Abstract
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the CNS. Conduction block in demyelinated axons underlies early neurological symptoms, but axonal transection and neuronal loss are believed to be responsible for more permanent chronic deficits. Several therapies are approved for treatment of relapsing-remitting MS, all of which are immunoregulatory and clinically proven to reduce the rate of lesion formation and exacerbation. However, existing approaches are only partially effective in preventing the onset of disability in MS patients, and novel treatments to protect myelin-producing oligodendrocytes and enhance myelin repair may improve long-term outcomes. Studies in vivo in genetically modified mice have assisted in the characterization of mechanisms underlying the generation of neuropathology in MS patients, and have identified potential avenues for oligodendrocyte protection and myelin repair. However, no treatments are yet approved that target these areas directly, and in addition, the relationship between demyelination and axonal transection in the lesions of the disease remain unclear. Here, we review translational research targeting oligodendrocyte protection and myelin repair in models of autoimmune demyelination, and their potential relevance as therapies in MS patients.
Keywords: multiple sclerosis, demyelination, axonal transection, regeneration, neuroprotection
A. Introduction
Multiple sclerosis (MS) presents in its initial form most commonly in young adults, especially women [1]. Conduction block in demyelinated axons is understood to be responsible for early neurological deficits [1], while axonal transection and neuronal loss underlie more permanent later disability [2]. Endogenous myelin repair is known to exist in early lesions, and leads to return of conduction and function [3,4], but this process gradually fails as the disease progresses [5,6]. For CNS remyelination to occur, progenitors of myelin-forming oligodendrocytes must be recruited into demyelinated areas, and then subsequently differentiate into mature, myelinating cells which each typically wrap multiple axons [7-9]. Consistent with this model, committed oligodendrocyte progenitors (OPCs) have been detected in developing brain, normal adult human brain, MS lesions and animal models [5,10-12]. Studies suggest that approaches protective for oligodendrocytes and that enhance myelin repair are likely to improve long-term outcomes and reduce the rate of axonal transection and neuronal loss. Moreover, such approaches could be employed in combination with currently available immunoregulatory treatments, for additive or synergistic effects [13]. However, all therapies currently approved for the treatment of relapsing-remitting MS are immunoregulatory, and do not directly address oligodendrocyte protection and remyelination. These areas are thus the subject of intense current research focus. The first neuroprotectant approved for use in MS will represent a significant advance in the field, and may also have far-ranging implications for the treatment of other neurodegenerative disorders.
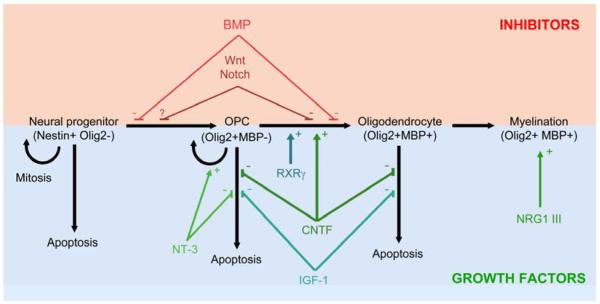
Studies in knockout mice and conditional mutants have accelerated our understanding of lesion pathogenesis in MS models, and have revealed pathways controlling oligodendrocyte survival and myelin repair [14]. Factors implicated in protection of oligodendrocytes or remyelination include neurotrophins, gp130 neurotrophic cytokines, insulin-like growth factors, and retinoid receptor signaling. By contrast, pathways shown to restrict repair have included LINGO-1, bone morphogenetic proteins, and canonical Wnt and Notch signaling [13-15] (Figure 1). However, despite these advances, no therapies have yet been approved that target these areas in MS patients. Moreover, the relationship between demyelination and axonal transection remains unclear, as does the potential for remyelination to restrict neuronal loss in MS lesions.
Figure 1. Regulation of oligodendrocyte progenitor mitosis, differentiation and apoptosis.
An outline of the progression of oligodendrocyte differentiation, from neural progenitor (left), through specified oligodendrocyte progenitor, to mature postmitotic oligodendrocyte capable of myelin formation (right). Mechanisms of action are shown of factors that augment (lower panel, blue/green) or restrict (upper panel, red) the number of mature myelinating cells. Retinoid X receptor signaling and the gp130 cytokines CNTF, IL-11 and LIF promote oligodendrocyte maturation (lower panel). The gp130 cytokines also improve the viability of mature oligodendrocytes or their precursors, a property they share with the insulin-like growth factors IGF-1 and IGF-2, and the neurotrophin, NT-3. Additionally, NT-3 potentiates precursor proliferation. At later stages of maturation, neuregulin 1 type III-ErbB signaling facilitates axonal ensheathment and myelin wrapping. Conversely, bone morphogenetic proteins inhibit oligodendrocyte lineage specification and subsequent progenitor differentiation (upper panel). Canonical Notch and Wnt signaling restrict maturation, and maintain the size of the progenitor pool.
Here, we review potential approaches for oligodendrocyte protection and remyelination in MS and its animal models. Obstacles to development of novel therapies in these areas will also be discussed.
B. Pathways promoting oligodendrocyte viability, maturation and myelination
B1. Gp130 neurotrophic cytokines
Gp130 was originally identified as a signal transducing component that associated with the interleukin 6 receptor (IL-6R) following ligand binding [16]. However, subsequent work defined gp130 as a receptor shared by all members of a large pleiotropic family of cytokines. The family includes IL-6, IL-11, IL-27, oncostatin M (OSM), leukemia inhibitory factor (LIF), cardiotrophin 1(CT-1), and ciliary neurotrophic factor (CNTF) [17]. IL-6R and IL-11Rα induce gp130 homodimerization [18], whereas LIF, CNTF, oncostatin M and CT-1 binding lead to heterodimerization with the LIF receptor (LIFR) [19]. Either homo- or heterodimerization triggers the activation of Janus kinase (JAK) tyrosine kinases [20,21], which leads to cytokine- and cell type-specific phosphorylation and nuclear translocation of Stat transcription factors [22]. Stat-independent signaling has also been described [22].
Members of the gp130 cytokine family play important roles in the immune, hemopoietic, cardiovascular and reproductive system [22]. Importantly, the family has also demonstrated strong trophic functions within the CNS, notably neuroprotection. For example, mice deficient in CNTF or CNTFR, and CT-1−/− mice, display loss of motoneurons [23,24], and CNTF is protective in neurodegenerative models in primates [25]. These reports have understandably stimulated research examining mechanisms underlying these effects, and the translational potential of gp130 signaling in CNS disease. Relevant to this review, investigation of effects on oligodendrocytes has focused primarily on CNTF, LIF and IL-11.
B1a. Neuroprotective roles of CNTF and LIF
CNTF promotes the final maturation of oligodendrocyte progenitors, and via gp130 and Jak signaling [26] leading to activation of STAT-1 and STAT-3 [27]. Soluble interleukin-6 (IL-6) receptor/IL-6 fusion protein similarly induces STAT1/3 phosphorylation and enhances differentiation of rodent oligodendrocyte progenitor cultures in vitro [28]. CNTF and LIF also promote oligodendrocyte survival [29,30]. CNTF−/− or LIFR+/−gp130+/− mice with the MS model, experimental autoimmune encephalomyelitis (EAE), display exacerbated disease and increased oligodendrocyte apoptosis, although LIF−/− mice also show an attenuated late phase of disease [31-33]. Administration of CNTF is not sufficient to promote oligodendrocyte remyelination in demyelinating lesions induced by microinjection of ethidium bromide in vivo [34], although transplantation of adult oligodendrocyte precursor cells expressing CNTF has been reported to promote remyelination and functional recovery following traumatic injury to the spinal cord [35]. Moreover, CNTF administration does protect mice from inflammatory pathology in EAE for the duration of treatment [36]. Endogenous LIF protects mature oligodendrocytes from demyelination and also enhances remyelination, and exogenous LIF has also been used successfully to limit the consequences of oligodendrocyte damage. [37,38].
Taken together, these findings implicate CNTF and LIF signaling as potential neuroprotective and pro-regenerative approaches for therapy of MS and other demyelinating diseases. However, despite successes of CNTF in studies in animal models of neurodegeneration [25,39], testing in neurodegenerative conditions in human patients was unsuccessful in [40,41], and these failures have significantly dampened enthusiasm for clinical trials in MS. Interestingly, however, the reasons for the ineffectiveness of CNTF in human trials have not been fully investigated, and it remains possible that lack of access to the CNS parenchyma through the blood-brain barrier may be responsible in part or whole for these negative findings.
B1b. IL-11 – oligodendrocyte protection and immunoregulation
Experiments in primary human cultures and mathematical modeling in rodent oligodendrocyte progenitors have shown that IL-11 enhances oligodendrocyte survival and maturation, and that these effects may be more potent than those of LIF [42-44] (Figure 1). IL-11 treatment is also associated with increased myelin formation in rodent CNS cocultures [43], and this effect appears to be due to predominantly to improved survival of oligodendrocyte lineage cells [44]. Interestingly, in contrast to the predominantly neuroprotective effects of CNTF and LIF, IL-11 displays significant immunoregulatory actions. Studies in vivo have shown that administration of recombinant IL-11 decreases the clinical course and neuropathology of EAE, whereas IL-11Rα−/− mice display increased demyelination and oligodendrocyte loss, and delayed remyelination, accompanied by exacerbated CNS inflammation [42,44]. While IL-11 directly increases the viability of oligodendrocyte lineage cells, its immunoregulatory properties depend on pro-apoptotic actions on antigen-presenting dendritic cells (DCs) [42,44]. Interestingly, both effects are dependent on lineage-specific activity of the transcription factors Stat1 versus Stat3. In both cell types, Stat3 is anti-apoptotic, whereas Stat1 promotes cell death. However, following IL-11 treatment, activity of Stat3 predominates over its relative in oligodendrocytes, whereas the pro-apoptotic effects of Stat1 prevail in DCs [44]. Collectively, these data reveal novel mechanisms underlying the actions of a neuroprotective and immunoregulatory member of the gp130 cytokine family in inflammatory demyelinating disease, and suggest new avenues to enhance oligodendrocyte survival and restrict CNS inflammation in MS.
B2. Insulin-like growth factors (IGFs)
IGFs are neuroprotective trophic factors for cells of the oligodendrocyte lineage [45], which encompass IGF-1 and IGF-2, and the regulatory IGF-binding proteins (IGFBPs). IGF-1 and IGF-2 signal through the IGF-1 receptor (IGF-1R) and IGF-2 receptor (IGF-2R), respectively. Interestingly, IGF-1 is expressed at high level in neuron-rich areas of the brain, whereas IGF-2 is found at high levels in myelinated tracts, and is the most abundant IGF in the CNS [46].
The phenotypes of mutants for the IGFs and their binding proteins have provided important insights into the roles of members of the family in CNS development. For example, mice deficient for IGF-1 display reduced brain size, hypomyelination, reduced oligodendrocyte numbers and severe neuronal loss [47]. IGF-1 exerts neurotrophic and neuroprotective effects in vitro, and enhances neuronal survival and differentiation. By comparison, the brains of transgenic mice overexpressing IGF-1 are 55% larger than controls, and show increased cell number and size, and myelin content in these mice is increased by 130% [48]. Moreover, the percentage of myelinated axons and myelin thickness are reduced in transgenic animals overexpressing IGFBP-1, [49]. IGF-1 enhances oligodendrocyte survival and supports differentiation in vitro, which is accompanied by enhanced process branching and increased production of myelin proteins [45,50].
Suggesting relevance to de- and remyelination, injection of IGF-1 into animals with EAE reduces the numbers and area of demyelinating lesions [51]. IGF-1 therapy has also been reported to increase the number of axons containing regenerating myelin segments, reduce blood-spinal cord barrier permeability, and enhance myelin gene expression, and these changes have been shown to be associated with rapid clinical and pathological recovery in the treated animals. However, although subsequent studies in chronic relapsing EAE have shown transient clinical amelioration and low-level remyelination after IGF-1 administration during the acute phase of disease, samples from acute phase-treated animals did not show potentiated remyelination at chronic time points [52].
The translational potential of IGF-1 also remains unfulfilled in MS. In active lesions from MS patients, IGF-1 expression localizes to reactive astrocytes, while IGF-2 is expressed by activated microglia [53]. However, a clinical trial examining the consequences of recombinant IGF-1 therapy in MS patients failed to produce statistically significant findings [54]. Similar to trials using CNTF, the extent to which this result was attributable to inability of IGF-1 to cross the BBB is unclear [54]. Restrictions in CNS access and issues with cell type specificity may once again represent a confound in clinical trial design in the case of IGF-1, confusing interpretation for another potentially neuroprotective and regenerative therapeutic pathway in MS.
B3. Neurotrophins
The neurotrophins (NTs) are a family of soluble mediators that include nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotophin-3 (NT-3) and neurotophin-4/5 (NT-4/5) [55]. Members of the family interact with two classes of transmembrane receptors: the receptor tyrosine kinase tropomyosin-related kinases (Trk), and the TNF-receptor superfamily p75 neurotrophin receptor p75NTR [56]. Each neurotrophin displays selective high-affinity interaction with specific Trk receptors, with NGF binding to TrkA, BDNF and NT-4/5 to Trk-B, and NT-3 to Trk-C. All bind additionally to p75NTR. The precursor form of NTs are known as proneurotrophins, and these are also found in abundance in the brain, and are believed to be biologically active [57,58].
Neurotrophins were originally characterized as playing important roles in the development and maturation of specific populations of CNS neurons [56]. However, they have also been found to exert strong effects on myelinating glia and on earlier uncommitted progenitors, and these will be summarized here, together with their translational potential.
B3a. Neurotrophins and myelination
Studies have revealed NGF as regulating myelination of TrkA-expressing dorsal root ganglion (DRG) neurons by both Schwann cells and oligodendrocytes in vitro, and have demonstrated that these effects are mediated via direct interaction between NGF and neuronal TrkA receptors [59]. However, the interaction between NGF and TrkA leads to differential outcomes in the two co-culture types, promoting myelin formation by Schwann cells but inhibiting oligodendrocyte myelination [59]. Thus, the impact of NGF on myelin formation includes important effects on neurons. However, additional investigations have shown that NGF also enhances oligodendrocyte survival, although it does not promote OPC proliferation unless coadministered with the mitogen fibroblast growth factor [60].
Unlike NGF, the impact of NT-3 on myelin formation encompasses potent glial effects. An important role in promoting mye lination is supported by the phenotype of NT-3 or TrkC-deficient mutants. NT-3 is essential for cardiovascular development and so most NT3−/− mice die within 3 weeks of birth [61], but at postnatal day 0 (P0) mice of this genotype exhibit a 35% reduction in the number of OPCs in the ventral spinal cord [62]. In addition, quantification of more mature oligodendrocyte lineage cells reveals significant reductions in the number of differentiated GalC+ oligodendrocytes (28% reduction) and myelin basic protein (MBP) mRNA+ (27% reduction) cells in spinal cords of NT-3−/− animals [62]. TrkC−/− mutants display comparable findings [62]. Experiments in vitro have described the effects of NT-3 on oligodendrocyte survival and proliferation, in addition to promoting the clonal expansion of oligodendrocyte progenitor cells when combined with the mitogen platelet-derived growth factor [29,63] (Figure 1). In addition, NT-3 has been shown to enhance myelination in co-cultures of OPCs and neurons, by inducing differentiation of OPCs into myelin-forming cells [64], and NT-3-transfected OPCs show increased MBP expression and enhanced myelination of hippocampal neurons [65]. In the optic nerve, neutralization of NT-3 by injection of hybridoma cells secreting anti-NT-3 antibody abrogates OPC proliferation and depletes the population of mature oligodendrocytes [63].
Interestingly, while NGF, NT-3 and BDNF have all been shown to promote differentiation of basal forebrain oligodendrocytes, only NGF and NT-3 promote maturation of cortical oligodendrocytes [66]. Whereas studies suggest relevance of BDNF in regulating myelination in vivo, the underlying mechanism is incompletely characterized. Most BDNF−/− mice show perinatal lethality, but a minority live for up to 21 days [67], and at P13 numbers of retinal ganglion cells and the thickness of the retina are normal in these survivors, but the proportion of myelinated axons in the optic nerve is reduced, by approximately 50% [67]. This reduction is maintained at P21, but is accompanied at this timepoint by a reduction in optic nerve axon diameter, suggesting that BDNF−/− mice may develop with a greater proportion of small unmyelinated axons. Moreover, the hippocampus and cortex of BDNF−/− mice display reduced levels of MBP and PLP mRNA [68]. However, since the phenotype of these mice combines alterations in both neuronal and glial compartments, it is not yet clear whether these events are mediated by BDNF acting directly on oligodendrocytes, or indirectly, via effects on neuronal TrkB receptors.
B3b. Translational potential of neurotrophins
Translational studies have explored the potential of neurotrophins to establish functional recovery following nerve injury and demyelination. Experiments in which fibroblasts expressing either BDNF or NT-3 have been transplanted into rodent spinal cords after injury have resulted in enhanced axonal growth, OPC proliferation and myelin formation [69]. Moreover, transplantation of Schwann cells expressing BDNF or NT-3 into demyelinated spinal cords leads to increased OPC proliferation and differentiation, remyelination and locomotor recovery in mice [70]. Interestingly, studies have also implied a functional role for BDNF in promoting axonal protection in autoimmune demyelination [71]. Remyelination and functional recovery have also been demonstrated following transplantation of glial-restricted precursor cells (GRPs) expressing a multi-neurotrophin of BDNF and NT-3 into the CNS of rats subjected to spinal cord injury [72]. However, although these reports have provided evidence that neurotrophins are effective at promoting spinal cord regeneration, the contributions of effects on neurons versus glia to these outcomes are not yet fully established.
B4. Neuregulin 1 type III
Members of the neuregulin 1 (NRG1) family play important roles in development and maintenance of the central and peripheral nervous systems [73]. They are ligands for Erb receptor tyrosine kinases located on glial cells, and more than 16 isoforms are known, encoded through multiple promoter utilization and alternative splicing [73,74]. These include neu differentiation factor (NDF), heregulin, acetylcholine receptor inducing activity (ARIA), and glial growth factor (GGF). All members of the family share a unique epidermal growth factor (EGF)-like domain, required for receptor activation.
The best understood function of NRG1 is in control of peripheral myelination, where axonal NRG1 is essential for neuronal survival, and for viability, proliferation and differentiation of myelinating Schwann cells [75]. In the developing PNS, a threshold level of axonal NRG1 type III expression is required to induce Schwann cell ensheathment [76], a nd both Schwann cell proliferation and myelination require glial ErbB2 receptors [77]. Subsequently, the level of NRG1 type III expression on myelinated axons determines myelin sheath thickness [78].
In the CNS, NRG1/ErbB signaling has been implicated in neuronal migration and in synaptic function [79]. In studies in vitro, NRG1 signaling also affects oligodendrocyte specification, differentiation and myelination [80-82], and heterozygous NRG1 type III mutants are hypomyelinated [83]. Oligodendrocytes and their progenitors express ErbB2, ErbB3 and ErbB4, and these two receptors have been suggested to control CNS myelination in vivo, [81]. Importantly, however, analysis conditional null mutants lacking NRG1 with onset at different stages of neural development has revealed that the CNS in these animals myelinates normally [84]. Experiments using disruption of ErbB signaling have produced similar findings. While oligodendrocytes express ErbB2 and ErbB4 homo/heterodimers, ErbB2 lacks ligand-binding activity. However, mice conditionally null for both ErbB3 and ErbB4 receptors myelinate normally in the absence of oligodendroglial neuregulin signaling [84]. Interestingly, however, transgenic overexpression of NRG1 type I or NRG1 type III leads to significant CNS hypermyelination [84]. Thus, collectively these data suggest that NRG1/ErbB signaling plays different roles in myelin formation in the PNS versus the CNS. Whereas neuregulin signaling regulates oligodendrocyte development and central myelin formation, control of oligodendrocyte myelination is significantly NRG/ErbB-independent. Thus, these findings significantly impact the translational potential for this pathway as a therapeutic avenue for myelin repair in MS.
B5. Retinoid X receptor signaling
Retinoid X receptors (RXRs) are nuclear receptors with important functions in regulation of cell proliferation and differentiation [85]. RXRα, RXRβ and RXRγ can form homodimers or heterodimers with other nuclear receptors, including retinoic acid receptors, thyroid hormone receptors, vitamin D receptors and peroxisome proliferator activator proteins, to control transcription of target genes. Suggesting potential translational relevance, all three members of the RXR family are highly expressed in lesions following CNS injury [86]. Interestingly, recent work has implicated RXRs as positive regulators of OPC differentiation [87]. Studies have shown that RXRγ is highly expressed in oligodendrocyte lineage cells during CNS remyelination in rodents, and that in acute and remyelinating MS lesions RXRγ is highly expressed by oligodendrocyte lineage cells, macrophages and astrocytes, but that expression is very low in chronic inactive lesions [87]. Importantly, knockdown of RXR-γ inhibits oligodendrocyte differentiation in vitro. Moreover, mice that lack RXR-γ display efficient repopulation of demyelinated lesions with OPCs, but delayed differentiation of these cells into mature oligodendrocytes [87]. In addition, administration of the RXR agonist 9-cis-retinoic acid to aged rats after demyelination results in an increase in remyelinated axons. Collectively, these data implicate RXR-γ as a regulator of OPC maturation and remyelination, and suggest it as a future avenue for regenerative therapy in MS.
C. Inhibitors of maturation and myelination
While committed oligodendrocyte progenitors (OPCs) have been detected in adult human brain and remyelination has been demonstrated in MS lesions [5,10-12], the capacity for regeneration in MS appears limited [88]. The mechanisms underlying this failure of repair are incompletely characterized [89,90]. Interestingly, recent studies have suggested that remyelinating cells may also arise from the proliferation and differentiation into mature oligodendrocytes of neural progenitor cells (NPCs) from germinative areas of the telencephalon such as the subventricular zone (SVZ) [91,92], thus NPCs may represent an additional source of remyelinating cells in MS. Like all progenitors, OPCs and NPCs are subject to precisely coordinated intrinsic and extrinsic signals that provide highly regulated control of the balance between and timing of proliferation and differentiation. Manipulation of these signals may facilitate expansion of the available pools of progenitors, and/or their subsequent differentiation into myelin-forming cells.
C1. Bone morphogenetic proteins
C1a. Role of BMPs in oligodendrocyte development
Bone morphogenetic proteins (BMPs) are a group of soluble proteins with more than 20 members, representing a major subclass of the transforming growth factor-β (TGFβ) superfamily. They are classified into five subgroups [93]. BMP ligands bind to a complex of the BMP receptor type 2 and Type 1 (a or b), leading to phosphorylation of R-Smads1, 5 and 8 and triggering their translocation to the nucleus with the co-Smad, Smad4 [94].
BMPs play pivotal roles in morphogenesis from gastrulation onwards [95]. For example, in the spinal cord, development of neuronal populations is dependent on the relative gradients of roof plate-derived dorsal BMPs, versus the ventralizing morphogen Sonic hedgehog (Shh), which is expressed by the notochord and floor plate [96,97]. The same cues later control oligodendrocyte specification and maturation. Generation of oligodendrocyte progenitor cells (OPCs) in the spinal cord occurs in two phases [98]. The first is ventral, with OPCs observed initially within the ventricular zone of the motor neuron progenitor (pMN) domain of neuroepithelium [99]. This requires Nkx6-regulated expression of the bHLH transcription factor Olig2, and occurs under the influence of Shh [100]. Conversely, the second phase occurs dorsally [101,102], and depends on inhibition of BMPs by the specific inhibitor Noggin [103,104]. BMPs restrict Shh-induced oligodendrocyte specification [103,105] and the differentiation of specified cells [104,106] (Figure 1). Blockade of BMP signaling induces oligodendrocyte generation both in vitro and in vivo [106,107].
C1b. BMP expression following CNS insult
Importantly, BMP expression is also upregulated following CNS injury or inflammation, and has been linked to glial scar formation [108-110]. BMPs are also significantly upregulated in models of disease resulting in demyelination, suggesting relevance to myelin repair in the adult CNS [106]. For example, both lysolethicin-induced demyelination in the spinal cord and ethidium bromide injection into the caudal cerebellar peduncle have been shown to result in upregulation of BMP4 [111,112]. Moreover, expression of BMPs 4, 6 and 7 is significantly increased in the spinal cord in EAE, correlating with disease severity [113]. BMP4 and 5 are also both detected in MS lesions in post mortem samples [114]. Inhibition of BMP signaling has therefore been suggested as a route to promotion of myelin repair [106]. Although still early in its translational development, such a strategy may represent a future avenue for understanding and manipulating glial responses in MS lesions.
C2. Canonical Notch pathway
The Notch signaling pathway is an important regulator of the balance between OPC proliferation and differentiation in the developing CNS [115,116]. The Notch family includes four cell surface receptors (Notch1-Notch4). Following binding of the receptor to its ligand, two cleavages, the first mediated by an ADAM metalloprotease and a the second by a γ-secretase complex, release the intracellular domain of the receptor (NICD) from the cell membrane, and it then translocates to the nucleus where it activates the transcription of target genes [117]. Contact-mediated activation of canonical Notch signaling by ligands including Jagged1 and Delta1 inhibits OPC differentiation and is permissive for proliferation [115,116] (Figure 1). Conversely, activation of a non-canonical Notch signaling pathway triggered by axonal ligands including F3/contactin has also been reported in OPC, and this pathway has been implicated in instructive effects on maturation [118]. Interestingly, studies have also implicated Notch signaling in maintenance of the size of the NPC pool in the subventricular zone [119-121], suggesting relevance to both progenitor populations that may generate remyelinating cells.
Interestingly, EAE and MS lesions have been shown to display high amounts of the Notch1 receptor and its canonical ligand Jagged1, and further characterization has revealed that Jagged-1 is expressed by reactive astrocytes under inflammatory conditions under the influence of the cytokine TGFβ1, while Notch-1 and its downstream effector Hes5 localize to OPCs [122,123]. Notch signaling has thus been suggested to act as a regulator of the balance between differentiation and maintenance of the progenitor pool in demyelinating conditions. However, studies examining myelin repair in vivo have reported contrasting findings. Initial experiments using PLPCreERT2:Notch1fl/fl animals did not report strong effects on remyelination [124]. Conversely, subsequent studies of lysolecithin-induced demyelinating lesions in Olig1Cre:Notch112f/12f mice revealed mildly potentiated OPC differentiation and accelerated remyelination [125]. Further complicating the field, it has also been proposed that canonical Notch signaling in OPCs may be countered in chronic MS lesions by the noncanonical pathway [126].
C3. Wingless (Wnt) signaling
In addition to extrinsic cues in lesions, progenitor maturation may also be regulated by intrinsic signals. For initiation of myelination, immature progenitors must exit the cell cycle and differentiate [127], and this transition has recently been shown to involve epigenetic regulation, which can control cell type-specific transcriptional states. How these factors participate to remyelination in the lesioned CNS remains only partially understood, but studies have reported the involvement of histone deacetylases (HDAC) in these events [128,129]. HDAC are a class of enzymes that antagonize the actions of histone acetyltransferases (HAT). Acetylation and deacetylation oppose each other, with acetylation functionally associated with active and open chromatin, whereas deacetylation correlates with inactive, closed chromatin [130]. Interestingly, interactions have been demonstrated between HDAC and Wingless (Wnt) signaling, in which canonical Wnt-mediated inhibition of OPC maturation was reversed by HDAC1 and 2 [131]. This molecular communication within OPC appears to be important for control of oligodendrocyte lineage commitment and differentiation [132].
The transcription factor YY1 (Yin-Yang 1) is one of the molecules implicated in control of the exit of OPCs from the cell cycle [133], and can act either as a transcriptional repressor or activator. Interestingly, conditional oligodendrocyte-specific ablation of YY1 results in impaired CNS myelination, accompanied by arrest of OPC differentiation [133]. This block has been linked to loss of recruitment of YY1 and inhibitory HDACs to the promoters of inhibitors such as the Wnt transcription factor 4 (Tcf4), and the helix-loop-helix factor Id4, resulting in potent restriction of OPC maturation.
Relevance of these mechanisms to myelin repair has been suggested by the recent demonstration that Wnt–cateninβ1 signaling is active in oligodendrocyte lineage cells surrounding MS lesions, and that dysregulation of Wnt signaling in OPCs results in delayed myelin formation and repair [134,135]. Unexpectedly, however, Tcf4-null mice display reduced rather than potentiated expression of oligodendrocyte terminal differentation genes [136]. Thus, the role of Wnt signaling in myelination and remyelination, and its potential as a novel translational avenue in MS, remain incompletely understood.
D. Future directions
Approaches directly targeting progenitors of myelinating oligodendrocytes may lead to improved long-term outcomes and delay accumulation of chronic demyelination and perhaps axonal transection in MS. They might be used in combination with currently-approved immunoregulators. However, these strategies have yet to meet with successful clinical trials in human patients, for reasons that are not fully understood. Potential confounds include incomplete CNS access, actions on additional lineages, and species differences. Moreover, the relationship between demyelination and axonal transection remains unclear, particularly important since the latter is reported to underlie permanent disability observed later in the disease course [2]. Notwithstanding these difficulties, protective and regenerative approaches represent an increasingly important translational focus. Their successful development is a critical goal for MS research.
Acknowledgements
Supported by USPHS Grants R01 NS046620, R01 NS062703 and R01 NS056074, and ARRA administrative supplement R01 NS056074-02S1 (to GRJ). Support for ATA comes from T32NS051147-03 (PI: Dr. Steven Levine/Dr. Stanley Tuhrim, MSSM). This work was also supported by National Multiple Sclerosis Society Research Grants RG3874 and RG4127 (to GRJ), and by the Jayne and Harvey Beker Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].McDonald WI, Sears TA. Effect of demyelination on conduction in the central nervous system. Nature. 1969;221:182–3. doi: 10.1038/221182a0. [DOI] [PubMed] [Google Scholar]
- [2].Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–85. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- [3].Smith EJ, Blakemore WF, McDonald WI. Central remyelination restores secure conduction. Nature. 1979;280:395–6. doi: 10.1038/280395a0. [DOI] [PubMed] [Google Scholar]
- [4].Prineas JW, Connell F. Remyelination in multiple sclerosis. Ann Neurol. 1979;5:22–31. doi: 10.1002/ana.410050105. [DOI] [PubMed] [Google Scholar]
- [5].Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346:165–73. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- [6].Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–52. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- [7].Redwine JM, Armstrong RC. In vivo proliferation of oligodendrocyte progenitors expressing PDGFalphaR during early remyelination. J Neurobiol. 1998;37:413–28. doi: 10.1002/(sici)1097-4695(19981115)37:3<413::aid-neu7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- [8].Watanabe M, Toyama Y, Nishiyama A. Differentiation of proliferated NG2-positive glial progenitor cells in a remyelinating lesion. J Neurosci Res. 2002;69:826–36. doi: 10.1002/jnr.10338. [DOI] [PubMed] [Google Scholar]
- [9].Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- [10].Armstrong RC, Dorn HH, Kufta CV, Friedman E, Dubois-Dalcq ME. Pre-oligodendrocytes from adult human CNS. J Neurosci. 1992;12:1538–47. doi: 10.1523/JNEUROSCI.12-04-01538.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Interaction between NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells is required for optimal response to PDGF. J Neurosci Res. 1996;43:315–30. doi: 10.1002/(SICI)1097-4547(19960201)43:3<315::AID-JNR6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- [12].Wolswijk G. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J Neurosci. 1998;18:601–9. doi: 10.1523/JNEUROSCI.18-02-00601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Aktas O, Kieseier B, Hartung HP. Neuroprotection, regeneration and immunomodulation: broadening the therapeutic repertoire in multiple sclerosis. Trends Neurosci. 33:140–52. doi: 10.1016/j.tins.2009.12.002. [DOI] [PubMed] [Google Scholar]
- [14].Van der Walt A, Butzkueven H, Kolbe S, Marriott M, Alexandrou E, Gresle M, Egan G, Kilpatrick T. Neuroprotection in multiple sclerosis: a therapeutic challenge for the next decade. Pharmacol Ther. 126:82–93. doi: 10.1016/j.pharmthera.2010.01.006. [DOI] [PubMed] [Google Scholar]
- [15].Lubetzki C, Williams A, Stankoff B. Promoting repair in multiple sclerosis: problems and prospects. Curr Opin Neurol. 2005;18:237–44. doi: 10.1097/01.wco.0000169739.83793.e0. [DOI] [PubMed] [Google Scholar]
- [16].Taga T, Hibi M, Hirata Y, Yamasaki K, Yasukawa K, Matsuda T, Hirano T, Kishimoto T. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58:573–81. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- [17].Anhuf D, et al. Signal transduction of IL-6, leukemia-inhibitory factor, and oncostatin M: structural receptor requirements for signal attenuation. J Immunol. 2000;165:2535–43. doi: 10.4049/jimmunol.165.5.2535. [DOI] [PubMed] [Google Scholar]
- [18].Murakami M, Hibi M, Nakagawa N, Nakagawa T, Yasukawa K, Yamanishi K, Taga T, Kishimoto T. IL-6-induced homodimerization of gp130 and associated activation of a tyrosine kinase. Science. 1993;260:1808–10. doi: 10.1126/science.8511589. [DOI] [PubMed] [Google Scholar]
- [19].Davis S, Aldrich TH, Stahl N, Pan L, Taga T, Kishimoto T, Ip NY, Yancopoulos GD. LIFR beta and gp130 as heterodimerizing signal transducers of the tripartite CNTF receptor. Science. 1993;260:1805–8. doi: 10.1126/science.8390097. [DOI] [PubMed] [Google Scholar]
- [20].Lutticken C, et al. Association of transcription factor APRF and protein kinase Jak1 with the interleukin-6 signal transducer gp130. Science. 1994;263:89–92. doi: 10.1126/science.8272872. [DOI] [PubMed] [Google Scholar]
- [21].Stahl N, et al. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science. 1994;263:92–5. doi: 10.1126/science.8272873. [DOI] [PubMed] [Google Scholar]
- [22].Ernst M, Jenkins BJ. Acquiring signalling specificity from the cytokine receptor gp130. Trends Genet. 2004;20:23–32. doi: 10.1016/j.tig.2003.11.003. [DOI] [PubMed] [Google Scholar]
- [23].Masu Y, Wolf E, Holtmann B, Sendtner M, Brem G, Thoenen H. Disruption of the CNTF gene results in motor neuron degeneration. Nature. 1993;365:27–32. doi: 10.1038/365027a0. [DOI] [PubMed] [Google Scholar]
- [24].Oppenheim RW, et al. Cardiotrophin-1, a muscle-derived cytokine, is required for the survival of subpopulations of developing motoneurons. J Neurosci. 2001;21:1283–91. doi: 10.1523/JNEUROSCI.21-04-01283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Emerich DF, et al. Protective effect of encapsulated cells producing neurotrophic factor CNTF in a monkey model of Huntington’s disease. Nature. 1997;386:395–9. doi: 10.1038/386395a0. [DOI] [PubMed] [Google Scholar]
- [26].Stankoff B, Aigrot MS, Noel F, Wattilliaux A, Zalc B, Lubetzki C. Ciliary neurotrophic factor (CNTF) enhances myelin formation: a novel role for CNTF and CNTF-related molecules. J Neurosci. 2002;22:9221–7. doi: 10.1523/JNEUROSCI.22-21-09221.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Marmur R, Kessler JA, Zhu G, Gokhan S, Mehler MF. Differentiation of oligodendroglial progenitors derived from cortical multipotent cells requires extrinsic signals including activation of gp130/LIFbeta receptors. J Neurosci. 1998;18:9800–11. doi: 10.1523/JNEUROSCI.18-23-09800.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Valerio A, Ferrario M, Dreano M, Garotta G, Spano P, Pizzi M. Soluble interleukin-6 (IL-6) receptor/IL-6 fusion protein enhances in vitro differentiation of purified rat oligodendroglial lineage cells. Mol Cell Neurosci. 2002;21:602–15. doi: 10.1006/mcne.2002.1208. [DOI] [PubMed] [Google Scholar]
- [29].Barres BA, Schmid R, Sendnter M, Raff MC. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993;118:283–95. doi: 10.1242/dev.118.1.283. [DOI] [PubMed] [Google Scholar]
- [30].Mayer M, Bhakoo K, Noble M. Ciliary neurotrophic factor and leukemia inhibitory factor promote the generation, maturation and survival of oligodendrocytes in vitro. Development. 1994;120:143–53. doi: 10.1242/dev.120.1.143. [DOI] [PubMed] [Google Scholar]
- [31].Butzkueven H, et al. LIF receptor signaling limits immune-mediated demyelination by enhancing oligodendrocyte survival. Nat Med. 2002;8:613–9. doi: 10.1038/nm0602-613. [DOI] [PubMed] [Google Scholar]
- [32].Linker RA, et al. CNTF is a major protective factor in demyelinating CNS disease: a neurotrophic cytokine as modulator in neuroinflammation. Nat Med. 2002;8:620–4. doi: 10.1038/nm0602-620. [DOI] [PubMed] [Google Scholar]
- [33].Linker RA, et al. Leukemia inhibitory factor deficiency modulates the immune response and limits autoimmune demyelination: a new role for neurotrophic cytokines in neuroinflammation. J Immunol. 2008;180:2204–13. doi: 10.4049/jimmunol.180.4.2204. [DOI] [PubMed] [Google Scholar]
- [34].Talbott JF, Cao Q, Bertram J, Nkansah M, Benton RL, Lavik E, Whittemore SR. CNTF promotes the survival and differentiation of adult spinal cord-derived oligodendrocyte precursor cells in vitro but fails to promote remyelination in vivo. Exp Neurol. 2007;204:485–9. doi: 10.1016/j.expneurol.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cao Q, et al. Transplantation of ciliary neurotrophic factor-expressing adult oligodendrocyte precursor cells promotes remyelination and functional recovery after spinal cord injury. J Neurosci. 30:2989–3001. doi: 10.1523/JNEUROSCI.3174-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kuhlmann T, et al. Continued administration of ciliary neurotrophic factor protects mice from inflammatory pathology in experimental autoimmune encephalomyelitis. Am J Pathol. 2006;169:584–98. doi: 10.2353/ajpath.2006.051086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Butzkueven H, Emery B, Cipriani T, Marriott MP, Kilpatrick TJ. Endogenous leukemia inhibitory factor production limits autoimmune demyelination and oligodendrocyte loss. Glia. 2006;53:696–703. doi: 10.1002/glia.20321. [DOI] [PubMed] [Google Scholar]
- [38].Marriott MP, et al. Leukemia inhibitory factor signaling modulates both central nervous system demyelination and myelin repair. Glia. 2008;56:686–98. doi: 10.1002/glia.20646. [DOI] [PubMed] [Google Scholar]
- [39].Sendtner M, Schmalbruch H, Stockli KA, Carroll P, Kreutzberg GW, Thoenen H. Ciliary neurotrophic factor prevents degeneration of motor neurons in mouse mutant progressive motor neuronopathy. Nature. 1992;358:502–4. doi: 10.1038/358502a0. [DOI] [PubMed] [Google Scholar]
- [40].Miller RG, et al. rhCNTF ALS Study Group A placebo-controlled trial of recombinant human ciliary neurotrophic (rhCNTF) factor in amyotrophic lateral sclerosis. Ann Neurol. 1996;39:256–60. doi: 10.1002/ana.410390215. [DOI] [PubMed] [Google Scholar]
- [41].ALS CNTF Treatment Study Group A double-blind placebo-controlled clinical trial of subcutaneous recombinant human ciliary neurotrophic factor (rHCNTF) in amyotrophic lateral sclerosis. Neurology. 1996;46:1244–9. doi: 10.1212/wnl.46.5.1244. [DOI] [PubMed] [Google Scholar]
- [42].Gurfein BT, Zhang Y, Lopez CB, Argaw AT, Zameer A, Moran TM, John GR. IL-11 regulates autoimmune demyelination. J Immunol. 2009;183:4229–40. doi: 10.4049/jimmunol.0900622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhang Y, Taveggia C, Melendez-Vasquez C, Einheber S, Raine CS, Salzer JL, Brosnan CF, John GR. Interleukin-11 potentiates oligodendrocyte survival and maturation, and myelin formation. J Neurosci. 2006;26:12174–85. doi: 10.1523/JNEUROSCI.2289-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhang J, et al. Proapoptotic and Antiapoptotic Actions of Stat1 versus Stat3 Underlie Neuroprotective and Immunoregulatory Functions of IL-11. J Immunol. doi: 10.4049/jimmunol.1004066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].McMorris FA, Smith TM, DeSalvo S, Furlanetto RW. Insulin-like growth factor I/somatomedin C: a potent inducer of oligodendrocyte development. Proc Natl Acad Sci U S A. 1986;83:822–6. doi: 10.1073/pnas.83.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chesik D, Wilczak N, De Keyser J. The insulin-like growth factor system in multiple sclerosis. Int Rev Neurobiol. 2007;79:203–26. doi: 10.1016/S0074-7742(07)79009-8. [DOI] [PubMed] [Google Scholar]
- [47].Beck KD, Powell-Braxton L, Widmer HR, Valverde J, Hefti F. Igf1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron. 1995;14:717–30. doi: 10.1016/0896-6273(95)90216-3. [DOI] [PubMed] [Google Scholar]
- [48].Carson MJ, Behringer RR, Brinster RL, McMorris FA. Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice. Neuron. 1993;10:729–40. doi: 10.1016/0896-6273(93)90173-o. [DOI] [PubMed] [Google Scholar]
- [49].Ye P, Carson J, D’Ercole AJ. In vivo actions of insulin-like growth factor-I (IGF-I) on brain myelination: studies of IGF-I and IGF binding protein-1 (IGFBP-1) transgenic mice. J Neurosci. 1995;15:7344–56. doi: 10.1523/JNEUROSCI.15-11-07344.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hsieh J, Aimone JB, Kaspar BK, Kuwabara T, Nakashima K, Gage FH. IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. J Cell Biol. 2004;164:111–22. doi: 10.1083/jcb.200308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yao DL, Liu X, Hudson LD, Webster HD. Insulin-Like Growth-Factor-I Treatment Reduces Demyelination and up-Regulates Gene-Expression of Myelin-Related Proteins in Experimental Autoimmune Encephalomyelitis. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:6190–6194. doi: 10.1073/pnas.92.13.6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Cannella B, Pitt D, Capello E, Raine CS. Insulin-like growth factor-1 fails to enhance central nervous system myelin repair during autoimmune demyelination. American Journal of Pathology. 2000;157:933–943. doi: 10.1016/S0002-9440(10)64606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gveric D, Cuzner ML, Newcombe J. Insulin-like growth factors and binding proteins in multiple sclerosis plaques. Neuropathology and Applied Neurobiology. 1999;25:215–225. doi: 10.1046/j.1365-2990.1999.00187.x. [DOI] [PubMed] [Google Scholar]
- [54].Frank JA, et al. A pilot study of recombinant insulin-like growth factor-1 in seven multiple sclerosis patients. Multiple Sclerosis. 2002;8:24–29. doi: 10.1191/1352458502ms768oa. [DOI] [PubMed] [Google Scholar]
- [55].Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1:549–53. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Rosenberg SS, Ng BK, Chan JR. The quest for remyelination: a new role for neurotrophins and their receptors. Brain Pathol. 2006;16:288–94. doi: 10.1111/j.1750-3639.2006.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Xiao J, Kilpatrick TJ, Murray SS. The role of neurotrophins in the regulation of myelin development. Neurosignals. 2009;17:265–76. doi: 10.1159/000231893. [DOI] [PubMed] [Google Scholar]
- [59].Chan JR, Watkins TA, Cosgaya JM, Zhang C, Chen L, Reichardt LF, Shooter EM, Barres BA. NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron. 2004;43:183–91. doi: 10.1016/j.neuron.2004.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Cohen RI, Marmur R, Norton WT, Mehler MF, Kessler JA. Nerve growth factor and neurotrophin-3 differentially regulate the proliferation and survival of developing rat brain oligodendrocytes. J Neurosci. 1996;16:6433–42. doi: 10.1523/JNEUROSCI.16-20-06433.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Tessarollo L, Vogel KS, Palko ME, Reid SW, Parada LF. Targeted mutation in the neurotrophin-3 gene results in loss of muscle sensory neurons. Proc Natl Acad Sci U S A. 1994;91:11844–8. doi: 10.1073/pnas.91.25.11844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kahn MA, Kumar S, Liebl D, Chang R, Parada LF, De Vellis J. Mice lacking NT-3, and its receptor TrkC, exhibit profound deficiencies in CNS glial cells. Glia. 1999;26:153–65. [PubMed] [Google Scholar]
- [63].Barres BA, Raff MC, Gaese F, Bartke I, Dechant G, Barde YA. A crucial role for neurotrophin-3 in oligodendrocyte development. Nature. 1994;367:371–5. doi: 10.1038/367371a0. [DOI] [PubMed] [Google Scholar]
- [64].Yan H, Wood PM. NT-3 weakly stimulates proliferation of adult rat O1(−)O4(+) oligodendrocyte-lineage cells and increases oligodendrocyte myelination in vitro. J Neurosci Res. 2000;62:329–35. doi: 10.1002/1097-4547(20001101)62:3<329::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- [65].Rubio N, Rodriguez R, Arevalo MA. In vitro myelination by oligodendrocyte precursor cells transfected with the neurotrophin-3 gene. Glia. 2004;47:78–87. doi: 10.1002/glia.20035. [DOI] [PubMed] [Google Scholar]
- [66].Du Y, Fischer TZ, Lee LN, Lercher LD, Dreyfus CF. Regionally specific effects of BDNF on oligodendrocytes. Dev Neurosci. 2003;25:116–26. doi: 10.1159/000072261. [DOI] [PubMed] [Google Scholar]
- [67].Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–99. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Cellerino A, Carroll P, Thoenen H, Barde YA. Reduced size of retinal ganglion cell axons and hypomyelination in mice lacking brain-derived neurotrophic factor. Mol Cell Neurosci. 1997;9:397–408. doi: 10.1006/mcne.1997.0641. [DOI] [PubMed] [Google Scholar]
- [69].McTigue DM, Horner PJ, Stokes BT, Gage FH. Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J Neurosci. 1998;18:5354–65. doi: 10.1523/JNEUROSCI.18-14-05354.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Girard C, Bemelmans AP, Dufour N, Mallet J, Bachelin C, Nait-Oumesmar B, Baron-Van Evercooren A, Lachapelle F. Grafts of brain-derived neurotrophic factor and neurotrophin 3-transduced primate Schwann cells lead to functional recovery of the demyelinated mouse spinal cord. J Neurosci. 2005;25:7924–33. doi: 10.1523/JNEUROSCI.4890-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Linker RA, et al. Functional role of brain-derived neurotrophic factor in neuroprotective autoimmunity: therapeutic implications in a model of multiple sclerosis. Brain. 133:2248–63. doi: 10.1093/brain/awq179. [DOI] [PubMed] [Google Scholar]
- [72].Cao Q, et al. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J Neurosci. 2005;25:6947–57. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- [74].Steinthorsdottir V, et al. Multiple novel transcription initiation sites for NRG1. Gene. 2004;342:97–105. doi: 10.1016/j.gene.2004.07.029. [DOI] [PubMed] [Google Scholar]
- [75].Nave KA, Salzer JL. Axonal regulation of myelination by neuregulin 1. Curr Opin Neurobiol. 2006;16:492–500. doi: 10.1016/j.conb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- [76].Taveggia C, et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–94. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Garratt AN, Voiculescu O, Topilko P, Charnay P, Birchmeier C. A dual role of erbB2 in myelination and in expansion of the schwann cell precursor pool. J Cell Biol. 2000;148:1035–46. doi: 10.1083/jcb.148.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Michailov GV, et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–3. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- [79].Lopez-Bendito G, et al. Tangential neuronal migration controls axon guidance: a role for neuregulin-1 in thalamocortical axon navigation. Cell. 2006;125:127–42. doi: 10.1016/j.cell.2006.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Canoll PD, Musacchio JM, Hardy R, Reynolds R, Marchionni MA, Salzer JL. GGF/neuregulin is a neuronal signal that promotes the proliferation and survival and inhibits the differentiation of oligodendrocyte progenitors. Neuron. 1996;17:229–43. doi: 10.1016/s0896-6273(00)80155-5. [DOI] [PubMed] [Google Scholar]
- [81].Vartanian T, Goodearl A, Viehover A, Fischbach G. Axonal neuregulin signals cells of the oligodendrocyte lineage through activation of HER4 and Schwann cells through HER2 and HER3. J Cell Biol. 1997;137:211–20. doi: 10.1083/jcb.137.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Fernandez PA, Tang DG, Cheng L, Prochiantz A, Mudge AW, Raff MC. Evidence that axon-derived neuregulin promotes oligodendrocyte survival in the developing rat optic nerve. Neuron. 2000;28:81–90. doi: 10.1016/s0896-6273(00)00087-8. [DOI] [PubMed] [Google Scholar]
- [83].Taveggia C, Thaker P, Petrylak A, Caporaso GL, Toews A, Falls DL, Einheber S, Salzer JL. Type III neuregulin-1 promotes oligodendrocyte myelination. Glia. 2008;56:284–93. doi: 10.1002/glia.20612. [DOI] [PubMed] [Google Scholar]
- [84].Brinkmann BG, et al. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron. 2008;59:581–95. doi: 10.1016/j.neuron.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Lefebvre P, Benomar Y, Staels B. Retinoid X receptors: common heterodimerization partners with distinct functions. Trends Endocrinol Metab. 21:676–83. doi: 10.1016/j.tem.2010.06.009. [DOI] [PubMed] [Google Scholar]
- [86].Schrage K, Koopmans G, Joosten EA, Mey J. Macrophages and neurons are targets of retinoic acid signaling after spinal cord contusion injury. Eur J Neurosci. 2006;23:285–95. doi: 10.1111/j.1460-9568.2005.04534.x. [DOI] [PubMed] [Google Scholar]
- [87].Huang JK, et al. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat Neurosci. 14:45–53. doi: 10.1038/nn.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–55. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- [89].Back SA, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–72. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- [90].Kuhlmann T, Miron V, Cui Q, Wegner C, Antel J, Bruck W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131:1749–58. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- [91].Nait-Oumesmar B, Decker L, Lachapelle F, Avellana-Adalid V, Bachelin C, Van Evercooren AB. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci. 1999;11:4357–66. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- [92].Nait-Oumesmar B, et al. Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors. Proc Natl Acad Sci U S A. 2007;104:4694–9. doi: 10.1073/pnas.0606835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Mehler MF, Mabie PC, Zhang D, Kessler JA. Bone morphogenetic proteins in the nervous system. Trends Neurosci. 1997;20:309–17. doi: 10.1016/s0166-2236(96)01046-6. [DOI] [PubMed] [Google Scholar]
- [94].Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19:1745–54. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Liu A, Niswander LA. Bone morphogenetic protein signalling and vertebrate nervous system development. Nat Rev Neurosci. 2005;6:945–54. doi: 10.1038/nrn1805. [DOI] [PubMed] [Google Scholar]
- [96].Liem KF, Jr., Tremml G, Jessell TM. A role for the roof plate and its resident TGFbeta-related proteins in neuronal patterning in the dorsal spinal cord. Cell. 1997;91:127–38. doi: 10.1016/s0092-8674(01)80015-5. [DOI] [PubMed] [Google Scholar]
- [97].Tanabe Y, Jessell TM. Diversity and pattern in the developing spinal cord. Science. 1996;274:1115–23. doi: 10.1126/science.274.5290.1115. [DOI] [PubMed] [Google Scholar]
- [98].Richardson WD, Kessaris N, Pringle N. Oligodendrocyte wars. Nat Rev Neurosci. 2006;7:11–8. doi: 10.1038/nrn1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Orentas DM, Miller RH. The origin of spinal cord oligodendrocytes is dependent on local influences from the notochord. Dev Biol. 1996;177:43–53. doi: 10.1006/dbio.1996.0143. [DOI] [PubMed] [Google Scholar]
- [100].Lu QR, et al. Sonic hedgehog--regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–29. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- [101].Cai J, et al. Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of Nkx6 regulation and Shh signaling. Neuron. 2005;45:41–53. doi: 10.1016/j.neuron.2004.12.028. [DOI] [PubMed] [Google Scholar]
- [102].Vallstedt A, Klos JM, Ericson J. Multiple dorsoventral origins of oligodendrocyte generation in the spinal cord and hindbrain. Neuron. 2005;45:55–67. doi: 10.1016/j.neuron.2004.12.026. [DOI] [PubMed] [Google Scholar]
- [103].Mekki-Dauriac S, Agius E, Kan P, Cochard P. Bone morphogenetic proteins negatively control oligodendrocyte precursor specification in the chick spinal cord. Development. 2002;129:5117–30. doi: 10.1242/dev.129.22.5117. [DOI] [PubMed] [Google Scholar]
- [104].Miller RH, Dinsio K, Wang R, Geertman R, Maier CE, Hall AK. Patterning of spinal cord oligodendrocyte development by dorsally derived BMP4. J Neurosci Res. 2004;76:9–19. doi: 10.1002/jnr.20047. [DOI] [PubMed] [Google Scholar]
- [105].Grinspan JB, Edell E, Carpio DF, Beesley JS, Lavy L, Pleasure D, Golden JA. Stage-specific effects of bone morphogenetic proteins on the oligodendrocyte lineage. J Neurobiol. 2000;43:1–17. [PubMed] [Google Scholar]
- [106].See J, Zhang X, Eraydin N, Mun SB, Mamontov P, Golden JA, Grinspan JB. Oligodendrocyte maturation is inhibited by bone morphogenetic protein. Mol Cell Neurosci. 2004;26:481–92. doi: 10.1016/j.mcn.2004.04.004. [DOI] [PubMed] [Google Scholar]
- [107].Gao L, Macklin W, Gerson J, Miller RH. Intrinsic and extrinsic inhibition of oligodendrocyte development by rat retina. Dev Biol. 2006;290:277–86. doi: 10.1016/j.ydbio.2005.11.007. [DOI] [PubMed] [Google Scholar]
- [108].Setoguchi T, Yone K, Matsuoka E, Takenouchi H, Nakashima K, Sakou T, Komiya S, Izumo S. Traumatic injury-induced BMP7 expression in the adult rat spinal cord. Brain Res. 2001;921:219–25. doi: 10.1016/s0006-8993(01)03123-7. [DOI] [PubMed] [Google Scholar]
- [109].Hall AK, Miller RH. Emerging roles for bone morphogenetic proteins in central nervous system glial biology. J Neurosci Res. 2004;76:1–8. doi: 10.1002/jnr.20019. [DOI] [PubMed] [Google Scholar]
- [110].Hampton DW, Asher RA, Kondo T, Steeves JD, Ramer MS, Fawcett JW. A potential role for bone morphogenetic protein signalling in glial cell fate determination following adult central nervous system injury in vivo. Eur J Neurosci. 2007;26:3024–35. doi: 10.1111/j.1460-9568.2007.05940.x. [DOI] [PubMed] [Google Scholar]
- [111].Zhao C, Fancy SP, Magy L, Urwin JE, Franklin RJ. Stem cells, progenitors and myelin repair. J Anat. 2005;207:251–8. doi: 10.1111/j.1469-7580.2005.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Fuller ML, DeChant AK, Rothstein B, Caprariello A, Wang R, Hall AK, Miller RH. Bone morphogenetic proteins promote gliosis in demyelinating spinal cord lesions. Ann Neurol. 2007;62:288–300. doi: 10.1002/ana.21179. [DOI] [PubMed] [Google Scholar]
- [113].Ara J, See J, Mamontov P, Hahn A, Bannerman P, Pleasure D, Grinspan JB. Bone morphogenetic proteins 4, 6, and 7 are up-regulated in mouse spinal cord during experimental autoimmune encephalomyelitis. J Neurosci Res. 2008;86:125–35. doi: 10.1002/jnr.21462. [DOI] [PubMed] [Google Scholar]
- [114].Deininger M, Meyermann R, Schluesener H. Detection of two transforming growth factor-beta-related morphogens, bone morphogenetic proteins-4 and -5, in RNA of multiple sclerosis and Creutzfeldt-Jakob disease lesions. Acta Neuropathol. 1995;90:76–9. doi: 10.1007/BF00294462. [DOI] [PubMed] [Google Scholar]
- [115].Wang S, Sdrulla AD, diSibio G, Bush G, Nofziger D, Hicks C, Weinmaster G, Barres BA. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 1998;21:63–75. doi: 10.1016/s0896-6273(00)80515-2. [DOI] [PubMed] [Google Scholar]
- [116].Genoud S, et al. Notch1 control of oligodendrocyte differentiation in the spinal cord. J Cell Biol. 2002;158:709–18. doi: 10.1083/jcb.200202002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].D’Souza B, Miyamoto A, Weinmaster G. The many facets of Notch ligands. Oncogene. 2008;27:5148–67. doi: 10.1038/onc.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Hu QD, et al. F3/contactin acts as a functional ligand for Notch during oligodendrocyte maturation. Cell. 2003;115:163–75. doi: 10.1016/s0092-8674(03)00810-9. [DOI] [PubMed] [Google Scholar]
- [119].Aguirre A, Rubio ME, Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature. 467:323–7. doi: 10.1038/nature09347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Nyfeler Y, Kirch RD, Mantei N, Leone DP, Radtke F, Suter U, Taylor V. Jagged1 signals in the postnatal subventricular zone are required for neural stem cell self-renewal. EMBO J. 2005;24:3504–15. doi: 10.1038/sj.emboj.7600816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351–5. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- [122].John GR, Shankar SL, Shafit-Zagardo B, Massimi A, Lee SC, Raine CS, Brosnan CF. Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat Med. 2002;8:1115–21. doi: 10.1038/nm781. [DOI] [PubMed] [Google Scholar]
- [123].Zhang Y, Z J, Navrazhina K, Argaw AT, Zameer A, Gurfein BT, Brosnan CF, John GR. TGFβ1 induces Jagged1 expression in astrocytes via ALK5 and Smad3 and regulates the balance between oligodendrocyte progenitor proliferation and differentiation. Glia. 2010 doi: 10.1002/glia.20978. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Stidworthy MF, Genoud S, Li WW, Leone DP, Mantei N, Suter U, Franklin RJ. Notch1 and Jagged1 are expressed after CNS demyelination, but are not a major rate-determining factor during remyelination. Brain. 2004;127:1928–41. doi: 10.1093/brain/awh217. [DOI] [PubMed] [Google Scholar]
- [125].Zhang Y, et al. Notch1 signaling plays a role in regulating precursor differentiation during CNS remyelination. Proc Natl Acad Sci U S A. 2009;106:19162–7. doi: 10.1073/pnas.0902834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Nakahara J, Kanekura K, Nawa M, Aiso S, Suzuki N. Abnormal expression of TIP30 and arrested nucleocytoplasmic transport within oligodendrocyte precursor cells in multiple sclerosis. J Clin Invest. 2009;119:169–81. doi: 10.1172/JCI35440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Taveggia C, Feltri ML, Wrabetz L. Signals to promote myelin formation and repair. Nat Rev Neurol. 6:276–87. doi: 10.1038/nrneurol.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Shen S, Li J, Casaccia-Bonnefil P. Histone modifications affect timing of oligodendrocyte progenitor differentiation in the developing rat brain. J Cell Biol. 2005;169:577–89. doi: 10.1083/jcb.200412101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Marin-Husstege M, Muggironi M, Liu A, Casaccia-Bonnefil P. Histone deacetylase activity is necessary for oligodendrocyte lineage progression. J Neurosci. 2002;22:10333–45. doi: 10.1523/JNEUROSCI.22-23-10333.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Bartova E, Krejci J, Harnicarova A, Galiova G, Kozubek S. Histone modifications and nuclear architecture: a review. J Histochem Cytochem. 2008;56:711–21. doi: 10.1369/jhc.2008.951251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Ye F, et al. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat Neurosci. 2009;12:829–38. doi: 10.1038/nn.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Li H, Richardson WD. Genetics meets epigenetics: HDACs and Wnt signaling in myelin development and regeneration. Nat Neurosci. 2009;12:815–7. doi: 10.1038/nn0709-815. [DOI] [PubMed] [Google Scholar]
- [133].He Y, et al. The transcription factor Yin Yang 1 is essential for oligodendrocyte progenitor differentiation. Neuron. 2007;55:217–30. doi: 10.1016/j.neuron.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Fancy SP, et al. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009;23:1571–85. doi: 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Feigenson K, Reid M, See J, Crenshaw EB, 3rd, Grinspan JB. Wnt signaling is sufficient to perturb oligodendrocyte maturation. Mol Cell Neurosci. 2009;42:255–65. doi: 10.1016/j.mcn.2009.07.010. [DOI] [PubMed] [Google Scholar]
- [136].Fu H, et al. A genome-wide screen for spatially restricted expression patterns identifies transcription factors that regulate glial development. J Neurosci. 2009;29:11399–408. doi: 10.1523/JNEUROSCI.0160-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]