Abstract
Introduction
Mineral Trioxide aggregate (MTA) is routinely used for pulp capping procedures. However, little is known about its direct interaction with the cells or whether MTA is capable of releasing soluble factors that could help in differentiating cells. There have been no previous studies demonstrating this aspect of MTA. Hence the aim of this study was to determine whether direct contact of the cells with MTA was necessary to help differentiate the pulp cells into odontoblast like cells.
Methods
Human dental pulp cells (DPCs) were cultured on Grey MTA, either in direct contact or away from the cells on a cell culture insert, and the levels of gene expression, secretion of Vascular Endothelial Growth Factor (VEGF) and the rates of cell proliferation were analyzed.
Results
MTA when placed in direct contact with the cells promoted upregulated the expression of important odontoblastic genes like Osteocalcin (OCN) and Dentin Sialoprotein (DSP), thereby demonstrating that direct contact of the cells with the MTA is necessary to promote differentiation of the pulp cells into odontoblast like cells which in turn are responsible for dentin bridge formation. MTA also induced an increase in secretion of VEGF when placed in direct contact with the cells.
Conclusion
Overall our studies support the fact that direct contact of the cells with the MTA is necessary to help differentiate them into odontoblast like cells which in turn will lead to a successful treatment outcome.
Keywords: Direct contact, MTA, Dental pulp stromal cells, odontoblasts
Introduction
The field of endodontics has evolved tremendously over the past decade. Advances in techniques and materials have lead to increased success rates of many procedures including pulp capping. Pulp capping is indicated for teeth that have had a pulp exposure following trauma or injury, which could include the process of caries excavation in developing or mature teeth. It can offer an alternative to root canal therapy when pulp is exposed with reversible injury or without signs of inflammation thereby offering a more conservative approach. Ultimately, the goal of treating the exposed pulp with an appropriate pulp capping material is to promote the dentinogenic potential of the pulpal cells (1). Historically, many different materials have been used for pulp capping which include resin-modified glass ionomer cements, tri-calcium phosphates, hydrophilic resins and calcium hydroxide. The success of different pulp capping materials have been measured by the thickness of the dentinal bridge, the morphology of the dentinal bridge, the intensity of pulpal inflammation, presence of odontoblasts cells, and biocompatibility. Calcium hydroxide has been considered the gold standard for pulp capping however previous research has shown that it is not ideally suited for this procedure (2). One of the more recent materials developed, Mineral Trioxide Aggregate (MTA), has drawn much interest due to its numerous applications. MTA has demonstrated significantly greater frequency of dentin bridge formation, thicker and less porous dentin, and less pulp inflammation compared to calcium hydroxide (2-5). MTA has also been shown to induce the recruitment and proliferation of undifferentiated cells to form a dentinal bridge, while reducing inflammation compared to calcium hydroxide (2). Other research has shown that MTA when placed in direct contact with the human dental pulp cells (DPCs) differentiated them into odontoblast like cells (6). However, little is known about the importance of this direct interaction with the cells. There have been no previous studies demonstrating whether MTA needs to be in direct contact with the pulp cells or whether it is capable of secreting any soluble substances that could exert the same effects on the pulp. We believe this is an important aspect during the process of direct pulp capping. Hence, the aim of this study was to compare the effectiveness of MTA when placed in direct contact with the DPCs versus the effectiveness of MTA when placed over a membrane (cell culture insert) that is in contact with the DPCs. This will help to determine if direct contact of the cells with MTA is necessary or if MTA is capable of releasing soluble factors which in turn would help in differentiating the DPCs.
Materials and Methods
Cell Culture
Human DPCs were obtained from the Somerman lab and maintained as previously described (6). The cells were originally derived from extracted human 3rd molars and those obtained from the lab for this study were from passage 2. The cells were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS) and penicillin, streptomycin, and L-glutamine (100 units/mL, 100 μg/mL, and 2 mM, respectively). Cells were incubated at 37°C in an atmosphere of 5% CO2.
DPCs for the experiments were cultured in 12-well plates in various conditions and analyzed at day 1, 4 and 7. Group 1 = control (DPCs were grown on a culture dish); Group 2= DPCs were grown on the set MTA (so the cells will be in direct contact with the MTA). Group 3= MTA was placed in a cell culture insert (BD Falcon, Franklin Lakes, NJ) and placed over the DPCs (so that they were not in direct contact with each other) as shown in figure 1.
Figure 1.
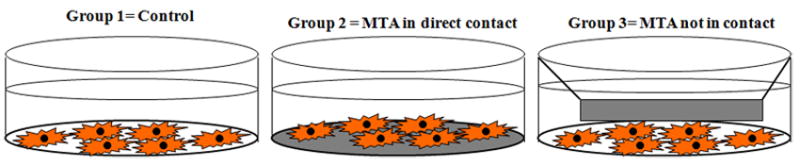
DPCs were cultured in 12-well plates under various: Group 1 = control (DPCs grown on a culture dish); Group 2 = DPCs was grown on the set MTA (so the cells will be in direct contact with the MTA). Group 3 = MTA was placed in a cell culture insert and placed over the DPCs (so that they were not in direct contact with each other)
Preparation of MTA
ProRoot Grey MTA (Tulsa Dental, Tulsa, Oklahoma) was mixed according to manufacturer’s instructions and plated in 12 well plates (Fisher Scientific, Pittsburg, PA) or in tissue culture dish inserts with a pore diameter of 0.4μm (BD Falcon, Franklin Lakes, NJ) as shown in figure 1. MTA was left to set for 48 hours at 37°C in a humidified 5% CO2, 95% air atmosphere after which the dental pulp cells were cultured as mentioned above.
Cell Proliferation
DPCs were cultured under the various conditions as stated previously in 12 well plates at a concentration of 2 × 104 cells/well in 1 mL of media. The proliferation rates were analyzed at days 1, 4 and 7 using the WST-1 Cell Proliferation Assay Kit from Millipore (Billerica, MA) according to the manufacturer’s recommendations. Samples were run in triplicates. In a separate set of experiments the number of cells was determined using a Cell Counting hemocytometer under a 50X magnification at the same time points.
RNA extraction
Human DPCs were plated in 12 well plates at a concentration of 1 × 105 cells/well and maintained in DMEM (Invitrogen; Carlsbad, CA) with 10% Fetal Bovine Serum (FBS). Cells were cultured under the above mentioned conditions for 1, 4 and 7 days. Total RNA was extracted using RNAease Micro Kit from Qiagen (Valencia, CA) at the various time points. The RNA was treated with DNase I (Qiagen, Valencia, CA) to ensure removal of the genomic DNA. The quality of RNA was measured using a Eppendorf biophotometer (A260/A280 ratio) (Fischer Scientific Inc., Pittsburg PA).
Real-time Reverse-Transcriptase Polymerase Chain Reaction (Real time PCR)
RNA was DNase-treated as mentioned above and cDNA was synthesized from 1.0 μg total RNA with a cDNA synthesis kit for RT-PCR (Roche Diagnostic, Indianapolis, IN). 2 μL of the resulting cDNA product were used per 20 μL reaction in the Lightcycler system (Roche Diagnostics, Mannheim, Germany). PCRs were carried out with the DNA Master SYBR Green I kit (Roche Diagnostic, Indianapolis, IN), with a total volume of 20 μL. Expression was analyzed for genes including Dentin Sialoprotein (DSP) and Ostecalcin (OCN) with Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) serving as a housekeeping/reference gene for normalization. Primer sequences have been stated previously (7). Relative quantification of PCR products was achieved by using LightCycler Relative Quantification Software, version 1.0 (Roche Diagnostics, Germany) to compare amplification of the target gene of interest to that of GAPDH, the reference gene (7).
ELISA
Cell supernatants were analyzed for the presence of Vascular Endothelial Growth Factor (VEGF). Human Quantikine ELISA kit for measuring VEGF was purchased from R&D Systems (Minneapolis, MN, USA). 200 μl of each sample was analyzed and the kit was used according to the manufacturer’s recommendations.
Statistical analysis
All experiments were performed in triplicates. Each value represents the mean ± standard deviation (SD). SigmaPlot 11.0 (Systat Software, Inc. San Jose, CA) was used for all the statistical testing. Data from proliferation, ELISA and RT-PCR experiments were analyzed by one-way analysis of variance (ANOVA) to determine differences among treatments, with further pairwise multiple comparisons made with the Holm Sidak test. Differences with p values (*) < 0.05 were considered significant.
Results
MTA when in contact with the DPCs did not induce significant proliferation over the other groups
Proliferation rates were measured at days 1, 4 and 7 as described in Materials and Methods. MTA did not induce any significant increase in proliferation of DPCs between groups 1, 2 and 3 (Figure 2A). These results were verified using WST-1 proliferation assay kit. The results obtained using the WST-1 proliferation assay for groups 2 and 3 are presented as percentages of the control group (group 1) for each time point (Figure 2B).
Figure 2.
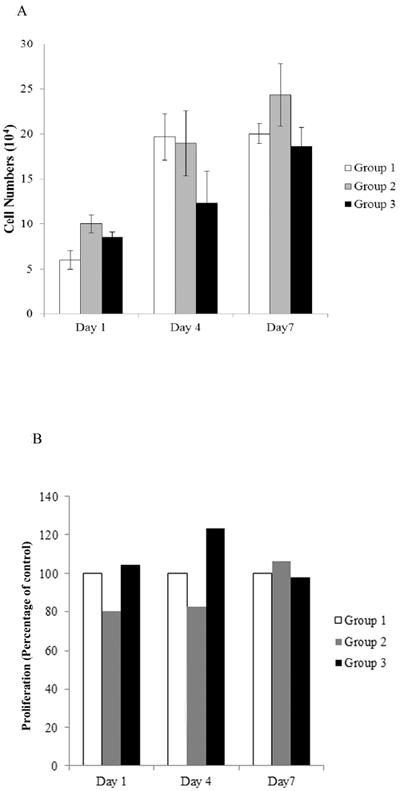
A) Cells were counted at the days 1, 4, 7 using a hemocytometer. No statistical differences (p > 0.05) were seen between the groups. B) Measurement of proliferation of DPCs was assayed at days 1, 4 and 7 using the WST-1 proliferation assay. The results are presented as percentage of the control group which was considered at 100% for each time point. There was no statistical difference (p > 0.05) in DPC proliferation between the groups.
MTA increased VEGF secretion in human DPCs when in direct contact
Cells were grown under the different conditions as stated in the Materials and Methods section. The supernatants were analyzed at 24 hours for VEGF secretion. As can be seen from Figure 3 the DPCs in direct contact with MTA (Group 2) secreted significantly more VEGF that was about 60% higher that of DPCs separated from MTA by the membrane (Group 3) and about 30% higher that of the baseline control group (Group 1) at day 1. The differences between groups 2 and 3 and groups 1 and 2 were statistically significant.
Figure 3.
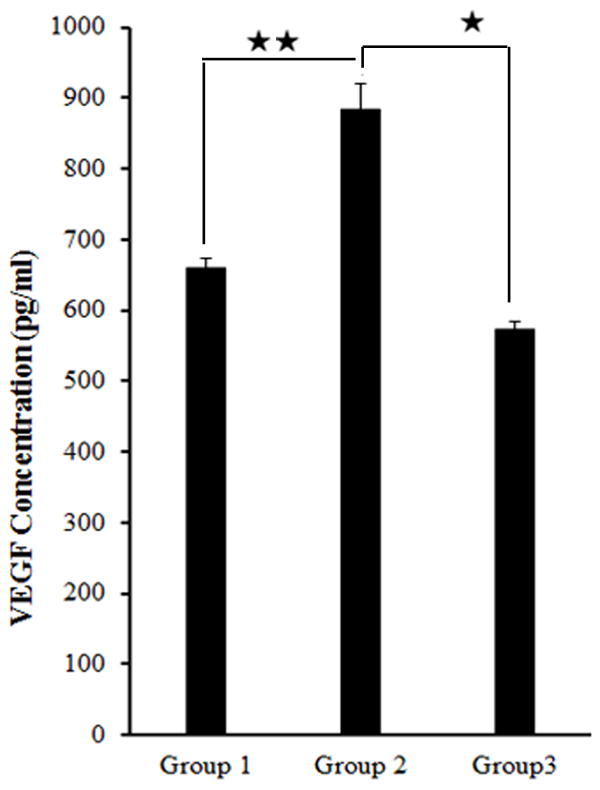
Human DPCs (2 × 105/ml) were cultured under the different conditions as seen in the figure and after an overnight incubation the supernatants were removed and assayed for VEGF secretion using a sensitive and specific ELISA. As can be seen the level of VEGF secretion of Group 2 was about 60% higher that of Group 3 and about 30% higher than that of Group 1 at day 1. One of two independent experiments is shown here. Results represent mean ± SD of triplicates. Statistically significant differences were seen between Groups 2 and 3 (*), and between Groups 2 and 1 (**) (p < 0.05), while there was no statistical difference between Groups 3 and 1 (p > 0.05).
MTA only when in direct contact with the DPCs induced osteo/dentinogenic gene expression in human pulp cells
Figures 4A and 4B demonstrate that the gene expression levels of DSP and OCN respectively, were significantly higher for group 2 when the cells were in contact with the MTA as compared to those of group 3, where the cells were not in direct contact with the MTA. There were no statistical differences between groups 1 and 3. The differences between groups 2 and 3 at day 7 for both genes were statistical significant. Results were normalized to GAPDH. It is interesting to note that the levels of DSP for Group 3 were lower than group 1 at days 1 and 4 and lower at all three time points for OCN as compared to group 1.
Figure 4.
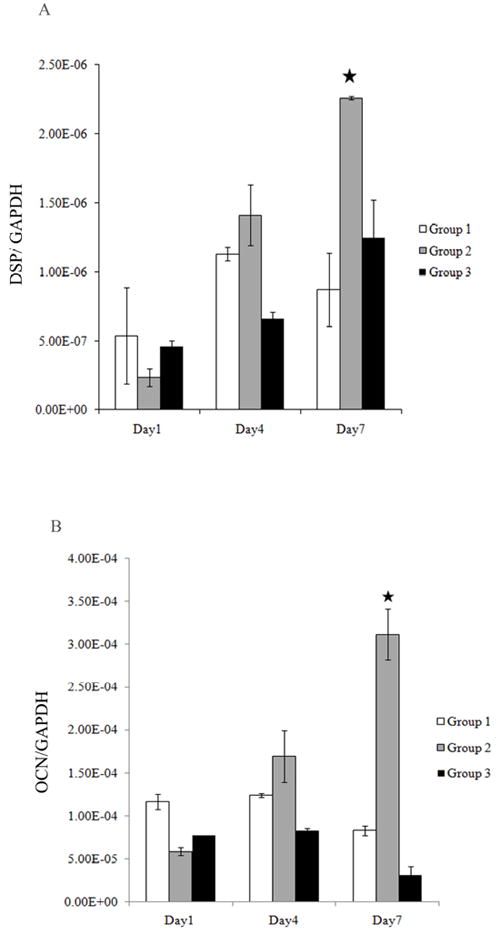
Human DPCs (5 × 105/ml) were cultured in 1 ml of media. The levels of DSP (Fig 4A) and OCN (Fig 4B) gene induction were determined by Real time RT-PCR at 3 different time points (day 1, 4, 7). Results were normalized to GAPDH as a reference gene. Results represent mean ± SD of quadruplicates. Cells in contact with the MTA (group 2) showed a significant upregulation of DSP and OCN at day 7 as compared to the cells that were not in contact with the MTA (group 3). The differences between the Group 2 and 3 for DSP and OCN at day 7 (*) were statistically significant (p < 0.05). One of three independent experiments is shown in this figure.
Discussion
MTA has been shown to be extremely effective in a number of different procedures which include pulp capping (8-10), perforation repair (2, 11), apexification (12), root-end filling (13, 14) and revascularization (15). In-vitro experiments have demonstrated that MTA upregulated the expression of type I collagen and OCN in osteoblasts after 24 hours (16). Other research studies have shown that MTA stimulates the proliferation of cementoblasts, fibroblasts, and osteoblasts (17). MTA has also been shown to permit cementoblast attachment and growth as well as the production of mineralized matrix gene and protein expression (18). Furthermore moisture or blood contamination does not affect the setting of MTA (19).
There have been numerous publications demonstrating the effectiveness of MTA as a pulp capping agent (20, 21). However, no previous studies have looked at the effects of direct contact of MTA versus placing it away from the cells. Hence, the primary goal of this study was to demonstrate how MTA works optimally and whether it was capable of releasing factors that could potentially pass through a membrane and in turn activate the DPCs or whether the MTA needed to be in direct contact with the cells for this purpose. Hence, we decided to use the tissue culture inserts with pore sizes of 0.4 μm which is about 100 times larger than those of a collagen membrane. By using a larger pore size we wanted to establish our results under the best case scenario.
There have been previous studies that have shown the differences between grey and white MTA (22, 23). Hence based on previous evidence, research studies (6) and taking into consideration that GMTA is used more often clinically we decided to use GMTA in our experiments.
After culturing the cells under the various conditions the rates of cellular proliferation and the levels of VEGF secretion were analyzed. Human DPCs secrete VEGF (24, 25). VEGF is a potent inducer of angiogenesis, vascular permeability, and edema and has been implicated in the regulation of dentin and dental pulp repair (24) and disruptions in the regulation of this angiogenic response have been correlated with delayed healing of wounds (26). Furthermore, the secreted levels of VEGF are closely correlated with the viability and functional competency of DPCs (24) which is why we chose to analyze this angiogenic factor. As can be seen in figure 3, the levels of VEGF were significantly increased when the DPCs that were in direct contact with the MTA (group 2) even though the proliferation rates were not significantly affected at day 1 (Fig 2A and 2B). Thus these results demonstrated that MTA when in contact activates the cells towards pulpal repair.
Next we looked at gene expression levels of the DPCs cultured under the various conditions. The expressions of OCN and DSP (6) were analyzed since previous research has shown that odontoblasts express specific proteins such as DSP and OCN (24, 27). Furthermore, DSP is a tooth specific protein expressed by odontoblasts cells (28). The distribution of DSP in the collagen matrix of forming dentin suggests that it plays an important role in regulation of mineral deposition (28). It also serves as a marker for reparative dentin (29). Furthermore, DSP has been shown to be an indication of functioning odontoblasts (30). As can be seen in figure 4A and 4B, the cells from Group 2 upregulated the levels OCN and DSP. These levels were significantly different from those of Group 3 at day 7. Upregulation of these genes correlates well with the differentiation of the human DPCs into odontoblast-like cells (24, 31). Furthermore, the differences between groups 1 and 3 were not statistically different. In fact gene expression of cells from group 3 were lower than those of group 1 at some time points suggestive that placing a membrane could possibly decrease activation of the DPCs. However this incidental finding was not investigated since the main aim of this study was to delineate the effects of MTA in contact and away from the cells. The effects of a membrane would need to be further investigated.
Based on the results obtained from this study, it is reasonable to suggest that when the DPCs are placed in direct contact with MTA they demonstrated higher levels of activation which in turn could translate into more effective pulpal repair and faster and more predictable formation of reparative dentin. We believe that this aspect of MTA is particularly important during the process of direct pulp capping. Overall the data presented in this study underscores the significance of MTA in direct contact with cells and would help practitioners to use this material in the optimal manner which in turn can lead to more successful treatment outcomes.
Acknowledgments
This research was supported by NIH/NIDCR Grant No. T32 DE007132.
We would also like to thank Tulsa Dental for providing us with the materials necessary for this study.
Footnotes
I affirm that I have no financial affiliation (e.g., employment, direct payment, stock holdings, retainers, consultantships, patent licensing arrangements or honoraria), or involvement with any commercial organization with direct financial interest in the subject or materials discussed in this manuscript, nor have any such arrangements existed in the past three years. Any other potential conflict of interest is disclosed.
The authors deny any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schroder U. Effects of calcium hydroxide-containing pulp-capping agents on pulp cell migration, proliferation, and differentiation. J Dent Res. 1985;64(Spec No):541–8. doi: 10.1177/002203458506400407. [DOI] [PubMed] [Google Scholar]
- 2.Holland R, Filho JA, de Souza V, Nery MJ, Bernabe PF, Junior ED. Mineral trioxide aggregate repair of lateral root perforations. J Endod. 2001;27:281–4. doi: 10.1097/00004770-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Nair PN, Duncan HF, Pitt Ford TR, Luder HU. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with Mineral Trioxide Aggregate: a randomized controlled trial. 2008. Int Endod J. 2009;42:422–44. doi: 10.1111/j.1365-2591.2009.01558.x. [DOI] [PubMed] [Google Scholar]
- 4.Ford TR, Torabinejad M, McKendry DJ, Hong CU, Kariyawasam SP. Use of mineral trioxide aggregate for repair of furcal perforations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:756–63. doi: 10.1016/s1079-2104(05)80313-0. [DOI] [PubMed] [Google Scholar]
- 5.Ford TR, Torabinejad M, Abedi HR, Bakland LK, Kariyawasam SP. Using mineral trioxide aggregate as a pulp-capping material. J Am Dent Assoc. 1996;127:1491–4. doi: 10.14219/jada.archive.1996.0058. [DOI] [PubMed] [Google Scholar]
- 6.Paranjpe A, Zhang H, Johnson JD. Effects of mineral trioxide aggregate on human dental pulp cells after pulp-capping procedures. J Endod. 2010;36:1042–7. doi: 10.1016/j.joen.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Foster BL, Nociti FH, Jr, Swanson EC, Matsa-Dunn D, Berry JE, Cupp CJ, et al. Regulation of cementoblast gene expression by inorganic phosphate in vitro. Calcif Tissue Int. 2006;78:103–12. doi: 10.1007/s00223-005-0184-7. [DOI] [PubMed] [Google Scholar]
- 8.Accorinte Mde L, Holland R, Reis A, Bortoluzzi MC, Murata SS, Dezan E, Jr, et al. Evaluation of mineral trioxide aggregate and calcium hydroxide cement as pulp-capping agents in human teeth. J Endod. 2008;34:1–6. doi: 10.1016/j.joen.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 9.de Souza Costa CA, Duarte PT, de Souza PP, Giro EM, Hebling J. Cytotoxic effects and pulpal response caused by a mineral trioxide aggregate formulation and calcium hydroxide. Am J Dent. 2008;21:255–61. [PubMed] [Google Scholar]
- 10.Nair PN, Duncan HF, Pitt Ford TR, Luder HU. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with Mineral Trioxide Aggregate: a randomized controlled trial. Int Endod J. 2009;42:422–44. doi: 10.1111/j.1365-2591.2009.01558.x. discussion 1. [DOI] [PubMed] [Google Scholar]
- 11.Arens DE, Torabinejad M. Repair of furcal perforations with mineral trioxide aggregate: two case reports. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:84–8. doi: 10.1016/s1079-2104(96)80382-9. [DOI] [PubMed] [Google Scholar]
- 12.Felippe WT, Felippe MC, Rocha MJ. The effect of mineral trioxide aggregate on the apexification and periapical healing of teeth with incomplete root formation. Int Endod J. 2006;39:2–9. doi: 10.1111/j.1365-2591.2005.01037.x. [DOI] [PubMed] [Google Scholar]
- 13.Torabinejad M, Rastegar AF, Kettering JD, Pitt Ford TR. Bacterial leakage of mineral trioxide aggregate as a root-end filling material. J Endod. 1995;21:109–12. doi: 10.1016/s0099-2399(06)80433-4. [DOI] [PubMed] [Google Scholar]
- 14.Torabinejad M, Watson TF, Pitt Ford TR. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J Endod. 1993;19:591–5. doi: 10.1016/S0099-2399(06)80271-2. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds K, Johnson JD, Cohenca N. Pulp revascularization of necrotic bilateral bicuspids using a modified novel technique to eliminate potential coronal discolouration: a case report. Int Endod J. 2009;42:84–92. doi: 10.1111/j.1365-2591.2008.01467.x. [DOI] [PubMed] [Google Scholar]
- 16.Tani-Ishii N, Hamada N, Watanabe K, Tujimoto Y, Teranaka T, Umemoto T. Expression of bone extracellular matrix proteins on osteoblast cells in the presence of mineral trioxide. J Endod. 2007;33:836–9. doi: 10.1016/j.joen.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Hakki SS, Bozkurt SB, Hakki EE, Belli S. Effects of mineral trioxide aggregate on cell survival, gene expression associated with mineralized tissues, and biomineralization of cementoblasts. J Endod. 2009;35:513–9. doi: 10.1016/j.joen.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Thomson TS, Berry JE, Somerman MJ, Kirkwood KL. Cementoblasts maintain expression of osteocalcin in the presence of mineral trioxide aggregate. J Endod. 2003;29:407–12. doi: 10.1097/00004770-200306000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review--Part III: Clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36:400–13. doi: 10.1016/j.joen.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Mente J, Geletneky B, Ohle M, Koch MJ, Friedrich Ding PG, Wolff D, et al. Mineral trioxide aggregate or calcium hydroxide direct pulp capping: an analysis of the clinical treatment outcome. J Endod. 2010;36:806–13. doi: 10.1016/j.joen.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 21.Reston EG, de Souza Costa CA. Scanning electron microscopy evaluation of the hard tissue barrier after pulp capping with calcium hydroxide, mineral trioxide aggregate (MTA) or ProRoot MTA. Aust Endod J. 2009;35:78–84. doi: 10.1111/j.1747-4477.2008.00131.x. [DOI] [PubMed] [Google Scholar]
- 22.Matt GD, Thorpe JR, Strother JM, McClanahan SB. Comparative study of white and gray mineral trioxide aggregate (MTA) simulating a one- or two-step apical barrier technique. J Endod. 2004;30:876–9. doi: 10.1097/01.don.0000136213.93171.45. [DOI] [PubMed] [Google Scholar]
- 23.Stefopoulos S, Tsatsas DV, Kerezoudis NP, Eliades G. Comparative in vitro study of the sealing efficiency of white vs grey ProRoot mineral trioxide aggregate formulas as apical barriers. Dent Traumatol. 2008;24:207–13. doi: 10.1111/j.1600-9657.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- 24.Paranjpe A, Cacalano NA, Hume WR, Jewett A. N-acetylcysteine protects dental pulp stromal cells from HEMA-induced apoptosis by inducing differentiation of the cells. Free Radic Biol Med. 2007;43:1394–408. doi: 10.1016/j.freeradbiomed.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mantellini MG, Botero T, Yaman P, Dennison JB, Hanks CT, Nor JE. Adhesive resin and the hydrophilic monomer HEMA induce VEGF expression on dental pulp cells and macrophages. Dent Mater. 2006;22:434–40. doi: 10.1016/j.dental.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 26.DiPietro LA, Nissen NN, Gamelli RL, Koch AE, Pyle JM, Polverini PJ. Thrombospondin 1 synthesis and function in wound repair. Am J Pathol. 1996;148:1851–60. [PMC free article] [PubMed] [Google Scholar]
- 27.Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166–71. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butler WT. Dentin matrix proteins. Eur J Oral Sci. 1998;106(Suppl 1):204–10. doi: 10.1111/j.1600-0722.1998.tb02177.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee YL, Liu J, Clarkson BH, Lin CP, Godovikova V, Ritchie HH. Dentin-pulp complex responses to carious lesions. Caries Res. 2006;40:256–64. doi: 10.1159/000092235. [DOI] [PubMed] [Google Scholar]
- 30.D’Souza RN, Bachman T, Baumgardner KR, Butler WT, Litz M. Characterization of cellular responses involved in reparative dentinogenesis in rat molars. J Dent Res. 1995;74:702–9. doi: 10.1177/00220345950740021301. [DOI] [PubMed] [Google Scholar]
- 31.Alliot-Licht B, Bluteau G, Magne D, Lopez-Cazaux S, Lieubeau B, Daculsi G, et al. Dexamethasone stimulates differentiation of odontoblast-like cells in human dental pulp cultures. Cell Tissue Res. 2005;321:391–400. doi: 10.1007/s00441-005-1115-7. [DOI] [PubMed] [Google Scholar]


