Abstract
Although reduction in leukocyte counts following hydroxyurea therapy in sickle cell disease (SCD) predicts fetal hemoglobin (HbF) response, the underlying mechanism remains unknown. We previously reported that leukocyte counts are regulated by granulocyte-macrophage colony-stimulating factor (GM-CSF) in SCD patients. Here we examined the roles of GM-CSF in the regulation of HbF expression in SCD. Upon the analysis of retrospective data in 372 patients, HbF levels were inversely correlated with leukocyte counts and GM-CSF levels in SCD patients without hydroxyurea therapy, while HbF increments after hydroxyurea therapy correlated with a reduction in leukocyte counts, suggesting a negative effect of GM-CSF on HbF expression. Consistently, in vitro studies using primary erythroblasts showed that addition of GM-CSF to erythroid cells decreased HbF expression. We next examined the intracellular signaling pathway through which GM-CSF reduced HbF expression. Treatment of erythroid cells with GM-CSF resulted in the reduction in intracellular cAMP levels and abrogated phosphorylation of cAMP response-element-binding-protein, suggesting attenuation of the cAMP-dependent pathway, while the phosphorylation levels of mitogen-activated protein kinases were not affected. This is compatible with our studies showing a role for the cAMP-dependent pathway in HbF expression. Together, these results demonstrate that GM-CSF plays a role in regulating both leukocyte counts and HbF expression in SCD. Reduction in GM-CSF levels upon hydroxyurea therapy may be critical for efficient HbF induction. The results showing the involvement of GM-CSF in HbF expression may suggest possible mechanisms for hydroxyurea resistance in SCD.
Keywords: sickle cell disease, leukocytosis, fetal hemoglobin, GM-CSF, cAMP
Introduction
Clinical manifestations of patients with sickle cell disease (SCD) are primarily due to the polymerization of sickle hemoglobin, leading to vaso-occlusive crisis, chronic hemolysis, and ischemia-reperfusion injury associated with inflammation [1–3]. Although SCD patients share a common mutation in the β-globin gene, the clinical severity of SCD is extremely heterogeneous. The mechanisms underlying this clinical heterogeneity remain unknown.
Fetal hemoglobin (HbF) is expressed in patients with SCD at variable levels and known to alleviate the clinical severity of SCD [4]. HbF expression has been shown to be regulated by single nucleotide polymorphisms (SNPs) of multiple genetic loci [5–7], and it is likely that these genetic loci play roles in determining the levels of HbF production in SCD patients [8]. Leukocytosis is frequently observed in untreated patients even in the absence of bacterial infection [9], and an elevated base-line leukocyte count is associated with an increased risk of early death [10]. Severe complications are also predicted later in life for sickle cell children with high leukocyte counts [11]. These clinical observations suggest that both HbF levels and leukocyte counts have significant effects on the clinical severity of SCD.
Pharmacological stimulation of HbF expression, especially with hydroxyurea, is now a recognized therapeutic option for SCD patients with a severe clinical phenotype. However, the HbF response to hydroxyurea varies among patients. Discovering predictors of HbF response to hydroxyurea would permit clinicians to identify patients who would respond to hydroxyurea therapy and would increase the clinical effectiveness of hydroxyurea, which in turn would minimize adverse effects of the drug. A study by Charache and associates showed that significant predictors of high post-therapy HbF levels are post-therapy plasma hydroxyurea level, initial leukocyte counts, and initial HbF concentration [12]. Ware et al. also demonstrated that changes in blood counts in SCD children after hydroxyurea therapy indicate the HbF response to hydroxyurea [13]. However, the mechanisms by which these clinical variables serve as predictors of HbF response remain unknown.
Earlier studies by Croizat and Nagel reported elevated plasma GM-CSF levels in SCD patients who have low HbF levels [14,15]. They concluded that hematopoietic stress associated with low HbF levels contributes to increased levels of GM-CSF [15]. We recently reported a positive correlation between leukocyte counts and plasma GM-CSF levels in SCD patients [16]. Subsequently we found that leukocyte counts correlate inversely with HbF levels in steady-state SCD patients, suggesting the possibility that HbF expression in SCD patients is negatively affected by the mechanisms that sustain leukocyte counts.
We here report that leukocyte counts and plasma GM-CSF levels inversely correlated with HbF levels in SCD patients, and that GM-CSF is a negative regulator for HbF expression, which is in sharp contrast to the conclusion by Croizat and Nagel [15]. Our in vitro studies using primary erythroid cells consistently showed that GM-CSF downregulates HbF expression. Moreover, we demonstrate that GM-CSF attenuates the cAMP-dependent pathway in both cytokine-dependent erythroid cells and primary erythroid cells; our previous studies showed critical roles of cyclic nucleotide-dependent pathways in sustaining HbF expression [17–19]. These results suggest that GM-CSF has regulatory roles in both leukocyte counts and HbF levels in SCD patients. GM-CSF is likely to downregulate HbF expression at least in part by attenuating the cAMP-dependent pathway. Potential mechanisms by which HbF response to hydroxyurea is regulated in the context of GM-CSF are discussed.
Materials and Methods
SCD patients
Retrospective data on 372 SCD patients who were homozygous for the βS mutation and were under clinical care at the Sickle Cell Center of Georgia Health Sciences University were analyzed in this study. Of these, 192 patients who had not received hydroxyurea or blood transfusions for at least the past 3 months were considered to be in steady-state, and 125 SCD patients who were receiving HU therapy (15 to 35 mg/kg/day) for at least 6 months were enrolled in this study. In addition, 63 pediatric SCD patients (Kuwait University) who had a mutation in the γ-globin gene promoter [20] and expressed HbF at high levels were included to examine the effects of leukocyte counts on HbF expression; none of these patients were receiving hydroxyurea. Clinical characteristics of these cohorts of SCD patients were shown in Supplementary Tables (Table S1 to S3). Hematologic data reported in this study were the average of individual data for at least 3 months. Informed consent was obtained from all subjects. The study was performed in accordance with the principles of the Declaration of Helsinki and approved by the institutional review boards of Georgia Health Sciences University and Kuwait University.
Measurement of plasma GM-CSF levels
Plasma levels of GM-CSF were measured as described previously [16]. Briefly, GM-CSF levels were determined using high sensitivity immunoenzymatic assay kits according to the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA).
Complete blood counts and HbF measurement
Complete blood counts were performed using an Advia Hematology System 120 (Bayer Diagnostics, Tarrytown, NY, USA). HbF quantification was performed by cation-exchange high-pressure liquid chromatography (Synchropak CM 300) as described previously [21]. Increases in HbF levels in patients on hydroxyurea was determined by deducting pre-therapy HbF levels from post-therapy HbF levels.
Culture of TF-1 cells and CD34+ cells and globin mRNA analysis
TF-1 cells (American Type Culture Collection, Manassas, VA, USA) were maintained in RPMI1640 medium containing 10% fetal bovine serum (FBS), 2 mM L-glutamine, and 10 mM HEPES. All cytokines were purchased from Peprotech (Rocky Hill, NJ) unless otherwise stated. TF-1 cells were cultured with 5 ng/ml GM-CSF or 2 U/ml erythropoietin (Amgen, Thousand Oaks, CA, USA) or both. CD34+ cells were provided by NHLBI PEGT Hematopoietic Cell Processing Core (Fred Hutchinson Cancer Research Center, Seattle, WA, USA). CD34+ cells were cultured by the method described previously [19,22]. Briefly, CD34+ cells were maintained for 14 days in Iscove’s modified Dulbecco’s medium containing 15% FBS, 15% human AB serum, 2 units/ml erythropoietin, 10 ng/ml stem cell factor, 10 mM β-mercaptoethanol, and 600 g/ml holo-transferrin. To examine effects of GM-CSF, stem cell factor was depleted from cultures on day 5 and 10 to 100 ng/ml GM-CSF was added for the last 9 days before cell harvesting. Eighty to 90 percent of cells grown in the cultures expressed glycophorin A (CD235a) and CD71 as determined by flow cytometry. Total cytoplasmic RNA was extracted from nucleated erythroblasts using the method described by Chomoczynski and Sacchi [23]. Globin mRNAs were quantified by primer extension using a previously described method [24] with modifications [25,26].
Measurement of intracellular cAMP levels in erythroid cells
Intracellular levels of cAMP in TF-1 cells or primary erythroblasts were determined as described previously [17,27]. Briefly, 2×106 cells were incubated with 1 mM 3-isobutyl-1-methylxanthine for 30 min at room temperature. cAMP was extracted by suspending the cells with 0.5 M hydrocholoric acid. The supernatant was removed and the pH was neutralized by the addition of 8 M KOH. Intracellular cAMP levels were determined using a cAMP ELISA kit (Cayman Chemicals, Ann Arbor, MI, USA).
Isolation of whole cell extracts from erythroblasts and Western blotting
Whole cellular extracts were prepared from TF-1 cells or primary erythroblasts, as described [18]. Briefly, 5 to 10 × 106 cells were suspended with 1 × RIPA buffer (Santa Cruz Biotechnology, Santa Cruz, CA, USA) supplemented by 1 mM PMSF, 0.1% SDS, and 10% (v/v) protein phosphatase inhibitor cocktail Set IV (EMD Chemicals, Gibbstown, NJ, USA). Western blotting was performed as described previously [28]. Briefly, 20 to 30 µg of the cellular extracts were separated on 12% SDS polyacrylamide gels and transferred to nitrocellulose membranes (Invitrogen, Carlsbad, CA, USA). All antibodies used for Western blot analyses were purchased from Cell Signaling Technology (Danvers, MA, USA).
Statistical analysis
For analysis of correlations with non-Gaussian distributed data, which included hematological data of SCD patients (Figs.1 & 2), the Spearman correlation coefficient (rs) was utilized. Assays for cAMP levels were performed in triplicate and the data were expressed as means ± SE. The data were analyzed by the Student t test. P values < 0.05 were considered to be statistically significant.
Figure 1.
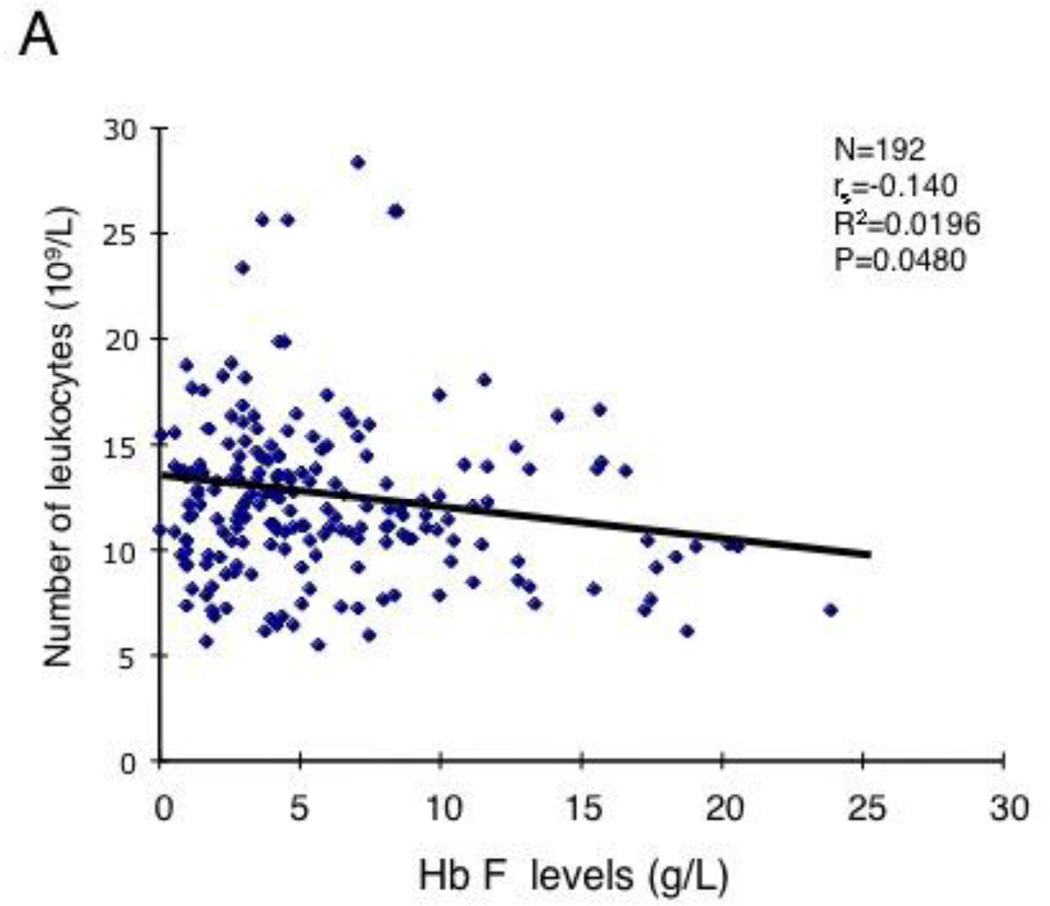
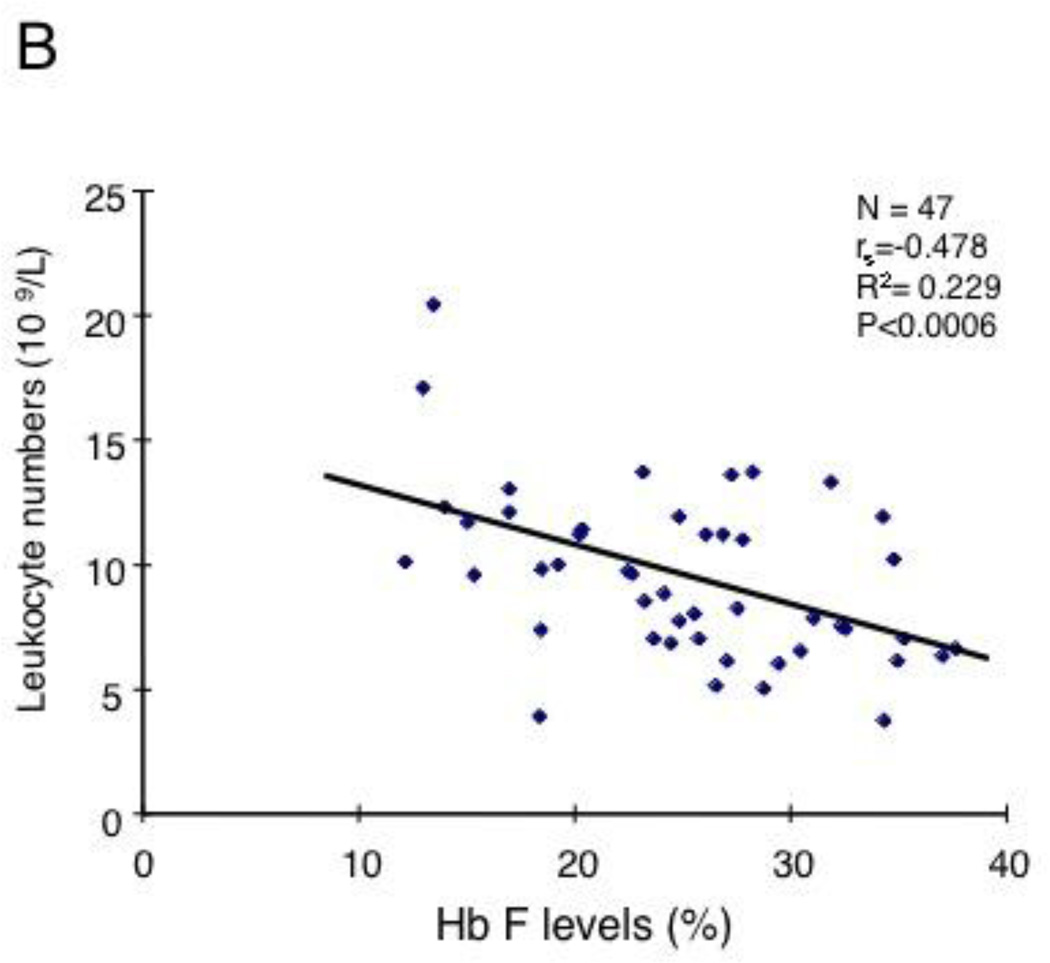
Correlation of HbF levels with leukocyte counts and with GM-CSF levels in SCD patients. (A) 200 SCD patients who were not receiving hydroxyurea (P<0.048, R2=0.0196). See Table S1 for the clinical characteristics. (B) 47 pediatric SCD patients without hydroxyurea treatment (P<0.0006, R2=0.229). See Table S2 for the clinical characteristics. (C) HbF levels inversely correlate with GM-CSF levels in 29 steady-state SCD patients (P<0.006, R2=0.139).
Figure 2.
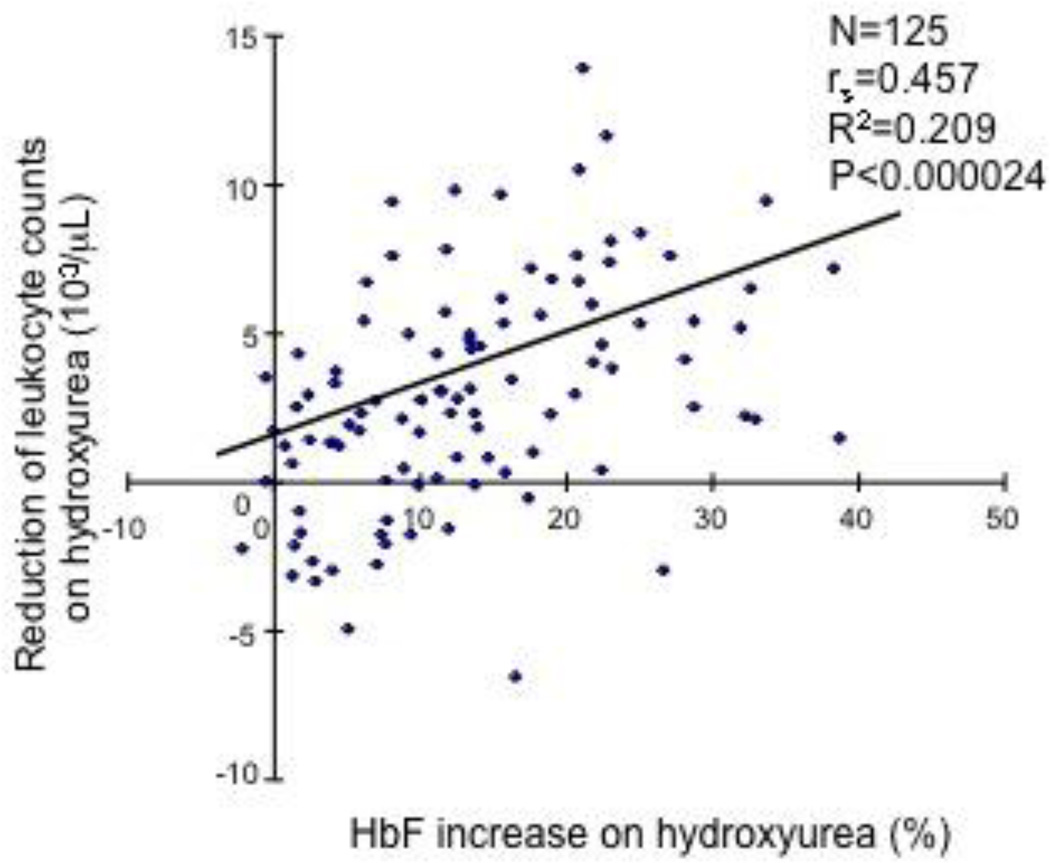
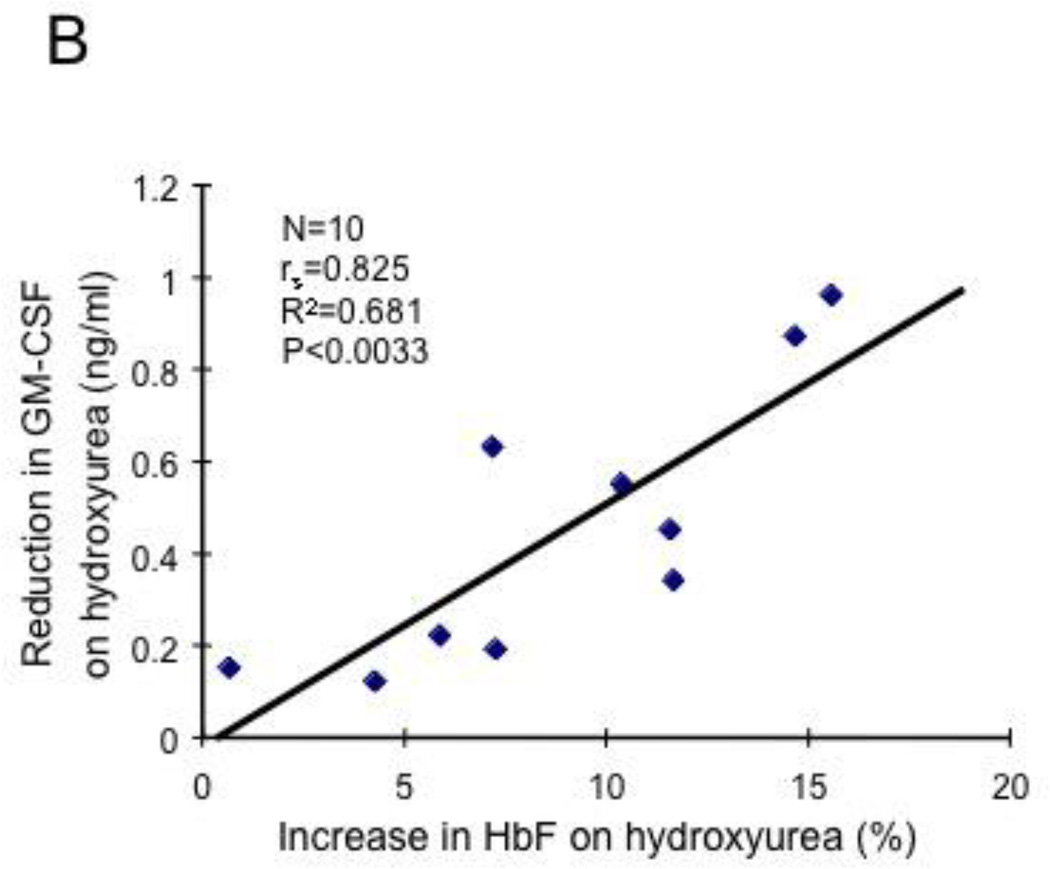
Levels of HbF induction by hydroxyurea correlate with reductions in leukocyte counts and levels of GM-CSF. (A) HbF levels and leukocyte counts were analyzed for 125 SCD patients who had received hydroxyurea for at least 3 months. Leukocyte counts and HbF levels were measured before and after hydroxyurea treatment. A significant correlation was observed (P<0.000024, R2=0.209). (B) HbF levels and GM-CSF levels were measured in 10 SCD patients who had received hydroxyurea for at least 3 months and a positive correlation was observed (P<0.0033, R2=0.681).
Results
HbF levels inversely correlate with leukocyte numbers and GM-CSF levels in SCD patients
To determine whether the levels of HbF expression in SCD patients are affected by the mechanisms underlying leukocytosis, we first investigated correlations between HbF levels and leukocyte counts for 192 steady-state SCD patients who were not receiving hydroxyurea therapy; the clinical characteristics of the patients were summarized in Table S1. A barely statistically significant inverse correlation was observed between the HbF levels and the leukocyte counts (Fig.1A, P=0.048, R2= 0.0196). We assumed that the weak correlation occured because the HbF levels of more than 85% of the patients analyzed were less than 10%. To more accurately determine the correlation between HbF levels and leukocyte counts, we next analyzed SCD patients with high HbF levels. We examined 47 steady-state pediatric SCD patients who were not taking hydroxyurea and had a C to T substitution at −158 base pair 5' to the cap site of the Gγ-globin gene and expressed HbF at high levels [20]; the hematological data of these patients are shown in Table S2. A strong inverse correlation was observed between the HbF levels and the leukocyte counts in this cohort of patients (Fig.1B, P<0.0006, R2= 0.229). On the basis of our previous study showing a major role for GM-CSF in leukocytosis [16], these results suggested a negative regulatory role for GM-CSF in HbF expression in steady-state SCD patients. To confirm the negative effect of GM-CSF on HbF expression, we examined a correlation between plasma GM-CSF levels and HbF levels in steady-state patients. The number of the patients involved in this analysis was relatively small, but we found a significant inverse correlation among them (Fig.1C, P<0.006, R2=0.139). Together, these clinical studies suggest a negative effect of GM-CSF on HbF expression in SCD.
Levels of HbF induction by hydroxyurea correlate with reduction levels of leukocyte counts
The mechanisms by which hydroxyurea induces HbF expression in SCD are not fully understood. We next examined whether a mechanism regulating leukocyte counts in SCD also has a consequence on HbF induction by hydroxyurea. We collected hematologic data from SCD patients before and after hydroxyurea therapy and examined the association between hydroxyurea-induced HbF changes and leukocyte counts; the clinical characteristics of these SCD patients are shown in Table S3. As shown in Fig.2A, we found a strong positive correlation between the levels of HbF induced by hydroxyurea and the reduction of leukocyte counts after hydroxyurea therapy (Fig.2A, P<0.000024, R2=0.209), demonstrating that reduction of leukocyte counts after hydroxyurea therapy is critical for HbF induction. This result is compatible with prior studies reporting the reduction of leukocyte counts following hydroxyurea therapy [12,13]. Also, these results suggested to us the possibility that GM-CSF may influence in hydroxyurea-induced HbF expression. To define the role for GM-CSF in the hydroxyurea-regulated HbF expression, we examined whether the reduction in GM-CSF after hydroxyurea therapy is associated with HbF induction. Although the number of SCD patients studied was small, we observed a significant correlation between reduced levels of GM-CSF and the levels of HbF (Fig.2B, P<0.0033, R2=0.681). Together, these results demonstrate that GM-CSF may have a negative regulatory effect on hydroxyurea-induced HbF expression in SCD.
GM-CSF downregulates HbF expression in both growth factor-dependent erythroid cells and primary erythroblasts
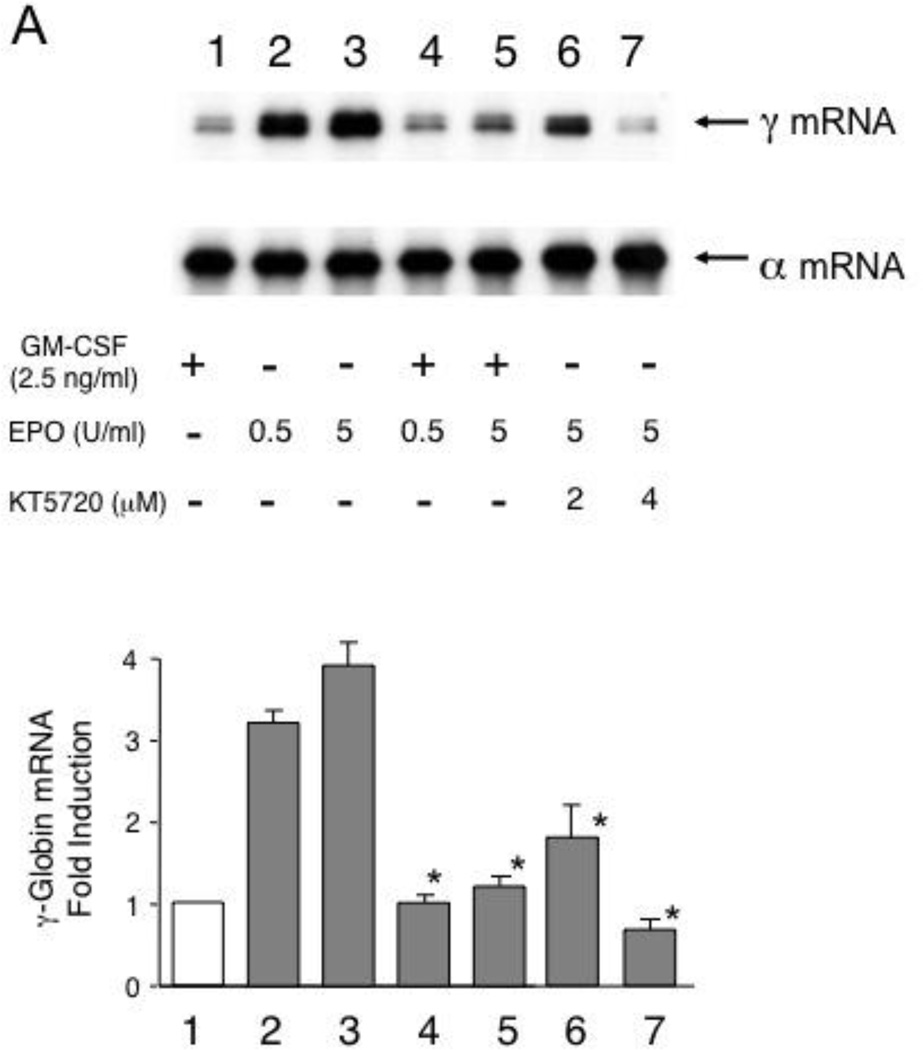
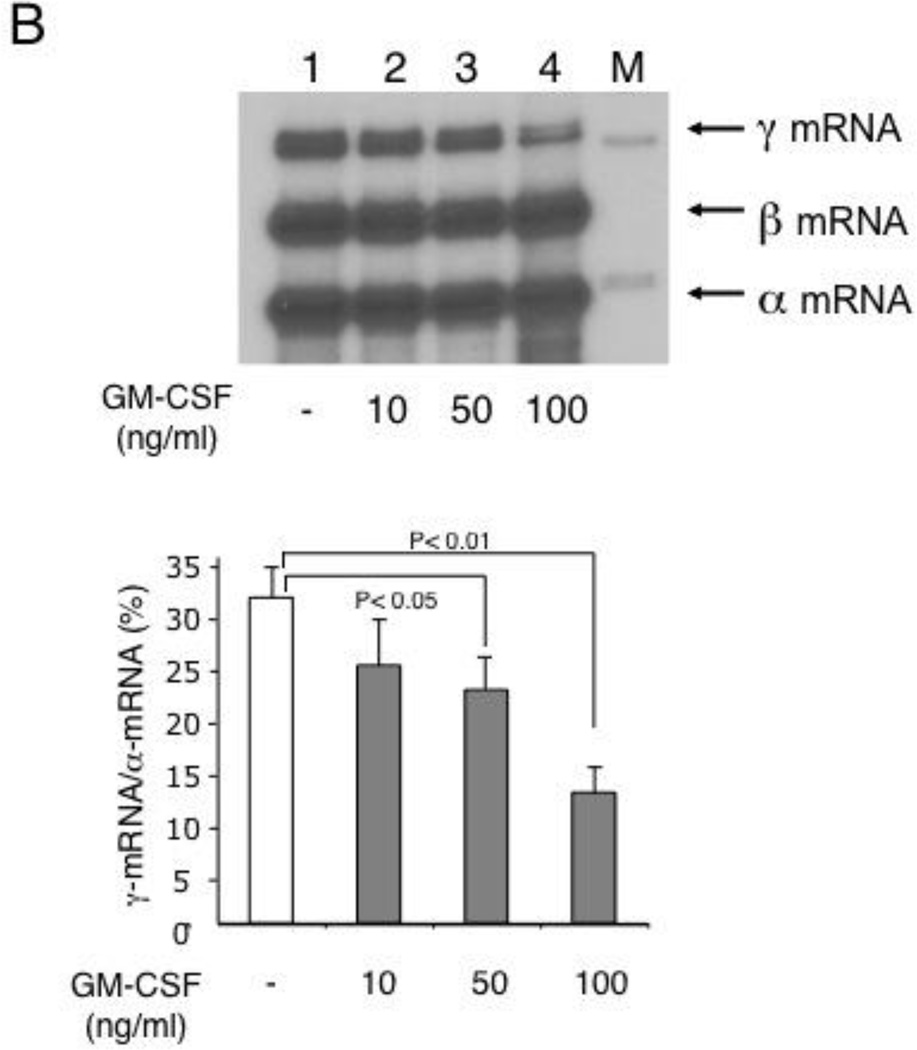
To determine the effects of GM-CSF on γ-globin gene expression in erythroid cells, we used cytokine-dependent TF-1 cells [29] as well as CD34+ cell-derived primary erythroblasts. TF-1 cells express human globin genes such as γ- and α-globin genes and grow in the presence of cytokines such as GM-CSF and erythropoietin [30]. TF-1 cells thus provide an excellent in vitro system to examine the effects of GM-CSF or erythropoietin on γ-globin expression, as we reported previously [19]. TF-1 cells grown with GM-CSF expressed γ-globin mRNA at a low level, but switching GM-CSF to erythropoietin induced γ-globin mRNA expression by 3- to 4-fold, suggesting that erythropoietin induces γ-globin gene expression in TF-1 cells (Fig.3A lanes 1 to 3). The positive effect of erythropoietin on HbF is supported by a previous clinical study with SCD patients [31]. However, the addition of GM-CSF to these cultures abrogated the induction of γ-globin mRNA expression, indicating that the GM-CSF has a dominant effect on γ-globin gene expression induced by erythropoietin (lanes 4 & 5). We previously showed that erythropoietin induces γ-globin gene expression by activating the cAMP-dependent pathway [19]. The addition of KT5720, which is an inhibitor of protein kinase A [32], consistently reduced erythropoietin-induced γ-globin mRNA expression (lanes 6 & 7). GM-CSF also downregulated γ-globin mRNA expression in a dose-dependent manner in primary erythroid cells that were prepared from CD34+ cells (Fig.3B). These results demonstrate that GM-CSF has a negative regulatory effect on γ-globin gene expression in both growth factor-dependent erythroid cells and primary erythroblasts.
Figure 3.
Downregulation of HbF expression by GM-CSF in erythroid cells. (A) Induction of γ-globin mRNA expression by erythropoietin is downregulated by GM-CSF. TF-1 cells were cultured with 0.5 or 5 U/ml erythropoietin in the absence or presence of 2.5ng/ml GM-CSF for 7 days. TF-1 cells grown with 5 U/ml erythropoietin were also incubated with or without the selective PKA inhibitor KT5720 (lanes 6 & 7). Total cytoplasmic RNA was purified and globin mRNAs were analyzed by primer extension. Summary of the result is shown below. Experiment was repeated 3 times and a representative result is shown. P values: *, P<0.05 compared with the cells treated with 5 U/ml erythropoietin (lane 3).
(B) GM-CSF downregulates expression of γ-globin mRNA in CD34+cell-derived primary erythroblasts. Cells were cultured in the presence of 10 to 100 ng/ml GM-CSF as described in “Design and Methods” and globin mRNAs were analyzed by primer extension. Summary of the result is shown below. Experiment was repeated 3 times and a representative result is shown.
GM-CSF attenuates the cAMP-dependent pathway in erythroid cells
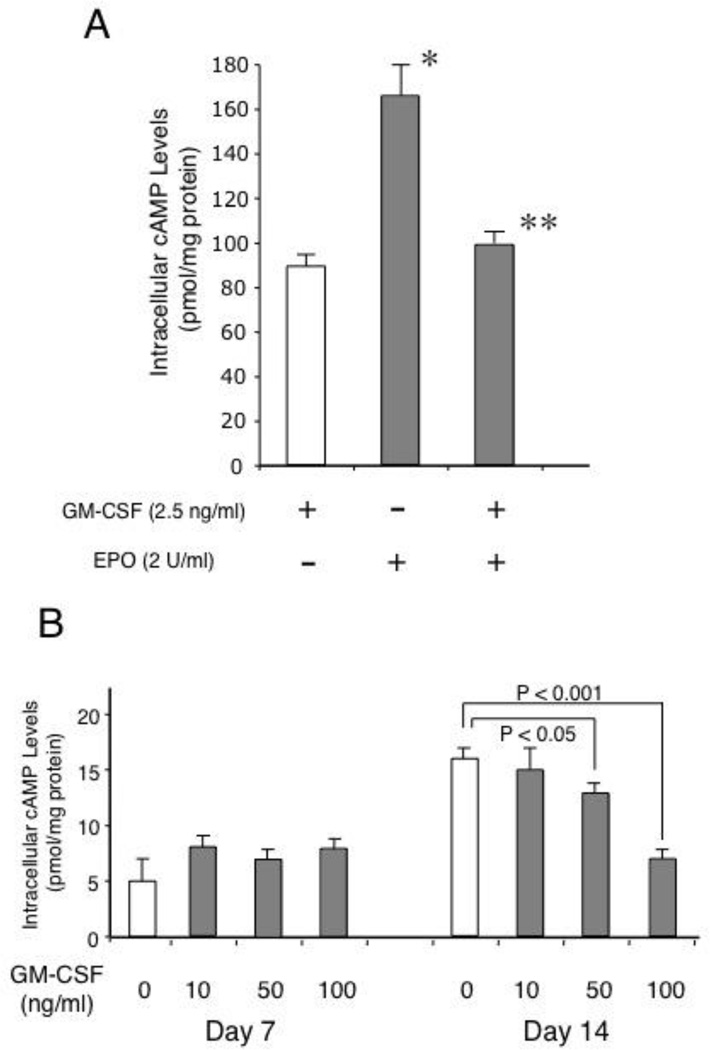
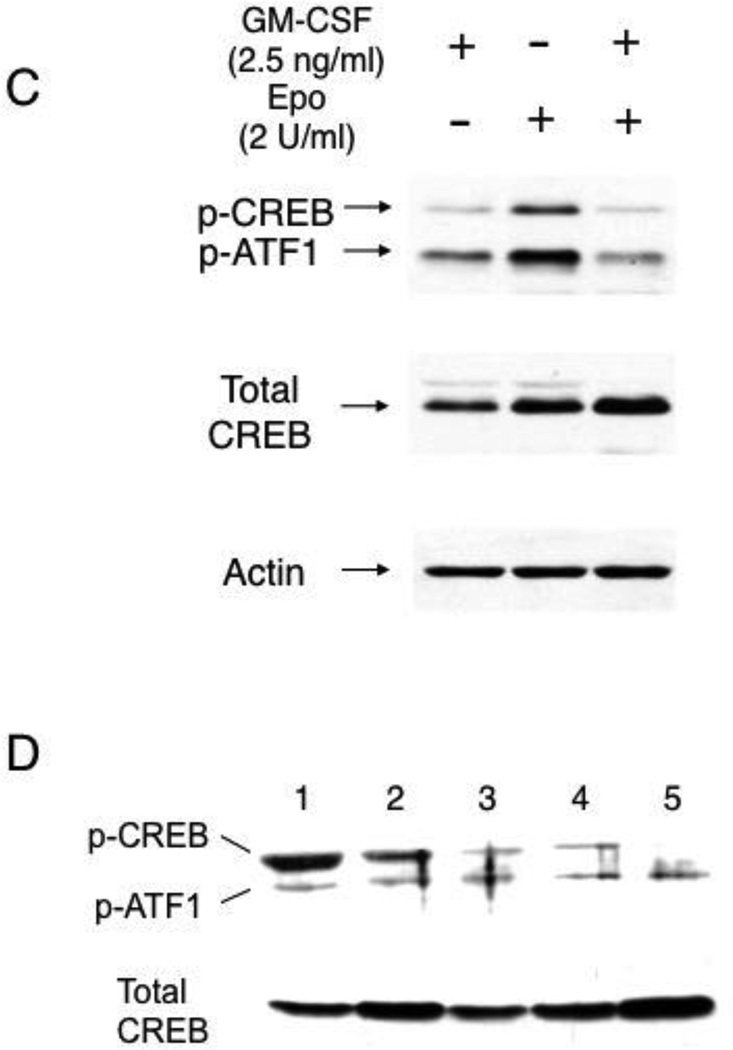
We next examined the mechanism by which GM-CSF downregulated γ-globin gene expression in erythroid cells. Our previous studies demonstrated a role for the cAMP-dependent pathway in sustaining γ-globin gene expression in primary erythroid cells from normal subjects [18] as well as in patients with β-thalassemia [19]. We first examined whether GM-CSF modulates intracellular cAMP levels in both TF-1 cells and CD34+ cell-derived primary erythroblasts. The intracellular cAMP levels of TF-1 cells on day 7 increased by 2-fold after switching the cytokine in cultures from GM-CSF to erythropoietin, but the addition of GM-CSF to these cultures decreased the cAMP levels to the levels that were similar to those of cells cultured with GM-CSF (Fig.4A). In primary erythroblasts, the intracellular cAMP levels of day 7 cultures were not influenced by GM-CSF, but a significant decrease in the intracellular cAMP levels was seen with the cells treated with GM-CSF on day 14 (Fig.4B). These results demonstrate that GM-CSF decreases intracellular cAMP levels in erythroid cells, suggesting an inhibitory effect of GM-CSF on the cAMP-dependent pathway. To examine whether decreases in intracellular cAMP levels by GM-CSF lead to modulation of the cAMP-dependent pathway, we examined phosphorylation of the cAMP response element binding (CREB) protein, which is a major target protein of cAMP-dependent protein kinase [33]. As shown in Fig.4C, the phosphorylation level of CREB was increased in TF-1 cells by switching the cytokine from GM-CSF to erythropoietin, suggesting that erythropoietin activates the cAMP-dependent pathway in this cells. However, addition of GM-CSF to these cultures completely abolished the increase in CREB phosphorylation by erythropoietin (Fig.4C right lane). These results are in accord with those for the intracellular cAMP levels in TF-1 cells (Fig.4A). Consistently, the addition of GM-CSF to primary erythroblasts also decreased the phosphorylation levels of CREB in a dose-dependent manner (Fig.4D, lanes 3–5). Collectively, these results show that GM-CSF attenuates the cAMP-dependent pathway in erythroid cells.
Figure 4.
GM-CSF attenuates the cAMP-dependent pathway in erythroid cells. (A) GM-CSF decreases intracellular cAMP levels in TF-1 cells. TF-1cells were grown for 7 days in 3 different cultures; with 2.5 ng/ml GM-CSF, with 2 U/ml erythropoietin, or with 2.5 ng/ml GM-CSF and 2 U/ml erythropoietin. Intracellular cAMP levels were measured using ELISA kits as described in "Design & Methods". P values: *, p<0.05 compared with GM-CSF cultures; **, P<0.05 compared with erythropoietin cultures.
(B) Reduction in intracellular cAMP levels of CD34+cell-derived primary erythroblasts cultured with GM-CSF. Primary erythroblasts were induced from CD34+ cells as described in "Design & Methods." The cAMP levels were measured in the cells of day 7 and those of day 14 as described above. Statistical analyses are shown above the figure.
(C) (D) GM-CSF decreases CREB phosphorylation in TF-1 cells and in CD34+cell-derived primary erythroblasts. TF-1 cells were cultured as described in Figure 5A. Primary erythroblasts were prepared from CD34+ cells for 14 days as described in "Design & Methods." Whole cell extracts (30 g) prepared from cells were analyzed by Western blotting. Experiment was repeated 3 times and a representative result is shown. Cytokines added to TF-1 cells are shown on top of figure. In Figure 5D, lanes are follows: 1, fetal liver erythroblasts; 2, primary erythroblasts (control, no GM-CSF); 3, primary erythroblasts cultured with 10 ng/ml GM-CSF; 4, primary erythroblasts cultured with 50 ng/ml GM-CSF; 5, primary erythroblasts cultured with 100 ng/ml GM-CSF.
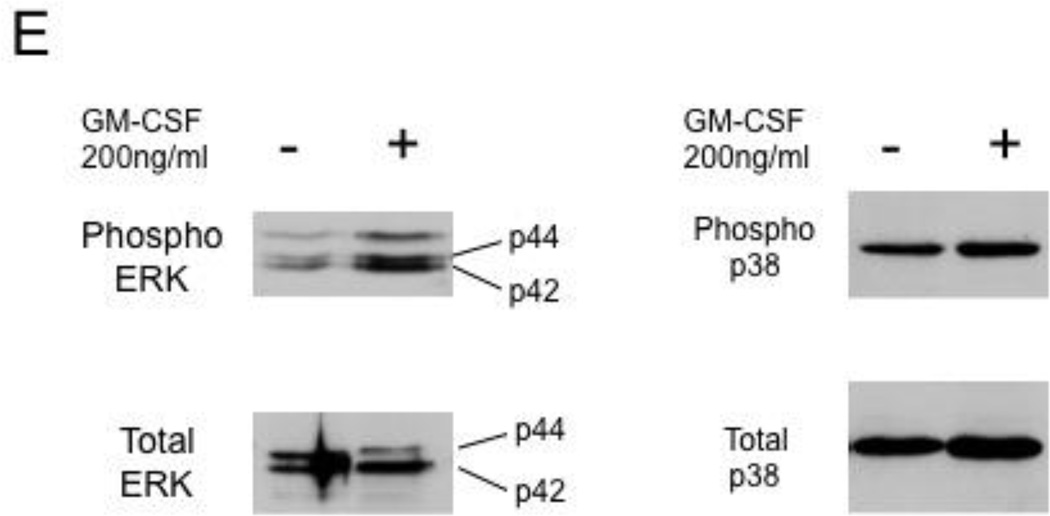
(E) Effects of GM-CSF on phosphorylation of mitogen-activated protein kinases. Primary erythroblasts were prepared from CD34+ cells for 14 days as described in "Design & Methods." Whole cell extracts (30 µg) prepared from cells were analyzed for ERKs and p38 kinases by Western blotting. Experiment was repeated 3 times and a representative result is shown.
Mitogen-activated protein kinases such as extracellular signal-regulated protein kinases (ERKs) and p38 kinases were demonstrated to play roles in HbF expression in primary erythroid cells [34,35]. No significant alterations in the phosphorylation levels of these protein kinases were observed in erythroid cells treated with 200 ng/ml GM-CSF (Fig.4E).
Discussion
Previous studies have provided important insight into the mechanisms regulating HbF expression in SCD. Changes in blood cell counts following hydroxyurea therapy predict a response of HbF to hydroxyurea [12,13]. Recent genome-wide association studies have revealed the presence of SNPs in a variety of genes that potentially have consequences on HbF expression in SCD [36]. However, it is not clear how changes in blood cell counts make HbF responsive to hydroxyurea [12,13]. The mechanistic aspects of such SNPs identified remain to be established.
To gain insight into the mechanisms by which HbF levels are regulated in the context of SCD, we have identified a soluble factor that downregulates HbF. In this study we have shown that GM-CSF, a proinflammatory cytokine that is elevated in SCD patients [14,16], plays roles in regulating both leukocyte counts and HbF expression in SCD; GM-CSF increases leukocyte counts but downregulates HbF expression. Regarding the mechanism for GM-CSF-mediated HbF repression, we have shown that GM-CSF downregulates HbF expression in erythroid cells by attenuating the cAMP-dependent pathway that is required to sustain HbF expression in β-thalassemic patients (Figs.3 & 4) [18,19]. We and other previously demonstrated a role for the cGMP-dependent pathway in the upregulation of γ-globin gene expression by HbF inducing chemicals [17,37]. It seems that both the cAMP- and cGMP-dependent pathways are likely to upregulate HbF expression, but the types of extracellular signals to which these pathways respond and their induction efficiency for HbF might be distinct from each other.
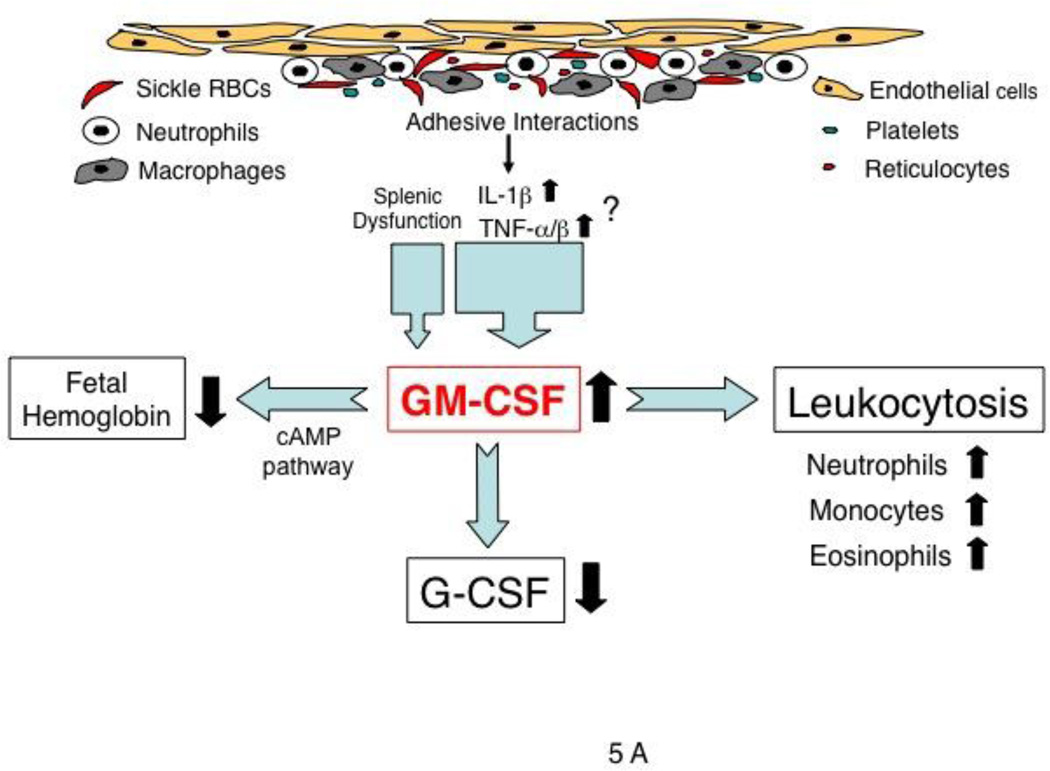
The mechanism by which plasma GM-CSF levels are elevated and vary in SCD patients remains unclear, but a few possible mechanisms are conceivable. Adhesion of monocytes or macrophages to endothelial cells is known to induce GM-CSF production in monocytes [38] (Fig.5A). Second, the proinflammatory cytokines IL-1β and TNF-α/β, both of which are increased in SCD [39], may be involved in increased production of GM-CSF by a transcriptional [40] or post-transcriptional mechanism [41] (Fig.5A). Third, splenectomized patients with spherocytosis had higher levels of GM-CSF than did healthy subjects, suggesting that the splenic dysfunction associated with SCD may contribute to elevated GM-CSF levels [16]. As previously reported [16], plasma granulocyte colony-stimulating factor (G-CSF) levels in SCD patients are significantly lower than those of normal subjects (Fig.5A). Such an antagonism between these two cytokines was previously found in the synovial fluid of patients with rheumatoid arthritis [42].
Figure 5.
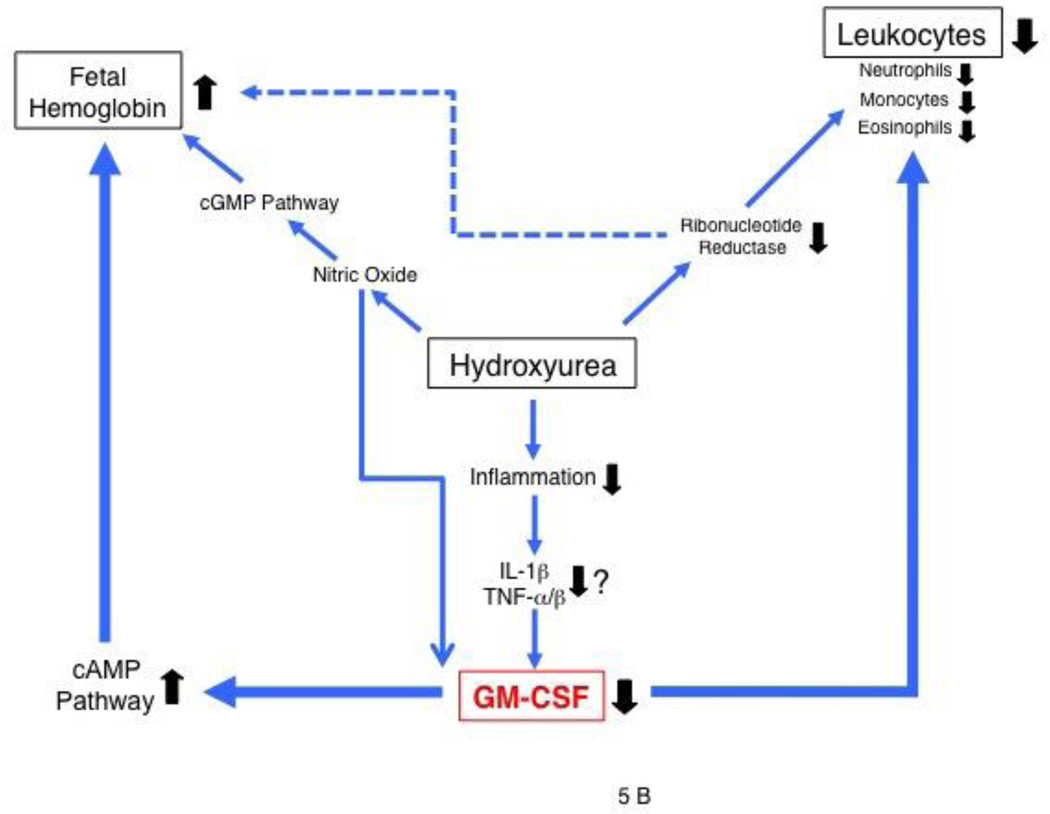
Potential roles of GM-CSF. (A) and hydroxyurea (B) in regulating leukocyte counts and HbF expression in SCD. (A) In SCD, GM-CSF levels can be increased by chronic inflammation resulting from adhesive interactions among many types of blood cells and endothelial cells, in part perhaps from splenic dysfunction [16]. GM-CSF likely regulates both leukocyte numbers and HbF expression, and possibly G-CSF production as well. (B) Hydroxyurea, a nitric oxide donor, is shown to increase HbF expression by altering erythroid regeneration kinetics (dotted line) [45,46] as well as via the cyclic GMP-dependent pathway [17,37]. Nitric oxide down-regulates GM-CSF production [49]. Leukocyte reduction by hydroxyurea may be a result of direct inhibition of cell proliferation by inhibiting ribonucleotide reductase, and down-regulation of GM-CSF production via nitric oxide or proinflammatory cytokines (IL-1β, TNFα/β).
Our finding that HbF levels in steady-state SCD patients inversely correlate with leukocyte counts and GM-CSF levels (Figs.1&2) has important implications for regulatory mechanisms for the γ-globin gene. Recently, SNPs at three gene loci such as BCL11A, HBS1L-MYB, and Xmn 1 have been shown to have major influences on determining HbF levels in SCD patients [8]. However, as shown in Fig.1B, pediatric SCD patients with high HbF of 30% to 40% due to the Xmn 1 mutation had much lower HbF levels of 10% to 20% when they had high leukocyte counts, suggesting that GM-CSF is a dominant effect over Xmn 1 mutation on HbF expression in SCD. Thus, SCD patients with high GM-CSF likely have high leukocyte counts and low HbF levels, possibly predisposing them to a clinically severe phenotype, as reported previously [11]. BCL11A silences the γ-globin gene through transcriptional mechanisms including the alteration of long-range interactions [43], while GM-CSF likely downregulates γ-globin gene expression by modulating activities of the cAMP-dependent pathway for the γ-globin gene (Figs.4&5). It is of particular interest to identify the transcription factors through which the cAMP-dependent pathway regulates γ-globin gene expression, which is currently under way in our laboratory.
Another striking finding in this study is that the reduction levels of leukocyte counts or plasma GM-CSF levels after hydroxyurea therapy positively correlate with the increase levels of HbF in SCD patients (Fig.2A & 2B), suggesting that GM-CSF is a critical cytokine that determines the magnitude of HbF response to hydroxyurea. This notion is supported by previous clinical evidence: SCD patients who have high leukocyte counts at baseline and exhibit a great reduction in the counts with hydroxyurea therapy tend to have large increases in HbF levels [44]. Our data suggest that such patients are likely to have high GM-CSF levels and that the great reduction in leukocyte counts is presumably associated with a drastic decrease in GM-CSF levels, which may be critical for permitting large increases in HbF expression following hydroxyurea therapy. A possible model with the roles of hydroxyurea in the regulation of HbF and leukocyte counts is shown in Fig.5B. Previous studies had shown that hydroxyurea induces HbF expression by altering erythroid regeneration kinetics after cytotoxic events owing to its inhibitory activity on ribonucleotide reductase [45] [46] (dotted line in Fig.5B). In addition, hydroxyurea was recently shown to induce HbF expression via the cGMP-dependent pathway [17,37], but efficient HbF induction may depend in part on whether hydroxyurea substantially decreases plasma levels of GM-CSF that attenuates the cAMP-dependent pathway, as demonstrated in this study. This may suggest that the cross-talk between the two pathways is important for high-level HbF expression [47]. Hydroxyurea may decrease GM-CSF production through the generation of nitric oxide, as nitric oxide is produced from the metabolism of hydroxyurea [48] and inhibits GM-CSF production in bronchial epithelia cells [49]. If this were the case, the pharmacokinetics of the metabolism of hydroxyurea in the liver could affect HbF levels in SCD patients [50].
Interestingly, the tristetraprolin (TTP) family of CCCH tandem zinc-finger proteins is an important regulator for GM-CSF [51]. These proteins bind to AU-rich elements within the mRNA encoding GM-CSF and act as negative regulators by destabilizing the mRNA. Mice deficient for ZFP36L2, which is a protein of the TTP family, have elevated levels of GM-CSF [52], verifying the role for TTP proteins in GM-CSF expression. Hydroxyurea increases TTP expression in K562 cells (T. Ikuta, unpublished data) and more importantly, these genes include a number of non-synonymous SNPs in their exons, some of which are specifically associated with African Americans [53]. The presence of such SNPs may account in part for variable levels of GM-CSF in SCD patients. Furthermore, hydroxyurea is postulated to reduce leukocyte numbers by inhibiting DNA synthesis of myeloid progenitors [54] (Fig.5B). Since hydroxyurea significantly decreases plasma GM-CSF levels [16], leukocyte reduction in SCD patients on hydroxyurea therapy might result, in part, from diminished levels of plasma GM-CSF, as GM-CSF mobilizes hematopoietic progenitors in the periphery [55]. Thus, dissecting the mechanisms by which hydroxyurea inhibits GM-CSF synthesis in the context of SCD patients may provide clues to the mechanism by which patients exhibit heterogeneous response to hydroxyurea.
A previous study by Gabbianelli et al. reported that GM-CSF stimulates HbF synthesis in primary erythroblasts cultured in vitro and suggested a possible use of GM-CSF as an HbF-inducing agent for SCD patients [56]. It is difficult to tease out the difference between their results and ours. Our results with negative effects of GM-CSF on HbF expression are supported by clinical studies showing inverse correlations between GM-CSF levels and HbF levels in distinct patient populations. Administration of GM-CSF in rhesus monkeys did not result in an increase in the number of F-cells, suggesting no positive effects of this cytokine on HbF expression [57]. Moreover, several clinical case reports documented that administration of myeloid growth factors including GM-CSF or G-CSF to SCD patients results in the induction of vaso-occlusive crisis [58,59], suggesting that these myeloid growth factors induce pain crises in SCD patients and thereby augment the clinical severity. It is uncertain whether GM-CSF administration induces HbF expression and thereby ameliorates the clinical severity of SCD.
Although the current study has shown a strong correlation between the reduction levels of total leukocyte counts and the increment levels of HbF in SCD patients on hydroxyurea therapy (Fig.2A), the roles for neutrophils in modulating the mortality rate of SCD are not established [60,61]. In our analysis, there were no significant correlations between post-therapy HbF levels and pre- or post-therapy neutrophil counts (data not shown). This may highlight a role for monocytes and lymphocytes, but not for neutrophils, in the regulation of HbF expression in SCD, as GM-CSF is expressed in monocytes and lymphocytes, but not in neutrophils [62].
In conclusion, this study has demonstrated important roles for GM-CSF in both leukocyte counts and HbF expression in SCD. Previous studies have identified various predictors of HbF response to hydroxyurea, but the mechanisms by which such predictors suggest HbF response to hydroxyurea remain unclear [12,13]. We have shown that GM-CSF downregulates HbF expression by inhibiting the cAMP-dependent pathway, which is an important pathway for HbF expression in β-hemoglobinopathies [19]. Studies to investigate an association between GM-CSF levels and the presence of SNPs in TTP genes may provide clues to the mechanisms underlying hydroxyurea resistance in this disorder.
Supplementary Material
Acknowledgements
We thank Yuichi Kuroyanagi and Akio Inoue for their contributions to earlier erythroid cell studies and Nicola Conran for sharing clinical data. We also wish to thank Hongyan Xu for advice on statistical analyses and Amgen for providing erythropoietin. This study was supported by National Institutes of Health (DK61806, HL73452, and P20 MD003383) (T.I.) and Southeastern Clinical and Translational Research Institute (T.I.) grants.
Abbreviations used
- CREB
cAMP response element binding protein
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HbF
fetal hemoglobin
- SCD
sickle cell disease
- SNP
single nucleotide polymorphism
- TTP
tristetrapolin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship and Disclosures
Contributions: TI designed and conducted the research, analyzed the data, and wrote the manuscript; ADA provided patients' samples and revised the manuscript; DRG, JBP, and SDY conducted mouse experiments, analyzed the data, and edited the manuscript; BC, AK, and NO provided clinical data and edited the manuscript; CAH, analyzed the data and revised the manuscript.
The authors declare no potential conflicts of interests.
References
- 1.Charache S, Barton FB, Moore RD, et al. Hydroxyurea and sickle cell anemia. Clinical utility of a myelosuppressive "switching" agent. The Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Medicine. 1996;75:300–326. doi: 10.1097/00005792-199611000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Kaul DK, Hebbel RP. Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice. J. Clin. Invest. 2000;106:411–420. doi: 10.1172/JCI9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat. Med. 2001;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 4.Miller BA, Salameh M, Ahmed M, et al. High fetal hemoglobin production in sickle cell anemia in the eastern province of Saudi Arabia is genetically determined. Blood. 1986;67:1404–1410. [PubMed] [Google Scholar]
- 5.Craig JE, Rochette J, Fisher CA, et al. Dissecting the loci controlling fetal haemoglobin production on chromosomes 11p and 6q by the regressive approach. Nat. Genet. 1996;12:58–64. doi: 10.1038/ng0196-58. [DOI] [PubMed] [Google Scholar]
- 6.Close J, Game L, Clark B, et al. Genome annotation of a 1.5 Mb region of human chromosome 6q23 encompassing a quantitative trait locus for fetal hemoglobin expression in adults. BMC Genomics. 2004;5:33–46. doi: 10.1186/1471-2164-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yavarian M, Karimi M, Bakker E, Harteveld CL, Giordano PC. Response to hydroxyurea treatment in Iranian transfusion-dependent β-thalassemia patients. Haematologica. 2004;89:1172–1178. [PubMed] [Google Scholar]
- 8.Lettre G, Sankaran VG, Bezerra MAC, et al. DNA polymorphisms at the BCL11A, HBS1L-MYB, and β-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proc. Natl. Acad. Sci. USA. 2008;105:11869–11874. doi: 10.1073/pnas.0804799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boggs DR, Hyde F, Srodes C. An unusual pattern of neutrophil kinetics in sickle cell anemia. Blood. 1973;41:59–65. [PubMed] [Google Scholar]
- 10.Platt OS, Dover GJ. Sickle cell disease. In: Oski FA, editor. Hematology of Infancy and Childhood. Philadelphia: Saunders; 1992. pp. 732–782. [Google Scholar]
- 11.Miller ST, Sleeper LA, Pegelow CH, et al. Prediction of adverse outcomes in children with sickle cell disease. N. Engl. J. Med. 2000;342:83–89. doi: 10.1056/NEJM200001133420203. [DOI] [PubMed] [Google Scholar]
- 12.Charache S, Dover GJ, Moore RD, et al. Hydroxyurea: effects on hemoglobin F production in patients with sickle cell anemia. Blood. 1992;79:2555–2565. [PubMed] [Google Scholar]
- 13.Ware RE, Eggleston B, Redding-Lallinger R, et al. Predictors of fetal hemoglobin response in children with sickle cell anemia receiving hydroxyurea therapy. Blood. 2002;99:10–14. doi: 10.1182/blood.v99.1.10. [DOI] [PubMed] [Google Scholar]
- 14.Croizat H. Circulating cytokines in sickle cell patients during steady state. Br. J. Haematol. 1994;87:592–597. doi: 10.1111/j.1365-2141.1994.tb08318.x. [DOI] [PubMed] [Google Scholar]
- 15.Croizat H, Nagel RL. Circulating cytokines response and the level of erythropoiesis in sickle cell anemia. Am. J. Hematol. 1999;60:105–115. doi: 10.1002/(sici)1096-8652(199902)60:2<105::aid-ajh4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 16.Conran N, Saad STO, Costa FF, Ikuta T. Leukocyte numbers correlate with plasma levels of granulocyte-macrophage colony-stimulating factor in sickle cell disease. Ann. Hematol. 2007;86:255–261. doi: 10.1007/s00277-006-0246-6. [DOI] [PubMed] [Google Scholar]
- 17.Ikuta T, Ausenda S, Cappellini MD. Mechanism for Fetal Globin Gene Expression:Role of the Soluble Guanylate Cyclase-cyclic GMP-Dependent Protein Kinase Pathway. Proc. Natl. Acad. Sci. USA. 2001;98:1847–1852. doi: 10.1073/pnas.041599798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuroyanagi Y, Kaneko Y, Muta K, et al. cAMP differentially regulates g-globin gene expression in erythroleukemic cells and primary erythroblasts through c-Myb expression. Biochem. Biophys. Res. Commun. 2006;344:1038–1047. doi: 10.1016/j.bbrc.2006.03.203. [DOI] [PubMed] [Google Scholar]
- 19.Bailey L, Kuroyanagi Y, Franco-Penteado CF, et al. Expression of the γ-globin gene is sustained by the cAMP-dependent pathway in β-thalassaemia. Br. J. Haematol. 2007;138:382–395. doi: 10.1111/j.1365-2141.2007.06673.x. [DOI] [PubMed] [Google Scholar]
- 20.Miller BA, Olivieri N, Salameh M, et al. Molecular analysis of the high-hemoglobin-F phenotype in Saudi Arabian sickle cell anemia. N. Engl. J. Med. 1987;316:244–250. doi: 10.1056/NEJM198701293160504. [DOI] [PubMed] [Google Scholar]
- 21.Huisman TH, Henson JB, Wilson JB. A new high-performance liquid chromatographic procedure to quantitate hemoglobin A1c and other minor hemoglobins in blood of normal, diabetic, and alcoholic individuals. J. Lab. Clin. Med. 1983;102:163–173. [PubMed] [Google Scholar]
- 22.Uddin S, Ah-Kang J, Ulaszek J, Mahmud D, Wickrema A. Differentiation stage-specific activation of p38 mitogen-activated protein kinase isoforms in primary human erythroid cells. Proc. Natl. Acad. Sci. USA. 2004;101:147–152. doi: 10.1073/pnas.0307075101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1989. [Google Scholar]
- 25.Ikuta T, Kan YW. In vivo protein-DNA interactions at the beta-globin gene locus. Proc. Natl. Acad. Sci. USA. 1991;88:10188–10192. doi: 10.1073/pnas.88.22.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikuta T, Kan YW, Swerdlow PS, Faller DV, Perrine SP. Alterations in protein-DNA interactions in the γ-globin gene promoter in response to butyrate therapy. Blood. 1998;92:2924–2933. [PubMed] [Google Scholar]
- 27.Conran N, Oresco-Santos C, Acosta HC, et al. Increased soluble guanylate cyclase activity in the red blood cells of sickle cell patients. Br. J. Haematol. 2004;124:547–554. doi: 10.1111/j.1365-2141.2004.04810.x. [DOI] [PubMed] [Google Scholar]
- 28.Inoue A, Kuroyanagi Y, Terui K, Moi P, Ikuta T. Negative regulation of γ-globin gene expression by cAMP-dependent pathway in erythroid cells. Exp. Hematol. 2004;32:244–253. doi: 10.1016/j.exphem.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Kitamura T, Tange T, Terasawa T, et al. Establishment and characterization of a unique human cell line that proliferates dependently on GM-CSF, IL-3, or erythropoietin. J. Cell. Physiol. 1989;140:323–334. doi: 10.1002/jcp.1041400219. [DOI] [PubMed] [Google Scholar]
- 30.Kitamura T, Tojo A, Kuwaki T, et al. Identification and analysis of human erythropoietin receptors on a factor-dependent cell line, TF-1. Blood. 1989;73:375–380. [PubMed] [Google Scholar]
- 31.Nagel RL, Vichinsky E, Shah M, et al. F reticulocyte response in sickle cell anemia treated with recombinant human erythropoietin: a double-blind study. Blood. 1993;81:9–14. [PubMed] [Google Scholar]
- 32.Kase H, Iwahashi K, Nakanishi S, et al. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem. Biophys. Res. Commun. 1987;142:436–440. doi: 10.1016/0006-291x(87)90293-2. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto KK, Gonzalez GA, Biggs WHd, Montminy MR. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988;334:494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]
- 34.Pace BS, Qian XH, Sangerman J, et al. p38 MAP kinase activation mediates gamma-globin gene induction in erythroid progenitors. Exp. Hematol. 2003;31:1089–1096. doi: 10.1016/s0301-472x(03)00235-2. [DOI] [PubMed] [Google Scholar]
- 35.Bhanu NV, Trice TA, Lee YT, Miller JL. A signaling mechanism for growth-related expression of fetal hemoglobin. Blood. 2004;103:1929–1933. doi: 10.1182/blood-2003-05-1624. [DOI] [PubMed] [Google Scholar]
- 36.Ma Q, Wyszynski DF, Farrell JJ, et al. Fetal hemoglobin in sickle cell anemia: genetic determinants of response to hydroxyurea. Pharmacogenomics J. 2007;7:386–394. doi: 10.1038/sj.tpj.6500433. [DOI] [PubMed] [Google Scholar]
- 37.Cokic VP, Smith RD, Beleslin-Cokic BB, et al. Hydroxyurea induces fetal hemoglobin by the nitric oxide-dependent activation of soluble guanylyl cyclase. J. Clin. Invest. 2003;111:231–239. doi: 10.1172/JCI16672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi M, Kitagawa S, Masuyama J-I, et al. Human Monocyte-Endothelial Cell Interaction Induces Synthesis of Granulocyte-Macrophage Colony-Stimulating Factor. Circulation. 1996;93:1185–1193. doi: 10.1161/01.cir.93.6.1185. [DOI] [PubMed] [Google Scholar]
- 39.Perelman N, Selvaraj SK, Batra S, et al. Placenta growth factor activates monocytes and correlates with sickle cell disease severity. Blood. 2003;102:1506–1514. doi: 10.1182/blood-2002-11-3422. [DOI] [PubMed] [Google Scholar]
- 40.Shannon MF, Coles LS, Vadas MA, Cockerill PN. Signals for activation of the GM-CSF promoter and enhancer in T cells. Crit. Rev. Immunol. 1997;17:301–323. doi: 10.1615/critrevimmunol.v17.i3-4.30. [DOI] [PubMed] [Google Scholar]
- 41.Thorens B, Mermod JJ, Vassalli P. Phagocytosis and inflammatory stimuli induce GM-CSF mRNA in macrophages through posttranscriptional regulation. Cell. 1987;48:671–679. doi: 10.1016/0092-8674(87)90245-5. [DOI] [PubMed] [Google Scholar]
- 42.Bell AL, Magill MK, McKane WR, Kirk F, Irvine AE. Measurement of colony-stimulating factors in synovial fluid: potential clinical value. Rheumatol. Int. 1995;14:177–182. doi: 10.1007/BF00262295. [DOI] [PubMed] [Google Scholar]
- 43.Xu J, Sankaran VG, Ni M, et al. Transcriptional silencing of γ-globin by BCL11A involves long-range interactions and cooperation with SOX6. Genes Dev. 2010;24:783–798. doi: 10.1101/gad.1897310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinberg MH. Drug Therapy: Management of Sickle Cell Disease. N. Engl. J. Med. 1999;340:1021–1030. doi: 10.1056/NEJM199904013401307. [DOI] [PubMed] [Google Scholar]
- 45.Papayannopoulou T, Torrealba de Ron A, Veith R, Knitter G, Stamatoyannopoulos G. Arabinosylcytosine induces fetal hemoglobin in baboons by perturbing erythroid cell differentiation kinetics. Science. 1984;224:617–619. doi: 10.1126/science.6200940. [DOI] [PubMed] [Google Scholar]
- 46.Galanello R, Stamatoyannopoulos G, Papayannopoulou T. Mechanism of Hb F stimulation by S-stage compounds. In vitro studies with bone marrow cells exposed to 5-azacytidine, Ara-C, or hydroxyurea. J. Clin. Invest. 1988;81:1209–1216. doi: 10.1172/JCI113437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polson JB, Strada SJ. Cyclic nucleotide phosphodiesterases and vascular smooth muscle. Annu. Rev. Pharmacol. Toxicol. 1996;36:403–427. doi: 10.1146/annurev.pa.36.040196.002155. [DOI] [PubMed] [Google Scholar]
- 48.Jiang J, Jordan SJ, Barr DP, et al. In vivo production of nitric oxide in rats after administration of hydroxyurea. Mol. Pharmacol. 1997;52:1081–1086. doi: 10.1124/mol.52.6.1081. [DOI] [PubMed] [Google Scholar]
- 49.Sanders SP, Kim J, Connolly KR, et al. Nitric oxide inhibits rhinovirus-induced granulocyte macrophage colony-stimulating factor production in bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2001;24:317–325. doi: 10.1165/ajrcmb.24.3.4131. [DOI] [PubMed] [Google Scholar]
- 50.Huang J, Yakubu M, Kim-Shapiro DB, King SB. Rat liver-mediated metabolism of hydroxyurea to nitric oxide. Free Radic. Biol. Med. 2006;40:1675–1681. [Google Scholar]
- 51.Blackshear PJ. Tristetraprolin and other CCCH tandem zinc-finger proteins in the regulation of mRNA turnover. Biochem. Soc. Trans. 2002;30:945–952. doi: 10.1042/bst0300945. [DOI] [PubMed] [Google Scholar]
- 52.Stumpo DJ, Broxmeyer HE, Ward T, et al. Targeted disruption of Zfp36l2, encoding a CCCH tandem zinc finger RNA-binding protein, results in defective hematopoiesis. Blood. 2009;114:2401–2410. doi: 10.1182/blood-2009-04-214619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blackshear PJ, Phillips RS, Vazquez-Matias J, Mohrenweiser H. Polymorphisms in the genes encoding members of the tristetraprolin family of human tandem CCCH zinc finger proteins. Prog. Nucleic Acid Res Mol. Biol. 2003;75:43–68. doi: 10.1016/s0079-6603(03)75002-8. [DOI] [PubMed] [Google Scholar]
- 54.Yarbro JW. Mechanism of action of hydroxyurea. Semin. Oncol. 1992;19:1–10. [PubMed] [Google Scholar]
- 55.Spitzer G, Adkins D, Mathews M, et al. Randomized comparison of G-CSF + GM-CSF vs G-CSF alone for mobilization of peripheral blood stem cells: effects on hematopoietic recovery after high-dose chemotherapy. Bone Marrow Transplant. 1997;20:921–930. doi: 10.1038/sj.bmt.1700999. [DOI] [PubMed] [Google Scholar]
- 56.Gabbianelli M, Pelosi E, Bassano E, et al. Granulocyte-macrophage colony-stimulating factor reactivates fetal hemoglobin synthesis in erythroblast clones from normal adults. Blood. 1989;74:2657–2667. [PubMed] [Google Scholar]
- 57.McDonagh KT, Dover GJ, Donahue RE, et al. Hydroxyurea-induced HbF production in anemic primates: augmentation by erythropoietin, hematopoietic growth factors, and sodium butyrate. Exp. Hematol. 1992;20:1156–1164. [PubMed] [Google Scholar]
- 58.Pieters RC, Rojer RA, Saleh AW, Saleh AE, Duits AJ. Molgramostim to treat SS-sickle cell leg ulcers. Lancet. 1995;345:528. [PubMed] [Google Scholar]
- 59.Abboud M, Laver J, Blau CA. Granulocytosis causing sickle-cell crisis. Lancet. 1998;351:28. doi: 10.1016/S0140-6736(05)60614-9. [DOI] [PubMed] [Google Scholar]
- 60.Steinberg MH, Barton F, Castro O, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA. 2003;289:1645–1651. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- 61.Bakanay SM, Dainer E, Clair B, et al. Mortality in sickle cell patients on hydroxyurea therapy. Blood. 2005;105:545–547. doi: 10.1182/blood-2004-01-0322. [DOI] [PubMed] [Google Scholar]
- 62.Stephen T, Baiqing L, Stephen D, et al. Secretion of the eosinophil-active cytokines interleukin-5, granulocyte/macrophage colonystimulating factor and interleukin-3 by bronchoalveolar lavage CD4+ and CD8+ T cell lines in atopics asthmatics, and atopic and nonatopic controls. Eur. J. Immunol. 1995;25:2727–2731. doi: 10.1002/eji.1830251002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.