Synopsis
This chapter reviews the development of the senses of taste and smell, which provide information on the flavor of foods, and discusses how innate predispositions interact with early-life feeding experiences to form children’s dietary preferences and habits. A basic understanding of the development and functioning of the chemical senses during early childhood may assist in forming evidence-based strategies to improve children’s diets, especially for those who experience a discontinuity or disruption in early flavor experiences.
Keywords: high risk neonate, gustatory system development, olfactory system development, infant feeding behaviors, flavor learning
Introduction
Our senses of taste and smell are intimately connected to nutrition and allow us to reject those foods that are harmful and to seek out those that are beneficial and pleasurable[1]. During the past several decades, researchers have begun to unravel some of the mysteries underlying the ontogeny of the function of these senses as well as the roles they play in food choice, health, and social interactions.
Building upon the scientific definition of flavor and the basic biology of taste and smell, we summarize insights gleaned from basic scientific research in the chemical senses, with a focus on the sensory capabilities of the human infant and the inherent contributions of genetic differences in taste perception and the plasticity of the chemical senses in the development of flavor and food preferences. We highlight differences between normal and high risk neonates with regard to early sensory experiences and its potential impact on learning and later feeding.
Definition of Flavor
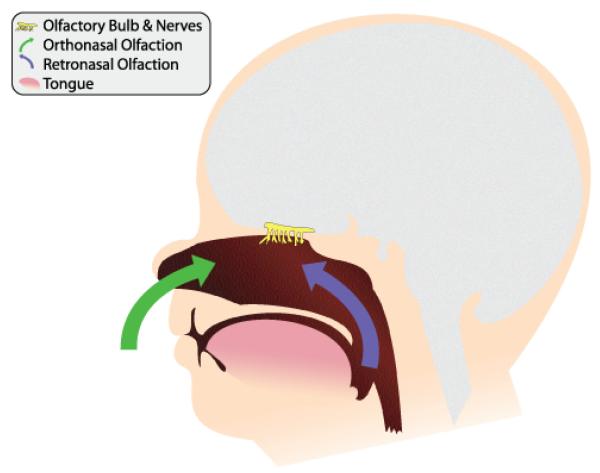
Flavor, a powerful determinant of human ingestion throughout the life span, is a product of several sensory systems, most notably those of the chemical senses, taste and smell. The perceptions arising from these two senses are often confused and misappropriated[2]. Sensations such as garlic, chocolate, anise, and lemon are erroneously attributed to the taste system per se, when in fact only a small number of primary taste qualities can be perceived by the tongue: sweet, salty, bitter, sour, and savory. Smell sensations, on the other hand, encompass thousands of diverse qualities, including the flavors noted above. As illustrated in Figure 1, the receptors for the olfactory system, located high in the nasal chambers, are stimulated not only during inhalation (orthonasal route) but also when infants suck and when children and adults swallow, as chemical constituents in foods and beverages reach the nasal receptors by passing from the oral cavity through the nasal pharynx (retronasal route). It is this retronasal stimulation arising from the molecules of foodstuffs that leads to the predominant flavor sensations.
Figure 1.
Orthonasal (green arrows) and retronasal (purple arrow) routes of olfaction.
Basic Flavor Biology
Taste occurs when chemicals come into contact with taste receptors on the tongue, palate, throat, epiglottis, or esophagus that then send signals to the brain. Taste receptor cells are the interface between the oral environment and the nervous system (reviewed in ref[3]). These cells, arranged in groups of 50 to 100 to form taste buds, contain the proteins necessary to recognize each of the five types of taste: sweet, salty, sour, bitter, and savory. Salty and sour foods are recognized by ion channels[4]. Salty taste is most commonly imparted by sodium ions in sodium chloride, but other sodium and nonsodium salts also convey a salty characteristic. Sour taste is generated by protons in acids. Sweet, bitter, and savory tastes are translated to the brain via G-protein-coupled receptors (GPCRs): type I GPCRs (T1R1, T1R2, and T1R3) are stimulated by sweet (T1R2+T1R3) and savory (T1R1+T1R3) compounds[5,6], while bitter compounds are recognized by type II GPCRs (T2Rs)[7,8]. T2Rs recognize a variety of unpleasant-tasting compounds and may have evolved as a warning to avoid toxins[9,10].
Odors are recognized by olfactory receptors, which are located on a small patch of tissue in the nasal cavity. Olfactory receptors are GPCRs that are generated by the largest mammalian gene superfamily, with more than 400 functional genes[11,12]. The olfactory system becomes tuned to respond to stimuli in different ways based on the experience of the individual and the context in which odors are experienced[13,14]. Olfactory signals combine with taste signals to communicate flavor to the brain[15,16].
Genetics of Taste
Polymorphisms in the genes that encode taste and odorant receptors result in differential sensory patterns in humans, by altering amino acid sequences of receptors, which alters their function, or by altering gene expression[17-28]. Although these mutations are found in a variety of receptor genes, few examples have been well characterized in the literature.
Polymorphisms in the bitter taste receptor gene TAS2R38 are the most studied of all taste receptor variants. Genetic variation in this receptor translates into individual differences in taste sensitivity for the synthetic compounds phenylthiocarbamide and propylthiouracil (PROP), as well as bitter-tasting compounds commonly found in cruciferous vegetables[29]. The polymorphisms result in changes to the amino acid sequence of the receptor from alanine-valineisoleucine (AVI) in nontasters to proline-alanine-valine (PAV) in tasters[17,30,31]. These polymorphisms allow homozygous AVI people to enjoy broccoli or turnips without perceiving the bitterness that heterozygous AVI/PAV and especially homozygous PAV people taste[32]. Studies in children and their mothers indicate that the phenotype-genotype relationship for PROP sensitivity varies with age, such that AVI/PAV heterozygous children are more sensitive to PROP than are heterozygous adults, with adolescents being intermediate[25,33]. These results imply that within the same genotype, taste sensitivity can change over the life span (from more to less bitter sensitivity).
A commonly cited example of individual variation in human olfaction is the perception of androstenone, a volatile steroid found in human perspiration, boar saliva, some pork products, truffles, and celery[34]). While some individuals describe this volatile as “sweaty and urinous,” others perceive it as smelling “sweet and floral,” or odorless[35-37]. The odorant receptor OR7D4 is activated by androstenone, and recently two polymorphisms were identified within the gene that change the amino acid sequence and impair the function of the receptor[21].
Individuals with the arginine-threonine variant smell androstenone, and those with the tryptophan-methionine variant find it to be odorless. Similar to bitterness sensitivity, the ability to detect androstenone seems to change with age[38-40].
Extraoral Taste and Nutrient Sensing
Although not much is known about their relative function, taste receptors have been found in many extraoral tissues, including the lungs, brain, gut, and reproductive system[41-44]. Sweet and bitter receptors are both found in the gut but have different functions. Sweet receptors regulate local glucose transporters to enhance glucose uptake[45], whereas one function of the bitter taste receptors is to regulate the absorption of toxic secondary plant compounds or other poisons[46]. Bitter receptors are also found in the upper and lower airways in mammals[47-50], and they are probably also present in humans. Their function in the airway is not known, but one possibility is that they sense bitter molecules secreted by bacteria and may evoke immune or other responses to clear the airway of pathogens[51]. The developmental trajectory of extraoral bitter and sweet receptors in gut, airway, and other tissues is not known, either in humans or in other species.
Pre- and Postnatal Development of Flavor
Both olfactory and taste receptors must be functional in order for a human fetus or infant to sense flavor. The primary olfactory receptors are formed by the 8th week of gestation (see [52] for a review) and are functional as early as the 24th week[53,54]. Taste cells also begin to form at 7 to 8 weeks of gestation[55,56]; by 13 to 15 weeks they look like mature receptor cells, and by around 17 weeks they are considered functionally mature. Fetal swallowing begins at approximately 12 weeks of gestation[57,58]. Around 18 weeks, gestational nonnutritive suckling begins, and the sucking and swallowing actions are coordinated by 35 to 40 weeks of gestation. Near the end of gestation the fetus swallows significant amounts of amniotic fluid. After 6 months of gestation, when the epithelial plugs no longer obstruct the air passages, amniotic fluid is also inhaled. The inhalation and swallowing of amniotic fluid are the first chemosensory experiences of the fetus and mark the beginning of flavor learning.
Amniotic fluid, the first food of infants, contains a wide range of nutrients that have particular tastes, such as glucose, fructose, lactic acid, fatty acids, and amino acids[59], as well as the flavors (for which the odors are perceived retronasally) of the foods consumed by the mother[60,61]. The fetus can detect these tastes and flavors: fetal swallowing frequency increases in response to the introduction of sweet solutions into the amniotic fluid and decreases in response to the introduction of bitter solutions[59,62]—this may be one of the first indications that our basic biology favors consumption of sweet tastes and avoidance of bitter tastes.
A similar response pattern is seen shortly after birth—within hours and days of being born, young infants react as would be expected to pleasurable and aversive taste stimuli[63-72]: provision of sweet or savory solutions to neonates elicits rhythmic tongue protrusions, lip smacks, lip and finger sucking, and elevation of the corners of the mouth, all of which have been interpreted as a positive or hedonic response[71,72]. In contrast, neonates gape, wrinkle their noses, shake their heads, flail their arms, and frown in response to a bitter solution[63,72]. Concentrated sour solutions elicit lip pursing and, to a certain extent, gaping, nose wrinkling, and arm flailing, as well as tongue protrusions and lip smacking[63,72,73]. Unlike the other basic tastes, salt taste receives a neutral reaction from neonates—the taste for salt does not emerge until later in infancy and then remains throughout childhood and adolescence[74].
These specific affective reactions to differing taste stimuli are strikingly similar across cultures[68,73,75] and species[72,76-79], suggesting a basic biological underpinning for the flavors and foods youngsters prefer and avoid. The convergence of research findings supports the conclusion that the innate preference for sweets and rejection of bitter tastes in humans are consequences of selection, favoring consumption of high-energy, vitamin-rich fruit and vegetable diets and avoidance of bitter, poisonous fruits and plants. Thus, when we examine children’s dietary patterns from the perspective of the development of taste, the foods children naturally prefer (e.g., sweet snacks) and those they dislike (e.g., bitter-tasting green vegetables) are not surprising and reflect their basic biology.
In addition to containing chemicals with distinct taste properties, amniotic fluid contains volatile chemicals (flavors) transmitted from the maternal diet[60,61,80], which, by at least the second trimester, appear to be detected by the fetus. Shortly after birth, infants will respond differently to flavors experienced in amniotic fluid, indicating that memories are formed from these early sensory experiences. For example, neonates whose mothers consumed an anise-flavored beverage or ate garlic-containing foods throughout pregnancy were more accepting of and interested in (as measured by mouthing and orienting) anise and garlic odors[80,81]. Similar findings were observed with alcohol odors[82].
Learning about the dietary choices of the mother continues when infants experience the flavors of the mother’s diet transmitted in breast milk. Young mammals first learn about the dietary choices of their mothers through transmitted flavor cues, a type of learning documented in a wide variety of species (see[83] for review). Following from this, researchers determined that many flavors (e.g., anise, garlic, ethanol, carrot, mint, vanilla, bleu cheese) pass from mother to offspring through breast milk[60,61,83-86]. Human infants detect the flavors in mother’s milk, as evidenced by changes in their suckling rate, patterning and duration of feeding and intake[60,85,86], and differential acceptance of similarly flavored foods at weaning and beyond[60,87-89]. Similarly, breastfed infants were more accepting of fruits and vegetables than were formula-fed infants, but only if their mothers regularly ate these foods themselves[87].
That these early flavor experiences can influence the acceptance of foods was first demonstrated in a randomized, controlled study of mothers who consumed carrot juice for several days each week during the last trimester of pregnancy or for a similar period during the first 3 months of lactation[88]. The control group drank water and avoided carrots and carrot juice during both pregnancy and lactation. When mothers weaned their infants around 6 months of age, the children were tested for acceptance of plain cereal on one day and carrot-flavored cereal on another. Infants who experienced the flavor of carrots in either amniotic fluid or mother’s milk responded more favorably (e.g., ate more, made fewer faces of distaste) to carrot-flavored cereal than did nonexposed control infants. Thus, as with many other mammals, human infants’ pre- and postnatal experiences with food flavors transmitted from the mother’s diet lead to greater acceptance and enjoyment of these foods during weaning.
Sensitive Period for Flavor Learning
Although the types of flavors that breastfed infants experience before their first taste of solid foods reflect the culinary practices of their mothers, which varies from infant to infant[60,87], formula-fed infants are usually exposed to constant flavors after birth and prior to weaning, since most formula-fed infants experience a single type of formula[90]. The absence of a robust experimental paradigm, like that employed for other sensory systems (e.g., vision, audition/language) and other animals, has inhibited progress in understanding whether human flavor programming exhibits age-related changes in functional plasticity, commonly referred to as sensitive periods. To address this gap, a model system was used that exploits the naturally occurring flavor variation in infant formulas[91,92].
In the United States, formulas are available for healthy term infants and for special medical purposes (such as preterm infants or infants with inborn errors of metabolism). Among the formulas for healthy term infants, one of the main distinctions is their protein source and/or degree of protein hydrolysis. Cow milk formula (CMF) is the most common formula consumed by infants, accounting for 76% of all U.S. infant formula sales in 2000[93]. Its protein usually includes combinations of intact casein and whey proteins[94,95]. Extensive protein hydrolysate formula (ePHF), a type of formula typically fed to infants who have cow milk protein allergy or intolerance to intact protein, is less prevalent in use than is CMF[93]. The milk proteins (i.e., whey, casein) in ePHF are treated with enzymes to break down the protein structure to reduce allergenicity; these formulas contain low-molecular-weight peptides and free amino acids. Partial protein hydrolysate formulas (pPHF) contain whey or casein milk proteins that are enzymatically treated but to a lesser extent than for ePHF. The varying composition and degree of hydrolysis among hydrolysate formulas affect formula flavor profiles[96]. To adults who were not fed ePHF during infancy, it is extremely unpalatable compared with CMF because of PHF’s distinctive, unpleasant flavors, including both volatile (odors) and nonvolatile (e.g., bitter and sour tastes) components.
Using the flavor differences between CMF and ePHF as a model system, a “window” of acceptance was identified during which young infants readily accept ePHF. Beginning around 4 months of age and continuing through adulthood, its flavor is rejected unless the individual was exposed to ePHF earlier in life (consistent with anecdotal pediatrician reports that it is difficult to begin feeding ePHF to infants 4 or more months of age). Thus, depending on an individual’s exposure to its flavor during the first few months of life, ePHF acquires a completely different “hedonic tone,” or perceived pleasantness[91].
A randomized trial was conducted to begin to characterize the effects of the timing and duration of early-life exposure when hedonic responses to PHF flavors are established. Infants were randomized to be fed ePHF for 1 month beginning at 1.5 months, 2.5 months, or 3.5 months or for 3 months beginning at 1.5 months[92]. All groups were then compared with control groups that had either no ePHF exposure or 7 months of ePHF exposure. At 7.5 months, infant acceptance of ePHF was tested with complete “meals” of both formulas. Among infants who began feeding ePHF at 1.5 months, those fed for 1 month were as accepting of ePHF as those fed for 3 months. That is, flavor experience of a relatively brief occurrence (1 month) before the baby is 3.5 months of age is sufficient to maintain acceptance. However, infants fed ePHF for 1 month were less accepting than infants exposed to ePHF for the entire 7 months. Early exposure is also important: infants exposed to ePHF for 1 month starting at 3.5 months were less accepting of ePHF at 7.5 months of age than were infants exposed at an earlier age. Maternal perceptions of infants’ enjoyment of the formulas and the frequency of facial expressions of distaste were consistent with both the exposure- and timing-related differences in intake. Early exposure eliminated the age-related rejection seen in unexposed infants and resulted in a complete shift in hedonic tone[92].
The effects of early exposure to ePHF are persistent, leading to heightened preferences for the taste and aroma of ePHF and foods containing similar volatiles or tastes (e.g., bitter, sour, savory) at weaning and several years after children’s last exposure to the formula. Children fed ePHF had an increased preference for sour-flavored apple juice[75,97] and savory-, bitter-, and sour-tasting and plain cereals compared with other children[98]. The mothers of these children were also more likely to list broccoli as one of their child’s preferred vegetables than were mothers of infants fed CMF[97].
Why should there be a sensitive period in the early acceptance of the flavor of ePHF? First, presuming there is an adaptive reason, it clearly has nothing directly to do with hydrolyzed protein formulas, which were introduced only a half-century ago. Indeed, these observations with formulas may conveniently expose a much more fundamental aspect of early mammalian flavor learning. We hypothesize that it is important for the human infant to accept and be particularly (but not exclusively) attracted to the flavors that are consumed by the culture and, more specifically, by the mother. All else being equal, these are the flavors that are associated with nutritious foods or, at the very least, foods the mother has access to—and the foods and flavors that the infant will experience at weaning and probably thereafter. Under this hypothesis, much of the normal exposure would occur in utero and during breastfeeding, as flavors mothers consume are transferred to these chemosensory environments. Additional research is needed to determine the extent to which early exposure (and the lack of early exposure) to these flavors, perhaps during sensitive periods of development, helps establish enduring preferences for foods and flavors.
Challenges for the High Risk Neonate
The first few months of life are an essential part of the flavor learning process for humans, and during this period the sensory experiences of the high risk neonate are drastically different from those of a typical infant, lacking continuity with prenatal sensory experiences. Preterm infants are often unable to coordinate sucking, swallowing, and breathing, so nasogastric or orogastric tube feeding is used to provide adequate nutrition[99]. When fed by a tube, infants likely have a relatively constrained olfactory and flavor experience in the context of feeding because their nutrition bypasses the oral and nasal cavities. Even those who are tube fed human milk may not have the opportunity to experience retronasally the flavors present in milk. Furthermore, it is unknown how the body responds when nutrients are sensed in the gut but have not been sensed in the oral cavity.
Tube-fed infants increase nonnutritive sucking when exposed to the smell of mother’s milk through an infant olfactometer, suggesting that exposure to maternal nutrient odor may assist in transition to oral feeding[100]. However, in the neonatal intensive care unit and normal infant wards in hospitals, infants are also exposed to (and learn about) unpleasant or noxious odors, including disinfectants, antibacterial compounds, and cleaning solutions[101]. The long-term consequences of this altered sensory environment remain unknown.
Tube feeding is generally done using either preterm formula or fortified human milk[102-104]. Greater efforts are being made to increase the amount of human milk given to preterm infants because human milk provides many benefits such as improved immune status and increased cognitive development, while successful expression of breast milk allows for more maternal involvement in feeding and increases maternal confidence[105-119]. We hypothesize that, given the presence of functional taste receptors in both the oral cavity and the gut[45], increasing intake of human milk is beneficial for future feeding behavior because the extraoral stimulation of milk on gut taste receptors may aid in the transition from tube feeding to breastfeeding. Encouraging the mother to pump breast milk also increases the likelihood that the child will eventually be able to transition to the breast and experience the flavors in mother’s milk within the sensitive window for flavor learning[106].
In addition to altered oral sensory exposure, infants who are tube fed do not have early experience with traditional feeding behaviors (sucking, swallowing, and chewing). However, we emphasize that there has been a paucity of experimental research in this area on how such altered sensory experiences affect later behaviors associated with feeding. Several case studies from the 1960s revealed that if children were not introduced to solid foods at the time when they are first able to chew, acceptance of these foods became very difficult[120]. In three of the cases, the children had esophageal atresia and were tube fed beginning days after birth. Repair of the esophagus did not happen until 16 to 22 months later, at which point two of the three patients readily accepted fluids, but all three had significant difficulty transitioning to solid food. Long-term tube feeding may affect the physical development of feeding behaviors, with consequences lasting into childhood.
When tube feeding occurs for a short period of time (15-20 days), in combination with nonnutritive sucking, infants generally transition well to oral feeding[121-124]. However, if the tube feeding lasts for a longer period of time (>45 days), it becomes much more difficult for the child to make the transition. A study of 9 infants who were tube fed for at least 2 months starting from birth, the infants refused all attempts at oral feeding and reacted with agitation, arching, tongue thrusting, gagging, and vomiting[124]. The infants also had an absent or deficient sucking reflex and a gag reflex that was triggered by any foreign object. To help wean the children, during tube feedings at regular intervals the infants were provided with stimulation to reproduce normal feeding as closely as possible. They were cradled in their mother’s arms, the gums and palate were massaged, and the tongue was stimulated with breast milk from the mother’s finger to stimulate the sucking reflex. Eight of the children eventually weaned from tube to oral feeding, with those who were tube fed the longest requiring the most time to establish normal eating behavior. The authors of the study hypothesize that stimulation during tube feeding helps inhibit the gag reflex, creates an association among tactile, olfactory, and taste sensations and the mechanical replenishing of the stomach, and establishes normal circadian rhythms[124].
High risk infants are also faced with a wide array of medical conditions that contribute to temporary or permanent alteration of taste and smell as adults. Many medications, including antibiotics and anti-inflammatory agents, have been shown to alter taste and smell, and these are commonly given to high risk neonates[125-127]. Feeding problems can lead to vitamin deficiencies, which has also been linked to altered taste and smell[128]. Gastroesophageal reflux disease is another common problem in preterm infants and results in a sour or bitter taste in the mouth from reflux of stomach acid up the esophagus and into the throat[129]. The long-term effects of these alterations on the development of flavor preferences in the child are not known.
Concluding Remarks
Every culture differs in the flavor principles that characterize its cuisine and the types of foods preferred by the families who identify with its traditions. Thus, cultural traditions guide the types of food individuals eat on a daily basis. Although many would argue that learning about these flavor principles, food preferences, and cultural traditions begins when parents serve their children cultural meals during family dinners, research demonstrates that this learning begins long before a child ever consumes solid food. Flavors of the mother’s diet are transmitted to the offspring through the amniotic fluid and breast milk, and infants more readily accept flavors they’ve already experienced through these two mediums when fed as solid foods at weaning. The recent discoveries of taste receptors in tissues outside of the oral cavity only add to the complexity of this system. Infants may also be sensing bitter stimuli in the airways or sweet stimuli in the gut, and the development of these sensory systems is not yet understood.
Because the senses of taste and smell are the major determinants of whether young children will accept a food (e.g., they eat only what they like), these senses take on greater significance in understanding the biological basis for children’s food choices. Not being exposed to the flavors of healthy foods early in life can have detrimental consequences. While there are innate responses to the basic tastes, and some individuals may be more sensitive to some tastes due to genotype, the development of these chemical senses has inherent plasticity that interacts with early-life experiences to shape and modify flavor and food preferences. Such functional plasticity, one of the main characteristics of the brain, highlights the ability to change behavior based on experience. In other words, our biology is not necessarily our destiny.
Although we are beginning to learn how the chemical senses develop during infancy and their impact on food choices and other behaviors, there are many gaps in our knowledge. In particular, we know little about the contingencies for early learning and how the absence of early postnatal chemosensory experience (e.g., absence of breastfeeding), disruptions in mother-infant attachment (e.g., tube feeding of high risk infants), or negative associations with early feeding (e.g., chemical smells in hospital settings) interfere with the acquisition of feeding skills. The increasing awareness of the importance of infant feeding behavior makes it imperative to determine the extent to which restoration of normal oral motor and sensory experiences affect feeding behavior and nutrition.
Clearly, more research is needed to develop evidence-based practices aimed at infant feeding difficulties, which constitute a medically and economically important complication for some neonatal diseases. Applying the knowledge gleaned from such research and clinical practice, which takes into account the developing sensory world of the child, could have long-term consequences in preventing eating disorders in early infancy. Moreover, understanding the development and functioning of these senses may assist in the development of evidence-based strategies to improve children’s diets, since many of the illnesses that plague modern society (e.g., obesity, diabetes, and hypertension) are often the consequence of poor food choices that start in childhood.
Acknowledgments
Preparation of this manuscript was supported in part by grant DC011287 and training grant T32-DC00014-32 from the National Institute of Deafness and Other Communication Disorders and by grant HD37119 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sarah V. Lipchock, Monell Chemical Senses Center, 3500 Market Street, Philadelphia, PA 19104-3308 USA, 215-898-2084 (fax), 267-519-4891 (phone), slipchock@monell.org (email).
Danielle R. Reed, Monell Chemical Senses Center, 3500 Market Street, Philadelphia, PA 19104-3308 USA, 215-898-2084 (fax), 267-519-4915 (phone), reed@monell.org (email).
References
- 1.Reed DR, Knaapila A. Genetics of taste and smell: poisons and pleasures. Prog Mol Biol Transl Sci. 2010;94:213–240. doi: 10.1016/B978-0-12-375003-7.00008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rozin P. “Taste-smell confusions” and the duality of the olfactory sense. Percept Psychophys. 1982;31(4):397–401. doi: 10.3758/bf03202667. [DOI] [PubMed] [Google Scholar]
- 3.Breslin PA, Spector AC. Mammalian taste perception. Curr Biol. 2008;18(4):R148–155. doi: 10.1016/j.cub.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Medler K, Kinnamon S. Transduction Mechanisms in Taste Cells. In: Frings S, Bradley J, editors. Transduction channels in sensory cells. Wiley-VCH; Weinheim, Germany: 2004. pp. 153–174. [Google Scholar]
- 5.Zhao GQ, Zhang Y, Hoon MA, et al. The receptors for mammalian sweet and umami taste. Cell. 2003;115(3):255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci U S A. 2002;99(7):4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandrashekar J, Mueller KL, Hoon MA, et al. T2Rs function as bitter taste receptors. Cell. 2000;100(6):703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 8.Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100(6):693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 9.Peyrot des Gachons C, Beauchamp GK, Stern RM, Koch KL, Breslin PA. Bitter taste induces nausea. Curr Biol. 2011;21(7):R247–248. doi: 10.1016/j.cub.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 10.Fox AL. The Relationship between Chemical Constitution and Taste. Proc Natl Acad Sci U S A. 1932;18(1):115–120. doi: 10.1073/pnas.18.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65(1):175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 12.Hasin-Brumshtein Y, Lancet D, Olender T. Human olfaction: from genomic variation to phenotypic diversity. Trends Genet. 2009;25(4):178–184. doi: 10.1016/j.tig.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Epple G, Herz RS. Ambient odors associated to failure influence cognitive performance in children. Dev Psychobiol. 1999;35(2):103–107. doi: 10.1002/(sici)1098-2302(199909)35:2<103::aid-dev3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Forestell CA, Mennella JA. Children’s hedonic judgments of cigarette smoke odor: effects of parental smoking and maternal mood. Psychol Addict Behav. 2005;19(4):423–432. doi: 10.1037/0893-164X.19.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Small DM, Gerber JC, Mak YE, Hummel T. Differential neural responses evoked by orthonasal versus retronasal odorant perception in humans. Neuron. 2005;47(4):593–605. doi: 10.1016/j.neuron.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 16.Bender G, Hummel T, Negoias S, Small DM. Separate signals for orthonasal vs. retronasal perception of food but not nonfood odors. Behav Neurosci. 2009;123(3):481–489. doi: 10.1037/a0015065. [DOI] [PubMed] [Google Scholar]
- 17.Bufe B, Breslin PA, Kuhn C, et al. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr Biol. 2005;15(4):322–327. doi: 10.1016/j.cub.2005.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen QY, Alarcon S, Tharp A, et al. Perceptual variation in umami taste and polymorphisms in TAS1R taste receptor genes. Am J Clin Nutr. 2009;90(3):770S–779S. doi: 10.3945/ajcn.2009.27462N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eriksson N, Macpherson JM, Tung J, et al. Web-based, participant-driven studies yield novel genetic asociations for common traits. PLoS Genet. 2010;6(6):e1000993. doi: 10.1371/journal.pgen.1000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fushan AA, Simons CT, Slack JP, Manichaikul A, Drayna D. Allelic polymorphism within the TAS1R3 promoter is associated with human taste sensitivity to sucrose. Curr Biol. 2009;19(15):1288–1293. doi: 10.1016/j.cub.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keller A, Zhuang H, Chi Q, Vosshall LB, Matsunami H. Genetic variation in a human odorant receptor alters odour perception. Nature. 2007;449(7161):468–472. doi: 10.1038/nature06162. [DOI] [PubMed] [Google Scholar]
- 22.Kim UK, Breslin PA, Reed D, Drayna D. Genetics of human taste perception. J Dent Res. 2004;83(6):448–453. doi: 10.1177/154405910408300603. [DOI] [PubMed] [Google Scholar]
- 23.Kim UK, Wooding S, Riaz N, Jorde LB, Drayna D. Variation in the human TAS1R taste receptor genes. Chem Senses. 2006;31(7):599–611. doi: 10.1093/chemse/bjj065. [DOI] [PubMed] [Google Scholar]
- 24.Menashe I, Abaffy T, Hasin Y, et al. Genetic elucidation of human hyperosmia to isovaleric acid. PLoS Biol. 2007;5(11):e284. doi: 10.1371/journal.pbio.0050284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mennella JA, Pepino MY, Reed DR. Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics. 2005;115(2):e216–222. doi: 10.1542/peds.2004-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelchat ML, Bykowski C, Duke FF, Reed DR. Excretion and perception of a characteristic odor in urine after asparagus ingestion: a psychophysical and genetic study. Chem Senses. 2010 doi: 10.1093/chemse/bjq081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed DR, Zhu G, Breslin PA, et al. The perception of quinine taste intensity is associated with common genetic variants in a bitter receptor cluster on chromosome 12. Hum Mol Genet. 2010;19(21):4278–4285. doi: 10.1093/hmg/ddq324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shigemura N, Shirosaki S, Ohkuri T, et al. Variation in umami perception and in candidate genes for the umami receptor in mice and humans. Am J Clin Nutr. 2009;90(3):764S–769S. doi: 10.3945/ajcn.2009.27462M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wooding S, Gunn H, Ramos P, Thalmann S, Xing C, Meyerhof W. Genetics and bitter taste responses to goitrin, a plant toxin found in vegetables. Chem Senses. 2010;35(8):685–692. doi: 10.1093/chemse/bjq061. [DOI] [PubMed] [Google Scholar]
- 30.Kim UK, Jorgenson E, Coon H, Leppert M, Risch N, Drayna D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299(5610):1221–1225. doi: 10.1126/science.1080190. [DOI] [PubMed] [Google Scholar]
- 31.Timpson NJ, Heron J, Day IN, et al. Refining associations between TAS2R38 diplotypes and the 6-n-propylthiouracil (PROP) taste test: findings from the Avon Longitudinal Study of Parents and Children. BMC Genet. 2007;8:51. doi: 10.1186/1471-2156-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandell MA, Breslin PA. Variability in a taste-receptor gene determines whether we taste toxins in food. Curr Biol. 2006;16(18):R792–794. doi: 10.1016/j.cub.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 33.Mennella JA, Pepino MY, Duke FF, Reed DR. Age modifies the genotype-phenotype relationship for the bitter receptor TAS2R38. BMC Genet. 2010;11:60. doi: 10.1186/1471-2156-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wysocki CJ, Dorries KM, Beauchamp GK. Ability to perceive androstenone can be acquired by ostensibly anosmic people. Proc Natl Acad Sci U S A. 1989;86(20):7976–7978. doi: 10.1073/pnas.86.20.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pollack MS, Wysocki CJ, Beauchamp GK, Braun D, Jr., Callaway C, Dupont B. Absence of HLA association or linkage for variations in sensitivity to the odor of androstenone. Immunogenetics. 1982;15(6):579–589. doi: 10.1007/BF00347052. [DOI] [PubMed] [Google Scholar]
- 36.Theimer ET, Yoshida T, Klaiber EM. Olfaction and molecular shape. Chirality as a requisite for odor. J Agric Food Chem. 1977;25(5):1168–1177. doi: 10.1021/jf60213a029. [DOI] [PubMed] [Google Scholar]
- 37.Wysocki CJ, Beauchamp GK. Ability to smell androstenone is genetically determined. Proc Natl Acad Sci U S A. 1984;81(15):4899–4902. doi: 10.1073/pnas.81.15.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bekaert KM, Tuyttens FA, Duchateau L, et al. The sensitivity of Flemish citizens to androstenone: influence of gender, age, location and smoking habits. Meat Sci. 2011;88(3):548–552. doi: 10.1016/j.meatsci.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 39.Dorries KM, Schmidt HJ, Beauchamp GK, Wysocki CJ. Changes in sensitivity to the odor of androstenone during adolescence. Dev Psychobiol. 1989;22(5):423–435. doi: 10.1002/dev.420220502. [DOI] [PubMed] [Google Scholar]
- 40.Wysocki CJ, Gilbert AN. National Geographic Smell Survey. Effects of age are heterogenous. Ann N Y Acad Sci. 1989;561:12–28. doi: 10.1111/j.1749-6632.1989.tb20966.x. [DOI] [PubMed] [Google Scholar]
- 41.Ren X, Zhou L, Terwilliger R, Newton SS, de Araujo IE. Sweet taste signaling functions as a hypothalamic glucose sensor. Front Integr Neurosci. 2009;3:12. doi: 10.3389/neuro.07.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Behrens M, Meyerhof W. Oral and extraoral bitter taste receptors. Results Probl Cell Differ. 2010;52:87–99. doi: 10.1007/978-3-642-14426-4_8. [DOI] [PubMed] [Google Scholar]
- 43.Iwatsuki K, Nomura M, Shibata A, et al. Generation and characterization of T1R2-LacZ knock-in mouse. Biochem Biophys Res Commun. 2010 doi: 10.1016/j.bbrc.2010.10.057. [DOI] [PubMed] [Google Scholar]
- 44.Kokrashvili Z, Mosinger B, Margolskee RF. T1r3 and alpha-gustducin in gut regulate secretion of glucagon-like peptide-1. Ann N Y Acad Sci. 2009;1170:91–94. doi: 10.1111/j.1749-6632.2009.04485.x. [DOI] [PubMed] [Google Scholar]
- 45.Margolskee RF, Dyer J, Kokrashvili Z, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104(38):15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jeon TI, Seo YK, Osborne TF. Gut Bitter Taste Receptor Signaling Induces ABCB1 through a Mechanism Involving CCK. Biochem J. 2011 doi: 10.1042/BJ20110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Finger TE, Bottger B, Hansen A, Anderson KT, Alimohammadi H, Silver WL. Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc Natl Acad Sci U S A. 2003;100(15):8981–8986. doi: 10.1073/pnas.1531172100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gulbransen BD, Clapp TR, Finger TE, Kinnamon SC. Nasal solitary chemoreceptor cell responses to bitter and trigeminal stimulants in vitro. J Neurophysiol. 2008;99(6):2929–2937. doi: 10.1152/jn.00066.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deshpande DA, Wang WC, McIlmoyle EL, et al. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med. 2010;16(11):1299–1304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tizzano M, Cristofoletti M, Sbarbati A, Finger TE. Expression of taste receptors in solitary chemosensory cells of rodent airways. BMC Pulm Med. 2011;11:3. doi: 10.1186/1471-2466-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tizzano M, Gulbransen BD, Vandenbeuch A, et al. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci U S A. 2010;107:3210–3215. doi: 10.1073/pnas.0911934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ganchrow JR, Mennella JA. The ontogeny of human flavor perception. In: Doty RL, editor. Handbook of Olfaction and Gustation, 2nd edition. 2nd edition ed. Marcel Dekker, Inc.; New York: 2003. pp. 823–946. [Google Scholar]
- 53.Chuah MI, Zheng DR. Olfactory marker protein is present in olfactory receptor cells of human fetuses. Neuroscience. 1987;23(1):363–370. doi: 10.1016/0306-4522(87)90296-x. [DOI] [PubMed] [Google Scholar]
- 54.Johnson EW, Eller PM, Jafek BW. Distribution of OMP-, PGP 9.5- and CaBP-like immunoreactive chemoreceptor neurons in the developing human olfactory epithelium. Anat Embryol (Berl) 1995;191(4):311–317. doi: 10.1007/BF00534683. [DOI] [PubMed] [Google Scholar]
- 55.Bradley RM, Stern IB. The development of the human taste bud during the foetal period. J Anat. 1967;101(Pt 4):743–752. [PMC free article] [PubMed] [Google Scholar]
- 56.Witt M, Reutter K. Scanning electron microscopical studies of developing gustatory papillae in humans. Chem Senses. 1997;22(6):601–612. doi: 10.1093/chemse/22.6.601. [DOI] [PubMed] [Google Scholar]
- 57.Pritchard JA. Deglutition by Normal and Anencephalic Fetuses. Obstet Gynecol. 1965;25:289–297. [PubMed] [Google Scholar]
- 58.Schaffer JP. The lateral wall of the cayum nasi in man with special reference to the various developmental stages. J Morphol. 1910;21:613–617. [Google Scholar]
- 59.Liley AW. Disorders of amniotic fluid. In: Assali NS, editor. Pathophysiology of gestation. Fetal placental disorders. Academic Press; New York: 1972. pp. 157–206. [Google Scholar]
- 60.Mennella JA, Jagnow CP, Beauchamp GK. Prenatal and postnatal flavor learning by human infants. Pediatrics. 2001;107(6):E88–E88. doi: 10.1542/peds.107.6.e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mennella JA, Johnson A, Beauchamp GK. Garlic ingestion by pregnant women alters the odor of amniotic fluid. Chem Senses. 1995;20(2):207–209. doi: 10.1093/chemse/20.2.207. [DOI] [PubMed] [Google Scholar]
- 62.DeSnoo K. Das trinkende kind im uterus. Monoats Geburtsh Gynaekol. 1937;105:88–97. [Google Scholar]
- 63.Desor JA, Maller O, Andrews K. Ingestive responses of human newborns to salty, sour, and bitter stimuli. J Comp Physiol Psychol. 1975;89(8):966–970. doi: 10.1037/h0077171. [DOI] [PubMed] [Google Scholar]
- 64.Beauchamp GK, Pearson P. Human development and umami taste. Physiol Behav. 1991;49(5):1009–1012. doi: 10.1016/0031-9384(91)90215-a. [DOI] [PubMed] [Google Scholar]
- 65.Desor JA, Maller O, Greene LS. Preference for sweet in humans: Infants, children and adults. In: Weiffenbach JM, editor. Taste and Development: The genesis of sweet preference. Government Printing Office; Washington DC: 1977. pp. 161–173. [Google Scholar]
- 66.Fox NA, Davidson RJ. Taste-elicited changes in facial signs of emotion and the asymmetry of brain electrical activity in human newborns. Neuropsychologia. 1986;24(3):417–422. doi: 10.1016/0028-3932(86)90028-x. [DOI] [PubMed] [Google Scholar]
- 67.Maller O, Desor JA. Effect of taste on ingestion by human newborns. Symp Oral Sens Percept. 1973;(4):279–291. [PubMed] [Google Scholar]
- 68.Rosenstein D, Oster H. Differential facial responses to four basic tastes in newborns. Child Dev. 1988;59(6):1555–1568. [PubMed] [Google Scholar]
- 69.Steiner JE. The gustofacial response: observation on normal and anencephalic newborn infants. Symp Oral Sens Percept. 1973;(4):254–278. [PubMed] [Google Scholar]
- 70.Steiner JE. In: Bosma JF, editor. The human gustofacial response; Fourth Symposium of Oral Sensation and Perception: Development in the fetus and infant; Bethesda, MD: Department of Health, Education and Welfare. 1973; pp. 254–278. [PubMed] [Google Scholar]
- 71.Steiner JE. What the human neonate can tell us about umami. In: Kawamura Y, Kare MR, editors. Umami: A Basic Taste. Marcel Dekker; New York: 1987. pp. 97–123. [Google Scholar]
- 72.Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci Biobehav Rev. 2001;25(1):53–74. doi: 10.1016/s0149-7634(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 73.Steiner JE. Facial expressions of the neonate infant indicating the hedonics of food related chemical stimuli. In: Weiffenbach JM, editor. Taste and Development: The genesis of sweet preference. Government Printing Office; Washington DC: 1977. pp. 173–189. [Google Scholar]
- 74.Beauchamp GK, Cowart BJ, Moran M. Developmental changes in salt acceptability in human infants. Dev Psychobiol. 1986;19(1):17–25. doi: 10.1002/dev.420190103. [DOI] [PubMed] [Google Scholar]
- 75.Liem DG, Mennella JA. Sweet and sour preferences during childhood: role of early experiences. Dev Psychobiol. 2002;41(4):388–395. doi: 10.1002/dev.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brining SK, Belecky TL, Smith DV. Taste reactivity in the hamster. Physiol Behav. 1991;49(6):1265–1272. doi: 10.1016/0031-9384(91)90361-q. [DOI] [PubMed] [Google Scholar]
- 77.Ganchrow JR, Steiner JE, Bartana A. Behavioral reactions to gustatory stimuli in young chicks (Gallus gallus domesticus) Dev Psychobiol. 1990;23(2):103–117. doi: 10.1002/dev.420230202. [DOI] [PubMed] [Google Scholar]
- 78.Grill HJ, Roitman MF, Kaplan JM. A new taste reactivity analysis of the integration of taste and physiological state information. The American Journal of Physiology. 1996;271(3 Pt 2):R677-687–R677-687. doi: 10.1152/ajpregu.1996.271.3.R677. [DOI] [PubMed] [Google Scholar]
- 79.Beauchamp GK, Mason JR. Comparative hedonics of taste. In: Bolles RC, editor. The hedonics of taste. Lawrence Earlbaum Associates, Inc.; Hillsdale, NJ: 1991. pp. 159–183. [Google Scholar]
- 80.Schaal B, Marlier L, Soussignan R. Human foetuses learn odours from their pregnant mother’s diet. Chem Senses. 2000;25(6):729–737. doi: 10.1093/chemse/25.6.729. [DOI] [PubMed] [Google Scholar]
- 81.Hepper PG. Adaptive fetal learning: prenatal exposure to garlic affects personal preferences. Anim Behav. 1988;36:935–936. [Google Scholar]
- 82.Faas AE, Sponton ED, Moya PR, Molina JC. Differential responsiveness to alcohol odor in human neonates: effects of maternal consumption during gestation. Alcohol. 2000;22(1):7–17. doi: 10.1016/s0741-8329(00)00103-8. [DOI] [PubMed] [Google Scholar]
- 83.Mennella JA. The chemical senses and the development of flavor preferences in humans. In: Hale TW, Hartmann PE, editors. Textbook on Human Lactation. Hale Publishing; Texas: 2007. pp. 403–414. [Google Scholar]
- 84.Mennella JA, Beauchamp GK. Maternal diet alters the sensory qualities of human milk and the nursling’s behavior. Pediatrics. 1991;88(4):737–744. [PubMed] [Google Scholar]
- 85.Mennella JA, Beauchamp GK. The transfer of alcohol to human milk. Effects on flavor and the infant’s behavior. N Engl J Med. 1991;325(14):981–985. doi: 10.1056/NEJM199110033251401. [DOI] [PubMed] [Google Scholar]
- 86.Mennella JA, Beauchamp GK. The effects of repeated exposure to garlic-flavored milk on the nursling’s behavior. Pediatr Res. 1993;34(6):805–808. doi: 10.1203/00006450-199312000-00022. [DOI] [PubMed] [Google Scholar]
- 87.Forestell CA, Mennella JA. Early determinants of fruit and vegetable acceptance. Pediatrics. 2007;120(6):1247–1254. doi: 10.1542/peds.2007-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mennella JA, Beauchamp GK. Experience with a flavor in mother’s milk modifies the infant’s acceptance of flavored cereal. Dev Psychobiol. 1999;35(3):197–203. doi: 10.1002/(sici)1098-2302(199911)35:3<197::aid-dev4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 89.Mennella JA, Beauchamp GK. Mothers’ milk enhances the acceptance of cereal during weaning. Pediatr Res. 1997;41(2):188–192. doi: 10.1203/00006450-199702000-00006. [DOI] [PubMed] [Google Scholar]
- 90.Nevo N, Rubin L, Tamir A, Levine A, Shaoul R. Infant feeding patterns in the first 6 months: an assessment in full-term infants. J Pediatr Gastroenterol Nutr. 2007;45(2):234–239. doi: 10.1097/MPG.0b013e31803e1706. [DOI] [PubMed] [Google Scholar]
- 91.Mennella JA, Griffin CE, Beauchamp GK. Flavor programming during infancy. Pediatrics. 2004;113(4):840–845. doi: 10.1542/peds.113.4.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mennella JA, Lukasewycz LD, Castor SM, Beauchamp GK. The timing and duration of a sensitive period in human flavor learning: a randomized trial. Am J Clin Nutr. 2011;93(5):1019–1024. doi: 10.3945/ajcn.110.003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.ERS/USDA . Infant Formula Trends. USDA; 2005. [Google Scholar]
- 94.Similac [Accessed January 10];Abbot Nutrition: Similac Advance Early Sheild Product Information. 2010 Available at: http://abbottnutrition.com/Products/similac-advance-earlyshield.
- 95.Hennigs JK, Burhenne N, Stahler F, et al. Sweet Taste Receptor Interacting Protein CIB1 Is a General Inhibitor of InsP(3)-Dependent Ca(2+)-Release In Vivo. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05563.x. [DOI] [PubMed] [Google Scholar]
- 96.Cook DA, Sarett HP. Design of infant formulas for meeting normal and special need; Pediatric nutrition: Infant feeding, deficiencies, disease; New York, NY: Marcel Dekker, Inc. 1982. [Google Scholar]
- 97.Mennella JA, Beauchamp GK. Flavor experiences during formula feeding are related to preferences during childhood. Early Hum Dev. 2002;68(2):71–82. doi: 10.1016/s0378-3782(02)00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mennella JA, Forestell CA, Morgan LK, Beauchamp GK. Early milk feeding influences taste acceptance and liking during infancy. Am J Clin Nutr. 2009;90(3):780S–788S. doi: 10.3945/ajcn.2009.27462O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Toce SS, Keenan WJ, Homan SM. Enteral feeding in very-low-birth-weight infants. A comparison of two nasogastric methods. Am J Dis Child. 1987;141(4):439–444. doi: 10.1001/archpedi.1987.04460040097025. [DOI] [PubMed] [Google Scholar]
- 100.Bingham PM, Churchill D, Ashikaga T. Breast milk odor via olfactometer for tube-fed, premature infants. Behav Res Methods. 2007;39(3):630–634. doi: 10.3758/bf03193035. [DOI] [PubMed] [Google Scholar]
- 101.Laudert S, Liu WF, Blackington S, et al. Implementing potentially better practices to support the neurodevelopment of infants in the NICU. J Perinatol. 2007;27(Suppl 2):S75–93. doi: 10.1038/sj.jp.7211843. [DOI] [PubMed] [Google Scholar]
- 102.Heiman H, Schanler RJ. Enteral nutrition for premature infants: the role of human milk. Semin Fetal Neonatal Med. 2007;12(1):26–34. doi: 10.1016/j.siny.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 103.Reali A, Greco F, Fanaro S, et al. Fortification of maternal milk for very low birth weight (VLBW) pre-term neonates. Early Hum Dev. 2010;86(Suppl 1):33–36. doi: 10.1016/j.earlhumdev.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 104.Fanaro S, Ballardini E, Vigi V. Different pre-term formulas for different pre-term infants. Early Hum Dev. 2010;86(Suppl 1):27–31. doi: 10.1016/j.earlhumdev.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 105.Anderson JW, Johnstone BM, Remley DT. Breast-feeding and cognitive development: a meta-analysis. Am J Clin Nutr. 1999;70(4):525–535. doi: 10.1093/ajcn/70.4.525. [DOI] [PubMed] [Google Scholar]
- 106.Black KA, Hylander MA. Breastfeeding the high risk infant: implications for midwifery management. J Midwifery Womens Health. 2000;45(3):238–245. doi: 10.1016/s1526-9523(00)00017-9. [DOI] [PubMed] [Google Scholar]
- 107.Hylander MA, Strobino DM, Dhanireddy R. Human milk feedings and infection among very low birth weight infants. Pediatrics. 1998;102(3):E38. doi: 10.1542/peds.102.3.e38. [DOI] [PubMed] [Google Scholar]
- 108.Kavanaugh K, Meier P, Zimmermann B, Mead L. The rewards outweigh the efforts: breastfeeding outcomes for mothers of preterm infants. J Hum Lact. 1997;13(1):15–21. doi: 10.1177/089033449701300111. [DOI] [PubMed] [Google Scholar]
- 109.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336(8730):1519–1523. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- 110.Lucas A, Morley R, Cole TJ, Lister G, Leeson-Payne C. Breast milk and subsequent intelligence quotient in children born preterm. Lancet. 1992;339(8788):261–264. doi: 10.1016/0140-6736(92)91329-7. [DOI] [PubMed] [Google Scholar]
- 111.Meier PP, Brown LP. State of the science. Breastfeeding for mothers and low birth weight infants. Nurs Clin North Am. 1996;31(2):351–365. [PubMed] [Google Scholar]
- 112.Schanler RJ. Suitability of human milk for the low-birthweight infant. Clin Perinatol. 1995;22(1):207–222. [PubMed] [Google Scholar]
- 113.Amin SB, Merle KS, Orlando MS, Dalzell LE, Guillet R. Brainstem maturation in premature infants as a function of enteral feeding type. Pediatrics. 2000;106(2 Pt 1):318–322. doi: 10.1542/peds.106.2.318. [DOI] [PubMed] [Google Scholar]
- 114.Bier JA, Oliver T, Ferguson AE, Vohr BR. Human milk improves cognitive and motor development of premature infants during infancy. J Hum Lact. 2002;18(4):361–367. doi: 10.1177/089033402237909. [DOI] [PubMed] [Google Scholar]
- 115.Blaymore Bier JA, Oliver T, Ferguson A, Vohr BR. Human milk reduces outpatient upper respiratory symptoms in premature infants during their first year of life. J Perinatol. 2002;22(5):354–359. doi: 10.1038/sj.jp.7210742. [DOI] [PubMed] [Google Scholar]
- 116.Hylander MA, Strobino DM, Pezzullo JC, Dhanireddy R. Association of human milk feedings with a reduction in retinopathy of prematurity among very low birthweight infants. J Perinatol. 2001;21(6):356–362. doi: 10.1038/sj.jp.7210548. [DOI] [PubMed] [Google Scholar]
- 117.Lucas A, Morley R, Cole TJ. Randomised trial of early diet in preterm babies and later intelligence quotient. BMJ. 1998;317(7171):1481–1487. doi: 10.1136/bmj.317.7171.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schanler RJ. The use of human milk for premature infants. Pediatr Clin North Am. 2001;48(1):207–219. doi: 10.1016/s0031-3955(05)70295-9. [DOI] [PubMed] [Google Scholar]
- 119.Schanler RJ, Shulman RJ, Lau C. Feeding strategies for premature infants: beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics. 1999;103(6 Pt 1):1150–1157. doi: 10.1542/peds.103.6.1150. [DOI] [PubMed] [Google Scholar]
- 120.Illingworth RS, Lister J. The Critical or Sensitive Period, with Special Reference to Certain Feeding Problems in Infants and Children. J Pediatr. 1964;65:839–848. doi: 10.1016/s0022-3476(64)80006-8. [DOI] [PubMed] [Google Scholar]
- 121.Bernbaum JC, Pereira GR, Watkins JB, Peckham GJ. Nonnutritive sucking during gavage feeding enhances growth and maturation in premature infants. Pediatrics. 1983;71(1):41–45. [PubMed] [Google Scholar]
- 122.Dunbar SB, Jarvis AH, Breyer M. The transition from nonoral to oral feeding in children. Am J Occup Ther. 1991;45(5):402–408. doi: 10.5014/ajot.45.5.402. [DOI] [PubMed] [Google Scholar]
- 123.Field T, Ignatoff E, Stringer S, et al. Nonnutritive sucking during tube feedings: effects on preterm neonates in an intensive care unit. Pediatrics. 1982;70(3):381–384. [PubMed] [Google Scholar]
- 124.Senez C, Guys JM, Mancini J, Paz Paredes A, Lena G, Choux M. Weaning children from tube to oral feeding. Childs Nerv Syst. 1996;12(10):590–594. doi: 10.1007/BF00261653. [DOI] [PubMed] [Google Scholar]
- 125.Ackerman BH, Kasbekar N. Disturbances of taste and smell induced by drugs. Pharmacotherapy. 1997;17(3):482–496. [PubMed] [Google Scholar]
- 126.Deems DA, Doty RL, Settle RG, et al. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch Otolaryngol Head Neck Surg. 1991;117(5):519–528. doi: 10.1001/archotol.1991.01870170065015. [DOI] [PubMed] [Google Scholar]
- 127.Schiffman SS. Taste and smell losses in normal aging and disease. JAMA. 1997;278(16):1357–1362. [PubMed] [Google Scholar]
- 128.Goodspeed RB, Gent JF, Catalanotto FA. Chemosensory dysfunction. Clinical evaluation results from a taste and smell clinic. Postgrad Med. 1987;81(1):251–257. 260. doi: 10.1080/00325481.1987.11699680. [DOI] [PubMed] [Google Scholar]
- 129.Poh CH, Allen L, Malagon I, et al. Riser’s reflux--an eye-opening experience. Neurogastroenterol Motil. 2010;22(4):387–394. doi: 10.1111/j.1365-2982.2009.01446.x. [DOI] [PubMed] [Google Scholar]