Abstract
Caspase-independent cell death is known to be important in physiological and pathological conditions, but its molecular regulation is not well-understood. Eiger is the sole fly ortholog of TNF. The ectopic expression of Eiger in the developing eye primordium caused JNK-dependent but caspase-independent cell death. To understand the molecular basis of this Eiger-induced nonapoptotic cell death, we performed a large-scale genetic screen in Drosophila for suppressors of the Eiger-induced cell death phenotype. We found that molecules that regulate metabolic energy production are central to this form of cell death: it was dramatically suppressed by decreased levels of molecules that regulate cytosolic glycolysis, mitochondrial β-oxidation of fatty acids, the tricarboxylic acid cycle, and the electron transport chain. Importantly, reducing the expression of energy production-related genes did not affect the cell death triggered by proapoptotic genes, such as reaper, hid, or debcl, indicating that the energy production-related genes have a specific role in Eiger-induced nonapoptotic cell death. We also found that energy production-related genes regulate the Eiger-induced cell death downstream of JNK. In addition, Eiger induced the production of reactive oxygen species in a manner dependent on energy production-related genes. Furthermore, we showed that this cell death machinery is involved in Eiger's physiological function, because decreasing the energy production-related genes suppressed Eiger-dependent tumor suppression, an intrinsic mechanism for removing tumorigenic mutant clones from epithelia by inducing cell death. This result suggests a link between sensitivity to cell death and metabolic activity in cancer.
Keywords: necroptosis, chromosomal deficiency screen, tumor, scribble
Recent studies have revealed that nonapoptotic cell death is important in both physiological and pathological conditions; however, its molecular mechanism is still largely unknown. Agonistic ligands of death receptors, such as tumor necrosis factor-α (TNFα), FasL, and TNF-related apoptosis-inducing ligand (TRAIL), generally induce caspase-dependent cell death, the typical form of apoptosis (1). Intriguingly, these ligands can also induce nonapoptotic cell death when caspases are inhibited or cannot be activated efficiently (2, 3).
A surprising finding that implied the importance of nonapoptotic cell death was that, except in the brain, mice mutant for core regulators of apoptosis do not exhibit severe embryonic tissue-sculpting defects such as interdigital tissue removal, a classic example of physiological cell death (although bax−/−bak−/− double mutant mice have some remaining interdigital tissue) (4). Interestingly, nonapoptotic cell death is observed in the interdigital region of apaf-1 mutant mice (5, 6). These results indicate that nonapoptotic cell death may function physiologically to remove unnecessary tissue or as an alternative mechanism to remove cells when the apoptotic machinery is inhibited. Caspase-independent or nonapoptotic cell death has also been observed in oncogenic ras-induced cell death (7) and under other pathological conditions (8).
We and other groups previously reported that the Drosophila genome encodes one pair of TNF/TNF receptor (TNFR) superfamily proteins: Eiger and its receptor, Wengen (9–12). The ectopic expression of Eiger induces cell death through the activation of c-Jun N-terminal kinase (JNK) signaling both in vivo (9, 10) and in vitro (12). Intriguingly, Eiger-induced cell death seems far less sensitive to the baculovirus-derived pan-caspase inhibitor p35 (9, 10) than other apoptotic cell deaths (13). Thus, the Eiger-induced cell death signaling in Drosophila can provide a powerful genetic model system for studying the conserved mechanism of TNF-induced, caspase-insensitive cell death signaling in vivo. Although several downstream molecules that mediate Eiger-Wengen signaling have been identified (9–12, 14–18), little is known about the mechanism by which these molecules mediate nonapoptotic cell death. In mammals, the signaling cascade of nonapoptotic cell death has been studied since the identification of necroptosis, which is induced by TNF in the presence of caspase inhibitor (19–21). However, no comprehensive genetic investigation to elucidate the mechanisms of nonapoptotic cell death, programmed necrosis, or necroptosis in vivo has been reported. Therefore, to examine the genetic control of nonapoptotic cell death, we chose a forward genetic screen for Eiger/TNF-induced cell death signaling in Drosophila.
In this study, we found that Eiger-induced nonapoptotic cell death signaling requires molecules related to the production of metabolic energy downstream of activated JNK. The ectopic activation of Eiger signaling resulted in the overproduction of reactive oxygen species (ROS) downstream of JNK signaling. We also found that this cell death system is, at least in part, used as an intrinsic tumor suppression system in endogenous Eiger signaling to remove tumorigenic scrib mutant cells from epithelia.
Results
Eiger-Induced Cell Death Does Not Require the Canonical Caspase Activation Pathway in Drosophila Developing Eyes.
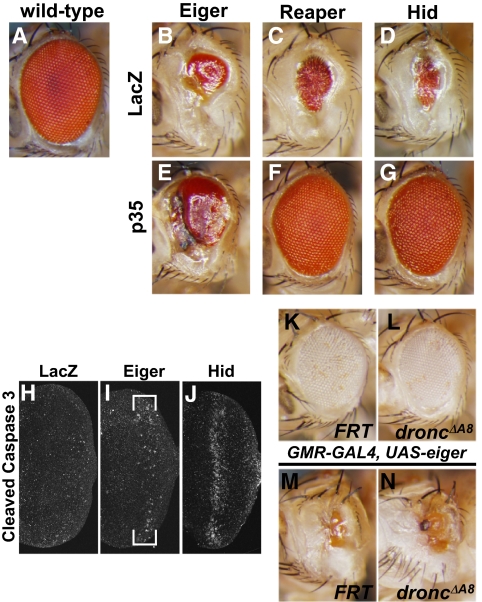
We previously showed that the sole Drosophila TNF superfamily ligand, Eiger, induces massive cell death when it is ectopically expressed in the developing eye primordium (eye imaginal disc). This cell death requires the activation of the Drosophila JNK, Basket (Bsk) (9, 10). Intriguingly, although Eiger has been shown to activate caspases (9, 10, 22, 23), the Eiger-induced eye phenotype was only slightly suppressed by coexpression of the pan-caspase inhibitor, p35 (Fig. 1 B and E) (9, 10), whereas p35 almost completely suppressed the cell death phenotype triggered by the ectopic expression of the proapoptotic gene reaper or head involution defective (hid) (Fig. 1 C, D, F, and G). This finding suggested that the Eiger-induced cell death is only partly dependent on caspases. Therefore, we first sought to clarify the requirement for caspase activation in Eiger-induced cell death. Immunostaining for cleaved caspase 3 revealed that the overexpression of Eiger only slightly increased the caspase 3-like protease activity (Fig. 1 H–J). In addition, because p35 blocks the effector caspases drICE and DCP1 but not the initiator caspase Dronc (24, 25), we evaluated Dronc's involvement in the Eiger-induced cell death by overexpressing Eiger in a dronc-null mutant background. However, no phenotypic suppression was observed under this condition (Fig. 1 K–N), consistent with a previous observation that the overexpression of a dominant negative form of Dronc does not affect the Eiger-induced eye phenotype (9). Thus, Eiger-induced cell death requires little, if any, activation of the canonical caspase pathway in the eye imaginal disc.
Fig. 1.
Eiger-induced cell death is caspase-independent in Drosophila eyes. (A–G) Genetic interactions between the pan-caspase inhibitor p35 and Eiger, Reaper, or Hid. Note that the coexpression of p35 with Eiger did not significantly suppress the reduction of the adult eye size. (H–J) Ectopic expression of Eiger did not induce a significant increase in caspase 3-like activity. (K–N) Loss of the Drosophila initiator caspase, dronc, did not suppress the Eiger-induced eye reduction. The dronc-null mutant allele is described in ref. 49. In all images, anterior is to the left.
Eighteen Deficiencies That Dramatically Suppress the Eiger-Induced Eye Phenotype Identified by a Genome-Wide Dominant Modifier Screen.
To understand the molecular mechanism of the Eiger-induced nonapoptotic cell death in vivo, we conducted a genome-wide screen using a series of chromosomal deficiency lines to identify dominant suppressors of Eiger-induced eye reduction (Materials and Methods). We screened more than 80% of the genome and recovered 18 deficiency alleles, which we named suppressors of Eiger (SEs 1–18) (Fig. S1 and Table S1). The responsible regions for the suppression in these deficiency alleles were narrowed down by using sets of small, partially overlapping deficiencies, and the responsible genes were determined by screening publicly available mutant lines. The Drosophila TNF receptor, Wengen, was identified in the deficiency region of SE1, as described previously (11).
Knockdown of Genes Related to Energy Production Suppresses the Eiger-Induced Small Eye Phenotype.
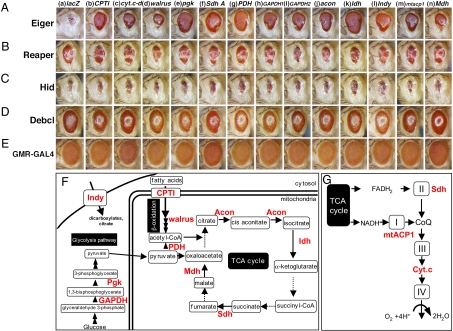
We noticed that genes involved or predicted to be involved in mitochondrial function were frequently found in the responsible regions of the SE lines (Table S1). For instance, the Eiger-induced small eye phenotype was significantly suppressed by P-element insertions into or near the loci of cytochrome c-d (cyt.c-d; it encodes one of two isoforms of Drosophila Cyt.c), Indy (a Krebs cycle intermediate transporter at the plasma membrane), Nmd (an ATPase), or CG18317 (a mitochondrial carrier protein) (Table S1). In addition, the locus of Isocitrate dehydrogenase, which encodes a mitochondrial tricarboxylic acid (TCA) cycle enzyme, was involved in a small deletion of Df(3L)66C-I65, which was recovered as the small deletion in SE8 (Table S1). Because the mitochondrion is the major energy-producing organelle, we next evaluated the role of metabolic energy production-related genes in the regulation of Eiger-induced cell death. To examine the contribution of energy homeostasis to Eiger-induced cell death, we knocked down energy production-related genes in Eiger-expressing imaginal discs. Strikingly, the knockdown of genes involved in cytoplasmic glycolysis, the mitochondrial TCA cycle, the β-oxidation of fatty acids, or the electron transport chain was effective in suppressing the Eiger-induced small eye phenotype (Fig. 2 A, F, and G). However, the knockdown of these genes had no effect on the caspase-dependent cell death triggered by the overexpression of Reaper, Hid, or Debcl (Fig. 2 B–D), indicating that regulation by energy production-related molecules is specific for the Eiger-induced nonapoptotic cell death. These results indicated that the energy production system is a crucial regulator of Eiger signaling.
Fig. 2.
Energy production-related proteins are specifically required to execute Eiger-induced cell death signaling. (A–E) Light micrographs of transgenic flies. The genetic background in A–E was w; UAS-eigerregg1/+; GMR-GAL4/+ (A), w; GMR-GAL4/+; GMR-rpr/+ (B), w; GMR- hid/+; GMR-GAL4/+ (C), w; GMR-GAL4/+; UAS-debcl/+ (D), and w; GMR-GAL4/+; +/+ (E). Columns a–n indicate the responsible genes for each UAS-dsRNA line. Anterior is to the left. (F and G) Schematic diagram of glycolysis, β-oxidation of fatty acids, and the TCA cycle (F) and diagram of the electron transport chain (G). Responsible genes for RNAi lines that suppressed the Eiger-induced phenotype are shown in red letters. Dashed arrows indicate that the corresponding genes have not been mapped to the chromosome yet or RNAi flies are not publicly available. Complete genotypes and abbreviations are listed in SI Materials and Methods.
Energy Production-Related Molecules Act Downstream of JNK Signaling.
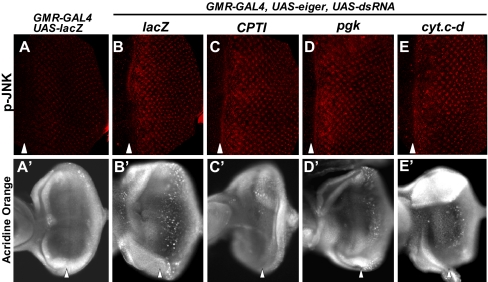
Because Eiger-induced cell death is mediated by the JNK pathway (9, 10), we next examined the epistasis between the JNK pathway and the energy production-related genes that we identified as suppressors of Eiger. The knockdown of carnitine-palmitoyl transferase-I (CPTI; believed to be the rate-limiting step of fatty acid oxidation), phosphoglycerate kinase (pgk; a transferase in glycolysis), or cyt.c-d (electron transport chain protein) significantly inhibited cell death (Fig. 3 A′–E′) without affecting JNK activation (Fig. 3 A–E), suggesting that these molecules mediate Eiger-induced cell death signaling downstream of JNK.
Fig. 3.
Energy production-related molecules act downstream of the JNK pathway. Eye imaginal discs of wandering third instar larvae stained with an anti-phospho-JNK antibody (A–E) and acridine orange (A′–E′). (A–E) Eiger-induced activation of JNK was not suppressed by the down-regulation of energy production-related molecules. (A′–E′) The extensive Eiger-induced cell death posterior to the morphogenetic furrow was suppressed by the down-regulation of energy production-related molecules. In all images, anterior is to the left, and arrowheads indicate the morphogenetic furrow.
Eiger-Induced Cell Death Is Accompanied by the Production of ROS.
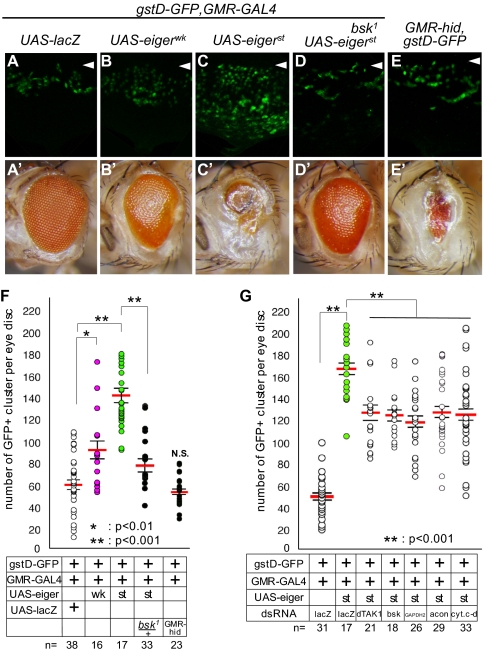
In some cell lines, such as the murine fibrosarcoma-derived L929, treatment with TNFα induces caspase-independent cell death through the production of ROS (26). We therefore examined if Eiger-induced cell death follows oxidative stress using flies that express Glutathione S transferase D1-GFP (gstD-GFP), a ROS-inducible gstD promoter-GFP reporter (27, 28). When Eiger was ectopically expressed in the eye imaginal discs of these flies, the cells showed an oxidative stress response, which was revealed by the increase in gstD-GFP expression (Fig. 4 A–C). The strength of this response correlated with the strength of the adult eye phenotype (Fig. 4 A–C and A′–C′). Furthermore, dihydroethidium staining of these eye discs, in which Eiger was expressed in small patches of the disc, indicated that the cells that ectopically expressed Eiger produced superoxide (O2−) (Fig. S2). In addition, the up-regulation of gstD-GFP reporter expression was strongly suppressed by reducing the gene dosage of bsk (Fig. 4 C, C′, D, D′, and F). Furthermore, the ectopic expression of a JNK kinase kinase, dTAK1, also increased the gstD-GFP signal (Fig. S3). These results suggest that oxidative stress is induced downstream of the activated JNK pathway.
Fig. 4.
Activation of Eiger signaling induces ROS production. (A–E) Eye imaginal discs of wandering third instar larvae. Anterior is to the top. Arrowheads indicate the morphogenetic furrow. (A′ –E′) Adult eye images of A–E. Anterior is to the left. (F) The number of proommatidial clusters that expressed GFP within the single eye discs shown in A–E was counted and analyzed statistically. Asterisks indicate statistical significance, which was determined by Student's t test. *P < 0.01; **P < 0.001. wk and st mean weak and strong alleles of UAS-eiger, respectively. (G) RNAi-based knockdown of JNK pathway components or mitochondria-related genes attenuated the production of ROS induced by the overexpression of Eiger. Each bar in the graphs of F and G represents the mean ± SEM. The number of independent data points in F and G is indicated as n.
We also found that the knockdown of energy production-related genes such as cyt.c-d, GAPDH2, or aconitase significantly attenuated the production of oxidative stress that was induced by the ectopic expression of Eiger to a similar extent as the knockdown of bsk or dTAK1 (Fig. 4G). In contrast, the overexpression of Hid did not increase the gstD-GFP signal (Fig. 4 E, E′, and F). These results, together with the findings shown in Fig. 3, suggest that the energy production-related molecules regulate the production of ROS downstream of JNK in the Eiger-induced cell death pathway.
Energy Production-Related Molecules Are Required for the Physiological Eiger-Induced Death of Tumorigenic Mutant Cells.
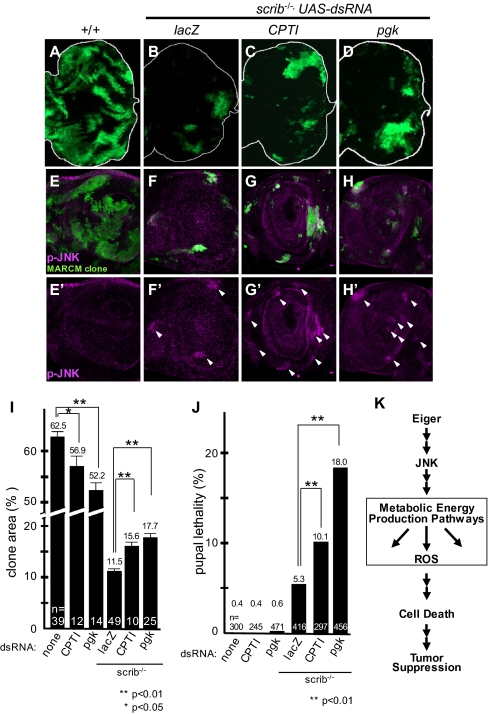
We next assessed whether these molecules contribute to the cell death triggered by endogenous Eiger. The imaginal tissue of mutants for evolutionarily conserved tumor suppressors, such as scribble (scrib) or discs large (dlg), overgrow and develop into tumors (29). However, when these tumorigenic mutant cells are surrounded by WT tissue, they do not overgrow but are, instead, eliminated from the tissue through Eiger–JNK-induced cell death (30, 31). Thus, physiological Eiger signaling functions as an intrinsic tumor suppressor to eliminate tumorigenic mutant cells. We therefore asked if energy production-related molecules regulate the endogenous Eiger signaling during the elimination of tumorigenic scrib mutant clones. To this end, we reduced the expression of CPTI (for fatty acid metabolism) or pgk (for glycolysis) in scrib mutant clones by the Mosaic Analysis with a Repressible Cell Marker (MARCM) method (32). The scrib mutant clones generated in the eye antennal discs were largely eliminated from the tissue during the larval and pupal stages (Fig. 5 A, B, E, and F) (31). However, when CPTI or pgk was knocked down, the area of the tumorigenic scrib mutant clones in the eye antennal discs increased significantly (Fig. 5 B–D and I), although strong JNK activation was still observed in these cells (Fig. 5 E–H and E′–H′). The knockdown of CPTI or pgk in the WT cells did not increase the clone area or pupal lethality; rather, it decreased the clone area, presumably because of the reduction in energy production (Fig. 5I). Therefore, the increased area of the tumorigenic scrib mutant clone was not an additive effect of the CPTI- or pgk-RNAi. Consistent with this result, a significantly greater number of animals carrying these cells than control animals died as pupae (Fig. 5J). Together, these results indicated that energy production-related genes are crucial for the endogenous Eiger-JNK-mediated cell death signaling that removes tumorigenic scrib cells from epithelia.
Fig. 5.
Energy production-related molecules are required for the endogenous Eiger-JNK–induced cell death signaling that removes tumorigenic scrib cells. GFP-labeled WT (A, E, and E′), scrib mutant clones (B, F, and F′), or scrib mutant clones with RNAi constructs against energy production-related genes (C, D, G, G′, H, and H′) were generated in eye antenna imaginal discs. E–H show the anti-phospho-JNK antibody staining (magenta) and MARCM clone (green). E′–H′ show the anti-phospho-JNK antibody staining of E–H. Arrowheads indicate JNK activation in the clone. A–D show eye discs. E–H and E′–H′ show antenna discs. Anterior is to the left. (I) RNAi of energy production-related genes had a survival effect on scrib cells. (J) Pupal lethality caused by the clones induced in eye antennal discs. Asterisks in I and J indicate statistical significance, determined by Student's t test. *, P < 0.05; **, P < 0.01. Each bar in the graphs of I and J represents the mean ± SEM. The number of independent data points in I and J is indicated as n. (K) Schematic diagram for the JNK-mediated cell death signaling triggered by Eiger. Eiger-stimulated signaling is transduced to the JNK pathway through a Wengen/dTRAF2/dTAB2/dTAK1-mediated signal (9–12, 16–18). The activated JNK signaling is mediated by the energy metabolism-related pathway as shown in this paper. The deregulation of mitochondrial energy homeostasis, involving ROS production, was induced downstream of the activated JNK and energy production. This molecular mechanism is also used by physiological Eiger signaling to remove tumorigenic scrib cells.
Discussion
Ectopic Expression of Eiger Induces Nonapoptotic Cell Death.
In this paper, we provide genetic evidence that metabolic energy production-related molecules are central to the Eiger/TNF-induced cell death (Fig. 5K). We also provide evidence that this cell death system is, at least in part, used in endogenous Eiger signaling to remove tumorigenic mutant cells from epithelia as an intrinsic tumor suppression system.
It was recently revealed that at least some nonapoptotic cell deaths can be categorized as necroptosis, in which cells undergo nonapoptotic cell death under apoptosis-deficient conditions when treated with agonistic ligands of death receptors, such as TNFα, FasL, or TRAIL (33). The Eiger-induced cell death shares features with necroptosis in that it is triggered by TNF family proteins, produces ROS, and is caspase-independent. Furthermore, the Drosophila homolog of a tumor suppressor protein, Cylindromatosis, one of the essential regulators of necroptosis (34, 35), has been shown to regulate JNK activation in Eiger-induced cell death signaling (16). However, despite the high conservation of most of the apoptotic machinery, blast search analysis has not identified Drosophila homologs of receptor-interacting protein (RIP) 1 or RIP3, the essential kinases for inducing necroptosis (19, 20, 36–39).
As we showed in Fig. S3, oxidative stress could be induced downstream of the activated JNK pathway. However, we also found that the knockdown of energy production-related genes such as cyt.c-d or GAPDH2 did not suppress the dTAK1- or HepCA-induced cell death phenotype (Fig. S4). This finding could be caused by the overexpression of dTAK1- or HepCA-induced additional pathways, because we found that dTAK1 or HepCA overexpression induced JNK activation much more strongly than Eiger overexpression (Fig. S5). Because dTAK1 also functions downstream of Imd in the innate immune response, Imd-related signaling was another possible mechanism for mediating Eiger signaling. Therefore, we examined the genetic interaction between Eiger-induced cell death and Imd or Imd-related genes. However, we did not find any significant interactions between them (Fig. S6). Therefore, it would be interesting to examine if other proteins can substitute for the functions of RIP1 or RIP3 in Eiger signaling or if fly TNF signaling uses other mechanisms to induce nonapoptotic cell death.
TNF-Induced Nonapoptotic Cell Death and Energy Production.
It has been reported that TNF-induced nonapoptotic cell death leads to the RIP3-dependent activation of glycogen phosphorylase, which is the rate-limiting enzyme in the degradation of glycogen and therefore, the key molecule for regulating energy production (39). In this context, ROS can be generated by the production of excess energy (39). This finding could explain why the down-regulation of energy production-related genes suppressed Eiger-induced cell death. However, we also observed that the amount of ATP in the eye antenna imaginal disc decreased when Eiger was overexpressed, and this decrease in ATP was cancelled by the knockdown of bsk, CPTI, pgk, or cyt.c-d (Fig. S7). This finding suggests that the activation of energy production by Eiger signaling could also trigger another mechanism that decreases the tissue ATP level. It is possible that the tissue loses ATP simply because of the massive cell death caused by Eiger expression. Alternatively, the work by Temkin et al. (40) reported that treatment with TNFα and the caspase inhibitor zVAD not only induces nonapoptotic cell death with ROS production but also decreases ATP because of the inhibition of adenine nucleotide translocase (ANT) by RIP1. A similar ANT-dependent inhibition mechanism could be involved in Eiger-induced cell death. Because neither RIP1 nor RIP3 has yet been identified in Drosophila, the mechanism by which the total ATP is regulated in Eiger-induced cell death remains to be elucidated.
Drosophila Model of Intrinsic Tumor Suppression.
When scrib or dlg mutant cells are induced as clones in otherwise WT eye imaginal discs, most of these mutant cells are eliminated by Eiger–JNK-dependent cell death during development (31, 41). This cell death could involve a caspase-independent mechanism, because the elimination of mutant cells is not fully suppressed by the overexpression of p35 compared with the blockage of JNK signaling (30, 31). Thus, the mode of cell death triggered in scrib mutant clones is analogous to the mode of cell death triggered by the overexpression of Eiger in imaginal discs. Our observations suggest that the regulation of energy production could be a crucial determinant of the susceptibility of tumor cells to cytotoxic stimuli.
Interestingly, tumor cells frequently produce ATP by glycolysis in the cytosol rather than in the mitochondria, which is known as the Warburg effect (42, 43). Because mitochondrial energy production generates cytotoxic ROS, cancer cells might increase their resistance to cytotoxic stimuli by reducing mitochondrial energy production (43). In this sense, mitochondrial energy production could act as a tumor suppressor. In fact, subunits of a TCA cycle enzyme, Succinate dehydrogenase (Sdh; SdhB, SdhC, and SdhD), are reported to be classical tumor suppressors in pheochromocytoma or paraganglioma (44). Furthermore, a specific isoform of pyruvate kinase, which is involved in glycolysis, is necessary for cellular metabolism to shift to aerobic glycolysis and the promotion of tumorigenesis (45). Similarly, the activity of pyruvate dehydrogenase (PDH), which links the glycolytic pathway to the TCA cycle by transforming pyruvate to acetyl-CoA, is suppressed in cancer cells, whereas the reactivation of PDH induces cell death in a solid tumor cell line and xenografts (46). Interestingly, we found that the knockdown of Drosophila PDH (which is encoded by CG7010) (47) strongly suppressed Eiger-induced cell death (Fig. 2A, g). Furthermore, the down-regulation of genes involved in glycolysis and the β-oxidation of fatty acids significantly suppressed the elimination of scrib cells from imaginal epithelia (Fig. 5). These observations suggest that the regulation of cellular energy production or even the source of energy could be critical for controlling the susceptibility of cancer cells to cytotoxic stimuli such as TNFα.
Materials and Methods
The deficiency kit flies were obtained from Bloomington Stock Center. Transgenic flies for RNAi experiments were obtained from the Vienna Drosophila RNAi Center and National Institute of Genetics. The UAS-eigerregg1 allele was described previously (9, 48). The gstD-GFP reporter fly (27, 28) was used to detect the antioxidant response. Experiments were approved by the committee on Living Modified Organisms of Keio University, Kobe University, and The University of Tokyo. Additional details are in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Shu Kondo for sharing unpublished data, Hiroka Aonuma and Takahiro Chihara for helpful support, and Shu Kondo and Hirotaka Kanuka for discussions. We also thank Rieko Shimamura, Toshie Naoi, Naoko Tokushige, and Yuki Yamamoto-Goto for technical support, Shizue Ohsawa for technical advice, Takeshi Yagi for kind support and cooperation, and current and former H.O. and M.M. laboratory members for helpful discussions and comments. We thank Toshiro Aigaki, Sharad Kumar, Helena Richardson, Dirk Bohmann, Darren Williams, Yasushi Hiromi, Hermann Steller, Makoto Nakamura, the Bloomington Stock Center, the Drosophila Genetic Resource Center, the National Institute of Genetics stock center (NIG-FLY), and the Vienna Drosophila RNAi center for fly stocks. This work was supported in part by grants from the Japanese Ministry of Education, Science, Sports, Culture, and Technology (MEXT; to H.K., T.I., H.O., and M.M.), the Japan Society for the Promotion of Science (to H.K.), the Keio Gijuku Academic Development Funds (to H.K.), the Keio University Grant-in-Aid for Encouragement of Young Medical Scientists (to H.K.), the Strategic Research Foundation Grant-Aided Project for Private Universities from MEXT (to H.K.), the Global Center of Excellence (G-COE) program for Global Center for Education and Research in Integrative Membrane Biology (to T.I.), the International Human Frontier Science Program (to T.I.), the Japan Science and Technology Agency (to T.I. and M.M.), and grant-in-aid for the G-COE program from MEXT to Keio University (to H.K. and H.O.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103242108/-/DCSupplemental.
References
- 1.Guicciardi ME, Gores GJ. Life and death by death receptors. FASEB J. 2009;23:1625–1637. doi: 10.1096/fj.08-111005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laster SM, Wood JG, Gooding LR. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol. 1988;141:2629–2634. [PubMed] [Google Scholar]
- 3.Vercammen D, et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;187:1477–1485. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindsten T, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chautan M, Chazal G, Cecconi F, Gruss P, Golstein P. Interdigital cell death can occur through a necrotic and caspase-independent pathway. Curr Biol. 1999;9:967–970. doi: 10.1016/s0960-9822(99)80425-4. [DOI] [PubMed] [Google Scholar]
- 6.Candé C, Cecconi F, Dessen P, Kroemer G. Apoptosis-inducing factor (AIF): Key to the conserved caspase-independent pathways of cell death? J Cell Sci. 2002;115:4727–4734. doi: 10.1242/jcs.00210. [DOI] [PubMed] [Google Scholar]
- 7.Kitanaka C, Kuchino Y. Caspase-independent programmed cell death with necrotic morphology. Cell Death Differ. 1999;6:508–515. doi: 10.1038/sj.cdd.4400526. [DOI] [PubMed] [Google Scholar]
- 8.Yuan J, Kroemer G. Alternative cell death mechanisms in development and beyond. Genes Dev. 2010;24:2592–2602. doi: 10.1101/gad.1984410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Igaki T, et al. Eiger, a TNF superfamily ligand that triggers the Drosophila JNK pathway. EMBO J. 2002;21:3009–3018. doi: 10.1093/emboj/cdf306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreno E, Yan M, Basler K. Evolution of TNF signaling mechanisms: JNK-dependent apoptosis triggered by Eiger, the Drosophila homolog of the TNF superfamily. Curr Biol. 2002;12:1263–1268. doi: 10.1016/s0960-9822(02)00954-5. [DOI] [PubMed] [Google Scholar]
- 11.Kanda H, Igaki T, Kanuka H, Yagi T, Miura M. Wengen, a member of the Drosophila tumor necrosis factor receptor superfamily, is required for Eiger signaling. J Biol Chem. 2002;277:28372–28375. doi: 10.1074/jbc.C200324200. [DOI] [PubMed] [Google Scholar]
- 12.Kauppila S, et al. Eiger and its receptor, Wengen, comprise a TNF-like system in Drosophila. Oncogene. 2003;22:4860–4867. doi: 10.1038/sj.onc.1206715. [DOI] [PubMed] [Google Scholar]
- 13.Bergmann A, Yang AY, Srivastava M. Regulators of IAP function: Coming to grips with the grim reaper. Curr Opin Cell Biol. 2003;15:717–724. doi: 10.1016/j.ceb.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Cai Y, Chia W, Yang X. Drosophila homologs of mammalian TNF/TNFR-related molecules regulate segregation of Miranda/Prospero in neuroblasts. EMBO J. 2006;25:5783–5793. doi: 10.1038/sj.emboj.7601461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brodsky MH, et al. Drosophila melanogaster MNK/Chk2 and p53 regulate multiple DNA repair and apoptotic pathways following DNA damage. Mol Cell Biol. 2004;24:1219–1231. doi: 10.1128/MCB.24.3.1219-1231.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xue L, et al. Tumor suppressor CYLD regulates JNK-induced cell death in Drosophila. Dev Cell. 2007;13:446–454. doi: 10.1016/j.devcel.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Geuking P, Narasimamurthy R, Basler K. A genetic screen targeting the tumor necrosis factor/Eiger signaling pathway: Identification of Drosophila TAB2 as a functionally conserved component. Genetics. 2005;171:1683–1694. doi: 10.1534/genetics.105.045534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geuking P, Narasimamurthy R, Lemaitre B, Basler K, Leulier F. A non-redundant role for Drosophila Mkk4 and hemipterous/Mkk7 in TAK1-mediated activation of JNK. PLoS One. 2009;4:e7709. doi: 10.1371/journal.pone.0007709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Degterev A, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 20.Degterev A, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 22.Smith-Bolton RK, Worley MI, Kanda H, Hariharan IK. Regenerative growth in Drosophila imaginal discs is regulated by Wingless and Myc. Dev Cell. 2009;16:797–809. doi: 10.1016/j.devcel.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuda M, et al. POSH promotes cell survival in Drosophila and in human RASF cells. FEBS Lett. 2010;584:4689–4694. doi: 10.1016/j.febslet.2010.10.048. [DOI] [PubMed] [Google Scholar]
- 24.Meier P, Silke J, Leevers SJ, Evan GI. The Drosophila caspase DRONC is regulated by DIAP1. EMBO J. 2000;19:598–611. doi: 10.1093/emboj/19.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar S. Migrate, differentiate, proliferate, or die: Pleiotropic functions of an apical “apoptotic caspase.”. Sci STKE. 2004;2004:pe49. doi: 10.1126/stke.2542004pe49. [DOI] [PubMed] [Google Scholar]
- 26.Fiers W, Beyaert R, Declercq W, Vandenabeele P. More than one way to die: Apoptosis, necrosis and reactive oxygen damage. Oncogene. 1999;18:7719–7730. doi: 10.1038/sj.onc.1203249. [DOI] [PubMed] [Google Scholar]
- 27.Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sawicki R, Singh SP, Mondal AK, Benes H, Zimniak P. Cloning, expression and biochemical characterization of one Epsilon-class (GST-3) and ten Delta-class (GST-1) glutathione S-transferases from Drosophila melanogaster, and identification of additional nine members of the Epsilon class. Biochem J. 2003;370:661–669. doi: 10.1042/BJ20021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bilder D. Epithelial polarity and proliferation control: Links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–1925. doi: 10.1101/gad.1211604. [DOI] [PubMed] [Google Scholar]
- 30.Brumby AM, Richardson HE. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 2003;22:5769–5779. doi: 10.1093/emboj/cdg548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Igaki T, Pastor-Pareja JC, Aonuma H, Miura M, Xu T. Intrinsic tumor suppression and epithelial maintenance by endocytic activation of Eiger/TNF signaling in Drosophila. Dev Cell. 2009;16:458–465. doi: 10.1016/j.devcel.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee T, Winter C, Marticke SS, Lee A, Luo L. Essential roles of Drosophila RhoA in the regulation of neuroblast proliferation and dendritic but not axonal morphogenesis. Neuron. 2000;25:307–316. doi: 10.1016/s0896-6273(00)80896-x. [DOI] [PubMed] [Google Scholar]
- 33.Christofferson DE, Yuan J. Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol. 2010;22:263–268. doi: 10.1016/j.ceb.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 35.Hitomi J, et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holler N, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 37.Cho YS, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He S, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 39.Zhang DW, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 40.Temkin V, Huang Q, Liu H, Osada H, Pope RM. Inhibition of ADP/ATP exchange in receptor-interacting protein-mediated necrosis. Mol Cell Biol. 2006;26:2215–2225. doi: 10.1128/MCB.26.6.2215-2225.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cordero JB, et al. Oncogenic Ras diverts a host TNF tumor suppressor activity into tumor promoter. Dev Cell. 2010;18:999–1011. doi: 10.1016/j.devcel.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 43.Kim JW, Dang CV. Cancer's molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 44.Gottlieb E, Tomlinson IP. Mitochondrial tumour suppressors: A genetic and biochemical update. Nat Rev Cancer. 2005;5:857–866. doi: 10.1038/nrc1737. [DOI] [PubMed] [Google Scholar]
- 45.Christofk HR, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 46.Bonnet S, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 47.Seegmiller AC, et al. The SREBP pathway in Drosophila: Regulation by palmitate, not sterols. Dev Cell. 2002;2:229–238. doi: 10.1016/s1534-5807(01)00119-8. [DOI] [PubMed] [Google Scholar]
- 48.Toba G, et al. The gene search system. A method for efficient detection and rapid molecular identification of genes in Drosophila melanogaster. Genetics. 1999;151:725–737. doi: 10.1093/genetics/151.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kondo S, Senoo-Matsuda N, Hiromi Y, Miura M. DRONC coordinates cell death and compensatory proliferation. Mol Cell Biol. 2006;26:7258–7268. doi: 10.1128/MCB.00183-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.