Abstract
We have evaluated the serum response to seasonal influenza vaccination in subjects of different ages and associated this with the specific B cell response to the vaccine in vitro. Although the serum response has previously been shown to decrease with age, this has largely been associated to decreased T cell functions. Our results show that in response to the vaccine, the specific response of B cells in vitro, as measured by AID (activation-induced cytidine deaminase), the in vivo serum HI (hemagglutination inhibition) response, and the in vivo generation of switch memory B cells are decreased with age, as evaluated in the same subjects. This is the first report to demonstrate that intrinsic B cell defects with age contribute to reduced antibody responses to the influenza vaccine. The level of AID in response to CpG before vaccination can also predict the robustness of the vaccine response. These results could contribute to developing more effective vaccines to protect the elderly as well as identifying those most at risk.
Keywords: Aging, B lymphocytes, immunoglobulin class switch recombination, influenza vaccination
1. Introduction
The social and economic impact of influenza represent a major issue for public health [1, 2]. Vaccination against influenza has convincingly been shown to be effective in preventing infection in healthy individuals [3]. However, evidence that it is also effective in reducing influenza-related mortality in elderly subjects is weak. Many of the elderly individuals who routinely receive the vaccine still contract the virus and have in fact secondary complications leading to hospitalization, debilitating complications, and death [1, 4–8].
The influenza vaccine induces an antiviral response in both B and T cells, resulting in humoral and cellular immunity, respectively [9]. Vaccine-activated T cells stimulate B cells to differentiate and secrete antibodies specific for a particular vaccine strain. The antibody response to the vaccine is the first line of defense against subsequent influenza infection. The specific antibodies bind to the surface glycoproteins hemagglutinin (HA) and neuraminidase (NA), to neutralize the virus particle [10, 11]. Secretory IgA and IgM provide protection against the establishment of initial infection, however, IgG neutralizes newly replicating virus once infection has been established [12]. Annual influenza vaccinations help the human host to make protective levels of antibodies against the currently circulating strains [13, 14]. Although for most pathogens pre-existing neutralizing antibodies provide the first line of defense, there is little or no pre-existing immunity to newly emerging influenza variants in human beings [13, 14].
The serologic response to influenza virus vaccine varies with age [13, 15–18]. Successive annual vaccinations increase protection against influenza [6, 19, 20], suggesting that cellular and humoral immune mechanisms are important for protection in elderly individuals. Antibody responses to influenza vaccination have previously been correlated with T cell function [21, 22].
Our research has focused on the characterization of B cell responses in healthy individuals of different ages, and in particular the response against both polyclonal and influenza-specific stimulation, as a means to evaluate general as well as antigen-specific B cell responses, respectively. Objectives of our work are to establish biomarkers of human B cell function and associate these with a biologically relevant immune response, such as the influenza vaccine. We have previously shown in mice [23] and humans [24] intrinsic B cell defects with aging in Ig class switch recombination (CSR), due to defects in the expression of both activation-induced cytidine deaminase (AID) and the E47 transcription factor. Our results from 2 influenza seasons indicate that AID can accurately track optimal immune responses and activity, and can be a valid predictor of vaccine effectiveness in humans.
2. Materials and Methods
2.1.Subjects
Experiments were conducted using PBMC isolated from healthy volunteers of different ages after appropriate signed consent. The study was conducted under approved IRB protocol #20070481. The individuals participating in the study were screened for diseases known to alter the immune response or for consumption of medications that could alter the immune response. In particular, the following categories were excluded: established diagnosis of diabetes; one or more of the following co-morbid conditions including malignancy (patients without a recurrence in the last 5 yrs have been allowed), Congestive Heart Failure, Cardiovascular Disease (unstable, <6 mo), Chronic Renal Failure, no renal or hepatic diseases, autoimmune diseases, infectious disease, recent (<3 mo) trauma or surgery, pregnancy, or documented current substance and/or alcohol abuse. The psychological status of all participants was within normal limits. Each individual was asked with a series of questions regarding the presence of symptoms associated with respiratory infections during the study. All subjects were influenza-free at the time of enrollment and at the time points of blood draws.
Participants were as follows. Season 2008–2009: 16 healthy subjects (age 22–83). There were 9 females and 7 males in this sample. Two were Black, and 14 White (of which 8 identified themselves as Hispanic Whites). Season 2009–2010: 21 healthy subjects (age 22–89). There were 11 females and 10 males in this sample. Three were Black, and 18 White (of which 11 identified themselves as Hispanic Whites). For the purpose of this study, “elderly” persons refer to those ≥65 years of age (n=8; mean age 77±3, age range 66–84 years). Young persons are 22–64 years of age (n=29; mean age 39±3). Only 4 out of 27 young subjects have been vaccinated previously (twice). Seven (of 8) elderly subjects were vaccinated each year in the 5 previous years. One elderly was vaccinated in the last 2 flu seasons.
2.2.Vaccination
For the 2008–2009 influenza season, the recommended commercially available influenza vaccine contained 15 μg HA each of the following components: H1N1: A/Brisbane/59/2007; H3N2: A/Brisbane/10/2007; B: B/Florida/4/2006-like (FLUARIX and FLULAVAL, both from GSK). Antigens H3N2 and B of the 2008–2009 season were already present in the 2006–2007 vaccine preparation.
For the 2009–2010 influenza season, the recommended commercially available influenza vaccine contained 15 μg HA each of the following components: H1N1: A/Brisbane/59/2007; H3N2: A/Brisbane/10/2007; B: B/Brisbane/60/2008 (FLUARIX from GSK and FLUVIRIN, from Novartis). Antigens H1N1 and H3N2 of the 2009–2010 season were already present in the 2008–2009 vaccine preparation.
We also evaluated the response of 7 individuals (5 young and 2 elderly) in the 2007–2008 influenza season. The results were not included here because we assayed the AID response to individual viral strains (from GSK) instead of the whole vaccine. Whole vaccine was used subsequently because the HI response was similar/representative to that seen against the individual viral strains, although we are aware that a conclusion based on 2 elderly subjects may represent a limitation in comparing the response to the whole vaccine with the response to the individual strains.
Pre-vaccination blood samples were collected immediately before vaccination (t0), while post-vaccination samples were obtained 1,2 and 4–6 weeks after immunization (t7, t14, t28). All subjects in the study had their blood drawn at t0 and t28. Only few of them also gave blood at the intermediate time points.
2.3.B cell enrichment
PBMC were collected by density gradient centrifugation on LSM Lymphocyte Separation Medium (ICN Biomedicals). Cells were then washed three times with PBS. B cells were isolated with anti-CD19 Microbeads (Miltenyi Biotech), according to the MiniMacs protocol (Miltenyi Biotech), briefly by incubation for 20 min at 4°C with 20 μl of beads/107 cells. Cells were then purified using magnetic columns. Comparable numbers of CD19+ cells were obtained after negative selection. After isolation, cells were maintained in serum-free medium for 1 h at 4°C to minimize potential effects of anti-CD19 antibodies on B cell activation.
2.4.B cell culture
B cells were cultured in complete medium (RPMI 1640, supplemented with 10% FCS, 10 μg/ml gentamicin, and 2 × 10−5 M 2-ME and 2 mM L-glutamine). B cells (1×106/ml complete medium) were stimulated for 7 days in 24-well culture plates with 5 μg/ml CpG (ODN 2006 In Vivogen), or with 2 μl/106 cells of influenza vaccine. For each subject, the vaccine used to stimulate B cells in vitro was the same that was given in vivo. At the end of this time, cells were harvested, and RNA extracted for quantitative (q)PCR.
2.5.Flow cytometry
One hundred μl of blood from each subject were stained with APC-conjugated anti-CD19 (BD Pharmingen 555415) for 20 min at 4°C. To detect naive B cells (IgG−/IgA−/CD27−), IgM memory B cells (IgG−/IgA−/CD27+), and switch memory B cells (IgG+/IgA+), blood cells were stained with PE-conjugated anti-CD27 (BD Pharmingen 555441), biotin-SP-conjugated AffiniPure F(ab′)2 fragment anti-human IgG (Jackson ImmunoResearch Laboratories.109-066-098), and biotin-SP-conjugated AffiniPure F(ab′)2 fragment anti-human IgA (Jackson ImmunoResearch Laboratories no.109-066-011). After washings, biotin-conjugated antibodies were revealed with PerCP-conjugated streptavidin (1/40 diluted; BD Pharmingen 554067). After staining, blood red cells were lyzed using the RBC Lysing Solution BD PharmLyse (BD 555899), according to the manufacturer’s instructions. Samples of 106 cells were analyzed on a LSRI flow cytometer (BD Biosciences) using logarithmic amplification. For four-color analysis, single color controls were included in every experiment to determine background fluorescence and gate set up.
For the subjects in the 2009–2010 influenza season, we also investigated the level of circulating antibody-secreting cells (ASC)/plasmablasts pre-vaccination and at day 7 after influenza vaccination. To detect ASC, PBMC were stained with FITC-conjugated anti-CD3 (BD Pharmingen 555339), PacBlue-conjugated anti-CD19 (Invitrogen MHCD1928), APC-H7-conjugated anti-CD20 (BD Pharmingen 641396), PE-conjugated anti-CD27 (BD Pharmingen 555441) and PerCP Cy5.5-conjugated anti-CD38 (BD Pharmingen 551400). ASC were CD3−/CD19+/CD20low/CD27bright/CD38bright. Because of the very low frequency of ASC, samples of at least 2×106 cells were acquired on an LSRII flow cytometer (BD Biosciences).
2.6.Hemagglutination inhibition (HI) assay
We measured the immunogenicity of the vaccine in young and elderly individuals before (t0) and after vaccination (t7, t14 and t28) by HI assay. Sera were stored frozen at −20°C. Paired pre- and post-immunization serum samples from the same individual were tested simultaneously, according to Hsiung et al. [25]. Briefly, sera were pretreated with receptor destroying enzyme (RDE, Denke Seiken Co Ltd) for 20 hours at 37°C; in order to inactivate this enzyme, sera were then heated at 56°C for 60 minutes. Two-fold serial dilutions were done; 25 μl of diluted sera were incubated with an equal volume of 4 HA units of whole vaccine, for 1 hour at room temperature and then 50 μl of a 1.25% suspension of human red blood cell, type O (HO) were added [25]. After two hours of incubation at room temperature titers were determined. A four-fold rise in titer at two different time points, pre (t0) to post-vaccination (t28) indicates a positive response to whole vaccine [9]. This criterion of fold-increase was used to normalize the data in the two seasons because the absolute titers differed (the 2008–2009 season was 3-fold higher). Human red blood cells (type O) have been shown to be as good as chicken, duck and guinea pig red blood cells, but better than cow, horse and pig, in viral agglutination, in particular of the human type A isolates [25, 26].
2.7.RNA extraction and RT-PCR
mRNA was extracted from stimulated B cells (106/ml) using the μMACS mRNA isolation kit (Miltenyi Biotec), according to the manufacturer’s protocol, eluted into 75 μl of preheated elution buffer, and stored at −80°C until use. Reverse transcriptase (RT) reactions were performed in a Mastercycler Eppendorf machine. Ten μl of mRNA were used as template for cDNA synthesis in the RT reaction.
2.8.Quantitative PCR (qPCR)
Two μl of cDNA were added to 10 μl of Taqman PCR Master mix (Applied Biosystems no.4369016), 0.5 μl of forward primer, 0.5 μl of reverse primer and probe, and deionized water in a final volume of 20 μl. Reactions were conducted in MicroAmp 96-well plates (Applied Biosystems, ABI no.N8010560), and run the ABI 7300 machine. Calculations were made with ABI software. Briefly, we determined the cycle number at which transcripts reached a significant threshold (Ct) for AID and GAPDH as control. A value of the target gene, relative to GAPDH, was calculated and expressed as ΔCt. Primers for PCR amplification of AID and GAPDH were the following (all from ABI): Hs00221068 (AID), Hs99999905 (GAPDH).
2.9.Statistical analyses
Before conducting the main analyses, we examined the data for normality of the distribution and outliers. Outliers were scores with >2 SDs above or below the mean. Two outliers were observed in AID mRNA expression (one from the 2008–2009 season and one from the 2009–2010 season): both belonged to the “young participants” group. Two outliers were also observed in HI (one from the 2008–2009 season and one from the 2009–2010 season): both were categorized as young. Because AID and HI data were not normally distributed (AID skewness=4.11, kurtosis=15.76; HI skewness=4.10, kurtosis=15.71), and therefore the assumptions for conducting parametric analyses were not met, non-parametric analyses were performed with these two variables. Values for the Spearman’s correlations and p values are shown in each figure legend. Geometric Mean Titers (GMT), which are the geometric mean of the reciprocal of the titers, were calculated as follows [27]. GMT=antilog (Σ log T)/n, where T is the reciprocal of the HI titer, and n is the number of samples. In order to compare young and elderly subjects for AID and HI, Mann-Withney U tests were conducted. For paired samples, statistical analyses were conducted using the Wilcoxon test.
3. Results
3.1.Age-related decrease in influenza-specific serum HI response
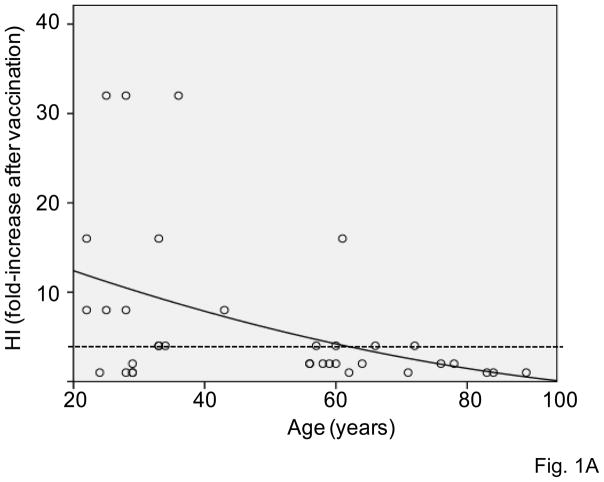
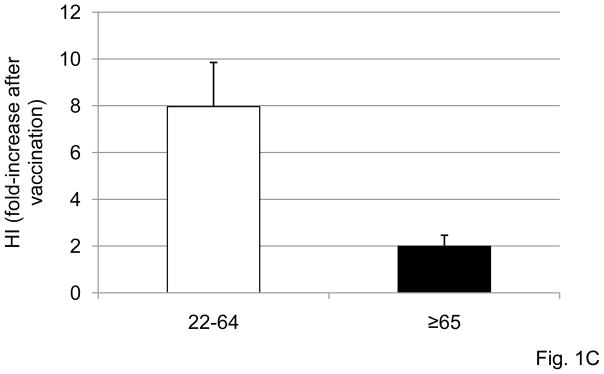
The HI response has previously been shown to decrease with age in both mice and humans [9, 15, 17]. We analyzed the HI response in the subjects in this study in order to test whether the in vivo response to the influenza vaccination (HI) was consistent with a decreased functional response in intrinsic B cells (AID). For both influenza seasons, sera isolated from subjects of different ages, before or after vaccination, were collected and analyzed by the HI assay to evaluate antibody production to the vaccine. The HI assay is based on the ability of certain viruses or viral components to hemagglutinate the red blood cells of specific animal species. Antibodies specific to influenza can inhibit this agglutination. The HI test is useful for the measurement of antibody titers of sera and is the most established correlate with vaccine protectiveness. Sera were isolated from the blood of subjects of different ages before (t0), or after (t28) influenza vaccination and then tested in HI assay. Fig. 1A shows the progressive and significant age-related decrease in the HI response of subjects of different ages that were vaccinated in the last 2 influenza seasons. The dashed horizontal line indicates the threshold of a positive response in the HI test, which corresponds to a 4-fold increase in the serum titer. Most of the young subjects (22–64) are on or above the line, whereas only 2 out of 8 elderly subjects are showing a 4-fold increase in their titers.
Figure 1. Serum HI response to influenza vaccination decreases with age.
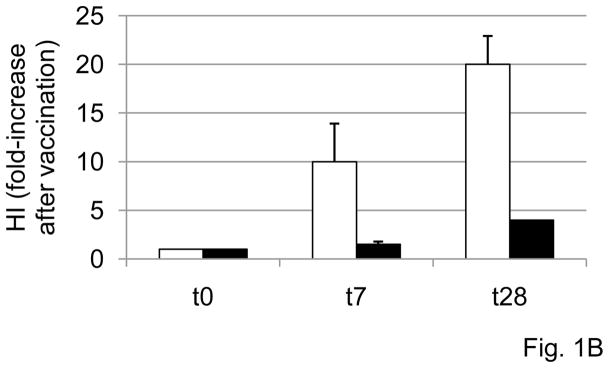
A. Sera isolated from subjects of different ages, before (t0) or after vaccination (t28), were collected and analyzed in HI assay to evaluate antibody production to vaccine. Results are expressed as fold-increase in titer after vaccination, calculated as follows: titer values after vaccination/titer values before vaccination. Thirty-seven subjects were evaluated, 29 young and 8 elderly. Two young subjects were not included because they were considered outliers (HI values >2 SDs above the mean). The dashed horizontal line indicates the threshold of a positive response in the HI test, which corresponds to 4-fold increase in the serum titer. Spearman’s rho =−0.449, p=0.005 (two-tailed). B. Sera isolated from the blood of 23 subjects of different ages (19 young and 4 elderly) at t0, t7 and t28 were tested in HI assay. Results are expressed as fold-increase in titer after vaccination, calculated as in A. The difference between young and old is significant at both t7 (p=0.040) and t28 (p=0.025), as evaluated by the Wilcoxon test. White columns: young. Black columns: elderly.
In further analyzing these HI data, three out of 8 elderly individuals had antibody titers at t0 higher than those of younger ones, with the GMT at t0 for young and elderly subjects being 307±240 and 3048±392, respectively. Two elderly had titers of 3840, 1 had 1920, 2 had 80 (all these did not respond to vaccination) and 3 had 40 (2 of these did respond to vaccination). This result can be partially explained by the fact that 7 out of 8 elderly subjects were vaccinated each year in the previous 5 influenza seasons, whereas 1 out of 8 was vaccinated only once before this season. Among the young subjects, 2 out of 27 had t0 titers of 3840 and 1920 and also did not respond to vaccination. An additional 10 young subjects were non-responders but had an initial titer of 1000 or less. Fifteen young subjects responded and also had an initial titer of 1000 or less. Even so, the correlation of fold-increase in HI and t0 HI titers was significant (p=0.031), suggesting that the initial titer could have negatively influenced the HI fold-increase. Nevertheless, because of the limited number of elderly samples, we would like to leave open the possible interpretation that the reduced response of the elderly to vaccination may not be due to their higher titers at t0 which could interfere with the effective antigen concentration. Additionally, others have previously found that the elderly, regardless of their initial HI titers, have decreased HI serum responses to the vaccine, or even with high initial HI titers can still give a good fold-increase to the vaccine [9].
Fig. 1B shows the kinetics of the serum response to vaccination in subjects of different ages. Briefly, sera were isolated from the blood of 23 subjects of different ages (19 young and 4 elderly), before (t0), or 1 and 4 weeks (t7 and t28) after influenza vaccination, respectively, and then tested in HI assay. A positive response to vaccination was observed in young individuals at t7 and to a higher extent at t28. In our 4 elderly subjects, conversely, a positive response was attained only at t28. Others have shown increased serum titers already at t7 in the elderly, which persist until 12 weeks post-vaccination [28]. This response of elderly individuals was significantly reduced as compared to that of young controls at both t7 and t28 (p<0.05). When we compared the group of 27 young versus the group of 8 elderly subjects in Fig. 1A, the difference between the 2 groups was significant (p=0.010, not shown). Similar results have been previously shown by others [13, 15–18], but these measurements were necessary to study the B cell component of the response in the same patients, as are given below.
3.2.Age-related decrease in influenza-specific AID mRNA induction
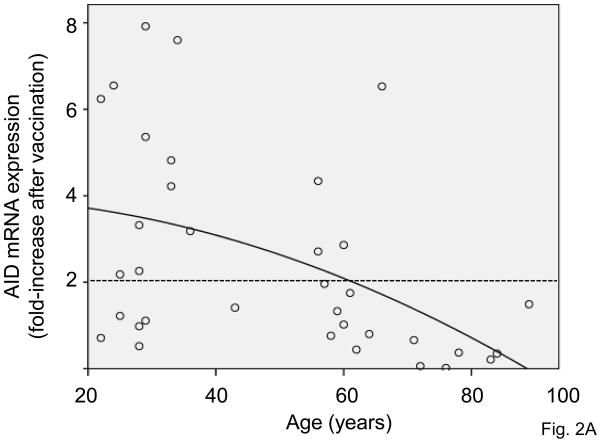
We have previously shown [24] that AID mRNA expression is induced by anti-CD40/IL-4 in human peripheral blood-derived B cells and is down-regulated with aging. AID is known to induce CSR and Ig SHM [29–32]. In the present study, B cells from the same individuals of different ages as above were stimulated in vitro with the influenza vaccine to induce AID mRNA expression. Briefly, B cells were isolated from the PBMC of subjects of different ages before (t0), or after (t28) influenza vaccination and then stimulated in vitro with the influenza vaccine for 7 days to induce optimal AID mRNA. We found that the influenza vaccine was able to stimulate purified B cells in the absence of antigen-presenting cells and T cells, likely because the vaccine itself has mitogenic activity. Fig. 2A shows the progressive and significant age-related decrease in the AID mRNA data from the 2 influenza seasons. We drew a dashed horizontal line to indicate the threshold of a positive response in AID mRNA expression after vaccination as compared to t0. We arbitrarily defined a 2 fold-increase as a positive response, based on the response seen in young subjects and the comparison with the HI curve. Results in Fig. 2A show that influenza vaccination induced an up-regulation of AID mRNA expression in almost all young subjects. However, only 1 elderly subject responded in this test. Therefore, this up-regulation was significantly higher in young than in elderly subjects. Only the influenza-specific AID response is down-regulated by age, whereas the CpG response stays the same before and after vaccination, as expected (data not shown). In correlating as above the initial titer at t0 with the fold-increase in AID, no significant correlation was found (p=0.408) and therefore the fold-increase in AID is not due to the initial HI titer.
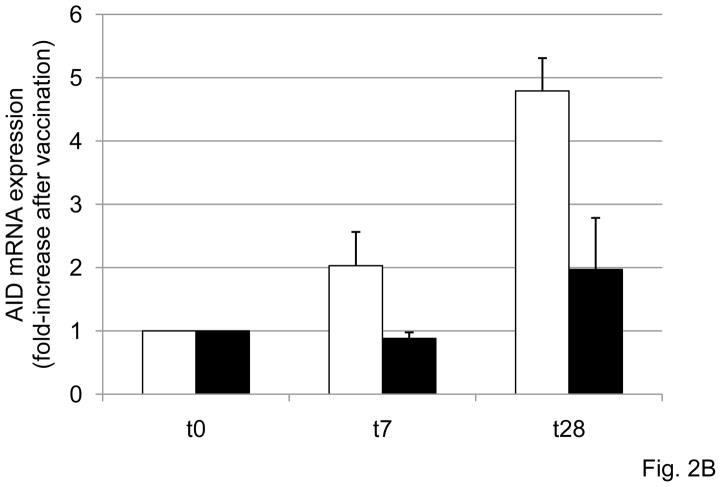
Figure 2. The in vitro AID response of B cells to influenza vaccination decreases with age.
A. B cells (106 cells/ml), isolated from the PBMC of subjects of different ages, before (t0) or after vaccination (t28), were cultured with the vaccine, for 7 days. At the end of this time, cells were processed as described in Materials and Methods. Thirty-seven subjects were evaluated, 29 young and 8 old. Two young subjects were not included because they were considered outliers (AID mRNA values >2 SDs above the mean). The dashed horizontal line indicates the threshold of a positive response in AID mRNA expression which was arbitrarily defined as 2-fold increase. Spearman’s rho =−0.527, p=0.001 (two-tailed). B. B cells (106 cells/ml), isolated from the PBMC of 10 subjects of different ages (6 young and 4 elderly), stimulated in vitro with the influenza vaccine for 7 days to induce optimal AID mRNA expression. At the end of this time, cells were harvested, RNA extracted and qPCR reactions performed to evaluate AID normalized to GAPDH transcripts. Results are expressed as fold-increase in AID mRNA expression after vaccination, calculated as follows: AID values after vaccination/AID values before vaccination. The difference between young and old is significant at both t7 (p=0.008) t28 (p=0.032), as evaluated by the Wilcoxon test. White columns: young. Black columns: elderly.
Kinetics of AID mRNA expression were also evaluated. Briefly, B cells were isolated from the PBMC of 10 subjects of different ages (6 young and 4 elderly), before (t0), or 1 and 4 weeks (t7 and t28) after influenza vaccination, respectively, and then stimulated in vitro with the influenza vaccine for 7 days to induce optimal AID mRNA. Fig. 2B shows the kinetics of AID mRNA expression in subjects of different ages. The stimulation of B cells with vaccine induced AID mRNA in a time-dependent manner, with the peak of expression being reached at t28 in all subjects. At both t7 and t28 (p<0.05), AID mRNA levels were significantly lower in elderly individuals as compared with younger controls. We therefore subsequently evaluated the fold-increase in AID mRNA expression at t28, and because the serum response is also optimal at that time. When we compared the group of 27 young versus the group of 8 elderly subjects in Fig. 2A, the difference between the 2 groups was significant (p=0.004, not shown).
For the subjects in the 2008–2009 influenza season, we also investigated the plasmablast response at t7 after influenza vaccination in 13 subjects (10 young and 3 elderly). We have determined that t7 is the peak for plasmablasts (not shown) as already published by others [14]. Although elderly subjects show lower percentages (2.77±0.39) of plasmablasts as compared to younger controls (4.36±0.94), this difference was not statistically significant (p=0.09) due to the limited number of subjects evaluated. In another study currently under investigation in our lab (Frasca et al., manuscript in preparation), we have shown that elderly subjects vaccinated against H1N1 influenza have significantly lower percentages of plasmablasts (1.41±0.33) as compared to those of younger controls (7.53±2.19) (p=0.03).
3.3.AID predicts HI response both before and after vaccination
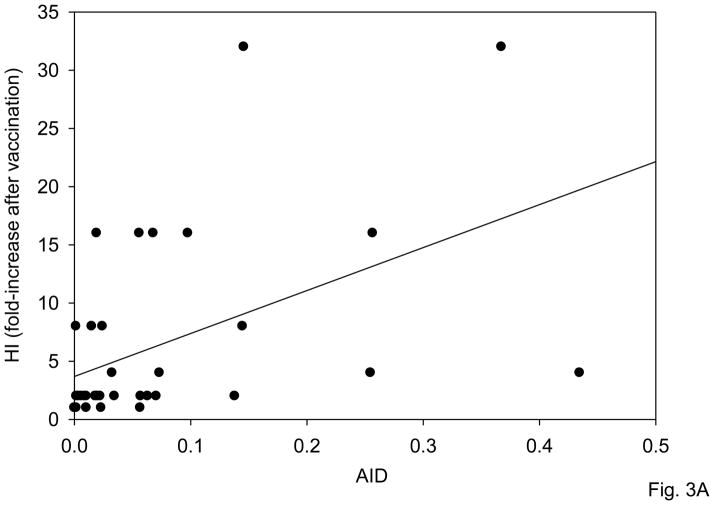
We have previously shown that the AID response of B cells stimulated with anti-CD40/IL-4 is decreased with age. In order to test whether AID could be used to predict the robustness of the influenza response, we measured AID in response to CpG at t0 and correlated this with the fold-increase in the HI response. The CpG AID response of B cells from young and elderly individuals was different, the elderly group mean was 35% of the group mean for young adults (data not shown). Results show that the HI response is correlated with AID (p<0.0001) (Fig. 3A). Therefore, B cell function, as measured by AID in stimulated B cells, can predict the ability to generate an optimal influenza vaccine response.
Figure 3. AID predicts HI response both before and after vaccination.
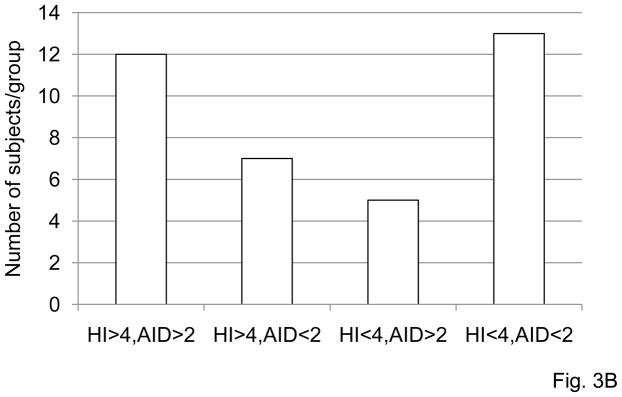
A. B cells (106 cells/ml), isolated from the PBMC of subjects of different ages before vaccination were cultured with CpG, for 7 days. At the end of this time, cells were processed as described in Materials and Methods. Thirty-seven subjects were evaluated, 29 young and 8 old. AID values are qPCR at t0. HI was measured at t0 and t28 as in Fig. 1 and fold-increase presented. Spearman’s rho=0.614, p=0.0001 (two-tailed). B. Same subjects as in A were analyzed for fold-increase in HI and correlated with the fold-increase in in vitro influenza-induced AID. Four categories were identified: HI>4/AID>2; HI<4/AID<2; HI>4/AID<2 and HI<4/AID>2. Most of the subjects (68%) fall within the two categories, hi HI/hi AID and low HI/low AID and is significant (p<0.05) by χ2. Kruskal-Wallis analysis was performed to examine whether there was a discernible pattern of age between groups HI>4/AID>2, HI>4/AID<2, HI<4/AID>2 and group HI<4/AID<2. The latter group, HI<4/AID<2, had most of the elderly in the study. This was statistically significant F(3,36)=6.53, p<0.001.
In order to find the best relationship between HI and AID data, we grouped the participants in this study in the following 4 categories: HI>4/AID>2; HI<4/AID<2; HI>4/AID<2 and HI<4/AID>2. Fig. 3B shows the number of subjects in each group and that the optimal HI response (>4) tracts with an optimal AID response (>2) in 12 of 19 individuals and a low HI response (<4) tracts with a low AID response (<2) in 13 of 18 cases. Kruskal-Wallis analysis was performed to examine whether there was a discernable pattern of age between groups. It was statistically significant: chi-square (3) = 11.39, p=0.01. Briefly, the mean age for participants in the different categories was: 37.4 (HI>4/AID>2), 64.2 (HI<4/AID<2), 44.9 (HI>4/AID<2) and 39.6 (HI<4/AID>2). Post hoc analysis showed that the HI<4/AID<2 group was statistically significantly older than the other 3 groups (Mean Rank 26.88 versus 13.13, 17.50, and 14.70). This means that participants in the category HI<4/AID<2 were significantly older than participants in each of the other 3 categories and these subjects are the ones that did not respond to influenza vaccination. These results also show that a high influenza-specific B cell AID response correlates with a high HI response and vice versa (low/low).
3.4.Age-related decrease in switch memory B cell induction
We have also analyzed the composition of the peripheral B cell pool in 21 influenza study subjects, only from the 2009–2010 season, before and after vaccination. We have evaluated the percentages of naive (IgG−/IgA−/CD27−), IgM memory (IgG−/IgA−/CD27+), or total switch memory (IgG+/IgA+) and CD27+ switch memory (IgG+/IgA+/CD27+) B cells in blood.
The results obtained with the blood staining show comparable age-related effects with the results obtained with peripheral blood-derived B cell staining, i.e. increase in naïve, no change in IgM memory and a decrease in both total and CD27+ switch memory B cells in the elderly [24]. However, the relative percentages of the different B cell subsets differed. On a larger number of subjects (n=112), we have shown by blood staining that the percentages of naïve B cells in young versus elderly are 56±2 versus 64±3 (p=0.001), those of IgM memory in young versus elderly are 33±2 versus 32±3 (p=0.6), those of total switch memory in young versus elderly are 12±1 versus 4±1 (p=0.0001), and those of CD27+ switch memory in young versus elderly are 4±0.4 versus 1±0.2 (p=0.0001) (Frasca et al., Aging Res Reviews, 2010 in press). These latter numbers agree with the blood staining in this report.
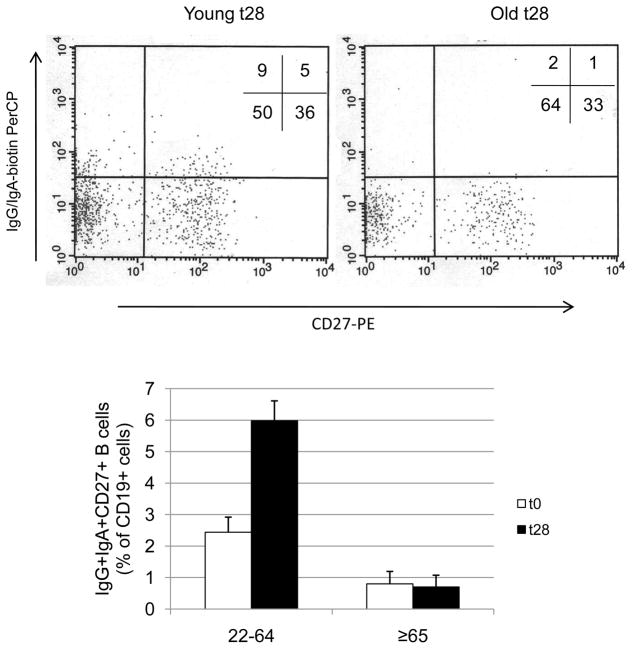
Our results show that influenza vaccination significantly increased the percentage of CD27+ switch memory B cells only in younger individuals (p=0.0045) (Fig. 4). The percentage of CD27+ switch memory B cells is also significantly decreased in the elderly, even at t0 (p=0.042). Naïve and IgM memory B cell percentages did not change between t0 and t28 in both young and elderly subjects. Percentages (t0 versus t28) were: 60±5 versus 61±5 (naïve young), 31±4 versus 30±5 (IgM memory young), 67±7 versus 64±5 (naïve elderly), 28±4 versus 30±4 (IgM memory elderly). The percentages of total switch memory B cells (IgG+/IgA+) also did not change between t0 and t28 in both young and elderly subjects. Percentages (t0 versus t28) were: 9±2 versus 9±1 (young) and 5±1 versus 6±2 (elderly). Again, in correlating as above the initial titer at t0 here with the fold-increase in the percentage of switch memory B cells, no significant correlation was found (p=0.114).
Figure 4. The percentages of IgG+IgA+CD27+ switch memory B cells increase after vaccination in young but not in elderly subjects.
One hundred μl of blood from young and elderly subjects at t0 and t28 were stained as described in Materials and Methods. A representative dot plot is shown for one young and one elderly subject, both at t28. Twenty-one subjects were evaluated, 16 young and 5 elderly. The difference between young and elderly at t0 and t28 is significant (U=17, p=0.048 and U=0.0001, p=0.001, respectively). The difference between t0 and t28 in young subjects is significant (p=0.0045), but in the elderly is not significant, as evaluated by the Wilcoxon test.
4. Discussion
Vaccination is an efficient way to reduce mortality and morbidity associated with influenza infection and is universally recommended for elderly people. The challenge of influenza is to design vaccines able to induce long-lasting humoral and cellular immunity against a pathogen that rapidly mutates (antigenic drift) and exchanges its genetic components (antigenic shift). There is significant evidence that the immune response to influenza vaccination declines significantly with age [9, 28, 33]. Most of the studies conducted so far have shown that the reduced response to vaccination in the elderly is correlated with the well-known decrease in T cell functions [21, 22, 34], in particular a reduction in naïve T cells and a concomitant increase in memory/effector T cells [35], loss in CD28 expression [36], and cytomegalovirus (CMV) positivity [37]. The decreased protective effect of vaccination in elderly people has been significantly correlated with the presence of CMV latency [34, 37, 38]. There are currently no studies that have evaluated possible age-related intrinsic defects in the B cell-specific response to influenza vaccination. Our data show that the AID response of B cells to the influenza vaccine is correlated with the serum HI response in most (68%) of the subjects. We also show that this AID response, as well as HI, is impaired with aging, thus contributing to the qualitatively and quantitatively reduced production of specific antibodies in the elderly. The control polyclonal response of both young and elderly B cells to CpG was not modified by influenza vaccination.
Others have shown that the frequencies of CD8+CD28− T cells can be useful biological markers of compromised immune competence, identifying individuals at risk for insufficient antibody responses [21]. We have previously shown that B cells activated with anti-CD40/IL-4 from elderly subjects have lower AID than younger subjects [24]. Here we have shown that the AID response to CpG at t0, which is 3-fold less in elderly than in young subjects, is correlated with the fold-increase in HI seen at t28 after vaccination in the same subjects. Thus, the function of B cells, and AID in stimulated B cells, is a predictor for an optimal vaccine response.
Human B lymphocyte differentiation progresses from naïve to IgM memory to switch memory B cells [24, 39, 40]. We have previously shown [24] an age-related decline in the percentage and numbers of switch memory B cells, no change in the percentage but a decline in the numbers of IgM memory B cells and an increase in the percentage but no change in the numbers of naive B cells, consistent with there being a defect both in vivo and in vitro [24] in generating AID and switch memory B cells. Both aged human naïve and IgM memory B cells show in vitro defects in CSR [24]. Therefore, aged switch memory B cells are deficient in number and function (for CSR), whereas naïve B cells are deficient functionally but not in numbers. We show herein that the percentage of switch memory B cells (IgG+/IgA+/CD27+) increased after influenza vaccination in young but much less in elderly subjects.
In an antigen-specific response, memory B cells undergo massive expansion and differentiate into short-lived plasma cells, some of which eventually become long-lived plasma cells if rescued in available niches, such as bone marrow [41]. Memory B cells can also proliferate and differentiate into plasma cells in response to polyclonal stimuli, such as CpG or homeostatic mechanisms [42]. In this homeostatic response, all memory B cells could undergo continuous proliferation and differentiation and become plasmablasts, contributing to a serum pool of specific antibodies which could theoretically be maintained throughout a human life-span. Although B cells can be activated by homeostatic mechanisms, the production of antibodies seems to remain specific, as reported for tetanus vaccination [43].
Our results showing that switch memory B cells are increased by influenza vaccination are of great importance because memory B cells can carry the history of the individual in terms of specific-immune responses [44]. We know that some elderly subjects have higher t0 HI values as compared to younger controls, due to the fact that they have undergone prior immunizations with related influenza virus antigens, but they do not show increased switch memory B cells after vaccination as younger people do. Therefore, a target for future generation vaccines is to potentiate this B cell subset in elderly subjects. Other potential limitations of this study are the small numbers of elderly subjects analyzed and the use of the whole vaccine instead of the 3 individual strains, H3N2, H1N1 and B analyzed separately.
We here show that switch memory B cells and influenza-specific serum responses as well as in vitro B cell responses are decreased by age. Another study performed on young/adults [45] has shown a correlation between fold-increase after vaccination in HI values and plasmablast B cell responses evaluated 7 days after vaccination. However, in this study no correlation was observed between HI values and memory B cell responses evaluated 4 weeks after vaccination. The discrepancy between these results and ours herein is probably due to different protocols used, i.e. PBMC stimulated with Pokeweed, CpG and Staphylococcus aureus Cowan and B cells stimulated with influenza vaccine. Our results obtained with purified B cells are important because they show that B cells from elderly individuals have an intrinsic defect in their ability to respond to influenza vaccination, and this defect is independent from defects exhibited by other cell types participating in the response to influenza.
In addition to the ability to induce antibody responses, the protective effects of the vaccine against influenza depend on the ability to stimulate T cell-mediated immune responses, in particular cytotoxic T cells which are responsible for the clearance of the virus once the infection has occurred [46] through the release of Granzyme B, a key cytolytic mediator, that induces the death of the virus-infected cells by apoptosis [47]. The levels of Granzyme B and the ratio of interferon-γ:interleukin-10 have been described to predict influenza illness among vaccinated elderly [48].
In conclusion, in this paper we have shown that the specific AID response of B cells to the influenza vaccine given in vitro, the in vivo serum HI response to vaccination, and the switch memory B cells are decreased with age. We also evaluated whether B cell-specific immunological parameters could predict poor anti-influenza virus vaccine responses and therefore can be used as biological markers of immune senescence. Our data demonstrate that the AID response correlates with an optimal HI serum response, at least in most (68%) of the subjects. We have also shown that the AID response to CpG at t0 can predict an optimal vaccine response. The observations provided herein could be used to design novel therapeutic approaches for improving B or T cells and the immune response in the elderly.
Acknowledgments
We are grateful to the people who participated as subjects in this study. We thank the personnel, doctors and nurses, of the Family Medicine Department at the University of Miami Miller School of Medicine, in particular Dr. Robert Schwartz (chairman), Ms. Dionne Brown (research coordinator), and Silia Batista (nurse), and Sandra Chen (Employee Health Manager). We thank Jim Phillips and the Sylvester Comprehensive Cancer Center Flow Cytometry Core Resource, and Michelle Perez for secretarial assistance. We also express our gratitude to Elisabeth R. Neumeier from GSK who helped us to get individual viral strains in the 2007–2008 flu season.
This work is supported by NIH AG-17618, AG-23717 and AG-28586 (BBB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simonsen L, Clarke MJ, Schonberger LB, Arden NH, Cox NJ, Fukuda K. Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J Infect Dis. 1998 Jul;178(1):53–60. doi: 10.1086/515616. [DOI] [PubMed] [Google Scholar]
- 2.Boraschi D, Del Giudice G, Dutel C, Ivanoff B, Rappuoli R, Grubeck-Loebenstein B. Ageing and immunity Addressing immune senescence to ensure healthy ageing. Vaccine. Apr 1; doi: 10.1016/j.vaccine.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 3.Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis. 2007 Oct;7(10):658–66. doi: 10.1016/S1473-3099(07)70236-0. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Racial/ethnic disparities in influenza and pneumococcal vaccination levels among persons aged > or = 65 years in the United States, 1989–2001. MMWR Morb Mortal Wkly Rep. 2003;52:958–62. [PubMed] [Google Scholar]
- 5.Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann Intern Med. 1995 Oct 1;123(7):518–27. doi: 10.7326/0003-4819-123-7-199510010-00008. [DOI] [PubMed] [Google Scholar]
- 6.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004 Sep 15;292(11):1333–40. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 7.Vu T, Farish S, Jenkins M, Kelly H. A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine. 2002 Mar 15;20(13–14):1831–6. doi: 10.1016/s0264-410x(02)00041-5. [DOI] [PubMed] [Google Scholar]
- 8.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003 Jan 8;289(2):179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 9.Murasko DM, Bernstein ED, Gardner EM, Gross P, Munk G, Dran S, et al. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp Gerontol. 2002 Jan–Mar;37(2–3):427–39. doi: 10.1016/s0531-5565(01)00210-8. [DOI] [PubMed] [Google Scholar]
- 10.Epstein SL, Misplon JA, Lawson CM, Subbarao EK, Connors M, Murphy BR. Beta 2-microglobulin-deficient mice can be protected against influenza A infection by vaccination with vaccinia-influenza recombinants expressing hemagglutinin and neuraminidase. J Immunol. 1993 Jun 15;150(12):5484–93. [PubMed] [Google Scholar]
- 11.Saelens X, Vanlandschoot P, Martinet W, Maras M, Neirynck S, Contreras R, et al. Protection of mice against a lethal influenza virus challenge after immunization with yeast-derived secreted influenza virus hemagglutinin. Eur J Biochem. 1999 Feb;260(1):166–75. doi: 10.1046/j.1432-1327.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- 12.Burlington DB, Clements ML, Meiklejohn G, Phelan M, Murphy BR. Hemagglutinin-specific antibody responses in immunoglobulin G, A, and M isotypes as measured by enzyme-linked immunosorbent assay after primary or secondary infection of humans with influenza A virus. Infect Immun. 1983 Aug;41(2):540–5. doi: 10.1128/iai.41.2.540-545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMurry JA, Johansson BE, De Groot AS. A call to cellular & humoral arms: enlisting cognate T cell help to develop broad-spectrum vaccines against influenza A. Hum Vaccin. 2008 Mar–Apr;4(2):148–57. doi: 10.4161/hv.4.2.5169. [DOI] [PubMed] [Google Scholar]
- 14.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008 May 29;453(7195):667–71. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Bruijn IA, Remarque EJ, Jol-van der Zijde CM, van Tol MJ, Westendorp RG, Knook DL. Quality and quantity of the humoral immune response in healthy elderly and young subjects after annually repeated influenza vaccination. J Infect Dis. 1999 Jan;179(1):31–6. doi: 10.1086/314540. [DOI] [PubMed] [Google Scholar]
- 16.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006 Feb 20;24(8):1159–69. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 17.McElhaney JE. Influenza vaccination in the elderly: seeking new correlates of protection and improved vaccines. Aging health. 2008 Dec 1;4(6):603–13. doi: 10.2217/1745509X.4.6.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLaren C, Verbonitz MW, Daniel S, Grubbs GE, Ennis FA. Effect of priming infection on serologic response to whole and subunit influenza virus vaccines in animals. J Infect Dis. 1977 Dec;136( Suppl):S706–11. doi: 10.1093/infdis/136.supplement_3.s706. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed AE, Nicholson KG, Nguyen-Van-Tam JS. Reduction in mortality associated with influenza vaccine during 1989–90 epidemic. Lancet. 1995 Sep 2;346(8975):591–5. doi: 10.1016/s0140-6736(95)91434-x. [DOI] [PubMed] [Google Scholar]
- 20.Keitel WA, Cate TR, Couch RB. Efficacy of sequential annual vaccination with inactivated influenza virus vaccine. Am J Epidemiol. 1988 Feb;127(2):353–64. doi: 10.1093/oxfordjournals.aje.a114809. [DOI] [PubMed] [Google Scholar]
- 21.Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O’Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 2001 Dec;75(24):12182–7. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saurwein-Teissl M, Lung TL, Marx F, Gschosser C, Asch E, Blasko I, et al. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002 Jun 1;168(11):5893–9. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- 23.Frasca D, Van der Put E, Riley RL, Blomberg BB. Reduced Ig class switch in aged mice correlates with decreased E47 and activation-induced cytidine deaminase. J Immunol. 2004 Feb 15;172(4):2155–62. doi: 10.4049/jimmunol.172.4.2155. [DOI] [PubMed] [Google Scholar]
- 24.Frasca D, Landin AM, Lechner SC, Ryan JG, Schwartz R, Riley RL, et al. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J Immunol. 2008 Apr 15;180(8):5283–90. doi: 10.4049/jimmunol.180.8.5283. [DOI] [PubMed] [Google Scholar]
- 25.Hsiung GD, Fong CKY, Landry ML, editors. Diagnostic Virology. 4. Yale University Press; 1994. [Google Scholar]
- 26.Ito T, Suzuki Y, Mitnaul L, Vines A, Kida H, Kawaoka Y. Receptor specificity of influenza A viruses correlates with the agglutination of erythrocytes from different animal species. Virology. 1997 Jan 20;227(2):493–9. doi: 10.1006/viro.1996.8323. [DOI] [PubMed] [Google Scholar]
- 27.McElhaney JE, Meneilly GS, Lechelt KE, Beattie BL, Bleackley RC. Antibody response to whole-virus and split-virus influenza vaccines in successful ageing. Vaccine. 1993;11(10):1055–60. doi: 10.1016/0264-410x(93)90133-i. [DOI] [PubMed] [Google Scholar]
- 28.Powers DC. Summary of a clinical trial with liposome-adjuvanted influenza A virus vaccine in elderly adults. Mech Ageing Dev. 1997 Feb;93(1–3):179–88. doi: 10.1016/s0047-6374(96)01809-x. [DOI] [PubMed] [Google Scholar]
- 29.Muramatsu M, Nagaoka H, Shinkura R, Begum NA, Honjo T. Discovery of activation-induced cytidine deaminase, the engraver of antibody memory. Advances in immunology. 2007;94:1–36. doi: 10.1016/S0065-2776(06)94001-2. [DOI] [PubMed] [Google Scholar]
- 30.Nussenzweig MC, Alt FW. Antibody diversity: one enzyme to rule them all. Nature medicine. 2004 Dec;10(12):1304–5. doi: 10.1038/nm1204-1304. [DOI] [PubMed] [Google Scholar]
- 31.Okazaki IM, Kinoshita K, Muramatsu M, Yoshikawa K, Honjo T. The AID enzyme induces class switch recombination in fibroblasts. Nature. 2002 Mar 21;416(6878):340–5. doi: 10.1038/nature727. [DOI] [PubMed] [Google Scholar]
- 32.Teng G, Papavasiliou FN. Immunoglobulin somatic hypermutation. Annu Rev Genet. 2007;41:107–20. doi: 10.1146/annurev.genet.41.110306.130340. [DOI] [PubMed] [Google Scholar]
- 33.Sambhara S, McElhaney JE. Immunosenescence and influenza vaccine efficacy. Curr Top Microbiol Immunol. 2009;333:413–29. doi: 10.1007/978-3-540-92165-3_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kovaiou RD, Herndler-Brandstetter D, Grubeck-Loebenstein B. Age-related changes in immunity: implications for vaccination in the elderly. Expert Rev Mol Med. 2007;9(3):1–17. doi: 10.1017/S1462399407000221. [DOI] [PubMed] [Google Scholar]
- 35.Pawelec G, Barnett Y, Forsey R, Frasca D, Globerson A, McLeod J, et al. T cells and aging, January 2002 update. Front Biosci. 2002 May 1;7:d1056–183. doi: 10.2741/a831. [DOI] [PubMed] [Google Scholar]
- 36.Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev. 2005 Jun;205:158–69. doi: 10.1111/j.0105-2896.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 37.Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A. Cytomegalovirus and human immunosenescence. Rev Med Virol. 2009 Jan;19(1):47–56. doi: 10.1002/rmv.598. [DOI] [PubMed] [Google Scholar]
- 38.Olsson J, Wikby A, Johansson B, Lofgren S, Nilsson BO, Ferguson FG. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech Ageing Dev. 2000 Dec 20;121(1–3):187–201. doi: 10.1016/s0047-6374(00)00210-4. [DOI] [PubMed] [Google Scholar]
- 39.Tangye SG, Tarlinton DM. Memory B cells: effectors of long-lived immune responses. Eur J Immunol. 2009 Aug;39(8):2065–75. doi: 10.1002/eji.200939531. [DOI] [PubMed] [Google Scholar]
- 40.Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu Rev Immunol. 2009;27:267–85. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- 41.Manz RA, Radbruch A. Plasma cells for a lifetime? Eur J Immunol. 2002 Apr;32(4):923–7. doi: 10.1002/1521-4141(200204)32:4<923::AID-IMMU923>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 42.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002 Dec 13;298(5601):2199–202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 43.Di Genova G, Roddick J, McNicholl F, Stevenson FK. Vaccination of human subjects expands both specific and bystander memory T cells but antibody production remains vaccine specific. Blood. 2006 Apr 1;107(7):2806–13. doi: 10.1182/blood-2005-08-3255. [DOI] [PubMed] [Google Scholar]
- 44.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007 Nov 8;357(19):1903–15. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 45.Sasaki S, He XS, Holmes TH, Dekker CL, Kemble GW, Arvin AM, et al. Influence of prior influenza vaccination on antibody and B-cell responses. PLoS One. 2008;3(8):e2975. doi: 10.1371/journal.pone.0002975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McMichael AJ, Gotch FM, Noble GR, Beare PA. Cytotoxic T-cell immunity to influenza. N Engl J Med. 1983 Jul 7;309(1):13–7. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- 47.McElhaney JE, Ewen C, Zhou X, Kane KP, Xie D, Hager WD, et al. Granzyme B: Correlates with protection and enhanced CTL response to influenza vaccination in older adults. Vaccine. 2009 Apr 21;27(18):2418–25. doi: 10.1016/j.vaccine.2009.01.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shahid Z, Kleppinger A, Gentleman B, Falsey AR, McElhaney JE. Clinical and immunologic predictors of influenza illness among vaccinated older adults. Vaccine. Jul 29; doi: 10.1016/j.vaccine.2010.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]