Abstract
Aims/hypothesis
Our objective was to examine whether longer duration of breast-feeding and later introduction of complementary foods are associated with lower glucose concentrations and insulin resistance (IR-HOMA) in Indian children.
Methods
Breast-feeding duration (6 categories from <3 to ≥18 months) and age at introduction of complementary foods (4 categories from <4 to ≥6 months) were recorded at 1, 2 and 3 year follow-up of 568 children from a birth cohort in Mysore, India. At 5- and 9.5-years of age 518 children were assessed for glucose tolerance and IR-HOMA.
Results
All the children were initially breast-fed; 90% were breast-fed for ≥6 months and 56.7% started complementary foods at or before the age of 4 months. Each category increase in breast-feeding duration was associated with lower fasting insulin concentration (β=−0.05 pmol/L (95% CI: −0.10, −0.004); P=0.03) and IR-HOMA (β=−0.05 (95% CI: −0.10, −0.001); P=0.046) at 5-years, adjusted for the child’s sex, age, current BMI, socio-economic status, parent’s education, rural/urban residence, birthweight and maternal gestational diabetes status. Longer duration of breastfeeding was associated with higher 120-minute glucose concentration at 5-years (β=0.08 mmol/L (95% CI: 0.001, 0.15; P=0.03) but lower 120-minute glucose concentration at 9.5-years (β=−0.09 (95% CI: −0.16, −0.03; P=0.006). Age at starting complementary foods was unrelated to the children’s glucose tolerance and IR-HOMA.
Conclusions/interpretation
Within this cohort, in which prolonged breast-feeding was the norm, there was evidence of a protective effect of longer duration of breast-feeding against glucose intolerance at 9.5-years. At 5-years longer duration of breast-feeding was associated with lower IR-HOMA.
Keywords: Breast-feeding, Children, Complementary foods, Glucose tolerance, India, Insulin resistance
Introduction
Children and adults who were breast-fed rather than bottle-fed, and breast-fed for a longer duration during infancy have lower rates of type 2 diabetes and lower insulin resistance (IR-HOMA) [1,2]. It has been suggested that differences between breast-fed and bottle-fed babies in the nutrient quality of the milk, patterns of infant weight gain, or in learned feeding behaviour influence later diabetes risk [1]. Few studies have examined diabetes risk in relation to the age at starting complementary foods in infancy.
In the Mysore Parthenon birth cohort study [3], data collected on infant feeding practices, and subsequent measurements of plasma glucose and insulin concentrations in the children enabled us to examine whether longer duration of breast-feeding and later introduction of complementary foods are associated with lower glucose concentrations and IR-HOMA in Indian children, and whether these associations change with age.
Research Design and Methods
As described previously [3], 830 pregnant women underwent an OGTT at 30±2 weeks of gestation (49 had gestational diabetes (GDM)). Of these, 663 delivered live normal babies at the Holdsworth Memorial Hospital, Mysore. The children had detailed anthropometry at birth, annually until the age of 5-years and every 6-months thereafter.
Infant feeding data were collected at 1-, 2- and 3-years of age by asking mothers: How was the baby fed from birth (breast, bottle, breast+bottle or other)?; If breast-fed, was the baby still being breast-fed?; If not, at what age (months) was breast-feeding stopped? At 1-year, mothers were asked the age in months at which their baby started taking solid foods regularly.
585 children (93% of survivors) were studied at 5-years and 539 (86%) at 9.5-years. Weight (Salter, UK) and height (Microtoise, CMS instruments, UK) was measured using standardized methods. After an overnight fast, blood samples were collected (fasting, and 30- and 120-minutes after an oral glucose load (1.75 g/kg body weight)). Insulin was analyzed by a time-resolved, fluoroimmunoassay (DELFIA) and plasma glucose concentrations by standard enzymatic method (Alcyon 3000, Abbott laboratories, USA). Inter-assay coefficients of variations for insulin and glucose were <10%.and <5% respectively. IR-HOMA was estimated using the HOMA equation [4]. Insulin increment was calculated [(30-minute insulin-fasting insulin)/30-minute glucose] [5].
The hospital’s Ethics Committee approved the study; informed consent/assent was obtained from parents and children.
Statistical Methods
Insulin (fasting, 30- and 120-minute), IR-HOMA and insulin increment were log transformed to normality. From a public health perspective (for example, having a bin for <3 months) and also from a statistical perspective (i.e. having enough subjects in each bin), to reduce the effect of outliers and maintain ordering, total duration of breast-feeding in months was split into 6 categories (<3, 3-5, 6-8, 9-11, 12-17 and 18+ months). Age at starting complementary foods was split into 4 categories (<4, 4, 5, ≥6 months). The associations of breast-feeding duration and age at starting complementary foods with outcomes and potential confounders were examined by multiple linear regression using Stata v10 (Stata Corporation, TX, USA).
Results
A total of 518 children had complete feeding data and outcomes at both 5- and 9.5-years (ESM-Figure1). All were initially breast-fed, 90% were breast-fed for ≥6 months and 64% for ≥12 months (Table 1). 56.7% of the children had started complementary foods by 4 months. At both time-points girls had higher insulin concentrations (fasting and 30-minute), insulin increment and IR-HOMA than boys (Table 1).
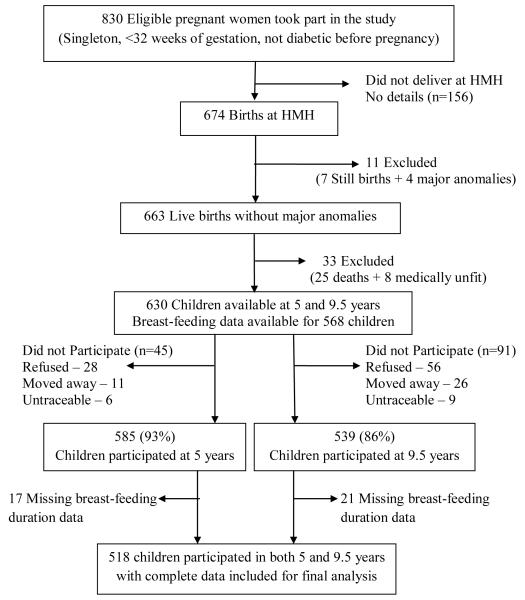
ESM-Figure 1.
Flow diagram depicting study participants
Table1.
General characteristics of the study cohort
| Boys (n=246) | Girls (n=272) | P | |||
|---|---|---|---|---|---|
| Breast-feeding duration categories: No (%) | |||||
| < 3months | 7 | (2.9) | 7 | (2.6) | 0.6 |
| 3-5 months | 23 | (9.4) | 19 | (7.0) | |
| 6-8 months | 26 | (10.6) | 23 | (8.5) | |
| 9-11 months | 34 | (13.8) | 45 | (16.5) | |
| 12-17 months | 105 | (42.7) | 131 | (48.2) | |
| 18+ months | 51 | (20.7) | 47 | (17.3 | |
| Age at starting regular solids: No (%) | |||||
| < 4 months | 43 | (18.7) | 51 | (20.2) | 0.5 |
| 4 months | 81 | (35.2) | 99 | (39.1) | |
| 5 months | 70 | (30.4) | 61 | (24.1) | |
| ≥ 6 months | 36 | (15.7) | 42 | (16.6) | |
| Children characteristics at 5 years-mean(SD) | |||||
| Age (years) | 5.0 | (0.04) | 5.0 | (0.03) | 0.09 |
| Height (cm) | 106.3 | (4.3) | 104.9 | (4.3) | 0.0004 |
| BMI(kg/m2) | 13.6 | (1.0) | 13.5 | (1.2) | 0.6 |
| Fasting glucose (mmol/l) | 4.9 | (0.5) | 4.8 | (0.3) | 0.3 |
| 30 min glucose (mmol/l) | 7.2 | (1.5) | 7.4 | (1.3) | 0.1 |
| 120 min glucose (mmol/l) | 5.9 | (1.0) | 5.9 | (0.9) | 0.5 |
| Fasting insulin (pmol/l)a | 18.6 | (11.4, 28.6) | 22.7 | (15.0, 22.7) | <0.0001 |
| 30 min insulin (pmol/l)a | 120.1 | (75.8, 211.1) | 168.4 | (108.0, 242.0) | 0.0006 |
| Insulin resistance (IR-HOMA)a | 0.6 | (0.4, 1.0) | 0.8 | (0.5, 1.2) | 0.0001 |
| Insulin increment a | 14.3 | (9.0, 24.7) | 19.5 | (12.8, 27.0) | 0.02 |
| Children characteristics at 9.5 years-mean(SD) | |||||
| Age (years) | 9.4 | (0.1) | 9.4 | (0.1) | 0.3 |
| Height (cm) | 131.3 | (5.5) | 130.3 | (5.9) | 0.07 |
| BMI(kg/m2) | 14.6 | (1.8) | 14.7 | (2.0) | 0.9 |
| Fasting glucose (mmol/l) | 4.7 | (0.4) | 4.7 | (0.4) | 0.1 |
| 30 min glucose (mmol/l) | 6.7 | (1.3) | 6.9 | (1.2) | 0.1 |
| 120 min glucose (mmol/l) | 5.0 | (1.0) | 5.2 | (0.8) | 0.067 |
| Fasting insulin (pmol/l)a | 19.2 | (12.0, 28.8) | 26.4 | (18.6, 36.0) | <0.0001 |
| 30 min insulin (pmol/l)a | 208.2 | (124.8, 342.0) | 270.0 | (166.8, 408.0) | <0.0001 |
| Insulin resistance (IR-HOMA)a | 0.7 | (0.4, 1.0) | 0.9 | (0.6, 1.3) | <0.0001 |
| Insulin increment a | 26.5 | (16.8, 45.5) | 35.3 | (21.6, 49.7) | 0.005 |
Log transformed variable; values are median and inter quartile range. P values for the differences between boys and girls derived using t-test or chi2 test.
Rural and GDM mothers breast-fed for longer than urban (p=0.001) and non-GDM mothers (p=0.05) respectively. There were no significant associations between breast-feeding duration and parental education or socio-economic status.
At 5-years, 120-minute glucose concentration increased while fasting insulin concentration and IR-HOMA decreased with increasing duration of breast-feeding, independent of potential confounders (Table 2). The 30-minute insulin concentration tended to fall with increasing breast-feeding duration, though this was non-significant. At 9.5-years, 120-minute glucose concentration decreased with increasing duration of breast-feeding. IR-HOMA was unrelated to breast-feeding duration (Table 2).
Table 2.
Associations of duration of breast-feeding and age at introduction of complementary foods with glucose/insulin concentrations at 5 and 9.5 years: Multiple linear regression analysis
| Breast-feeding duration | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Glucose/insulin oncentrations | 5 Years | 9.5 Years | ||||||
|
| ||||||||
| β (95% CI) | Pb | Pc | Pd | β (95% CI) | Pb | Pc | Pd | |
| Glucose | ||||||||
| Fasting glucose (mmol/l) | 0.01 (−0.02,0.05) | 0.4 | 0.6 | 0.4 | 0.002 (−0.02, 0.03) | 0.9 | 0.9 | 0.9 |
| 120 min glucose (mmol/l) | 0.08 (0.02,0.15) | 0.02 | 0.04 | 0.03 | −0.06 (−0.13, 0.0003) | 0.051 | 0.02 | 0.02 |
| Insulin | ||||||||
| Fasting insulin (pmol/l)a | −0.06 (−0.11, −0.02) | 0.006 | 0.01 | 0.03 | 0.001 (−0.04, 0.04) | 0.9 | 0.7 | 0.9 |
| 30 min insulin (pmol/l)a | −0.04 (−0..10, 0.01) | 0.1 | 0.1 | 0.3 | 0.02 (−0.03, 0.07) | 0.4 | 0.5 | 0.4 |
| Insulin resistance (IR)a | −0.06 (−0.11, −0.01) | 0.01 | 0.02 | 0.046 | 0.001 (−0.04, 0.04) | 0.9 | 0.7 | 0.9 |
| Insulin increment | −0.01 (−0.07, 0.05) | 0.8 | 0.8 | 0.9 | 0.01 (−0.04, 0.06) | 0.6 | 0.6 | 0.4 |
|
| ||||||||
| Age at introduction of complementary foods | ||||||||
|
| ||||||||
| Glucose | ||||||||
| Fasting glucose (mmol/l) | −0.02 (−0.06, 0.03) | 0.5 | 0.7 | 0.7 | 0.01 (−0.02, 0.05) | 0.5 | 0.5 | 0.4 |
| 120 min glucose (mmol/l) | 0.05 (−0.04, 0.14) | 0.3 | 0.2 | 0.1 | −0.01 (−0.09, 0.07) | 0.8 | 0.9 | 1.0 |
| Insulin | ||||||||
| Fasting insulin (pmol/l)a | 0.01 (−0.05,0.07) | 0.7 | 0.7 | 0.6 | 0.01 (−0.04, 0.07) | 0.6 | 0.4 | 0.1 |
| 30 min insulin (pmol/l)a | 0.04 (−0.03, 0.11) | 0.3 | 0.1 | 0.09 | −0.01(−0.08, 0.05) | 0.7 | 0.8 | 0.5 |
| Insulin resistance (IR)a | 0.01 (−0.06, 0.07) | 0.8 | 0.8 | 0.6 | 0.02 (−0.04, 0.07) | 0.5 | 0.3 | 0.1 |
| Insulin increment | 0.01 (−0.07, 0.09) | 0.8 | 0.7 | 0.6 | 0.01 (−0.08, 0.06) | 0.8 | 0.6 | 0.4 |
Log transformed variable; Regression co-efficient (β) is the effect size per category increase in breast-feeding duration (<3, 3-5, 6-8., 9-11, 12-17 and 18+ months) or age at starting complementary foods (<4, 4, 5, and ≥6 months); Pb adjusted for sex and child’s current age; Pc further adjusted for confounders (socio-economic status, parents’ education, rural/urban residence maternal GDM status and birthweight); Pd further adjusted for child’s current BMI
Breast-feeding duration correlated with the age at starting complementary foods (Spearman r=0.1; p=0.02). Earlier introduction of complementary foods was associated with higher BMI at 9.5-years (p=0.04) and maternal education (p=0.051). There were no significant associations, at either 5- or 9.5-years, of age at starting complementary foods with glucose tolerance or IR-HOMA (Table 2).
The findings were unchanged when we re-analysed our data using breast-feeding duration or age at starting complementary foods as continuous or normalised variables, or if the categories were changed; when breast-feeding duration and age at starting complementary foods were included simultaneously in the regression; when preterm (n=34) and offspring of GDM mothers (n=33) were excluded and when weight at 1-year was included with all the other confounders (data not shown). The associations were similar in boys and girls and there were no non-linear associations.
Discussion
We have shown in a cohort of Indian children, among whom prolonged breast-feeding was the norm across all socio-economic groups, that longer breast-feeding duration was associated with lower fasting insulin concentrations and IR-HOMA at 5-years, but not at 9.5-years. Longer breast-feeding duration was associated with higher 120-min glucose concentration at 5-years, but lower 120-min glucose at 9.5-years. These associations were independent of all potential confounding factors measured. There were no significant associations of age at starting complementary foods with glucose/insulin concentrations.
A strength of the study was that breast-feeding data were obtained prospectively. Limitations were a lack of information on the exclusivity and frequency of breast-feeding, the nutritional quality of the breast milk or the type of milk used after stopping breast-feeding, the type and nutritional quality of complementary foods, and current diet and physical activity.
Longer breast-feeding duration was associated with lower fasting insulin concentrations and IR-HOMA at 5- but not 9.5-years, suggesting that this association changes with age. There are no previous reports of serial measures of insulin in relation to breast-feeding. A meta-analysis of studies in developed countries (4 in adults and 2 in children) [2] showed no differences in fasting insulin concentrations among those who were breast-fed or not. Similarly, 2 studies in adults [1,2] and 3 in children [1,2,6] reported no association of exclusive breast-feeding and/or its duration with IR-HOMA [1,2,]. However, a recent study reported an inverse association between breast-feeding duration and IR-HOMA in adult men [7].
A notable finding in our study was that the association between breast-feeding duration and 120-minute glucose concentrations changed in direction between 5- and 9.5-years. Children who stopped breast-feeding earlier had lower 120-min glucose concentrations at 5-years, but higher 120-min glucose at 9.5-years. In addition to having higher fasting insulin concentrations, they tended to have higher 30-minute insulin concentrations. This may be analogous to the phenomenon observed in animal studies, in which offspring of rat dams exposed to a nutritional insult (high carbohydrate or low protein diets) during pregnancy have fewer and smaller pancreatic islets, long-term changes in the structure and function of insulin-sensitive tissues (liver, adipocytes and muscle) and reduced insulin signalling protein expression. These changes are associated with hyperinsulinaemia, enhanced glucose tolerance or reactive hypoglycaemia in the young animal and to glucose intolerance later [8]. Such a progression has not previously been reported in humans, though few have serial data like ours through childhood.
Consistent with findings from many studies including a meta-analysis, mainly from high-income countries, our data at 9.5-years suggest that longer breast-feeding is associated with better glucose tolerance, and possibly lower diabetes risk, in later life. Whether these associations are causal is unknown, but animal studies have demonstrated that the high long-chain polyunsaturated fatty acid content of breast milk may suppress pro-inflammatory cytokine production, regulate neurotransmitter function, enhance insulin receptor numbers in the brain and other tissues, and decrease IR-HOMA [9]. Whether these changes could persist up to the age of 5- or 9.5-years is unknown. Breast-fed babies are thought to develop better satiety sensing than formula-fed babies and gain less weight during infancy (1), both of which may protect against later obesity and thus diabetes.
Few studies have examined the association of age at starting complementary foods with later IR-HOMA and glucose tolerance. Consistent with our findings, a recent study found no association among young adults [10]. Since growth in infancy influences later health, timely introduction and nutritional quality of complementary foods are important for promoting optimal growth and lifelong health.
To conclude, in this cohort of healthy Indian children, there was evidence of a possible protective effect of longer breast-feeding duration against later diabetes, suggesting that promoting WHO guidelines (exclusive breast-feeding for 6-months, introduction of nutritious complementary foods from 6-months, and continued breast-feeding up to 2-years) [11] may contribute to reducing the escalating incidence of diabetes in developing countries. This improved glucose tolerance may be associated with a transient period of lower IR-HOMA and higher glucose concentrations in early childhood.
Acknowledgements
We are grateful to the families who participated in the study. We acknowledge the contribution made to the study by the research team (Epidemiology Research Unit, HMH, Mysore), and SNEHA-India for its support. The study was funded by the Parthenon Trust, Switzerland, the Wellcome Trust, UK and the Medical Research Council, UK.
Abbreviations
- GDM
Gestational diabetes mellitus
- IR-HOMA
Insulin resistance
Footnotes
Disclosure: Authors have no conflict of interest to declare.
References
- 1.Horta BL, Bahl R, Martinés JC, Victora CG. Evidence on the long-term effects of breastfeeding. WHO; 2007. [Google Scholar]
- 2.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. Am J Clin Nutr. 2006;84:1043–1054. doi: 10.1093/ajcn/84.5.1043. [DOI] [PubMed] [Google Scholar]
- 3.Krishnaveni GV, Hill JC, Leary SD, et al. Anthropometry, glucose tolerance, and insulin concentrations in Indian children: relationships to maternal glucose and insulin concentrations during pregnancy. Diabetes Care. 2005;28:2919–2925. doi: 10.2337/diacare.28.12.2919. [DOI] [PubMed] [Google Scholar]
- 4.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 5.Wareham NJ, Phillips DIW, Byrne CD, Hales CN. The 30 minute insulin incremental response in an oral glucose tolerance test as a measure of insulin secretion. Diabet Med. 1995;12:684–688. doi: 10.1111/j.1464-5491.1995.tb00399.x. [DOI] [PubMed] [Google Scholar]
- 6.Corvalán C, Kain J, Weisstaub G, Uauy R. Impact of growth patterns and early diet on obesity and cardiovascular risk factors in young children from developing countries. Proc Nutr Soc. 2009;68:327–337. doi: 10.1017/S002966510900130X. [DOI] [PubMed] [Google Scholar]
- 7.Pearce MS, Unwin NC, Parker L, Alberti KG. Life course determinants of insulin secretion and sensitivity at age 50 years: the Newcastle thousand families study. Diabetes Metab Res Rev. 2006;22:118–125. doi: 10.1002/dmrr.573. [DOI] [PubMed] [Google Scholar]
- 8.Jones RH, Ozanne SE. Intra-uterine origins of type 2 diabetes. Arch Physiol Biochem. 2007;113:25–29. doi: 10.1080/13813450701318484. [DOI] [PubMed] [Google Scholar]
- 9.Lombardo YB, Chicco AG. Effects of dietary polyunsaturated n-3 fatty acids on dyslipidemia and insulin resistance in rodents and humans. J Nutr Biochem. 2006;17:1–13. doi: 10.1016/j.jnutbio.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Fall CH, Borja JB, Osmond C, et al. Infant-feeding patterns and cardiovascular risk factors in young adulthood: data from five cohorts in low- and middle-income countries. Int J Epidemiol. 2011;40:47–62. doi: 10.1093/ije/dyq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. UNICEF . Global strategy for infant and young child feeding. WHO; Geneva: 2003. [Google Scholar]