Summary
Uterine vascular tone significantly decreases while uterine blood flow dramatically increases during pregnancy. However, the complete molecular mechanisms remain elusive. We hypothesized that increased BKCa channel activity contributes to the decreased myogenic tone of uterine arteries in pregnancy. Resistance-sized uterine arteries were isolated from nonpregnant and near-term pregnant sheep. Electrophysiological studies revealed a greater whole-cell K+ current density in pregnant than nonpregnant uterine arteries. Tetraethylammonium and iberoitoxin inhibited K+ currents to the same extent in uterine arterial myocytes. The BKCa channel current density was significantly increased in pregnant uterine arteries. In accordance, tetraethylammonium significantly increased pressure-induced myogenic tone in pregnant uterine arteries and abolished the difference in myogenic responses between pregnant and nonpregnant uterine arteries. Activation of protein kinase C produced a similar effect to tetraethylammonium by inhibiting BKCa channel activity and increasing myogenic tone in pregnant uterine arteries. Chronic treatment of nonpregnant uterine arteries with physiologically relevant concentrations of 17β-estradiol and progesterone caused a significant increase in the BKCa channel current density. Western blot analyses demonstrated a significant increase of the β1, but not α, subunit of BKCa channels in pregnant uterine arteries. In accordance, steroid treatment of nonpregnant uterine arteries resulted in an upregulation of the β1, but not α, subunit expression. The results indicate that the steroid hormone-mediated upregulation of the β1 subunit and BKCa channel activity may play a key role in attenuating myogenic tone of the uterine artery in pregnancy.
Keywords: uterine artery, pregnancy, BKCa channel, myogenic tone, steroids, protein kinase C
Introduction
Uterine blood flow increases substantially during pregnancy, which is essential both for the growth and survival of the fetus and for cardiovascular well-being of the mother. Maladaptation of the uteroplacental circulation during pregnancy is associated with high incidence of clinical complications including preeclampsia and fetal intrauterine growth restriction. While the mechanisms underlying the adaptation of uterine circulation to pregnancy are complex and poorly understood, recent studies have demonstrated that pressure-dependent myogenic reactivity plays a pivotal physiological role in the regulation of uterine circulation, and that decreased uterine arterial myogenic tone contributes significantly to the adaptation of uterine vascular hemodynamics in pregnancy.1,2
The molecular mechanisms underlying this attenuated myogenic tone of uterine arteries in pregnancy remain elusive. Previous studies have suggested a possible role of large-conductance Ca2+-activated K+ (BKCa) channels in the regulation of uterine circulation during pregnancy.3–5 The BKCa channel in vascular smooth muscle contains pore-forming α subunit (encoded by KCNMA1) and up to four accessory identical β1 subunits (encoded by KCNMB1).6 The β1 subunit is predominantly expressed in smooth muscle7–9 and enhances the Ca2+ sensitivity of BKCa channels.10 It has been shown that BKCa channels play an important role in regulating the resting membrane potential of vascular smooth muscle, and channel blockade by iberoitoxin (IBTX) or tetraethylammonium (TEA) depolarizes the membrane resulting in vasoconstriction.11 The importance of BKCa channel in the regulation of vascular smooth muscle function was further demonstrated with the gene deletion of BKCa channel, and α or β1 subunit knockout mice displayed increased vascular tone and elevated blood pressure,8,12 although uterine vascular function was not examined.
Little is known about the functional role of BKCa channels in regulating pressure-dependent myogenic tone of uterine arteries and its adaptation to pregnancy. In the present study, we test the hypothesis that upregulation of BKCa channel expression and activities accounts for attenuated myogenic tone of uterine arteries in pregnancy. This was achieved by investigating the myogenic reactivity and BKCa channel current density and protein expression in uterine arteries obtained from nonpregnant and near-term pregnant sheep using functional, electrophysiological and biochemical approaches. Given our recent findings that sex steroid hormones played an important role in downregulating pressure-dependent myogenic tone of the uterine artery in pregnancy,2 we further test the hypothesis that sex steroid hormones upregulate BKCa channel expression and activities in uterine arteries.
Materials and Methods
An expanded Materials and Methods section is available in the online data supplement at http://hyper.ahajournals.org.
Tissue Preparation and Treatment
Resistance-sized uterine arteries were isolated from nonpregnant and near-term pregnant sheep.2,13,14 For hormonal treatment, arteries were incubated in phenol red-free DMEM with 1% charcoal-stripped FBS for 48 hours at 37°C in a humidified incubator with 5% CO2/95% air in the absence or presence of 17β-estradiol and/or progesterone. All procedures and protocols were approved by the Institutional Animal Care and Use Committee and followed the guidelines by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Measurement of Myogenic Tone
Pressure-dependent myogenic tone of resistance-sized uterine arteries were measured as described previously.2,13,14
Measurement of BKCa Channel Current
Smooth muscle cells were enzymatically dissociated from resistance-sized uterine arteries, and whole-cell K+ currents were recorded and normalized to cell capacitance as picoampere per picofarad (pA/pF).15,16 Only relaxed and spindle-shaped myocytes were used for recording. The BKCa channel current was determined as the difference between the whole-cell K+ current in the absence and presence of IBTX or TEA.
Western Immunoblotting Analysis
Protein abundance of BKCa channel α and β1 subunits was measured in freshly isolated resistance-sized, endothelium-intact uterine arteries and after the hormonal treatment by Western blot analysis, as described previously.2,13,14.
Data Analysis
Results were expressed as means ± SEM obtained from the number of experimental animals given. Differences were evaluated for statistical significance (P<0.05) by ANOVA or t test, where appropriate.
Results
Pregnancy Upregulates BKCa Channel Function in Uterine Arteries
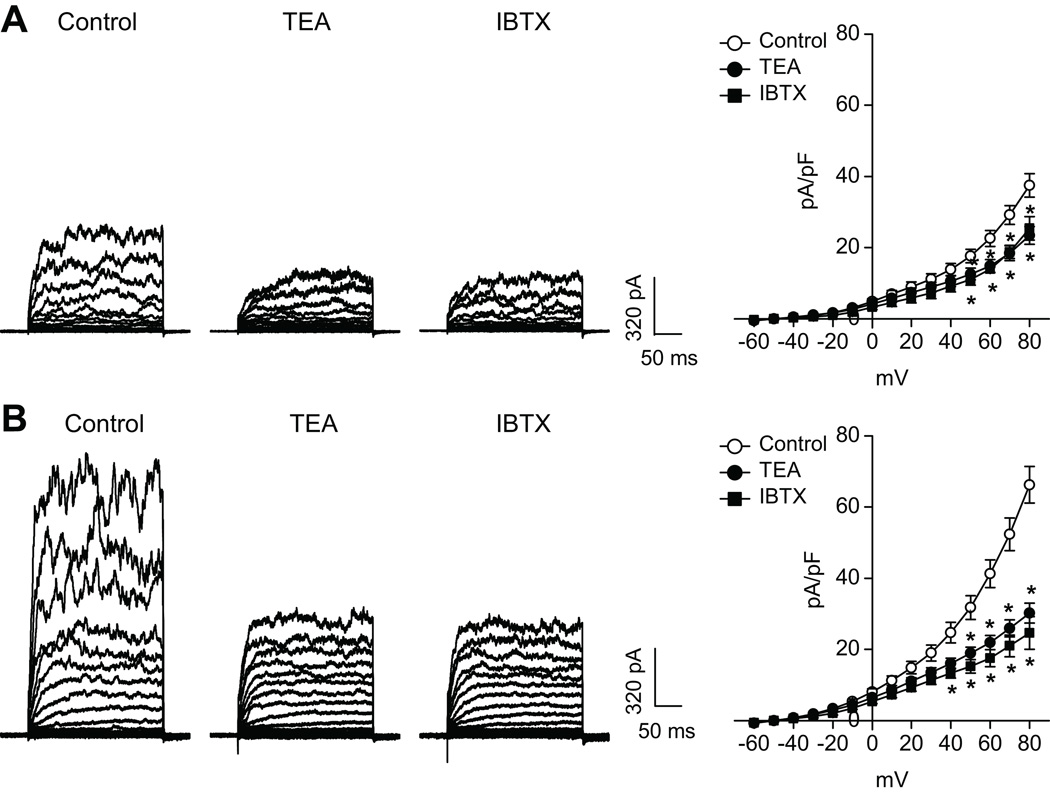
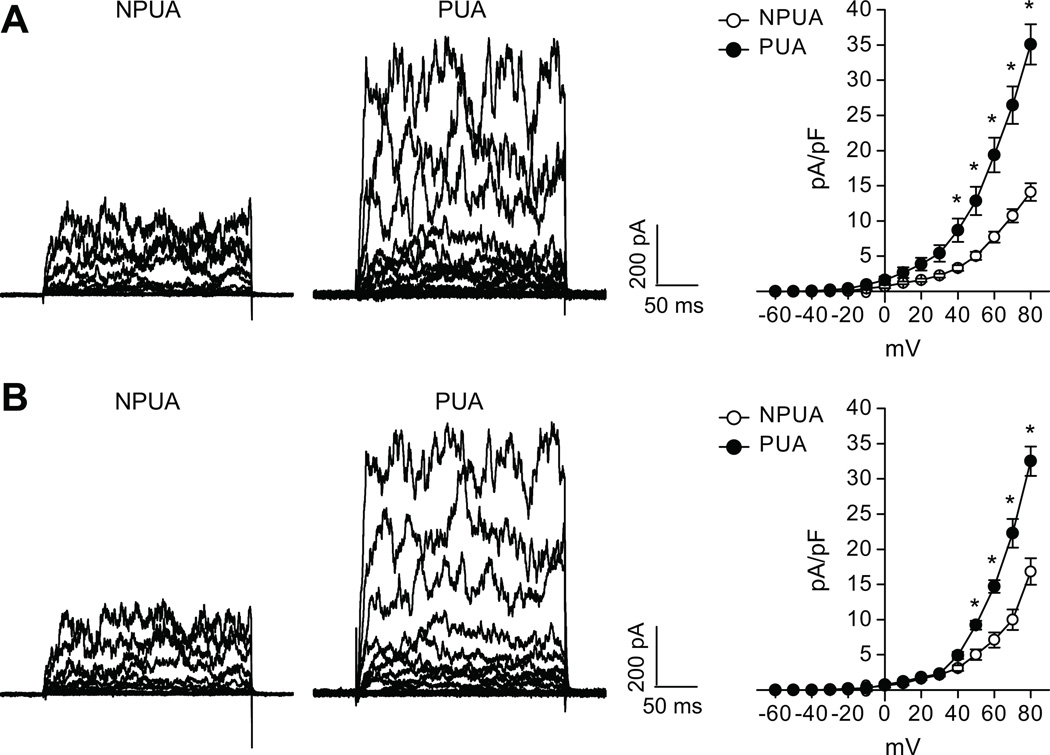
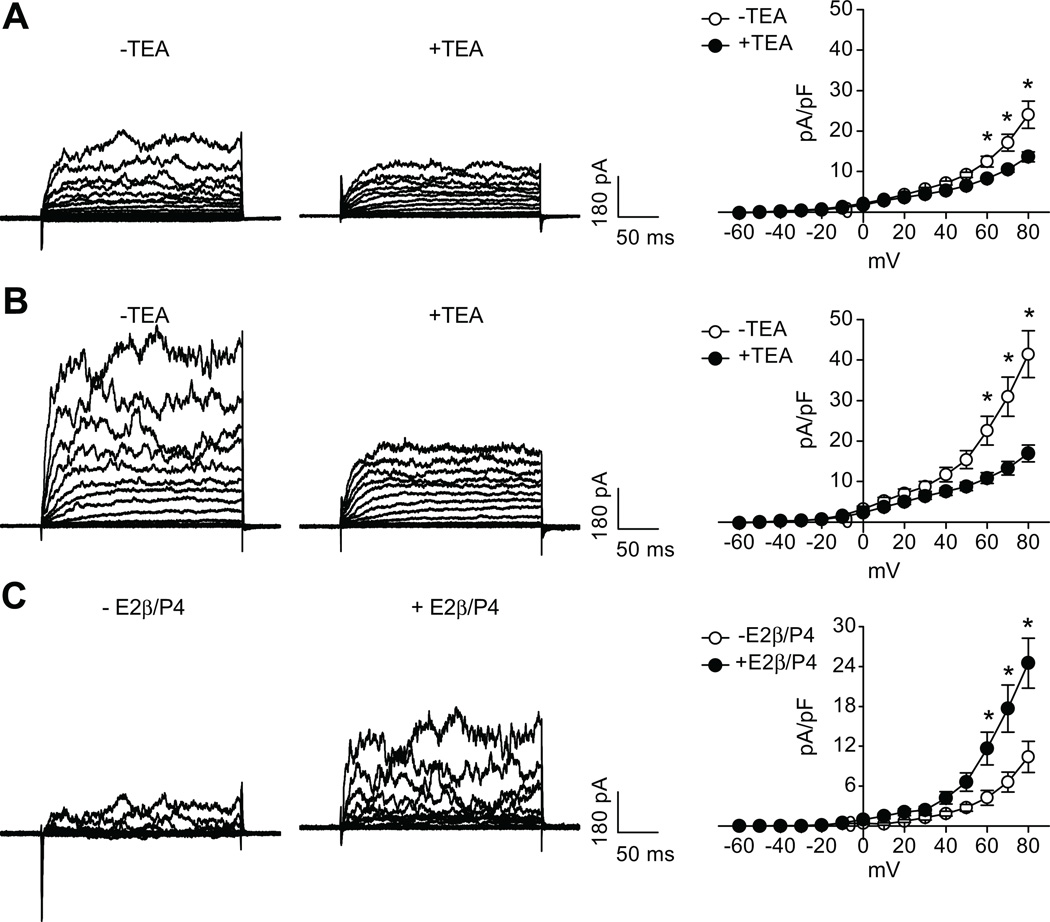
The whole-cell K+ current density in uterine arterial myocytes of pregnant sheep (66.3±5.2 pA/pF) was significantly greater than that in nonpregnant animals (37.5±3.3pA/pF) at +80 mV (P< 0.05) (Figure 1). TEA at concentrations ≤ 1.0 mmol/L selectively blocks BKCa channels with negligible effects on voltage-gated K+ channels.17–19 Exposure of the myocytes to TEA (1.0 mmol/L) or IBTX (100.0 nmol/L) significantly depressed the macroscopic K+ currents (Figure 1), indicating the functional presence of BKCa channels in uterine arteries from both nonpregnant and pregnant sheep. As shown in Figure 1, TEA and IBTX inhibited the K+ currents to the same extent in uterine arterial myocytes. The TEA- and IBTX-sensitive components of K+ currents in the myocytes at +80 mV were 38.5±2.5% and 40.0±3.1% (P>0.05) in nonpregnant sheep, and 53.2±1.8% and 55.7±2.7% (P>0.05) in pregnant animals, respectively. The finding that TEA and IBTX inhibited the K+ currents to the same extent in uterine arterial myocytes validated the specificity of TEA action on the BKCa channel. The BKCa current density determined by TEA (35.1±2.9 pA/pF in pregnant myocytes versus 14.1±1.3 pA/pF in nonpregnant myocytes, P<0.05) or by IBTX (32.5±2.1 pA/pF in pregnant myocytes versus 16.8±1.9 pA/pF in nonpregnant myocytes, P<0.05) at +80 mV was significantly increased in pregnant uterine arteries (Figure 2).
Figure 1. Effect of TEA and IBTX on K+ currents in uterine arteries.
Smooth muscle cells were freshly isolated from nonpregnant (panel A) and pregnant (panel B) uterine arteries and whole-cell K+ currents were recorded in the absence or presence of TEA (1.0 mmol/L) or IBTX (100.0 nmo/L). Data are means ± SEM of 4–12 cells from 4–7 animals of each group. * P < 0.05, versus control.
Figure 2. Effect of pregnancy on BKCa current density in uterine arteries.
Smooth muscle cells were freshly isolated from nonpregnant (NPUA) and pregnant (PUA) uterine arteries. BKCa current density was determined in the presence of TEA (panel A) or IBTX (panel B). Data are means ± SEM of 4–12 cells from 4–7 animals of each group. *P < 0.05, versus NPUA.
Inhibition of BKCa Channels Increases Pressure-Dependent Myogenic Tone in Uterine Arteries
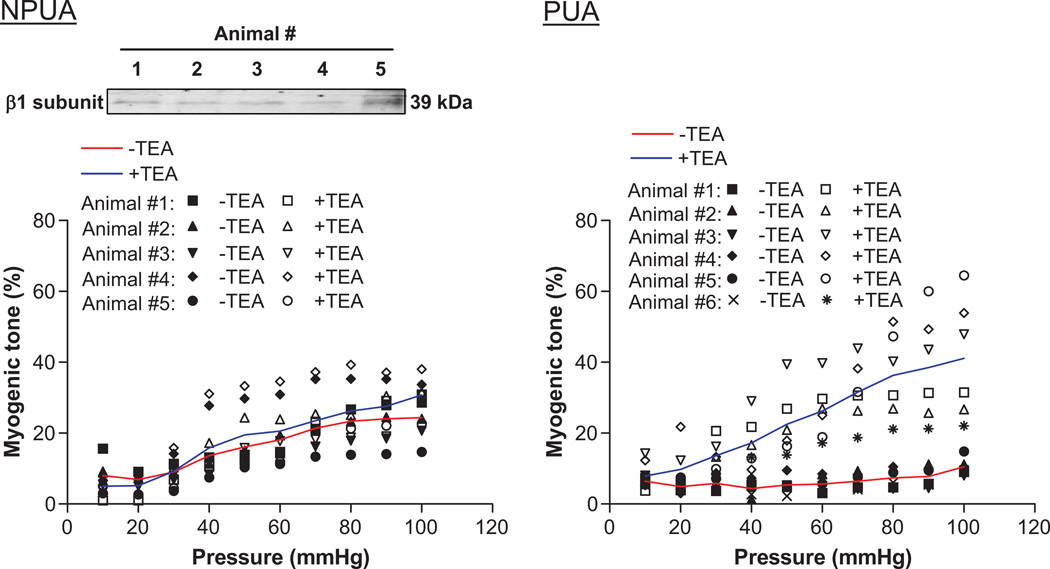
To determine the functional importance of heightened BKCa channel activity in regulating uterine arterial vascular tone, pressure-dependent myogenic responses of resistance-sized uterine arteries were measured in the absence or presence of the BKCa channel inhibitor TEA. Consistent with the previous studies,1 pressure-dependent myogenic tone in uterine arteries of pregnant sheep was significantly lower than that in uterine arteries of nonpregnant sheep over the physiological range of intravascular pressures (Figure 3). TEA significantly increased the myogenic tone in uterine arteries of pregnant sheep (Figure 3). In uterine arteries of nonpregnant animals, TEA showed no significant effect on the myogenic reactivity when the data were analyzed as groups (Figure 3). However, paired analysis of data in each animal revealed that TEA appeared to increase the myogenic tone in one out of five animals (Animal #5) (Figure 3). Consisting with this finding, it appeared that the uterine arteries from Animal #5 had higher expression level of the β1 subunit of BKCa channels (Figure 3). In the presence of TEA, there were no significant differences in pressure-dependent myogenic tone of uterine arteries between nonpregnant and pregnant animals.
Figure 3. Effect of TEA on myogenic tone in uterine arteries.
Pressure-dependent myogenic tone of nonpregnant (NPUA) and pregnant (PUA) uterine arteries was determined in the absence or presence of TEA (1.0 mmol/L for 20 minutes) in each animal in a pair wise fashion. Protein abundance of BKCa channel β1 subunit was determined by Western blot analysis. *P < 0.05, presence of TEA versus absence of TEA.
Activation of Protein Kinase C (PKC) Inhibits BKCa Channels and Increases Myogenic Tone in Uterine Arteries
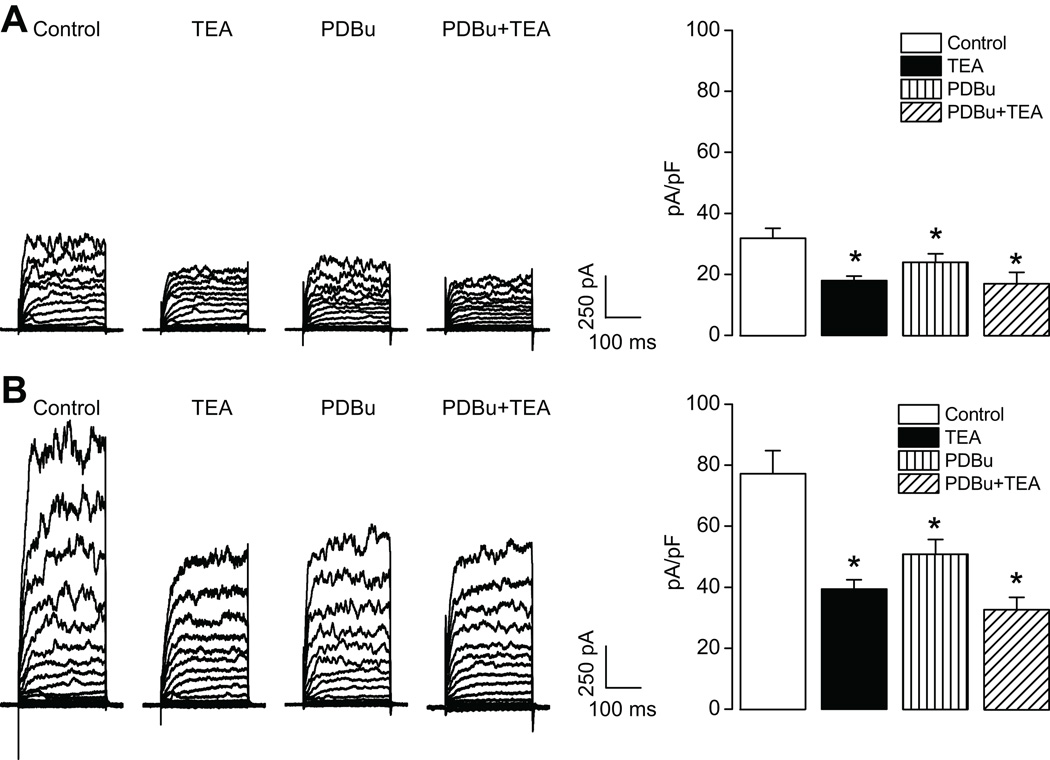
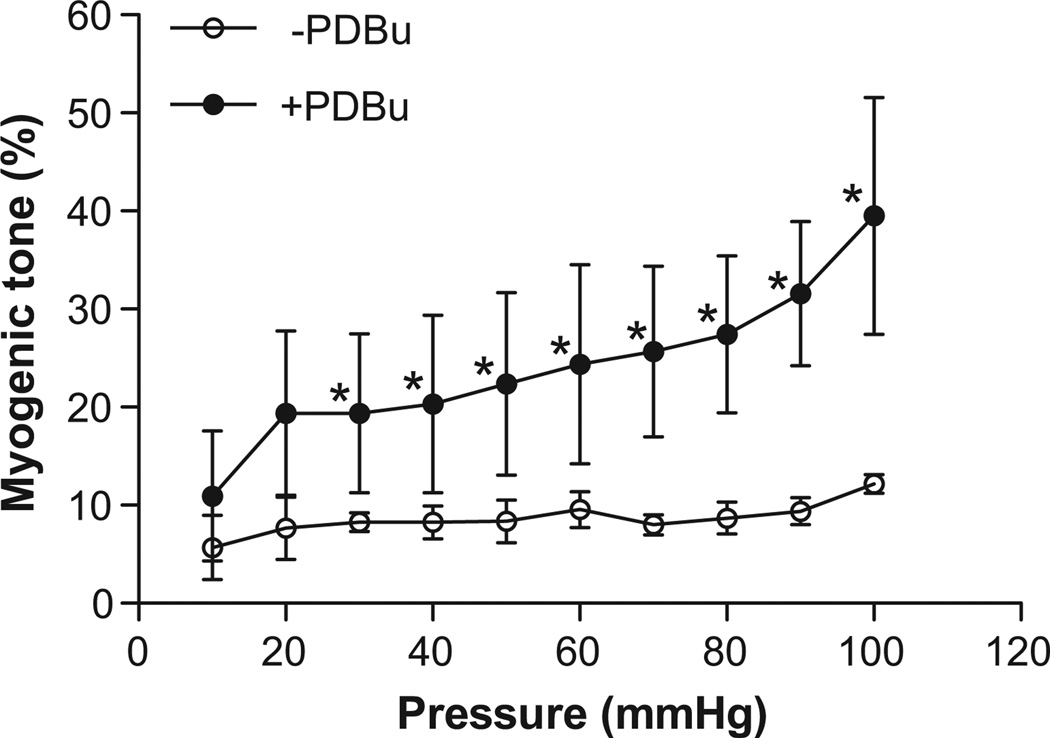
As shown in Figure 4, the activation of PKC by phorbol 12,13-dibutyrate (PDBu) significantly inhibited whole-cell K+ currents in uterine arterial myocytes. The inhibition of K+ currents at +80 mV by PDBu was significantly greater in the myocytes of pregnant sheep (34.0±2.6%) than that in nonpregnant animals (20.9±4.0%) (Figure 4, P< 0.05). However, there were no significant differences among TEA-, PDBu-, and PDBu plus TEA-produced inhibitions of K+ currents in either nonpregnant or pregnant uterine arterial myocytes, respectively (Figure 4), indicating that the PDBu-induced reduction of K+ currents is predominantly mediated by inhibiting the BKCa channel. In accordance, PDBu significantly increased pressure-dependent myogenic tone in uterine arteries of pregnant sheep (Figure 5) to the similar extent as that seen with TEA in Figure 3.
Figure 4. Effect of PDBu on K+ currents in uterine arteries.
Smooth muscle cells were freshly isolated from nonpregnant (panel A) and pregnant (panel B) uterine arteries and whole-cell K+ currents were recorded in the absence or presence of TEA (1.0 mmol/L), PDBu (1.0 µmol/L) or PDBu plus TEA, respectively. Bar graphs present the effects of TEA, PDBu or PDBu plus TEA on K+ current densities obtained at +80 mV. Data are means ± SEM of 5 cells from 5 animals of each group. * P < 0.05, versus control.
Figure 5. Effect of PDBu on myogenic tone in pregnant uterine arteries.
Pressure-dependent myogenic tone of pregnant uterine arteries was measured in the absence or presence of PDBu (100.0 nmol/L for 15 minutes). Data are means ± SEM of tissues from 5–6 animals of each group. *P < 0.05, versus −PDBu.
Steroid Hormones Increase the BKCa Channel Activity in Uterine Arteries
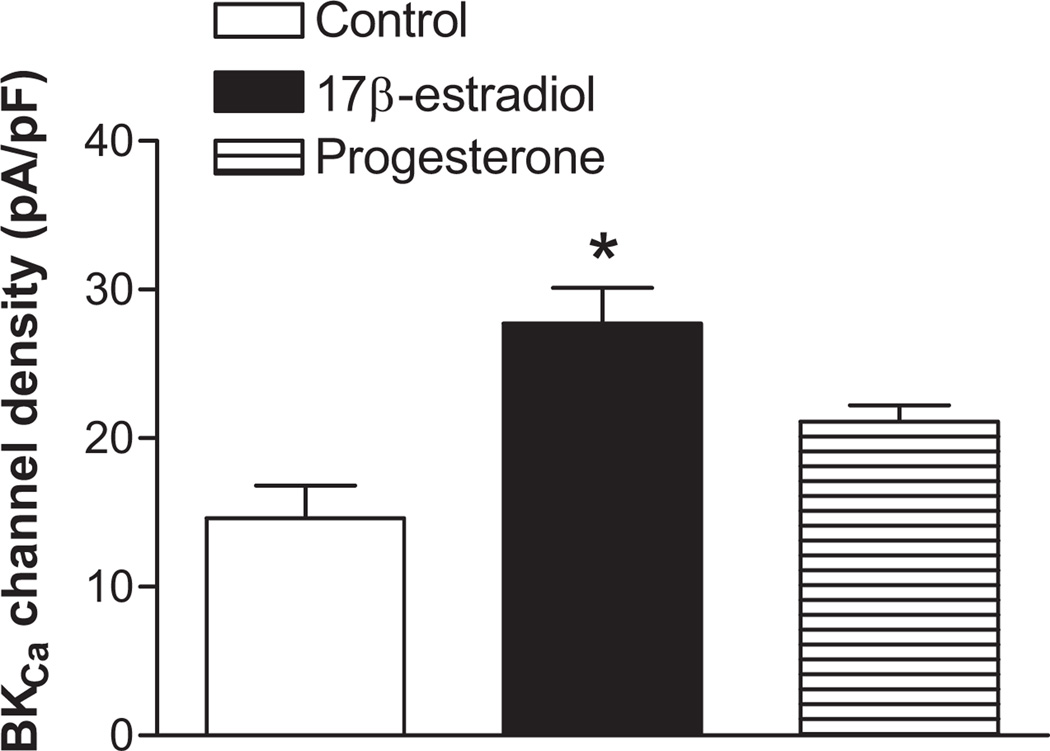
Previous studies demonstrated that 17β-estradiol and progesterone treatment for 48 hours resulted in a significant decrease in myogenic responses in nonpregnant uterine arteries.2 The effect of steroid hormones on the BKCa channel activity was thus determined by the treatment of nonpregnant uterine arteries with 17β-estradiol (0.3 nmol/L) and progesterone (100.0 nmol/L) for 48 hours. As shown in Figure 6, the steroid hormone treatment significantly increased macroscopic whole-cell K+ currents in uterine arterial myocytes. Compared with the control myocytes, the current density was increased by ~ 2-fold at +80 mV in hormone-treated myocytes (41.5±5.8 pA/pF versus 24.0±3.4 pA/pF, P< 0.05). Whole-cell K+ currents of myocytes isolated from both control and steroid hormone-treated uterine arteries were subject to the TEA inhibition. However, TEA produced a significantly greater inhibition of K+ currents in hormone-treated myocytes (58.6±1.7%) than that in the control (41.3±3.5%) (P<0.05). In accordance, the steroid hormone treatment resulted in ~2.5-fold increase in the current density of BKCa channels (24.5±3.7 pA/pF versus 10.4±2.4 pA/pF, P< 0.05) (Figure 6). To determine the specific effect of 17β-estradiol and progesterone, nonpregnant uterine arteries were treated with 17β-estradiol (0.3 nmol/L) or progesterone (100.0 nmol/L), respectively, for 48 hours. As shown in Figure 7, 17β-estradiol, but not progesterone, produced the similar effect seen with the combined steroids and significantly increased the BKCa channel current density by 2-fold in uterine arterial myocytes.
Figure 6. Effect of steroid hormone treatment on BKCa channel activity in nonpregnant uterine arteries.
Nonpregnant uterine arteries were treated with 17β-estradiol (E2β, 0.3 nmol/L) plus progesterone (P4, 100.0 nmol/L) for 48 hours. Myocytes were then isolated and whole-cell K+ currents were recorded in the absence or presence of TEA (1.0 mmol/L). Panel A: K+ currents in control myocytes. * P < 0.05, −TEA versus +TEA; Panel B: K+ currents in hormone-treated myocytes. * P < 0.05, −TEA versus +TEA; Panel C: BKCa current densities in control and hormone-treated myocytes. * P < 0.05, +E2β/P4 versus −E2β/P4. Data are means ± SEM of 7 cells from 4 animals of each group.
Figure 7. Specific effect of 17β-estradiol and progesterone on BKCa channel activity in nonpregnant uterine arteries.
Nonpregnant uterine arteries were treated with 17β-estradiol (E2β, 0.3 nmol/L) or progesterone (P4, 100.0 nmol/L) separately for 48 hours. Myocytes were then isolated and BKCa current densities were determined at +80 mV in the presence of TEA. Data are means ± SEM of 4 cells from 4 animals of each group. * P < 0.05, versus control.
Pregnancy and Hormonal Treatment Increase BKCa Channel β1 Subunit Expression in Uterine Arteries
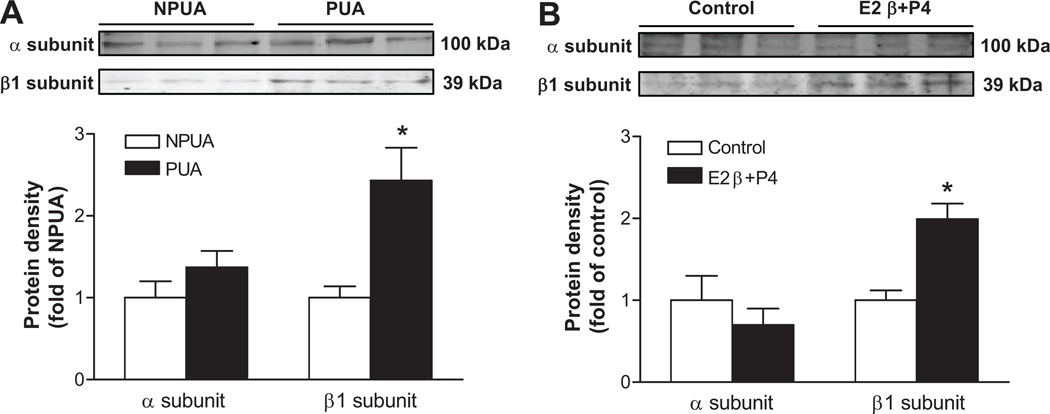
As shown in Figure 8, protein abundance of the α subunit detected at about 100 kDa was not significantly different between nonpregnant and pregnant uterine arteries. The β1 subunit was detected at 39 kDa as seen in the previous findings5,20 and its expression levels were significantly greater in pregnant uterine arteries than that in nonpregnant uterine arteries. In accordance, 17β-estradiol (0.3 nmol/L) and progesterone (100.0 nmol/L) treatment for 48 hours significantly upregulated β1, but not α, subunit expression in nonpregnant uterine arteries (Figure 8).
Figure 8. Effect of pregnancy and steroid hormones on BKCa channel subunit expression in uterine arteries.
Protein abundance of BKCa channel α and β1 subunits was determined by Western blot analyses. Panel A: Freshly isolated nonpregnant (NPUA) and pregnant (PUA) uterine arteries. * P < 0.05, PUA versus NPUA. Panel B: Nonpregnant uterine arteries were treated with 17β-estradiol (E2β, 0.3 nmol/L) plus progesterone (P4, 100.0 nmol/L) for 48 hours. * P < 0.05, E2β/P4 versus control. Data are means ± SEM of tissues from 5–6 animals of each group.
Discussion
The present study provides direct evidence for the first time that the BKCa channel activity in uterine arteries is significantly enhanced during pregnancy. Previous studies demonstrated that intra-arterial infusion of TEA into the uterine artery circulation of late gestation sheep caused a decrease of basal uterine blood flow from 50% to 80% in the absence of systemic effects or a change in contralateral uterine blood flow.3,4 This is consistent with the present finding that TEA inhibited K+ currents by 53% in pregnant uterine arteries. The findings that TEA significantly increased pressure-dependent myogenic tone in uterine arteries of pregnant sheep, and that it abrogated the difference in the myogenic response between nonpregnant and pregnant uterine arteries indicate that the pregnancy-induced attenuation in uterine arterial myogenic tone is conferred primarily by enhanced BKCa channel activity. BKCa channels have been shown to play an important role in the regulation of resting membrane potential and control of vascular tone.21 These findings support the notion that the heightened BKCa channel activity is a predominant mechanism in maintaining uteroplacental blood flow in pregnancy. The finding that the β1 subunit was higher in the nonpregnant animal that also showed the effect of TEA is intriguing, and suggests that the animal was in the follicular phase as shown by Kahn et al.20 This finding further supports the notion that elevated estrogen levels increase the β1/α stoichiometry and BKCa channel activity,20,22 which contributes to the downregulation of myogenic tone of uterine arteries. In agreement, previous studies in overiectomized nonpregnant sheep demonstrated that TEA had no significant effects on basal uterine vascular resistance and blood flow, but it inhibited estrogen-induced rise in uterine blood flow.23 These findings suggest that the regulation of myogenic tone of uterine arteries by BKCa channels is modulated by sex steroids.
The question arises as to how pregnancy might affect BKCa channel function and its regulation of uterine arterial myogenic tone. Previous studies have demonstrated that PKC plays an important role in the regulation of pressure-dependent myogenic response of resistance arteries,1, 24, 25, and a decrease in the PKC signaling pathway accounts for the attenuated myogenic tone of the uterine artery in pregnancy.1, 2,13,14,26–30 The BKCa channel is subject to modulation by PKC and activation of PKC has been shown to inhibit BKCa channels in vascular smooth muscle cells.31–34 The present finding that activation of PKC inhibited BKCa channel activity and increased pressure-dependent myogenic tone in pregnant uterine arteries provides a functional link of the BKCa channel in pregnancy-mediated downregulation of PKC and myogenic tone of uterine arteries. The work suggests a mechanism of attenuated PKC in the heightened BKCa channel activity in pregnant uterine arteries. Whereas ANOVA and post-hoc analysis indicated no significant differences between PDBu- and PDBu plus TEA-produced inhibition of the total K+ currents in uterine arterial myocytes, it appeared that the combination might produce a slightly greater inhibition than the PDBu alone, which would suggest that PDBu might not completely inhibit BKCa channels under the current conditions studied.
Consisting with the previous finding of a direct genomic effect of sex steroid hormones in attenuating the PKC activity and myogenic tone of the uterine artery,2,13,14 the present study demonstrated that the enhanced BKCa channel activity seen in pregnant uterine arteries was mimicked by the treatment of nonpregnant uterine arteries with 17β-estradiol and progesterone for 48 hours in an ex vivo tissue culture system. The acute and nongenomic effects of estrogen in activating BKCa channels have been previously reported at high nanomolar to micromolar concentrations that are substantially greater than physiological concentrations,23,35–37 To our knowledge, the present study is the first to demonstrate that physiologically relevant concentrations of 17β-estradiol and progesterone have direct genomic effects on upregulating the BKCa channel activity in vascular smooth muscle cells. Whereas both estrogen and progesterone receptors have been identified in uterine artery vascular smooth muscle,13,38,39 the present study demonstrated that 17β-estradiol alone was sufficient to increase the BKCa channel activity in uterine arterial myocytes. The conditions of ovarian cycle of luteal or follicular phases and systemic blood steroid levels were not determined in nonpregnant animals in the present study, and thus we cannot exclude the possibility that prior steroid exposure in vivo may have primed the uterine arteries to respond to estrogen. In agreement to the present study, similar findings were obtained in a neuronal cell line GT1-7 in which 17β-estradiol at physiological concentrations augmented BKCa channel currents in a genomic manner.40
The BKCa channel in vascular smooth muscle consists of pore-forming α subunit and up to four accessory β1 subunits.6 In ovine uterine arteries, both α and β1 subunits were detected exclusively in vascular smooth muscle cells with no evidence of their existence in the endothelium.5,20,22 In the present study, we found that the α subunit was not significantly different in uterine arteries between nonpregnant and pregnant sheep, suggesting no changes in the BKCa channel density by pregnancy. This is somewhat different from the previous finding showing about 60% increase in protein abundance of the α subunit in pregnant uterine arteries, although the mRNA was not significantly changed.5 One possible reason for this apparent difference may be because the previous study used larger diameter first and second generation uterine arteries while the present study used resistance-sized uterine arteries. In agreement with the present finding, it has been shown in sheep that increased endogenous estrogen in the follicular phase or infusion of exogenous estrogen in ovariectomized nonpregnant ewes have no significant effect on α subunit levels in uterine arteries.20,22 The present finding of increased β1 subunit in pregnant uterine arteries as compared with nonpregnant uterine arteries is consistent with the previous study in sheep.5 Although the β2 subunit was present in uterine artery smooth muscle, the expression was minimal and unchanged by pregnancy.5 Unlike the effect of pregnancy, both β1 and β2 subunits were found elevated in follicular as compared with luteal phase of the ovarian cycle in nonpregnant sheep, probably due to relatively high estrogen and low progesterone that was produced endogenously by the ovaries.20 Given that pregnancy is a state with substantially higher levels of estrogen and progesterone as compared with the nonpregnant state, it is possible that high progesterone inhibits the expression of β2 subunit. Similar findings were obtained in the ovariectomized estrogen-treated sheep showing an increase in the β1 subunit although the β2 subunit was not examined.22
The increase in β1 subunit expression of the BKCa channel would have functional consequences. It has been shown that β1 subunits of BKCa channels play a pivotal role in regulating vascular tone and blood pressure.8 The association of β1 subunits with α subunits are critical in regulating the Ca2+ sensitivity of BKCa channels.10,41 Downregulation of the β1 subunit and altered α:β1 stoichiometry decrease the Ca2+ sensitivity of the channel, resulting in decreased BKCa channel activity.42 In contrast, upregulation of the β1 subunit increases the Ca2+ sensitivity of BKCa channel and channel activity in vascular smooth muscle.43 In the present study, the increased β1:α subunit stoichiometry in the uterine arteries of pregnant animals is likely to augment the Ca2+ sensitivity of the BKCa channel and facilitate the activation of the channel and the consequent reduction in myogenic tone of the uterine arteries in pregnancy. The previous findings of the increased β1 subunit mRNA and protein levels in uterine arteries and myometrial smooth muscle after prolonged exposure of ovariectomized sheep or mice to estrogen suggested a possible role of the steroid hormone in modulating BKCa channel expression. The present study provides clear evidence of the direct effect of steroid hormones on upregulating the β1 subunit in the uterine artery. Although the molecular mechanisms remain elusive, the estrogen responsive elements have been identified at the promoter of ovine β1 subunit gene.
PERSPECTIVES
The present study suggests a possible mechanism of 17β-estradiol-mediated upregulation of the β1 subunit in the heightened BKCa channel activity in uterine arteries during pregnancy, resulting in the reduced myogenic tone of uterine artery in pregnancy. Given that the BKCa channel plays a pivotal role in regulating vascular tone and thus blood flow and pressure, dysregulation of the stoichiometric composition of BKCa channels and the channel activity in the uterine artery is likely to contribute significantly to the maladaptation of uterine vascular hemodynamics in pregnancies complicated by preeclampsia. Indeed, reductions in uteroplacental blood flow and chronic uteroplacental ischemia in a variety of animal models lead to a hypertension state that closely resembles preeclampsia in women.44,45 The present findings provide an understanding of the mechanisms of the BKCa channel in uterine vascular adaptation to pregnancy, and may suggest new insights of therapeutic strategies by enhancing the BKCa channel activity in vascular smooth muscle that may be beneficial for pregnant women with preeclampsia. Additionally, the present study offers insights into the mechanisms in the hormonal regulation of the BKCa channel activity and myogenic tone of resistance arteries in general, and improves our understanding of vascular benefits of hormone replacement therapy in postmenopausal women, given the well-established finding that premenopausal women are at lower risk of developing hypertension and coronary heart disease than men of the same age and that the cardiovascular risk increases only after the cessation of ovarian function.
Supplementary Material
Acknowledgments
Sources of Funding: This work was supported by National Institutes of Health Grants HL89012 (LZ) and DA025319 (SY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None.
References
- 1.Xiao D, Buchholz JN, Zhang L. Pregnancy attenuates uterine artery pressure-dependent vascular tone: role of PKC/ERK pathway. Am J Physiol Heart Circ Physiol. 2006;290:H2337–H2343. doi: 10.1152/ajpheart.01238.2005. [DOI] [PubMed] [Google Scholar]
- 2.Xiao D, Huang X, Yang S, Zhang L. Direct chronic effect of steroid hormones in attenuating uterine arterial myogenic tone: role of protein kinase c/extracellular signal-regulated kinase 1/2. Hypertension. 2009;54:352–358. doi: 10.1161/HYPERTENSIONAHA.109.130781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenfeld CR, Cornfield DN, Roy T. Ca2+-activated K+ channels modulate basal and E2β-induced rises in uterine blood flow in ovine pregnancy. Am J Physiol Heart Circ Physiol. 2001;28:H422–H431. doi: 10.1152/ajpheart.2001.281.1.H422. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld CR, Roy T, DeSpain K, Cox BE. Large-conductance Ca2+-dependent K+ channels regulate basal uteroplacental blood flow in ovine pregnancy. J Soc Gynecol Investig. 2005;12:402–408. doi: 10.1016/j.jsgi.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Rosenfeld CR, Liu XT, DeSpain K. Pregnancy modifies the large conductance Ca2+-activated K+ channel and cGMP-dependent signaling pathway in uterine vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2009;296:H1878–H1887. doi: 10.1152/ajpheart.01185.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka Y, Meera P, Song M, Knaus HG, Toro L. Molecular constituents of maxi KCa channels in human coronary smooth muscle: predominant alpha + beta subunit complexes. J Physiol. 1997;502:545–557. doi: 10.1111/j.1469-7793.1997.545bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knaus HG, Garcia-Calvo M, Kaczorowski GJ, Garcia ML. Subunit composition of the high conductance calcium-activated potassium channel from smooth muscle, a representative of the mSlo and slowpoke family of potassium channels. J Biol Chem. 1994;269:3921–3924. [PubMed] [Google Scholar]
- 8.Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 9.Pluger S, Faulhaber J, Furstenau M, Lohn M, Waldschutz R, Gollasch M, Haller H, Luft FC, Ehmke H, Pongs O. Mice with disrupted BK channel β1 subunit gene feature abnormal Ca2+ spark/STOC coupling and elevated blood pressure. Circ Res. 2000;87:E53–E60. doi: 10.1161/01.res.87.11.e53. [DOI] [PubMed] [Google Scholar]
- 10.Cox DH, Aldrich RW. Role of the β1 subunit in large-conductance Ca2+-activated K+ channel gating energetics. Mechanisms of enhanced Ca2+ sensitivity. J Gen Physiol. 2000;116:411–432. doi: 10.1085/jgp.116.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990;259:C3–C18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- 12.Sausbier M, Arntz C, Bucurenciu I, Zhao H, Zhou XB, Sausbier U, Feil S, Kamm S, Essin K, Sailer CA, Abdullah U, Krippeit-Drews P, Feil R, Hofmann F, Knaus HG, Kenyon C, Shipston MJ, Storm JF, Neuhuber W, Korth M, Schubert R, Gollasch M, Ruth P. Elevated blood pressure linked to primary hyperaldosteronism and impaired vasodilation in BK channel-deficient mice. Circulation. 2005;112:60–68. doi: 10.1161/01.CIR.0000156448.74296.FE. [DOI] [PubMed] [Google Scholar]
- 13.Chang K, Xiao D, Huang X, Xue Z, Yang S, Longo LD, Zhang L. Chronic hypoxia inhibits sex steroid hormone-mediated attenuation of ovine uterine arterial myogenic tone in pregnancy. Hypertension. 2010;56:750–757. doi: 10.1161/HYPERTENSIONAHA.110.155812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao D, Huang X, Yang S, Longo LD, Zhang L. Pregnancy downregulates actin polymerization and pressure-dependent myogenic tone in ovine uterine arteries. Hypertension. 2010;56:1009–1015. doi: 10.1161/HYPERTENSIONAHA.110.159137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu XQ, Singh N, Mukhopadhyay D, Akbarali HI. Modulation of voltage-dependent Ca2+ channels in rabbit colonic smooth muscle cells by c-Src and focal adhesion kinase. J Biol Chem. 1998;273:5337–5342. doi: 10.1074/jbc.273.9.5337. [DOI] [PubMed] [Google Scholar]
- 16.Hu XQ, Zhang L, Stewart RR, Weight FF. Arginine 222 in the pre-transmembrane domain 1 of 5-HT3A receptors links agonist binding to channel gating. J Biol Chem. 2003;278:46583–46589. doi: 10.1074/jbc.M308974200. [DOI] [PubMed] [Google Scholar]
- 17.Langton PD, Nelson MT, Huang Y, Standen NB. Block of calcium-activated potassium channels in mammalian arterial myocytes by tetraethylammonium ions. Am J Physiol. 1991;260:H927–H934. doi: 10.1152/ajpheart.1991.260.3.H927. [DOI] [PubMed] [Google Scholar]
- 18.Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- 19.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 20.Khan LH, Rosenfeld CR, Liu XT, Magness RR. Regulation of the cGMP-cPKG pathway and large-conductance Ca2+-activated K+ channels in uterine arteries during the ovine ovarian cycle. Am J Physiol Endocrinol Metab. 2010;298:E222–E228. doi: 10.1152/ajpendo.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill MA, Yang Y, Ella SR, Davis MJ, Braun AP. Large conductance, Ca2+-activated K+ channels (BKCa) and arteriolar myogenic signaling. FEBS Lett. 2010;584:2033–2042. doi: 10.1016/j.febslet.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagar D, Liu XT, Rosenfeld CR. Estrogen regulates β1-subunit expression in Ca2+-activated K+ channels in arteries from reproductive tissues. Am J Physiol Heart Circ Physiol. 2005;289:H1417–H1427. doi: 10.1152/ajpheart.01174.2004. [DOI] [PubMed] [Google Scholar]
- 23.Rosenfeld CR, White RE, Roy T, Cox BE. Calcium-activated potassium channels and nitric oxide coregulate estrogen-induced vasodilation. Am J Physiol Heart Circ Physiol. 2000;279:H319–H328. doi: 10.1152/ajpheart.2000.279.1.H319. [DOI] [PubMed] [Google Scholar]
- 24.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- 25.Osol G, Laher I, Cipolla M. Protein kinase C modulates basal myogenic tone in resistance arteries from the cerebral circulation. Circ Res. 1991;68:359–367. doi: 10.1161/01.res.68.2.359. [DOI] [PubMed] [Google Scholar]
- 26.Xiao D, Zhang L. ERK MAP kinases regulate smooth muscle contraction in ovine uterine artery: effect of pregnancy. Am J Physiol Heart Circ Physiol. 2002;282:H292–H300. doi: 10.1152/ajpheart.2002.282.1.H292. [DOI] [PubMed] [Google Scholar]
- 27.Xiao D, Zhang L. Adaptation of uterine artery thick- and thin-filament regulatory pathways to pregnancy. Am J Physiol Heart Circ Physiol. 2005;288:H142–H148. doi: 10.1152/ajpheart.00655.2004. [DOI] [PubMed] [Google Scholar]
- 28.Kanashiro CA, Cockrell KL, Alexander BT, Granger JP, Khalil RA. Pregnancy-associated reduction in vascular protein kinase C activity rebounds during inhibition of NO synthesis. Am J Physiol Regul Integr Comp Physiol. 2000;278:R295–R303. doi: 10.1152/ajpregu.2000.278.2.R295. [DOI] [PubMed] [Google Scholar]
- 29.Magness RR, Rosenfeld CR, Carr BR. Protein kinase C in uterine and systemic arteries during ovarian cycle and pregnancy. Am J Physiol. 1991;260:E464–E470. doi: 10.1152/ajpendo.1991.260.3.E464. [DOI] [PubMed] [Google Scholar]
- 30.Farley DB, Ford SP. Evidence for declining extracellular calcium uptake and protein kinase C activity in uterine arterial smooth muscle during gestation in gilts. Biol Reprod. 1992;46:315–321. doi: 10.1095/biolreprod46.3.315. [DOI] [PubMed] [Google Scholar]
- 31.Bonev AD, Jaggar JH, Rubart M, Nelson MT. Activators of protein kinase C decrease Ca2+ spark frequency in smooth muscle cells from cerebral arteries. Am J Physiol. 1997;273:C2090–C2095. doi: 10.1152/ajpcell.1997.273.6.C2090. [DOI] [PubMed] [Google Scholar]
- 32.Schubert R, Noack T, Serebryakov VN. Protein kinase C reduces the KCa current of rat tail artery smooth muscle cells. Am J Physiol. 1999;276:C648–C658. doi: 10.1152/ajpcell.1999.276.3.C648. [DOI] [PubMed] [Google Scholar]
- 33.Taguchi K, Kaneko K, Kubo T. Protein kinase C modulates Ca2+-activated K+ channels in cultured rat mesenteric artery smooth muscle cells. Biol Pharm Bull. 2000;23:1450–1454. doi: 10.1248/bpb.23.1450. [DOI] [PubMed] [Google Scholar]
- 34.Barman SA, Zhu S, White RE. Protein kinase C inhibits BKCa channel activity in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2004;286:L149–L155. doi: 10.1152/ajplung.00207.2003. [DOI] [PubMed] [Google Scholar]
- 35.White RE, Han G, Maunz M, Dimitropoulou C, El-Mowafy AM, Barlow RS, Catravas JD, Snead C, Carrier GO, Zhu S, Yu X. Endothelium-independent effect of estrogen on Ca2+-activated K+ channels in human coronary artery smooth muscle cells. Cardiovasc Res. 2002;53:650–661. doi: 10.1016/s0008-6363(01)00428-x. [DOI] [PubMed] [Google Scholar]
- 36.Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI, Mann GE, Vergara C, Latorre R. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the β subunit. Science. 1999;285:1929–1931. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- 37.Darkow DJ, Lu L, White RE. Estrogen relaxation of coronary artery smooth muscle is mediated by nitric oxide and cGMP. Am J Physiol. 1997;272:H2765–H2773. doi: 10.1152/ajpheart.1997.272.6.H2765. [DOI] [PubMed] [Google Scholar]
- 38.Byers MJ, Zangl A, Phernetton TM, Lopez G, Chen DB, Magness RR. Endothelial vasodilator production by ovine uterine and systemic arteries: ovarian steroid and pregnancy control of ERα and ERβ levels. J Physiol. 2005;565:85–99. doi: 10.1113/jphysiol.2005.085753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perrot-Applanat M, Groyer-Picard MT, Garcia E, Lorenzo F, Milgrom E. Immunocytochemical demonstration of estrogen and progesterone receptors in muscle cells of uterine arteries in rabbits and humans. Endocrinology. 1988;123:1511–1519. doi: 10.1210/endo-123-3-1511. [DOI] [PubMed] [Google Scholar]
- 40.Nishimura I, Ui-Tei K, Saigo K, Ishii H, Sakuma Y, Kato M. 17beta-estradiol at physiological concentrations augments Ca2+ -activated K+ currents via estrogen receptor beta in the gonadotropin-releasing hormone neuronal cell line GT1-7. Endocrinology. 2008;149:774–782. doi: 10.1210/en.2007-0759. [DOI] [PubMed] [Google Scholar]
- 41.Nimigean CM, Magleby KL. The beta subunit increases the Ca2+ sensitivity of large conductance Ca2+-activated potassium channels by retaining the gating in the bursting states. J Gen Physiol. 1999;113:425–440. doi: 10.1085/jgp.113.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amberg GC, Bonev AD, Rossow CF, Nelson MT, Santana LF. Modulation of the molecular composition of large conductance, Ca2+ activated K+ channels in vascular smooth muscle during hypertension. J Clin Invest. 2003;112:717–724. doi: 10.1172/JCI18684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao G, Zhao Y, Pan B, Liu J, Huang X, Zhang X, Cao C, Hou N, Wu C, Zhao KS, Cheng H. Hypersensitivity of BKCa to Ca2+ sparks underlies hyporeactivity of arterial smooth muscle in shock. Circ Res. 2007;101:493–502. doi: 10.1161/CIRCRESAHA.107.157271. [DOI] [PubMed] [Google Scholar]
- 44.Alexander BT, Bennett WA, Khalil RA, Granger JP. Preeclampsia: linking placental ischemia with cardiovascular-renal dysfunction. News Physiol Sci. 2001;16:282–286. doi: 10.1152/physiologyonline.2001.16.6.282. [DOI] [PubMed] [Google Scholar]
- 45.Sholook MM, Gilbert JS, Sedeek MH, Huang M, Hester RL, Granger JP. Systemic hemodynamic and regional blood flow changes in response to chronic reductions in uterine perfusion pressure in pregnant rats. Am J Physiol Heart Circ Physiol. 2007;293:H2080–H2084. doi: 10.1152/ajpheart.00667.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.