Abstract
Background
The Infant Aphakia Treatment Study (IATS) is a randomized trial comparing the treatment of unilateral congenital cataract with primary intraocular lens (IOL) implantation versus aphakic contact lens (CL). The purpose of this study was to compare the outcomes for infants with lens opacity associated with persistent fetal vasculature (PFV) to those without.
Methods
Retrospective subgroup analysis of grating visual acuity at 1 year of age and adverse events up to 1 year after surgery in eyes identified intraoperatively as having evidence of mild PFV from the IATS.
Results
Of 83 infants, 18 (22%: 11 CL, 7 IOL) had PFV. Median logMAR visual acuity was 0.88 for patients with PFV and 0.80 for patients without PFV (P = 0.46). One or more adverse events up to one year after surgery occurred in 12 infants (67%) with PFV and 30 infants (46%) without PFV (P = 0.18). The incidence of adverse events was significantly higher in patients with PFV compared to patients without PFV in the CL group (55% vs 20%, P = 0.049) but not in the IOL group (86% vs 71%, P = 0.65), possibly because all children receiving IOLs had higher rates of adverse events when compared to aphakic children (73% vs 29%, P < 0.001).
Conclusions
Aphakic infants with mild PFV treated with CL had a higher incidence of adverse events following lensectomy compared to children with other forms of unilateral congenital cataract; nevertheless, similar visual outcomes at one year after surgery were obtained.
The Infant Aphakia Treatment Study (IATS) is a randomized, multicenter trial comparing visual outcomes and adverse events after the treatment of aphakia caused by unilateral congenital cataract extraction with primary intraocular lens (IOL) implantation versus contact lens (CL).1 The results at 1 year of age revealed that children receiving CLs and IOLs had similar visual outcomes, although those receiving IOLs had significantly higher rates of adverse events.2
Unilateral congenital cataract is often associated with persistent fetal vasculature (PFV).3–9 Infants with evidence of stretched ciliary processes, severe microphthalmia, or retinal traction were excluded from the IATS since it was felt that visual potential was poor; however, children with unilateral cataract associated with mild forms of PFV were included in the study. Mild PFV was defined as the presence of a retrolental membrane with or without visible vessels or a visible patent or nonpatent hyaloid artery. The inclusion of children with PFV gave rise to the hypothesis that visual and structural outcomes in eyes with mild PFV may differ from those of the overall IATS study group. The purpose of this study was to evaluate the visual outcomes and adverse events of children from the IATS study with PFV-associated cataracts.
Methods
The IATS study was approved by the institutional review boards of all the participating institutions and was in compliance with the Health Insurance Portability and Accountability Act. IATS study eligibility criteria included the presence of a unilateral cataract prior to 7 months of age, cornea diameter greater than 9 mm, and no systemic condition (including severe prematurity) that may be associated with cataract or visual developmental delay. Children with mild PFV, such as retrolental membrane or visible persistent hyaloid artery, were allowed in the study. Cataracts associated with PFV were excluded if the following signs were present: visible ciliary process stretching, corneal diameter <9 mm, or posterior disease with tractional involvement of the optic nerve or retina. These factors were determined at an examination under anesthesia prior to randomization for the study. For all participants, surgical techniques for IOL and CL groups were standardized as were patching and optical correction regimens and follow-up examination procedures.1 Follow-up results for adverse events and additional intraocular operations until 1 year of age were reviewed. Monocular grating visual acuity was measured by 2 traveling examiners, one of whom performed the majority of the assessments (74%) at 1-year of age (±2 months) using Teller Acuity Cards (Stereo Optical, Chicago, IL).1
The design of the IATS has been described elsewhere.1 Briefly, a total of 114 children were enrolled in the study; 83 had surgical videos submitted for review. PFV was defined as the presence of a retrolental membrane with or without visible vessels or a visible patent or nonpatent hyaloid artery. The diagnosis of PFV was based upon video review rather than preoperative clinical examination as it was felt that video evidence would be more accurate in diagnosing fetal remnants when compared to biomicroscopy in infants.
The median visual acuity at one year after surgery was compared between patients with and without PFV using the Wilcoxon rank sum test. A nonparametric test was used on account of the skewed distribution of the data and the assignment of visual acuity values for patients with vision below the level detectable with Teller Acuity Cards.1 The Wilcoxon rank sum test was also used to compare age at surgery between patients with and without PFV. The percentage of patients with grating visual acuity of 1.0 logMAR or better, the percentage who experienced one or more adverse events, and the percentage who underwent additional intraocular operations were compared between patients with and without PFV using the Fisher exact test. The Fisher exact test was used to compare the incidence of PFV between the treatment groups. A 95% confidence interval for percentages was determined using the exact binomial method. A 95% confidence interval for the difference between percentages was calculated using the normal approximation. The agreement of a diagnosis of PFV between the local investigator and the central reviewers was assessed using the kappa statistic. All statistical tests were two-sided. No adjustment was made for multiple testing. P < 0.05 was deemed statistically significant.
Results
Of the 83 children included in the study (CL group, 42; IOL group, 41), 18 (22%; 95% CI, 13%–32%) were determined to have evidence of PFV by video review. The video review diagnosis of the presence or absence of PFV agreed with that of the local investigator in 72 patients (87%; κ = 0.62; 95% CI, 0.41–0.82). Of those that were not in agreement, five without a clinical diagnosis of PFV had evidence of persistent fetal remnants on video review, while six diagnosed by the local investigator did not meet video review criteria for PFV.
The rate of PFV did not differ significantly between the treatment groups (CL, 11/42 [26%]; IOL, 7/41 [17%]; P = 0.43; 95% CI for difference between percentages, −9% to 27%). One child from each of the treatment groups had stretching of the ciliary processes, an exclusion criterion that was unrecognized prior to surgery. As these 2 children had already been randomized, they were included in the IATS outcome results. Median age at surgery was 2.0 months for those with PFV and 1.8 for those without (P = 0.71).
Table 1 compares the rates of intraoperative complications for the PFV and non-PFV groups during initial cataract surgery according to treatment groups. (The specific intraoperative complications are provided in e-Supplement 2, available at jaapos.org). The percentage of patients for whom an intraoperative complication occurred during the initial cataract surgery did not differ between patients with (17%) and without (23%) PFV (P = 0.75). As previously reported,2 intraoperative complications were more frequent in the IOL group; however, within the treatment groups, the occurrence of at least one intraoperative complication was not significantly different between patients with and without PFV.
Table 1.
Occurrence of intraoperative complications during initial cataract surgery according to PFV diagnosis and treatment groups
| Number of patients (%) with intraoperative complicationa |
P valueb | 95% CI for difference between percents |
||
|---|---|---|---|---|
| No PFV | PFV | |||
| Treatment groups combined |
15/65 (23%) | 3/18 (17%) | 0.75 | −14% to −26% |
| Treatment = CL | 5/31 (16%) | 1/11 (9%) | 0.99 | −14% to 28% |
| Treatment = IOL | 10/34 (29%) | 2/7 (29%) | 0.99 | −36% to 38% |
CI, confidence interval; CL, contact lens; IOL, intraocular lens; PFV, persistent fetal vasculature.
The patient had one or more complications during the initial cataract surgery.
Fisher exact test comparing percentages between patients with and without a cataract diagnosis involving PFV.
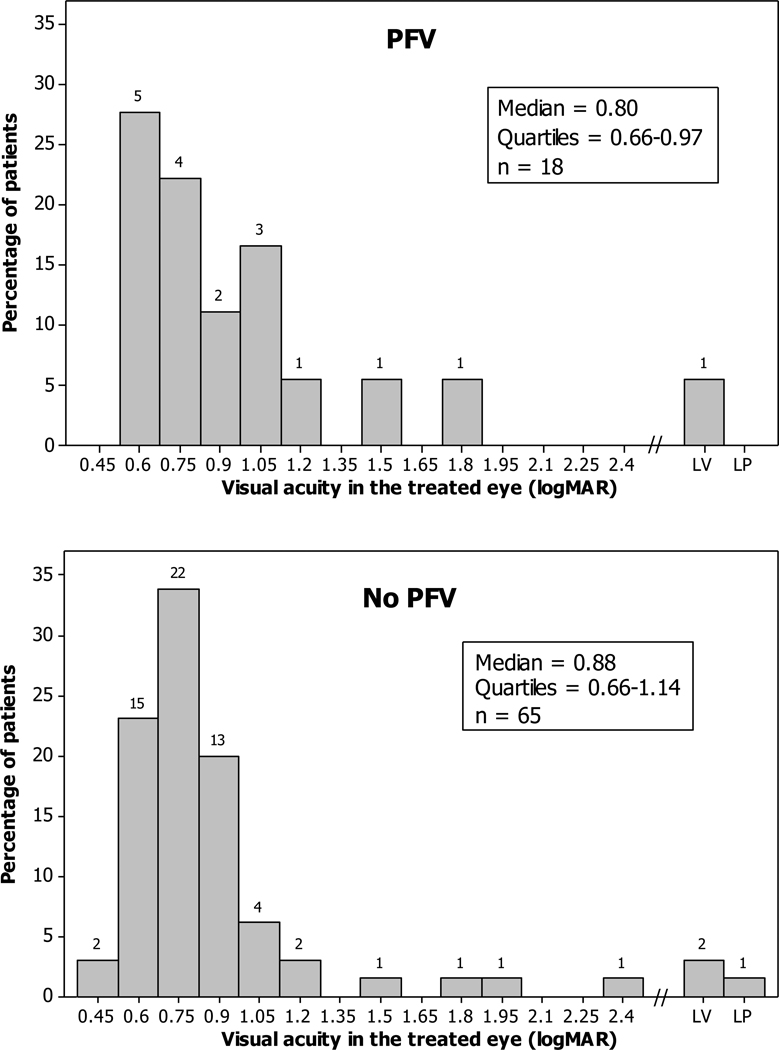
Visual acuity at one year of age did not differ between those with and without PFV. Median logMAR visual acuity was 0.88 in children with PFV and 0.80 in those without PFV (P = 0.46, 95% CI for difference between medians, −0.18 to 0.13 logMAR) (Figure 1). Of 65 children without PFV, 52 (80%) achieved a final logMAR of 1.0 (20/200 Snellen equivalent) or better, whereas 11 of 18 with PFV (61%) achieved this level of visual acuity (P = 0.12, 95% CI for difference between percentages, −6% to 43%).
FIG 1.
Grating visual acuity results at 1-year after surgery.
Table 2 details adverse events reported up to the first year after cataract surgery. In the overall study group, adverse events occurred in the first year after surgery in 67% of children with PFV and in 46% of children without PFV (P = 0.18). This difference was not statistically significant. In the IOL group, 6 of 7 patients with PFV (86%) had an adverse event, whereas 24 of 34 without PFV (71%) had an adverse event (P = 0.65). In the CL group, 6 of 11 children with PFV (55%) had an adverse event, whereas 6 of 31 without PFV (20%) had an adverse event (P = 0.049). The specific adverse events that occurred are provided in e-Supplement 3 (available at jaapos.org) according to treatment group and PFV diagnosis.
Table 2.
Occurrence of adverse events during the first year after initial cataract surgery according to PFV diagnosis and treatment
| Number of patients (%) with adverse eventa |
P valueb | 95% CI for difference between percentages |
||
|---|---|---|---|---|
| No PFV | PFV | |||
| Treatment groups combined |
30/65 (46%) | 12/18 (67%) | 0.18 | −45% to 4% |
| Treatment = CL | 6/31 (19%) | 6/11 (55%) | 0.049 | −68% to −3% |
| Treatment = IOL | 24/34 (71%) | 6/7 (86%) | 0.65 | −45% to 15% |
CI, confidence interval; CL, contact lens; IOL, intraocular lens; PFV, persistent fetal vasculature.
The patient had one or more adverse events during the first year after the initial cataract surgery.
Fisher exact test comparing percentages between patients with and without a cataract diagnosis involving PFV.
Table 3 compares the number of additional intraocular surgeries performed in the PFV and non-PFV groups up to the first year after cataract surgery. There was no significant difference in the percentage of patients with additional ocular surgery up to the first year after surgery in children with PFV compared to children without PFV (35% vs 39%; P = 0.79). Within each of the treatment groups, the occurrence of additional ocular surgery did not differ according to PFV diagnosis.
Table 3.
Occurrence of additional intraocular surgery during the first year after initial cataract surgery according to PFV diagnosis and treatment
| Number of patients (%) with one or more additional intraocular surgeriesa |
P valuea | 95% CI for difference between percentages |
||
|---|---|---|---|---|
| No PFV | PFV | |||
| Treatment groups combined |
23/65 (35%) | 7/18 (39%) | 0.79 | −29% to 22% |
| Treatment = CL | 4/31 (13%) | 3/11 (27%) | 0.35 | −43% to 14% |
| Treatment = IOL | 19/34 (56%) | 4/7 (57%) | 0.99 | −41% to 39% |
CI, confidence interval; CL, contact lens; IOL, intraocular lens PFV, persistent fetal vasculature.
Fisher exact test comparing percentages between patients with and without a cataract diagnosis involving PFV.
Discussion
The IATS study is unique in evaluating pediatric cataract in that it was a prospective, randomized trial with clear inclusion and exclusion criteria that included video documentation of lens morphology at the time of surgery. While the effect of PFV on visual outcome was not a primary outcome measure, much information can be gained from analysis of the data. First, children with mild PFV had similar visual outcomes to children without evidence of PFV. In our study, grating visual acuity at 1 year of age in children treated for unilateral congenital cataract associated with PFV did not differ from visual acuity in children with other forms of unilateral cataract. Mullner-Eidenbock and colleagues9 reported that 100% of children with unilateral cataract showed evidence of minimal fetal vascular remnants and concluded that all unilateral congenital cataracts occur as a result of a mild form of PFV. This hypothesis was not examined in our study. We only analyzed data from infants with evidence of hyaloid remnants that are classically associated with the diagnosis of PFV and not those with other putative forms of fetal remnants.
Many authors have reported their results in the treatment of congenital cataract associated with PFV.10–23 Visual and structural outcome results are highly variable, as are treatment strategies for children with mild or more severe (posterior) forms of PFV. Many children were managed nonsurgically. Best-corrected visual acuity of 20/400 or better has been reported in 20% to 71% of children with PFV after cataract surgery.16–23 A successful visual outcome has generally been limited to children with milder forms of PFV. In 1986 Karr and Scott12 reported 2 patients (of 48) that exhibited a mild form of PFV and had visual outcomes of 20/30 and 20/100. In 1991 Pollard13 reported 48 patients treated for PFV: 17% attained a vision of 20/100 or better, all of whom had a mild, anterior form of PFV. Alexandrakis and colleagues17 reported 30 children with PFV that underwent vitreoretinal surgery. In that series, 5 aphakic children had vision of 20/80 or better.17 In our study, the median visual acuity outcome was logMAR 0.88 (20/150 Snellen equivalent); this was not statistically different from the visual acuity outcome for eyes without PFV.
Very few authors have reported the use of IOLs in children with PFV.18,19,21 Anteby and colleauges18 reported the largest series with 30 pseudophakic eyes. Visual acuity of 20/50 or better was reported in 12.6% of all study patients (pseudophakic and aphakic); however, Anteby and colleagues18 reported a lower rate of no light perception vision in children with pseudophakia, despite similar rates of glaucoma and secondary reproliferation of lens cortex (31%) between pseudophakic and aphakic children. Since their study was not a randomized trial, it is likely that there was a selection bias for children with milder forms of PFV to receive an IOL. Additionally, the age at surgery was 1.4 years, as opposed to 2 months in our group. Early implantation of an IOL in a child with anterior PFV yielded no visual benefit at one year in our study, which does, however, demonstrate that early implantation is technically feasible. We should emphasize that IATS study data is only applicable to mild forms of anterior PFV.
Finally, PFV was associated with an increased risk of adverse events in children treated with contact lenses. In the primary IATS outcome report,2 additional intraocular surgeries were higher in the IOL group (12% in the CTL group vs 63% in the IOL group, P < 0.001). The presence of PFV did not significantly increase the amount of additional intraocular surgeries in children who received an IOL; however, the presence of PFV did increase the chance of adverse events occurring in children with aphakia. Infants in the CL group had the posterior capsulotomy and vitrectomy performed from an anterior (limbal) approach, whereas a pars plana approach was used for this part of the procedure in children receiving IOLs. An anterior surgical approach may be less effective in removing PFV remnants; however, Sisk and colleagues23 have reported that limbal versus pars plana incision did not have a statistically significant effect on final visual acuity or complication rate in their patients with PFV. Alternatively, it is possible that the removal of hyaloid remnants releases red blood cells into the anterior vitreous and therefore causes greater postoperative inflammation. Since adverse events were relatively uncommon in aphakic children without PFV, this putative increase in inflammation may have caused more adverse events in children with PFV. The lack of a significant difference in the IOL group was likely due to the fact that all children receiving an IOL had more postoperative inflammation; thus factors other than PFV may have played a more prominent role in the occurrence of adverse events.
Our study has several limitations. The IATS was not powered to study differing causes of unilateral, infantile cataract; therefore, the difference between patients with and without PFV would have to be quite large for this post hoc analysis to result in a statistically significant finding. For example, if the true answer were that 50% of patients with PFV have an adverse event, then the true percentage of patients without PFV having the adverse event would have to be 17% (33% lower) for the current study, with 18 patients with PFV and 65 patients without PFV to have 80% power. Documentation of PFV was made by the presence of specific criteria on video review of surgery rather than by local investigator diagnosis, which may not include all factors associated with PFV. Grating acuity in a 1-year-old patient measures resolution acuity and may not accurately access recognition acuity. Additionally, the long-term effects of each form of visual correction (IOL vs CL) is ongoing, and results at one year of age may differ from those obtained when optotype visual acuity testing is possible. Finally, our results only apply to the limited inclusion criteria of our study and are not applicable to other forms of PFV outside IATS inclusion criteria.
In conclusion, the presence of mild PFV did not appear to alter the visual acuity outcome for unilateral congenital cataracts at 1 year of age. Postoperative complications were more common in eyes with PFV left aphakic and equally common when an IOL was implanted compared to eyes with no evidence of PFV. Surgeons should be aware of the relatively higher likelihood of postoperative adverse events in aphakic eyes with anterior PFV.
Supplementary Material
Acknowledgments
This study was supported by grants U10 EY13272 and U10 EY013287 from the National Institutes of Health and in part by an unrestricted grant from Research to Prevent Blindness, Inc., New York, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Infant Aphakia Treatment Study Group. Lambert SR, Buckley EG, Drews-Botsch C, et al. The infant aphakia treatment study: Design and clinical measures at enrollment. Arch Ophthalmol. 2010;128:21–27. doi: 10.1001/archophthalmol.2009.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Infant Aphakia Treatment Study Group. Lambert SR, Buckley EG, Drews-Botsch C, et al. A randomized clinical trial comparing contact lens with intraocular lens correction of monocular aphakia during infancy: Grating acuity and adverse events at age 1 year. Arch Ophthalmol. 2010;128:810–818. doi: 10.1001/archophthalmol.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reese AB. Persistent hyperplastic primary vitreous. The Edward Jackson Memorial Lecture. Am J Ophthalmol. 1955;40:317–331. doi: 10.1016/0002-9394(55)91866-3. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd IC, Goss-Sampson M, Jeffrey BG, et al. Neonatal cataract: Aetiology, pathogenesis, and management. Eye. 1992;6:184–196. doi: 10.1038/eye.1992.37. [DOI] [PubMed] [Google Scholar]
- 5.Lambert SR, Drack AV. Infantile cataracts. Surv Ophthalmol. 1996;40:427–458. doi: 10.1016/s0039-6257(96)82011-x. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg MF. Persistent fetal vasculature (PFV): An integrated interpretation of signs and symptoms associated with persistent hyperplastic primary vitreous (PHPV). The Edward Jackson Memorial Lecture. Am J Ophthalmol. 1997;124:587–626. doi: 10.1016/s0002-9394(14)70899-2. [DOI] [PubMed] [Google Scholar]
- 7.Rahi JS, Dezateux C. British Congenital Cataract Interest Group. Congenital and infantile cataract in the United Kingdom: Underlying or associated factors. Invest Ophthalmol Vis Sci. 2000;41:2108–2114. [PubMed] [Google Scholar]
- 8.Wirth MG, Russell-Eggitt IM, Craig JE, et al. Aetiology of congenital and paediatric cataract in an Australian population. Br J Ophthalmol. 2002;86:782–786. doi: 10.1136/bjo.86.7.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mullner-Eidenbock A, Amon M, Moser E, Klebermass N. Persistent fetal vasculature and minimal fetal remnants: A frequent cause of unilateral congenital cataracts. Ophthalmology. 2004;111:906–913. doi: 10.1016/j.ophtha.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Federman JL, Shields JA, Altman B, Koller H. The surgical and nonsurgical management of persistent hyperplastic primary vitreous. Ophthalmology. 1982;89:20–24. doi: 10.1016/s0161-6420(82)34854-x. [DOI] [PubMed] [Google Scholar]
- 11.Stark WJ, Lindsey PS, Fagadau WR, Michels RG. Persistent hyperplastic primary vitreous: Surgical treatment. Ophthalmology. 1983;90:452–457. doi: 10.1016/s0161-6420(83)34531-0. [DOI] [PubMed] [Google Scholar]
- 12.Karr DJ, Scott WE. Visual acuity results following treatment of persistent hyperplastic primary vitreous. Arch Ophthalmol. 1986;104:662–667. doi: 10.1001/archopht.1986.01050170052020. [DOI] [PubMed] [Google Scholar]
- 13.Pollard ZF. Results of treatment of persistent hyperplastic primary vitreous. Ophthalmic Surg. 1991;22:48–52. [PubMed] [Google Scholar]
- 14.Cheung JC, Summers CG, Young TL. Myopia predicts better outcome in persistent hyperplastic primary vitreous. J Pediatr Ophthalmol Strabismus. 1997;34:170–176. doi: 10.3928/0191-3913-19970501-08. [DOI] [PubMed] [Google Scholar]
- 15.Mittra RA, Huynh LT, Ruttum MS, et al. Visual outcomes following lensectomy and vitrectomy for combined anterior and posterior persistent hyperplastic primary vitreous. Arch Ophthalmol. 1998;116:1190–1194. doi: 10.1001/archopht.116.9.1190. [DOI] [PubMed] [Google Scholar]
- 16.Dass AB, Trese MT. Surgical results of persistent hyperplastic primary vitreous. Ophthalmology. 1999;106:280–284. doi: 10.1016/S0161-6420(99)90066-0. [DOI] [PubMed] [Google Scholar]
- 17.Alexandrakis G, Scott IU, Flynn HW, et al. Visual acuity outcomes with and without surgery in patients with persistent fetal vasculature. Ophthalmology. 2000;107:1068–1072. doi: 10.1016/s0161-6420(00)00100-7. [DOI] [PubMed] [Google Scholar]
- 18.Anteby I, Cohen E, Karshai I, BenEzra D. Unilateral persistent hyperplastic primary vitreous: Course and outcome. J AAPOS. 2002;6:92–99. doi: 10.1067/mpa.2002.121324. [DOI] [PubMed] [Google Scholar]
- 19.Soheilian M, Vistamehr S, Rahmani B, et al. Outcomes of surgical (pars plicata and limbal lensectomy, vitrectomy) and nonsurgical management of persistent fetal vasculature (PFV): An analysis of 54 eyes. Eur J Ophthalmol. 2002;12:523–533. doi: 10.1177/112067210201200613. [DOI] [PubMed] [Google Scholar]
- 20.Cheng LS, Kuo HK, Lin SA, Kuo ML. Surgical results of persistent fetal vasculature. Chang Gung Med J. 2004;27:602–608. [PubMed] [Google Scholar]
- 21.Hunt A, Rowe N, Lam A, Martin F. Outcomes in persistent hyperplastic primary vitreous. Br J Ophthalmol. 2005;89:859–863. doi: 10.1136/bjo.2004.053595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen YC, Hu AC, Rosenbaum A, et al. Long-term results of early contact lens use in pediatric unilateral aphakia. Eye Contact Lens. 2010;36:19–25. doi: 10.1097/ICL.0b013e3181c6dfdc. [DOI] [PubMed] [Google Scholar]
- 23.Sisk RA, Berrocal AM, Feuer WJ, Murray TG. Visual and anatomic outcomes with or without surgery in persistent fetal vasculature. Ophthalmology. 2010;117:2178–2183. doi: 10.1016/j.ophtha.2010.03.062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.