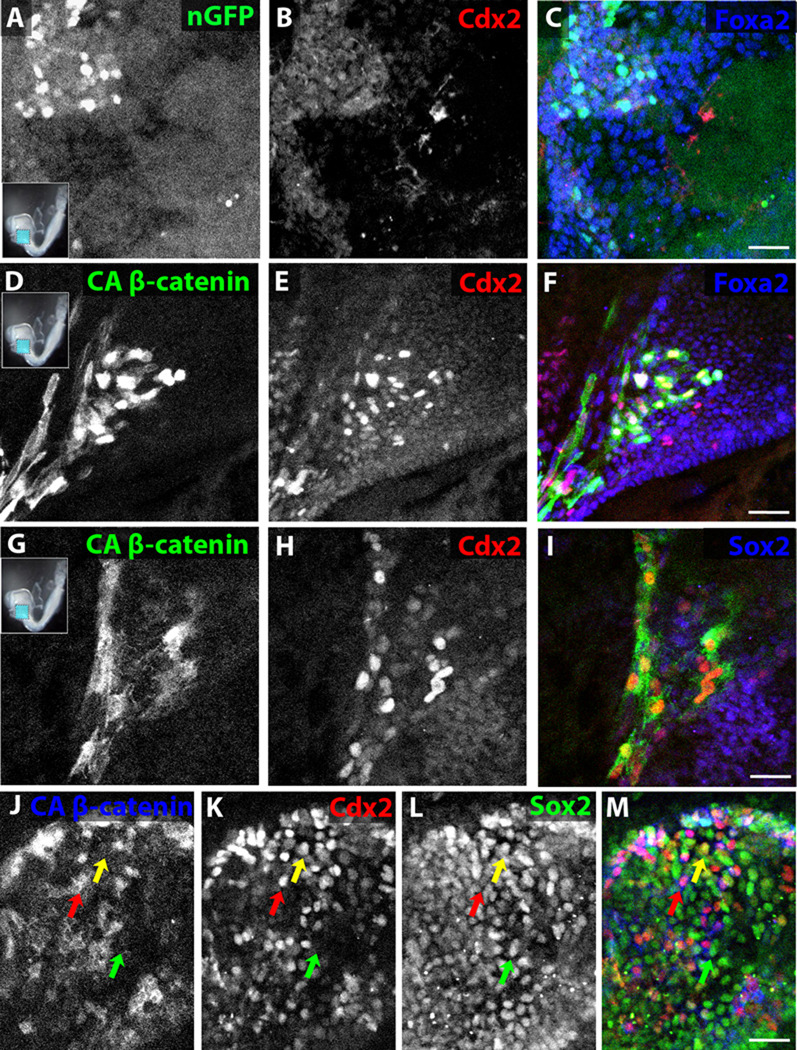
To determine the effect of Wnt signaling on endodermal organ fate specification, a whole embryo electroporation strategy was used to introduce DNA into the E8.25 foregut endoderm (Pierreux et al., 2005). After electroporation of the foregut with a control GFP-expressing vector and 24 hours of subsequent embryo culture, electroporated cells can be detected specifically in the foregut endoderm and, as expected, do not express Cdx2 (0/10 embryos have GFP+Cdx2+ cells, Figure 2A–C). When a vector expressing constitutively active beta catenin (CA β-catenin), which activates the Wnt signaling pathway (Toyofuku et al., 2000), fused to GFP was electroporated, a majority of electroporated cells express Cdx2 (10/10 embryos have GFP+Cdx2+ cells, Figure 2D–F). Cells transfected with CA β-catenin tend to cluster and can be found within or nearby the endogenous Cdx2+ posterior endodermal domain even though the electroporated domain in control GFP electroporations is around 20 cell diameters from the endogenous Cdx2+ domain.
Figure 2. Electroporation of activated β-catenin induces Cdx2 and represses Sox2 in foregut endoderm.
(A–K) Wholemount confocal immunofluorescence images of E8.25 foregutelectroporated with nuclear GFP (A–C) or activated β-catenin (D–M) and cultured for 24 hours in whole embryo culture (A–F) or foregut explant culture (G–M). Electroporated embryos were all stained for GFP (A, D, G, J) and Cdx2 (B, E, H, K) and some also for Foxa2 (C, F) or Sox2 (I, L). (J–M) Red arrow refers to a Cdx2+Sox2−cell, green arrow to a Sox2+Cdx2−cell and yellow arrow to a rare Cdx2+Sox2+ cell. Scale bar equals 100 µm in C and F and 50 µm in I and M.
In the CA β-catenin electroporations, GFP is first detected within 4 hours without significant Cdx2 co-expression (Supplemental Figure 2). By 8 hours, about half of the GFP-expressing cells co-express Cdx2 (Supplemental Figure 2), and by 12 hours, most GFP+ cells co-express Cdx2 (Supplemental Figure 2). In the first 8 hours, less than 5% of control GFP-electroporated and 25% of CA β-catenin-electroporated cells can be found within 5 cell layers of the endogenous hindgut Cdx2+ domain, whereas at and after 12 hours, 90% of CA β-catenin-electroporated cells are found within 5 cell layers of the endogenous hindgut Cdx2+ domain, which may be the result of selective survival or posterior migration (Supplemental Figure 2), and explant culture of the nascent gut tube for an additional 48 hours following the 24 hour embryo culture reveals a persistence of electroporated cells selectively in the Cdx2+ intestinal epithelium (Supplemental Figure 2).
To determine whether Wnt is capable of inducing Cdx2 in the absence of endogenous Cdx2-expressing endoderm, the anterior half of the embryo was explanted after foregut electroporation of CA β-catenin. In the absence of CA β-catenin electroporation, the foregut is devoid of Cdx2 expressing cells and uniformly expresses Sox2 (data not shown; (Sherwood et al., 2009)). After 24 hours in vitro, GFP+ cells express Cdx2 and the vast majority display markedly reduced expression of the anterior endoderm marker Sox2 (Figure 2G–M), although rare co-expression of Cdx2 and Sox2 can be detected (Figure 2J–M). Electroporation of CA β-catenin into E7.75 anterior endoderm yields significant co-expression of GFP and Cdx2 (Supplemental Figure 2); however, electroporation of CA β-catenin into E9.5 stomach does not yield any induction of Cdx2 (Supplemental Figure 2), suggesting that CA β-catenin (Wnt signaling) is able to induce endodermal Cdx2 expression in endoderm from E7.5 to about E8.5.
In vivo genetic and in vitro chemical activation of Wnt signaling induce Cdx2 expression in definitive endoderm
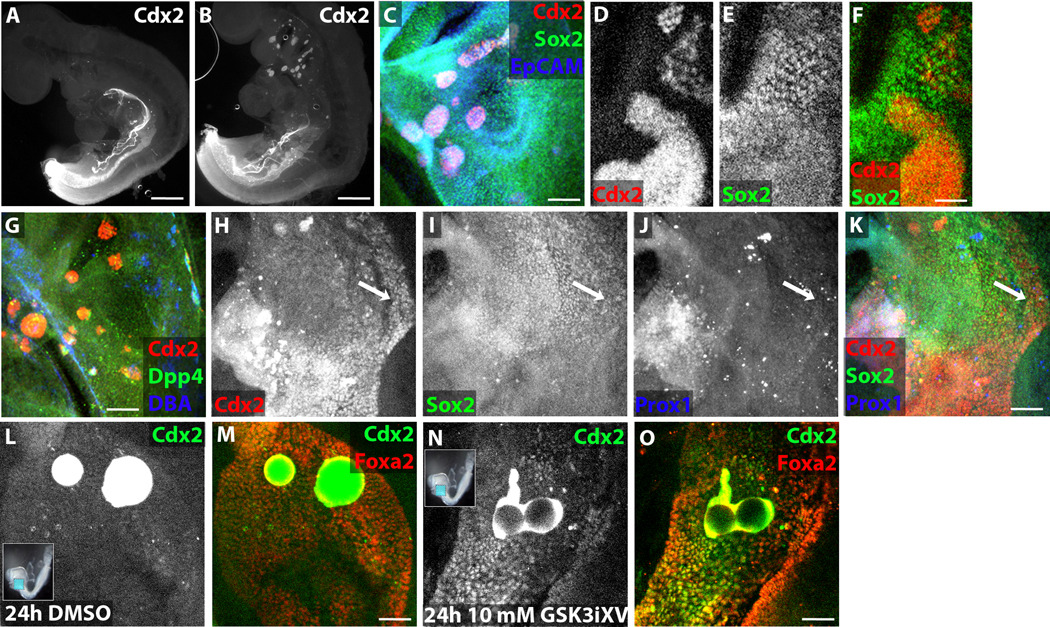
To confirm that Wnt signals can induce Cdx2 expression in definitive endoderm, in vivo genetic and in vitro chemical activation of Wnt signaling were employed. Mice engineered to express CreER from the Sox17 locus were crossed with mice containing loxP sites flanking exon 3 of β-catenin, which when excised creates a constitutively active form. Tamoxifen was administered at E6.5, a time point found to cause mosaic, endoderm-exclusive recombination in all anterior-posterior regions (manuscript in preparation). At E9.5, no gross abnormalities can be detected, yet upon wholemount immunofluorescence analysis, clusters of Cdx2-expressing cells are found throughout the anterior endoderm in half of the embryos (12/24), while no Cdx2-expressing cells are detected in the foregut of the other half of the embryos (12/24) (Figure 3A–C). Some Cdx2+ cells within these clusters downregulate the anterior endoderm marker Sox2 while others retain Sox2 expression (Figure 3D–F). A subset of these ectopic Cdx2+ cells express the intestinal markers Dpp4 and DBA (Figure 3G). While most embryos segment their endodermal organs normally, one of 12 mutant embryos examined had a missing dorsal pancreatic domain replaced by Cdx2-expressing cells (Figure 3H–K), a phenomenon that may be explained by variable recombination frequency and location. A similar phenotype of ectopic clusters of Cdx2-expressing cells is detected at E10.5 (data not shown), but mutant embryos cannot be recovered at later timepoints, presumably due to Tamoxifen toxicity at doses sufficient to induce recombination (Danielian et al., 1998), precluding analysis of intestinal maturation in the ectopic clusters.
Figure 3. Genetic and chemical activation of Wnt signaling leads to intestine induction in the foregut.
(A–B) Wholemount immunofluorescence images of E9.5 Sox17CreER X β-catenin+/+ wildtype littermate (A) and Sox17CreER X β-cateninEx3/+ (B) embryos stained for Cdx2. (C–E) Wholemount confocal immunofluorescence images of E9.5 Sox17CreER X β-cateninEx3/+ foregut (C–G) or stomach-intestine border (H–K) stained for Cdx2 (C, D, G, H), Sox2 (C, E, I), EpCAM (C), Dpp4 (G), DBA (G), and Prox1 (J). Arrow in H–K indicates missing dorsal pancreatic domain replaced by Cdx2 expression. (L–O) Wholemount confocal immunofluorescence images of E8.25 explanted foreguts cultured for 24 hours before fixation and stained for Cdx2 (L, N) and Foxa2 (M, O). Foreguts were implanted with beads soaked in DMSO (L–M) or 10 mM GSK3iXV (N–O). Scale bar equals 500 µm in A–B, 200 µm in C, G, and K, and 100 µm in F, M, and O.
To establish a method to allow for controlled manipulation of Wnt signaling in embryos, beads soaked in a highly selective chemical GSK3 inhibitor [GSK3 inhibitor XV (GSK3iXV)] (Atilla-Gokcumen et al., 2006), which activates Wnt signaling (Siegfried et al., 1994), were placed in the foregut cavity of E8.25 embryos. Immediately after bead implantation, the anterior half of the embryo was explanted and cultured for 24 hours. Cdx2+ cells are never detected in the foregut in the absence of beads or surrounding DMSO-soaked beads (n=10, Figure 3F–G), while large numbers of Cdx2+Foxa2+ endodermal cells can be found surrounding GSK3iXV-soaked beads in all embryos examined (n=20, Figure 3H–I)+. Cdx2 protein can be detected between 6 and 8 hours after treatment with GSK3iXV beads (data not shown). Consistent with the electroporation results, GSK3iXV soaked beads induce Cdx2 expression when placed in the nascent foregut of E8.0 embryos for 24 hours but are unable to induce Cdx2 expression in the E9.5 oral cavity or stomach after 24 or 48 hours (data not shown).
Characterization of intestinal induction of Wnt pathway
While Cdx2 is a key regulator of intestinal formation, we sought to determine whether activation of Wnt signaling more broadly can induce intestinal fates. To obtain a global view of gene expression changes induced by the Wnt pathway, microarray analysis was performed on anterior explants with either DMSO- or GSK3iXV-soaked beads placed in the foregut cavity. At two time points, after 6 and 24 hours in vitro, the pooled anterior explants were dissociated, and definitive endoderm was isolated by expression of the pan-endodermal cell surface protein EpCAM and minimal expression of the extraembryonic endoderm-specific lectin DBA (Sherwood et al., 2007).
A small number of genes are differentially expressed at 6 hours, and a much larger set of genes becomes differentially expressed at 24 hours (selected genes highlighted in Table 1). As expected, Cdx2 mRNA is highly upregulated by GSK3iXV beads within 6 hours (9.5-fold at 6 hours, 21.8-fold at 24 hours). Additionally, several positive and negative regulators of the Wnt pathway are strongly induced, including Dkk1, 3 and 4, Axin2, Fzd1 and 10, Wnt6 and 10a. Crucially, GSK3iXV beads downregulate expression of transcription factors involved in anterior endoderm development, starting with a subset of factors including Hhex, Otx2 and Sox2 at 6 hours and progressing to most known anterior endodermal transcription factors by 24 hours (Table 1). To obtain a more comprehensive view of the intestinal induction of GSK3iXV, a set of 50 genes unique to the intestine within the E11.5 endoderm (Sherwood et al., 2009) were selected. Intestinal gene expression after treatment with GSK3iXV versus control beads was relatively unchanged after 6 hours (mean foldchange 1.27, median foldchange 0.89, standard deviation 1.81) but dramatically induced by 24 hours (mean 3.25, median 1.24, standard deviation 10.3). This upregulation of intestinal genes, combined with the downregulation of genes involved in anterior endodermal development, provides strong evidence that Wnt pathway activation induces an intestinal gene expression program.
Table 1. Transcription factors and select other genes significantly affected by GSK3iXV treatment in E8.25 foregut endoderm.
Expression of these genes is changed >2-fold for 6 hour timepoint and >2.5-fold for 24 hour timepoint in EpCAM+ endoderm by treatment of E8.25 foregut endoderm with 10 mM GSK3iXV beads.
| Selected genes upregulated within 6 hours |
Selected genes upregulated between 6 and 24 hours |
Selected genes downregulated within 6 hours |
Selected genes downregulated between 6 and 24hours |
|---|---|---|---|
| Defcr-rs2 | Hoxd8 | Hhex | Rarb |
| Dkk4 | Hoxd9 | Wnt8a | Foxe1 |
| Evx1 | Hoxc9 | Hesx1 | Hoxb1 |
| Cdx2 | Hoxa7 | Gsc | Tbx1 |
| Sox17 | Fzd10 | Sox2 | Foxa1 |
| Axin2 | Sct | Pax1 | |
| Wnt6 | Sp7 | ||
| Cdx1 | T | ||
| Tcf7 | |||
| Klf5 | |||
| Lef1 | |||
| Sp6 | |||
| Tbx3 |
Wnt signaling induces intestinal differentiation in mouse embryonic stem cell-derived endoderm
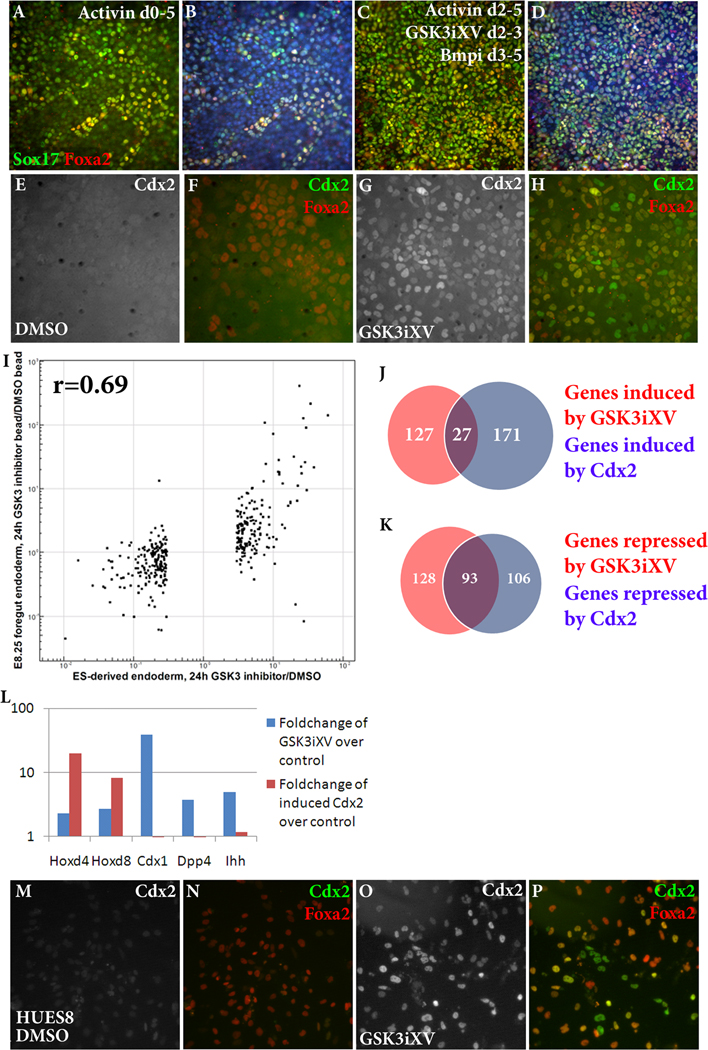
As our experiments demonstrated a role for Wnt signaling in embryonic intestinal specification from endoderm, we sought to determine whether Wnt signaling could be used to direct a similar differentiation in ES cell derivatives. To this end, we modified existing protocols to improve the efficiency of mouse ES cell differentiation into definitive endoderm. TGFβ ligands, in particular Activin A, have been widely used in endoderm differentiation of ES cells (Kubo et al., 2004; Tada et al., 2005; Gadue et al., 2006(Hansson et al., 2009)); however, addition of Activin A to ES cells for 5 days yields a heterogeneous population with only about half of the cells expressing the endodermal markers Sox17 and Foxa2 (Figure 4A–B).
Figure 4. Wnt induces intestinal differentiation from mouse and human ES cell-derived endoderm.
(A–D) Immunofluorescence analysis of mouse ES cells treated with Activin A from days 0–5 (A–B) or Activin A from days 2–5, GSK3iXV from day 2–3 and Dorsomorphin from days 3–5 (C–D) before fixation and stained for Sox17 (green) and Foxa2 (red). Nuclei are stained with Hoechst 33342 (B, D). (E–J) Immunofluorescence analysis of mouse ES-derived endoderm treated with DMSO (E–F) or 50 nM GSK3iXV (G–H) or 1 µg/mL Wnt3a (I–J) for 24 hours before fixation and stained for Cdx2 (white in E, G, I, green in F, H, J) and Foxa2 (red in F, H, J). (K) Graph comparing the microarray foldchange induced by 24 hours of GSK3iXV treatment in ES-derived endoderm (X-axis) and E8.25 endoderm (Y-axis). Only genes significantly affected by GSK3iXV in E8.25 endoderm are displayed, and the correlation coefficient (r) is shown. (L–M) Venn diagrams displaying the fraction of genes induced (L) or downregulated (M) by GSK3iXV treatment (red) and Cdx2 induction (blue) of ES-derived endoderm as determined by microarray analysis. (N) Graph displaying the microarray foldchange in certain genes induced by 24 hours of GSK3iXV treatment (blue) or 24 hours of Cdx2 induction (red). (O–R) Immunofluorescence analysis of HUES8 human ES-derived endoderm treated with DMSO (O–P) or 125 nM GSK3iXV (Q–R) for 24 hours before fixation and stained for Cdx2 (white in O, Q, green in P, R) and Foxa2 (red in P, R).Scale bar equals 100 µm in B and D, 50 µm in F, H, J, P, and R.
By evaluating candidate signaling pathways to enhance or inhibit Activin A-induced endodermal differentiation, an optimized endodermal induction protocol was developed such that a near-uniform population of definitive endoderm can be derived from ES cells within 5 days. Culturing embryonic stem cells in serum-free media without growth factors for two days prior to addition of Activin A improves endodermal yield (Supplemental Figure 3). From day 2–3, activating Wnt signaling, achieved by low-dose GSK3iXV, increases endodermal efficiency and antagonizing Wnt signaling impairs endoderm formation (Supplemental Figure 3). From day 3–5, inhibiting Bmp signaling with the small molecule Dorsomorphin increases endodermal differentiation whereas Bmp4 strikingly inhibits endoderm differentiation (Supplemental Figure 3), as has been found previously (Hansson et al., 2009). By combining all of these components, a near-uniform population of endoderm can be obtained after 5 days of differentiation (Figure 4C–D).
The effect of Wnt induction on ES cell-derived endoderm was then analyzed. Treatment of ES cell-derived endoderm with GSK3iXV induces a striking induction of Cdx2 (Figure4E–H). In the absence of GSK3iXV, fewer than 1% of Foxa2-expressing endoderm cells express Cdx2 (Figure 4E–F, Supplemental Figure 3), whereas 24 hours after addition of 100 nM GSK3iXV, over 99% of Foxa2-expressing endoderm cells express Cdx2 (Figure 4G–H, Supplemental Figure 3). Cdx2 induction occurred within a range of 20 nM–500 nM GSK3iXV (data not shown). Treatment with Wnt3a is also able to induce Cdx2 expression, although only in around 40% of endoderm, likely due to low bioactivity of Wnt3a (Figure 4I–J, Supplemental Figure 3).
Microarray analysis was performed on ES-derived endoderm treated with DMSO or GSK3iXV for 24 hours. As expected, GSK3iXV treatment induces transcripts of intestinal marker genes such as Cdx2 and Dpp4 and downregulates anterior endodermal genes such as Hhex and Hesx1 (selected genes highlighted in Table 2 with a complete list in Supplemental Table 2). GSK3iXV treatment of ES-derived endoderm produces strikingly similar gene expression changes to E8.25 GSK3iXV bead implantation. Using the set of genes significantly upregulated or downregulated by E8.25 GSK3iXV bead as compared to DMSO bead implantation (3-fold cutoff), foldchange in this bead implantation setting was plotted versus foldchange of ES-derived endoderm treated with GSK3iXV as compared to DMSO (Figure 4I). The correlation between gene expression changes in E8.25 embryonic endoderm and ES-derived endoderm is highly significant (r=0.69), suggesting that Wnt signaling plays a similar role in embryo-derived and ES-derived endoderm.
Table 2. Transcription factors and select other genes significantly affected by GSK3iXV treatment in ES cell-derived endoderm.
Expression of these genes is changed >3-fold by treatment of ES cell-derived endoderm with 50 nM GSK3iXV.
| Selected genes upregulated in GSK3iXV- treated ES-derived endoderm |
Selected genes downregulated in GSK3iXV- treated ES-derived endoderm |
|---|---|
| Axin2 | Foxd4 |
| Cdx1 | Gata3 |
| Cdx2 | Gsc |
| Defcr-rs2 | Hesx1 |
| Dkk4 | Hhex |
| Dpp4 | Isl1 |
| Evx1 | Nkx2–3 |
| Gbx2 | Otx2 |
| Sp8 | Tbx1 |
| Tcf2 | |
| Wnt6 |
Comparison of Wnt intestinal induction and Cdx2 induction
Cdx2 is known to be a key regulator of intestinal fate (Gao et al., 2009), so we investigated whether induction of Cdx2 alone in ES-derived endoderm would be sufficient to induce an intestinal gene program. An ES cell line that allows doxycycline-inducible expression of genes (Iacovino et al., 2009) was used to affect uniform induction of Cdx2 after ES cell endodermal differentiation. Microarray analysis was performed on ES cell-derived endoderm treated for 24 hours with either GSK3iXV alone, Doxycycline-inducible Cdx2 alone, or both. Only 27 of the 154 (18%) of the genes induced by GSK3iXV are also induced by Doxycycline-induced expression of Cdx2 (Figure 4J, full list in Supplemental Table 4). A larger degree of overlap exists between genes downregulated by GSK3iXV and Cdx2 (93/221, 42%, Figure 4K, full list in Supplemental Table 4). The lack of overlap between induced genes can be partially explained by the abundance of Wnt pathway feedback genes induced by GSK3iXV. To refine understanding of the role of Cdx2-independent Wnt induction in intestinal gene expression, the set of genes uniquely expressed in E11.5 intestinal endoderm, long after Wnt-mediated induction has ceased according to BAT-GAL reporter expression, was analyzed. While a large majority of E11.5 intestinal genes are induced by both GSK3iXV and Cdx2, including the intestinal Hox genes (Figure 4L), such as Cdx1, Ihh and Dpp4 are induced by Wnt signaling and not Cdx2 (Figure 4L). Together, this analysis indicates that the role of Wnt in intestinal induction is not simply the induction of Cdx2.
Wnt induces Cdx2 in human ES-derived endoderm
To address whether Wnt induction of intestinal fate is species-specific, the role of Wnt signaling was also examined in human ES-derived endoderm. The human ES cell line HUES8 was differentiated into definitive endoderm (Chen et al., 2009) with ~50–70% efficiency (Supplemental Figure 3). In the absence of GSK3iXV, <5% of Foxa2+ cells express Cdx2 (Figure 4M–N), whereas treatment of HUES8 ES-derived endoderm with GSK3iXV for 48 hours induces Cdx2 in >80% of Foxa2+ endoderm (Figure 4O–P). Thus, Wnt signaling induces intestinal fate in both mouse and human ES-derived endoderm.
Wnt signaling affects anterior-posterior intestinal domain specification
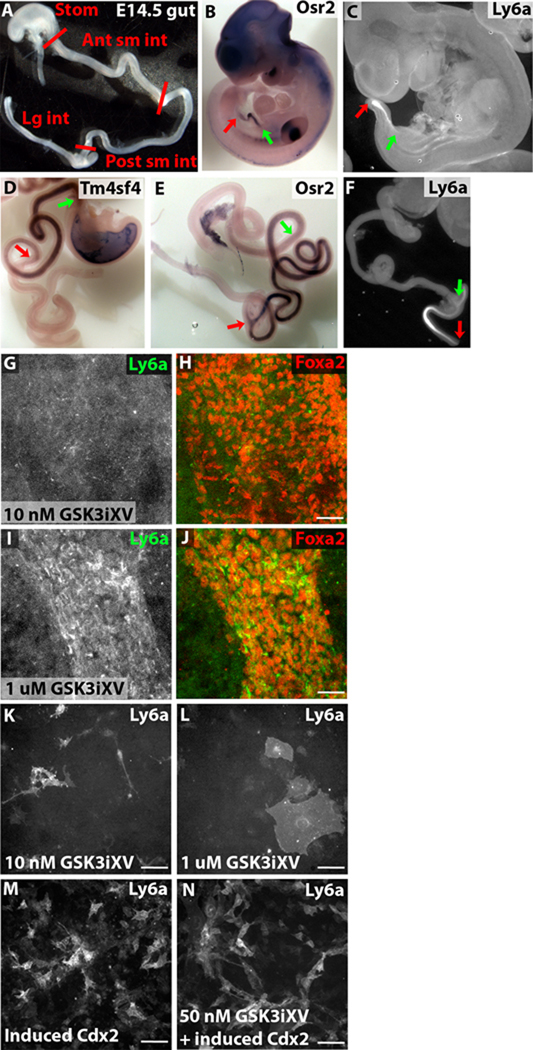
The intestine becomes subdivided into anterior-posterior domains, each with unique morphogenetic, enzymatic and absorptive properties. By E12.5, the cecum is clearly discernable morphologically, but there is no clear visual distinction among the segments of the small intestine or large intestine until late gestation. As there is a paucity of known molecular markers for these intestinal regions during midgestation, we first sought molecular markers of distinct intestinal regions. At E14.5, the large intestine posterior to and including the cecum, the anterior half of the small intestine, the posterior half of the small intestine, and the stomach were dissected (Figure 5A), dissociated, and endoderm was isolated on the basis of EpCAM expression. Microarray analysis was performed on these four endodermal populations, and a set of differentially expressed genes was determined (selected genes highlighted in Table 3 with a complete list in Supplemental Table 3). Several genes that displayed preferential expression in one E14.5 intestinal segment were further analyzed by in situ hybridization or immunofluorescence analysis. Genes confirmed to be enriched in anterior small intestine, in addition to the previously documented Pdx1 (Ahlgren et al., 1996; Offield et al., 1996) and Onecut2 (Jacquemin et al., 2003), include Anpep and Tm4sf4 (Figure 5D and Supplemental Figure 3). Cib2, Fzd10, and Osr2 showed preferential expression in posterior small intestinal endoderm from E10.5 through E14.5 (Figure 5B, E and Supplemental Figure 4), and Hoxd10, Ly6a (Sca-1), and Xpnpep2 displayed preferential expression in the large intestine (Figure 5C, F and Supplemental Figure 4).
Figure 5. Intestinal molecular regionalization occurs by E10.5 and can be affected by Wnt and Cdx2 concentration.
(A) Wholemount image of E14.5 gut with the different anterior-posterior divisions dissected for microarray analysis labeled. Distinction between anterior and posterior small intestine was chosen as the midpoint of the small intestine. (B–F) Wholemount in situ hybridization (B, D, E) or immunofluorescence (C, F) images of E10.5 embryos (B–C) or E14.5 gut (D–F) showing expression of posterior small intestine-specific Osr2 (B, E), large intestine-specific Ly6a (C, F), and anterior small intestine-specific Tm4sf4 (D). Green arrows refer to the anterior border of the intestinal staining and red arrows refer to the posterior border of the intestinal staining. (G–J) Wholemount confocal immunofluorescence images of E8.25 foreguts cultured for 24 hours before fixation and stained for Ly6a (white in G, I, green in H, J) and Foxa2 (red in H, J). Foreguts were treated with soluble GSK3iXV at 10 nM (G–H) or 1 µM (I–J). (K–M) Immunofluorescence images of inducible Cdx2 ES-derived endoderm treated with 10 nM GSK3iXV (K), 1 µM GSK3iXV (L), 1 µg/mL Doxycycline (M), or 50 nM GSK3iXV and 1 µg/mL Doxycycline (N) for 24 hours before fixation and stained for Ly6a. Scale bar equals 50 µm in H, J, K–N.
Table 3. Selected genes preferentially expressed in specific E14.5 gut segments.
These genes have expression >3-fold higher in one E14.5 gut segment as compared to all other segments.
| stomach | Anterior small intestine |
Posterior small intestine |
Large intestine |
|---|---|---|---|
| Bace2 | Anpep | Cib2 | Evx1 |
| Corin | Dlk1 | Fzd10 | Gna14 |
| Evi1 | Gpx3 | Guca2a | Hoxa7 |
| Gsta4 | Edg7 | Hoxd4 | Hoxb7 |
| Idb4 | Ipf1 | Olfml1 | Hoxd10 |
| Ly6h | Onecut2 | Osr2 | Ly6a |
| Sox21 | Slc40a1 | Rnase4 | Slc5a8 |
| Vsig2 | Tm4sf4 | Sdh1 | Xpnpep2 |
To determine whether Wnt signaling specifies a particular intestinal domain, we utilized the genes found by microarray to be specific to particular domains. Comparative expression in anterior endoderm treated for 24 hours with GSK3iXV beads versus with DMSO beads was calculated for each domain-specific gene set. As predicted, genes expressed in the stomach are on average downregulated by Wnt pathway activity (mean foldchange 0.90, median foldchange 0.76). Genes expressed in anterior small intestine are unaffected by Wnt signaling (mean 0.97, median 0.95), whereas genes expressed in posterior small intestine (mean 4.45, median 1.31) and large intestine (mean 3.24, median 1.34) are significantly upregulated by Wnt signaling.
This result leaves open the possibility that Wnt signaling specifically induces posterior small intestine and large intestine fate or that Wnt signaling can specify multiple intestinal domains at different concentrations or times. To investigate the role of Wnt in intestinal subtype specification in more detail, E8.25 anterior explants were cultured for 48 hours with either 1 µM or 10 nM GSK3iXV in the media. While both concentrations efficiently induced Cdx2 in the endoderm, 1 µM but not 10 nM GSK3iXV induced large intestine-specific Ly6a (Figure 5G–J), suggesting that higher levels of Wnt signaling preferentially induce more posterior intestinal genes.
The ability of Wnt dosage to affect intestinal patterning in ES-derived endoderm was also investigated. Neither high nor low doses of GSK3iXV were able to turn on expression of Ly6a (Figure 5K–L). However, Doxycycline-mediated induction of Cdx2 alone or in the context of GSK3iXV treatment yielded extensive expression of Ly6a (Figure 5M–N). Microarray profiling confirmed that Doxyxycline-induced Cdx2 or the combination of GSK3iXV and ectopicCdx2 induced an extensive set of large intestine-specific genes not induced by GSK3iXV alone, including Hoxa7, Lpl, and Xpnpep2 (Supplemental Figure 5). While we have as yet been unable to induce robust large intestinal gene expression from ES cells using exogenous factors, Cdx2 dosage appears to be crucial in this process.
Discussion
While genetic evidence has previously suggested a role for Wnt signaling in intestinal development, this report establishes a clear, direct and multifaceted role for Wnt signaling in intestinal specification and patterning. Wnt signaling acts directly on definitive endoderm to induce Cdx2, and application of Wnt to the early foregut endoderm is sufficient to downregulate the anterior endoderm program and to induce a gene expression program that resembles early embryonic intestine. As further proof of its role in intestinal specification, Wnt signaling is sufficient to induce ES cell-derived endoderm to an intestinal fate. Wnt signaling is involved not only in specification of the intestine but in its anterior-posterior patterning, as high-level Wnt and Cdx2 appear to be necessary for large intestine specification.
The effect of Wnt signaling on endoderm is transient. While Wnt possesses the ability to induce Cdx2 between E7.5 and E8.5, electroporation and bead experiments both demonstrate that Wnt signaling after E8.5 is no longer capable of inducing Cdx2. This loss of competence correlates with the time at which BAT-gal mice cease displaying midgut endodermal activity (Figure 1I), and this loss of active posterior endodermal Wnt signaling is seen at a similar embryonic stage in Xenopus (Schohl and Fagotto, 2002). E8.5 is also the stage at which Cdx2 and Sox2 expression domains meet at the stomach-intestine border and at which hepatic and pancreatic genes begin to be expressed at this border (Sherwood et al., 2009). Thus, at E8.5, posterior foregut ceases to receive Wnt signals or inhibitor activity outweighs ligand presence, and the endoderm loses competence to activate intestinal genes in response to such signaling. It has recently been shown that Wnt signaling in the E9.5 endoderm is vital for lung specification (Goss et al., 2009), and Wnt also plays an important role in the development of the pharyngeal pouches (Balciunaite et al., 2002), liver and pancreas (Verzi and Shivdasani, 2008). As the Wnt-Cdx2 axis is dominant over anterior fates, the loss of competence to activate Cdx2 in response to Wnt signaling is a crucial step in endodermal development.
Both the BAT-gal expression in the endoderm (Figure 1) and the anterior creeping of endodermal Cdx2 (Sherwood et al., 2009) suggest a model in which a posterior source of Wnt diffuses anteriorly, and progressively more anterior cells reach a threshold to activate Cdx2. Our study suggests direct activation of Cdx2 by Wnt signaling, as Cdx2 mRNA is seen within six hours of Wnt treatment in embryonic and ES-derived endoderm, and Tcf/Lef factors have been demonstrated to act directly on the promoters of Cdx1 and Cdx4 (Pilon et al., 2006; Prinos et al., 2001), so direct action on Cdx2 is a likely mechanism. The relationship between Wnt and Cdx2 in the nascent intestine may be more complex, however, as evidence from other developmental stages suggests that Cdx2 can induce Wnt signaling (Young et al., 2009), Cdx2 and the Wnt-responsive transcription factor Tcf4 co-occupy a large number of regulatory sequences (Verzi et al.), and Cdx2 has been shown to inhibit Wnt responsiveness (Guo et al.). This model of posterior Wnts patterning the intestinal endoderm provides a convenient explanation for the observation that only higher doses of Wnt in embryos and Cdx2 in ES-derived endoderm are able to induce large intestinal gene expression. However, the inability of high-dose Wnt alone to induce large intestinal gene expression in ES-derived endoderm suggests either that additional signaling mechanisms are required in concert with high-dose Wnt to induce high-level Cdx2 expression and large intestinal fates or that the protocol used for ES cell intestinal endoderm induction fails to provide the temporal competence window required for this induction.
Several experiments suggest that an additional aspect of intestinal organogenesis involves differential adhesion and possibly cell migration. After electroporation of CA β-catenin into foregut endoderm, electroporated cells are found at more posterior positions after longer periods of embryo culture, and after 24 hours, a significant percentage of cells are found near to the endogenous hindgut Cdx2+ domain (Figure 2D–F, Supplemental Figure 2). In the endoderm-specific genetic activation of β-catenin, ectopic Cdx2+ cells are found as clusters in the pharyngeal endoderm and as strands in the midgut region anterior to and connected to the endogenous Cdx2+ intestinal domain (Figure 3). It is clear from the cluster formation in the genetic activation experiments that Wnt-induced Cdx2+ cells have differential adhesion that allows them to cluster and at times disengage from the pharyngeal endodermal epithelium as protrusions, an interesting finding in light of the known role of Cdx2 in controlling cell polarity and cytoskeletal arrangement (Gao and Kaestner). It is also tempting to speculate that the lack of ectopic clusters in the midgut occurs because Cdx2+ cells close enough to the endogenous intestinal domain migrate to and adhere to the endogenous intestinal domain, which could provide an explanation as well for the electroporation results. These experiments cannot rule out selective survival or proliferation as alternative explanations, so future timelapse imaging experiments will be informative to determine whether migration and differential adhesion play a role in the formation of a strict boundary for the nascent intestinal domain.
Induction of intestinal fate by Wnt seems to rely heavily on Cdx2. Cdx2 is one of the earliest genes activated in the endoderm by activation of Wnt signaling. Wnt signaling has been shown to directly induce Cdx1 in ES cells (Lickert et al., 2000), and our results strongly suggest a direct activation of Cdx2 in endoderm, as Cdx2 mRNA is induced within 6 hours and Cdx2 protein is induced within 8 hours. As has been recently documented in Cdx2-deficient endoderm (Gao et al., 2009), Cdx2 orchestrates a network of intestinal transcription, so it is unsurprising that 24 hours after Wnt activation, gene expression has altered significantly toward that of native embryonic intestinal endoderm. Wnt signaling, however, has a more extensive role than simply activating Cdx2, as is demonstrated in experiments comparing genes induced by Wnt or by direct induction of Cdx2 in ES cell-derived endoderm. One potentially crucial difference is that Wnt induces Cdx1 along with Cdx2, and these two genes could have distinct targets during intestinal development. Additionally, Wnt signaling appears to induce Indian Hedgehog independently of Cdx2, and Hedgehog signaling is known to be important in the coordination of intestinal endoderm and mesoderm development (Roberts et al., 1998).
In the process of analyzing the role of Wnt in intestinal regional patterning, we have identified several genes differentially expressed in intestinal segments. This process has revealed two surprising results. The first is that, while the small intestine is traditionally divided into three anterior-posterior segments by morphology, gene expression patterns suggest two molecularly distinct small intestinal regions. The duodenum has unique expression of genes such as Ipf1, Onecut1 and 2, Anpep and Tm4sf4, while Osr2, Fzd10 and Cib2 have graded anterior borders at the presumed duodenum-jejunum boundary and are expressed until their graded posterior boundaries slightly past the cecum (Figure 5E, Supplemental Figure 3). The large intestine-specific genes identified span the entire large intestine posterior to the cecum (Figure 5F, Supplemental Figure 3), although reports of specific roles for Hox13 members in the anorectal endoderm (Warot et al., 1997) suggest that some genes may be expressed only in subregions of the large intestine. Second, these regional expression patterns are established at least as early as E10.5 and possibly earlier (Figure 5B–C, Supplemental Figure 3) even though morphological distinction among regions starts around E12.5 with the development of the cecum and does not become clear until villus formation after E14.5. The early establishment of regionalization is potentially a consequence of its reliance on differential Wnt levels, as Wnt acts in intestinal specification exclusively between E7.0 and E8.5.
Finally, we demonstrate that activation of Wnt signaling is sufficient to induce intestinal differentiation of ES cell-derived endoderm (Figure 4). This induction is highly efficient, as greater than 95% of ES cell-derived endoderm cells begin to express Cdx2 after treatment with GSK3iXV. While GSK3 inhibition has been found to affect pathways other than Wnt (Wu and Pan, 2010), the concordance of phenotypes using GSK3iXV, activated β-catenin, and Wnt3a in multiple assays lends confidence to the conclusion that GSK3 inhibition is exerting its intestinal inductive action primarily through activation of Wnt signaling. Recently, a protocol has been developed demonstrating three-dimensional intestinal organoid differentiation from human embryonic stem cell-derived endoderm by the addition of Fgf4 and Wnt3a (Spence et al., 2011). In these experiments, Fgf and Wnt signals are required in conjunction to allow for Cdx2 expression and intestinal differentiation; however, equivalent experiments in this work demonstrate that strong Wnt stimulation through GSK3 inhibition is sufficient to induce uniform Cdx2 (Figure 4), and the discrepancy is likely a result of the increased effectiveness of small molecule GSK3 inhibitors over Wnt3a (Figure 4). In Spence et al’s work (Spence et al., 2011), human ES-derived mesoderm is also present and becomes incorporated into the organoids, so Fgf signaling may be acting to pattern this mesenchyme. It will be interesting to replicate these experiments using ES cell-derived populations containing small and large intestinal marker expression to assess the differential morphological and transcriptional properties of these populations.
The gene expression changes induced by Wnt pathway activation are strikingly similar between embryo-derived endoderm and ES cell-derived endoderm. The few differences could result from differences in timing, as the embryonic experiments were performed in E8.25 foregut, which already expresses some specialized genes involved in anterior endoderm organogenesis whereas ES cell-derived endoderm appears by gene expression to resemble early embryonic endoderm, or from indirect effects of non-endodermal tissues in the bead experiments. This finding is significant for the ES cell field, as it lends confidence to the fact that ES cells do respond equivalently to embryonic cells when exposed to extracellular signals.
It will be interesting to investigate ES cell-derived intestine further to determine whether it possesses the ability to differentiate down mature intestinal lineages or to adopt adult intestinal stem cell activity. Derivation of intestinal populations from ES cells will lead to greater understanding of intestinal diseases and will hopefully lead to in vitro models of intestinal diseases and tissue replacement therapy.
Highlights.
Wnt acts directly on endoderm to specify intestinal fate
Wnt induces the intestinal master regulator Cdx2
Wnt signaling induces intestinal gene expression in ES-derived endoderm
Wnt, through Cdx2, activates large intestinal gene expression at high doses and small intestinal gene expression at lower doses
Supplementary Material
Acknowledgements
D.A.M. is an investigator of the Howard Hughes Medical Institute. R.I.S. was supported by a National Science Foundation Graduate Research Fellowship and the Sternlicht Fellowship. E.O.M. is the David and Sylvia Lieb Fellow of the Damon Runyon Cancer Research Foundation, DRG-1937-07. This work was supported by the N.I.H. The authors would like to thank Brian Tilton, Anastasie Kweudjeu, George Kenty, and the Molecular Genetics Core Facility at Children's Hospital Boston for technical assistance. The G8.8 antibody was obtained from the Developmental Studies Hybridoma Bank.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlgren U, Jonsson J, Edlund H. The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF1/PDX1-deficient mice. Development. 1996;122:1409–1416. doi: 10.1242/dev.122.5.1409. [DOI] [PubMed] [Google Scholar]
- Atilla-Gokcumen GE, Williams DS, Bregman H, Pagano N, Meggers E. Organometallic compounds with biological activity: a very selective and highly potent cellular inhibitor for glycogen synthase kinase 3. Chembiochem. 2006;7:1443–1450. doi: 10.1002/cbic.200600117. [DOI] [PubMed] [Google Scholar]
- Balciunaite G, Keller MP, Balciunaite E, Piali L, Zuklys S, Mathieu YD, Gill J, Boyd R, Sussman DJ, Hollander GA. Wnt glycoproteins regulate the expression of FoxN1, the gene defective in nude mice. Nat Immunol. 2002;3:1102–1108. doi: 10.1038/ni850. [DOI] [PubMed] [Google Scholar]
- Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck F, Erler T, Russell A, James R. Expression of Cdx-2 in the mouse embryo and placenta: possible role in patterning of the extra-embryonic membranes. Dev Dyn. 1995;204:219–227. doi: 10.1002/aja.1002040302. [DOI] [PubMed] [Google Scholar]
- Borowiak M, Maehr R, Chen S, Chen AE, Tang W, Fox JL, Schreiber SL, Melton DA. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell. 2009;4:348–358. doi: 10.1016/j.stem.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillet P, Oulad-Abdelghani M, Ward SJ, Bronner S, Chambon P, Dolle P. A new mouse member of the Wnt gene family, mWnt-8, is expressed during early embryogenesis and is ectopically induced by retinoic acid. Mech Dev. 1996;58:141–152. doi: 10.1016/s0925-4773(96)00569-2. [DOI] [PubMed] [Google Scholar]
- Chawengsaksophak K, de Graaff W, Rossant J, Deschamps J, Beck F. Cdx2 is essential for axial elongation in mouse development. Proc Natl Acad Sci U S A. 2004;101:7641–7645. doi: 10.1073/pnas.0401654101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Borowiak M, Fox JL, Maehr R, Osafune K, Davidow L, Lam K, Peng LF, Schreiber SL, Rubin LL, Melton D. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol. 2009;5:258–265. doi: 10.1038/nchembio.154. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Gao N, Kaestner KH. Cdx2 regulates endo-lysosomal function and epithelial cell polarity. Genes Dev. 24:1295–1305. doi: 10.1101/gad.1921510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N, White P, Kaestner KH. Establishment of intestinal identity and epithelial-mesenchymal signaling by Cdx2. Dev Cell. 2009;16:588–599. doi: 10.1016/j.devcel.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM, Yamaguchi TP, Morrisey EE. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell. 2009;17:290–298. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorieff A, Grosschedl R, Clevers H. Hindgut defects and transformation of the gastro-intestinal tract in Tcf4(−/−)/Tcf1(−/−) embryos. EMBO J. 2004;23:1825–1833. doi: 10.1038/sj.emboj.7600191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo RJ, Funakoshi S, Lee HH, Kong J, Lynch JP. The intestine-specific transcription factor Cdx2 inhibits beta-catenin/TCF transcriptional activity by disrupting the beta-catenin-TCF protein complex. Carcinogenesis. 31:159–166. doi: 10.1093/carcin/bgp213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson M, Olesen DR, Peterslund JM, Engberg N, Kahn M, Winzi M, Klein T, Maddox-Hyttel P, Serup P. A late requirement for Wnt and FGF signaling during activin-induced formation of foregut endoderm from mouse embryonic stem cells. Dev Biol. 2009;330:286–304. doi: 10.1016/j.ydbio.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller RS, Dichmann DS, Jensen J, Miller C, Wong G, Madsen OD, Serup P. Expression patterns of Wnts, Frizzleds, sFRPs, and misexpression in transgenic mice suggesting a role for Wnts in pancreas and foregut pattern formation. Dev Dyn. 2002;225:260–270. doi: 10.1002/dvdy.10157. [DOI] [PubMed] [Google Scholar]
- Iacovino M, Hernandez C, Xu Z, Bajwa G, Prather M, Kyba M. A conserved role for Hox paralog group 4 in regulation of hematopoietic progenitors. Stem Cells Dev. 2009;18:783–792. doi: 10.1089/scd.2008.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemin P, Pierreux CE, Fierens S, van Eyll JM, Lemaigre FP, Rousseau GG. Cloning and embryonic expression pattern of the mouse Onecut transcription factor OC-2. Gene Expr Patterns. 2003;3:639–644. doi: 10.1016/s1567-133x(03)00110-8. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BM, Buchner G, Miletich I, Sharpe PT, Shivdasani RA. The stomach mesenchymal transcription factor Barx1 specifies gastric epithelial identity through inhibition of transient Wnt signaling. Dev Cell. 2005;8:611–622. doi: 10.1016/j.devcel.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Lickert H, Domon C, Huls G, Wehrle C, Duluc I, Clevers H, Meyer BI, Freund JN, Kemler R. Wnt/(beta)-catenin signaling regulates the expression of the homeobox gene Cdx1 in embryonic intestine. Development. 2000;127:3805–3813. doi: 10.1242/dev.127.17.3805. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLin VA, Rankin SA, Zorn AM. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134:2207–2217. doi: 10.1242/dev.001230. [DOI] [PubMed] [Google Scholar]
- Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- Okubo T, Hogan BL. Hyperactive Wnt signaling changes the developmental potential of embryonic lung endoderm. J Biol. 2004;3:11. doi: 10.1186/jbiol3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierreux CE, Poll AV, Jacquemin P, Lemaigre FP, Rousseau GG. Gene transfer into mouse prepancreatic endoderm by whole embryo electroporation. JOP. 2005;6:128–135. [PubMed] [Google Scholar]
- Pilon N, Oh K, Sylvestre JR, Bouchard N, Savory J, Lohnes D. Cdx4 is a direct target of the canonical Wnt pathway. Dev Biol. 2006;289:55–63. doi: 10.1016/j.ydbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Prinos P, Joseph S, Oh K, Meyer BI, Gruss P, Lohnes D. Multiple pathways governing Cdx1 expression during murine development. Dev Biol. 2001;239:257–269. doi: 10.1006/dbio.2001.0446. [DOI] [PubMed] [Google Scholar]
- Ratineau C, Duluc I, Pourreyron C, Kedinger M, Freund JN, Roche C. Endoderm- and mesenchyme-dependent commitment of the differentiated epithelial cell types in the developing intestine of rat. Differentiation. 2003;71:163–169. doi: 10.1046/j.1432-0436.2003.t01-1-710203.x. [DOI] [PubMed] [Google Scholar]
- Roberts DJ, Smith DM, Goff DJ, Tabin CJ. Epithelial-mesenchymal signaling during the regionalization of the chick gut. Development. 1998;125:2791–2801. doi: 10.1242/dev.125.15.2791. [DOI] [PubMed] [Google Scholar]
- Schohl A, Fagotto F. Beta-catenin, MAPK and Smad signaling during early Xenopus development. Development. 2002;129:37–52. doi: 10.1242/dev.129.1.37. [DOI] [PubMed] [Google Scholar]
- Sherwood RI, Chen TY, Melton DA. Transcriptional dynamics of endodermal organ formation. Dev Dyn. 2009;238:29–42. doi: 10.1002/dvdy.21810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood RI, Jitianu C, Cleaver O, Shaywitz DA, Lamenzo JO, Chen AE, Golub TR, Melton DA. Prospective isolation and global gene expression analysis of definitive and visceral endoderm. Dev Biol. 2007;304:541–555. doi: 10.1016/j.ydbio.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Siegfried E, Wilder EL, Perrimon N. Components of wingless signalling in Drosophila. Nature. 1994;367:76–80. doi: 10.1038/367076a0. [DOI] [PubMed] [Google Scholar]
- Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger BZ, Datar R, Murtaugh LC, Melton DA. Direct regulation of intestinal fate by Notch. Proc Natl Acad Sci U S A. 2005;102:12443–12448. doi: 10.1073/pnas.0505690102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian V, Meyer BI, Gruss P. Disruption of the murine homeobox gene Cdx1 affects axial skeletal identities by altering the mesodermal expression domains of Hox genes. Cell. 1995;83:641–653. doi: 10.1016/0092-8674(95)90104-3. [DOI] [PubMed] [Google Scholar]
- Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 1994;8:174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- Theodosiou NA, Tabin CJ. Wnt signaling during development of the gastrointestinal tract. Dev Biol. 2003;259:258–271. doi: 10.1016/s0012-1606(03)00185-4. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Hong Z, Kuzuya T, Tada M, Hori M. Wnt/frizzled-2 signaling induces aggregation and adhesion among cardiac myocytes by increased cadherin-beta-catenin complex. J Cell Biol. 2000;150:225–241. doi: 10.1083/jcb.150.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Akker E, Forlani S, Chawengsaksophak K, de Graaff W, Beck F, Meyer BI, Deschamps J. Cdx1 and Cdx2 have overlapping functions in anteroposterior patterning and posterior axis elongation. Development. 2002;129:2181–2193. doi: 10.1242/dev.129.9.2181. [DOI] [PubMed] [Google Scholar]
- van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- Verzi MP, Hatzis P, Sulahian R, Philips J, Schuijers J, Shin H, Freed E, Lynch JP, Dang DT, Brown M, Clevers H, Liu XS, Shivdasani RA. TCF4 and CDX2, major transcription factors for intestinal function, converge on the same cis-regulatory regions. Proc Natl Acad Sci U S A. 107:15157–15162. doi: 10.1073/pnas.1003822107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzi MP, Shivdasani RA. Wnt signaling in gut organogenesis. Organogenesis. 2008;4:87–91. doi: 10.4161/org.4.2.5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warot X, Fromental-Ramain C, Fraulob V, Chambon P, Dolle P. Gene dosage-dependent effects of the Hoxa-13 and Hoxd-13 mutations on morphogenesis of the terminal parts of the digestive and urogenital tracts. Development. 1997;124:4781–4791. doi: 10.1242/dev.124.23.4781. [DOI] [PubMed] [Google Scholar]
- Whitcomb DC, Lowe ME. Human pancreatic digestive enzymes. Dig Dis Sci. 2007;52:1–17. doi: 10.1007/s10620-006-9589-z. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Nieto MA. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 1993;225:361–373. doi: 10.1016/0076-6879(93)25025-w. [DOI] [PubMed] [Google Scholar]
- Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci. 2010;35:161–168. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Young T, Rowland JE, van de Ven C, Bialecka M, Novoa A, Carapuco M, van Nes J, de Graaff W, Duluc I, Freund JN, Beck F, Mallo M, Deschamps J. Cdx and Hox genes differentially regulate posterior axial growth in mammalian embryos. Dev Cell. 2009;17:516–526. doi: 10.1016/j.devcel.2009.08.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.