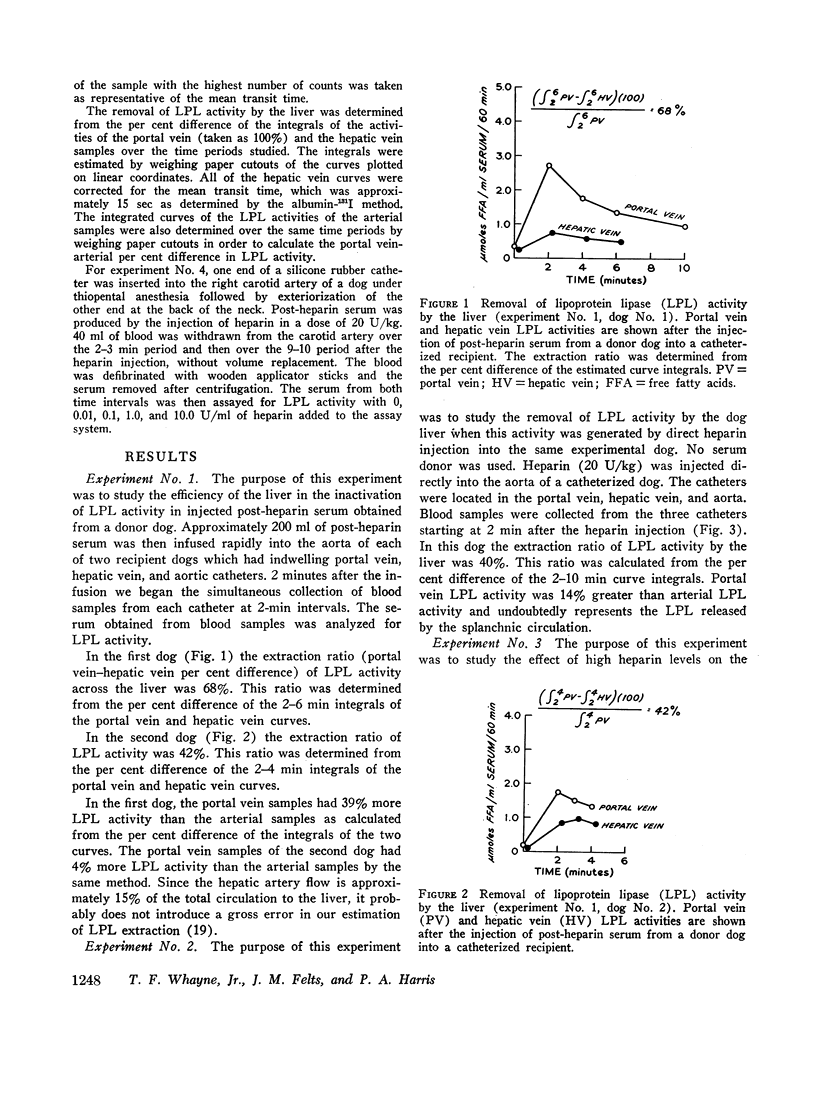
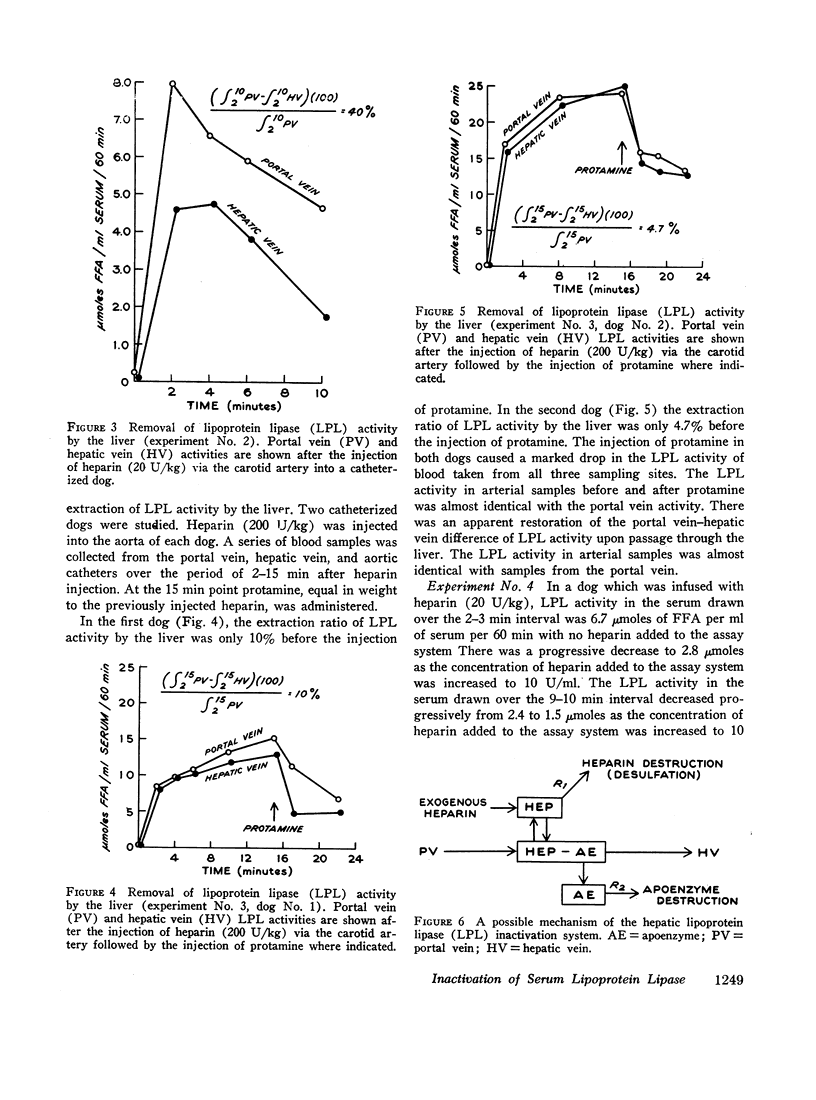
Abstract
The injection of heparin into the circulation produces a rapid increase in circulating serum lipoprotein lipase. The lipolysis system apparently circulates as a heparin-apoenzyme complex. Lipoprotein lipase activity disappears from the circulation in an exponential fashion. Available evidence suggests that a major site of removal of lipoprotein lipase activity is the liver. We have evaluated the efficiency of the inactivation system in catheterized unanesthetized dogs by studying the portal vein-hepatic vein difference in lipoprotein lipase activity. Our results demonstrate the high efficiency of the inactivation system in vivo. The results of this study also show that high levels of heparin can block the inactivation system and suggest a possible two-step mechanism. The first step in inactivation may involve the destruction of heparin by a liver heparinase. This step may induce dissociation of the active complex. After dissociation, the apoenzyme is apparently removed in a second step.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER S. P., LEVINE H., TURNER L., DUBIN A. Lipoprotein lipase response in Laennec's cirrhosis. Proc Soc Exp Biol Med. 1958 Dec;99(3):670–672. doi: 10.3181/00379727-99-24458. [DOI] [PubMed] [Google Scholar]
- BOBERG J., CARLSON L. A., NORMELL L. PRODUCTION OF LIPOLYTIC ACTIVITY BY THE ISOLATED, PERFUSED DOG LIVER IN RESPONSE TO HEPARIN. Life Sci. 1964 Sep;3:1011–1019. doi: 10.1016/0024-3205(64)90113-4. [DOI] [PubMed] [Google Scholar]
- CONDON R. E., TOBIAS H., DATTA D. V. IMPORTANCE OF LIVER IN RELEASE AND DEGRADATION OF LIPOPROTEIN LIPASE. Surg Forum. 1964;15:92–93. [PubMed] [Google Scholar]
- CONDON R. E., TOBIAS H., DATTA D. V. THE LIVER AND POSTHEPARIN PLASMA LIPOLYTIC ACTIVITY IN DOG AND MAN. J Clin Invest. 1965 May;44:860–869. doi: 10.1172/JCI105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONNOR W. E., ECKSTEIN J. W. The removal of lipoprotein lipase from the blood by the normal and diseased liver. J Clin Invest. 1959 Oct;38:1746–1755. doi: 10.1172/JCI103953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONSTANTINIDES P., SO Y., JOHNSTONE F. R. Role of liver and kidney in development of heparin-induced lipemia clearing activity. Proc Soc Exp Biol Med. 1959 Feb;100(2):262–264. doi: 10.3181/00379727-100-24593. [DOI] [PubMed] [Google Scholar]
- CRASS M. F., 3rd, MENG H. C. SERUM REQUIREMENT FOR RELEASE OF HEPARIN-INDUCED LIPASE FROM PERFUSED RAT HEART. Am J Physiol. 1964 Mar;206:610–614. doi: 10.1152/ajplegacy.1964.206.3.610. [DOI] [PubMed] [Google Scholar]
- Datta D. V. Removal of postheparin plasma "lipoprotein lipase" by the normal and diseased liver. Gastroenterology. 1965 Nov;49(5):515–520. [PubMed] [Google Scholar]
- Datta D. V. The mechanism of low postheparin plasma lipolytic activity (PHLA) in patients with cirrhosis of the liver. J Lab Clin Med. 1966 Mar;67(3):461–472. [PubMed] [Google Scholar]
- Hahn P. F. ABOLISHMENT OF ALIMENTARY LIPEMIA FOLLOWING INJECTION OF HEPARIN. Science. 1943 Jul 2;98(2531):19–20. doi: 10.1126/science.98.2531.19. [DOI] [PubMed] [Google Scholar]
- JEFFRIES G. H. The sites at which plasma clearing activity is produced and destroyed in the rat. Q J Exp Physiol Cogn Med Sci. 1954;39(4):261–270. doi: 10.1113/expphysiol.1954.sp001080. [DOI] [PubMed] [Google Scholar]
- KORN E. D. Clearing factor, a heparin-activated lipoprotein lipase. I. Isolation and characterization of the enzyme from normal rat heart. J Biol Chem. 1955 Jul;215(1):1–14. [PubMed] [Google Scholar]
- KORN E. D. Clearing factor, a heparin-activated lipoprotein lipase. II. Substrate specificity and activation of coconut oil. J Biol Chem. 1955 Jul;215(1):15–26. [PubMed] [Google Scholar]
- LEQUIRE V. S., HAMILTON R. L., ADAMS R., MERRILL J. M. LIPASE ACTIVITY IN BLOOD FROM THE HEPATIC AND PERIPHERAL VASCULAR BEDS FOLLOWING HEPARIN. Proc Soc Exp Biol Med. 1963 Oct;114:104–107. doi: 10.3181/00379727-114-28598. [DOI] [PubMed] [Google Scholar]
- Mayes P. A., Felts J. M. The functional status of lipoprotein lipase in rat liver. Biochem J. 1968 Jul;108(3):483–487. doi: 10.1042/bj1080483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTE D., Jr, WILLIAMS R. H. POST-HEPARIN LIPOLYTIC ACTIVITY FOLLOWING INTRAVENOUS HEPARIN-S35. Proc Soc Exp Biol Med. 1965 Mar;118:639–643. doi: 10.3181/00379727-118-29926. [DOI] [PubMed] [Google Scholar]
- REES J. R., REDDING V. J., ASHFIELD R. HEPATIC BLOOD-FLOW MEASUREMENT WITH XENON 133. EVIDENCE FOR SEPARATE HEPATIC-ARTERIAL AND PORTAL-VENOUS PATHWAYS. Lancet. 1964 Sep 12;2(7359):562–563. doi: 10.1016/s0140-6736(64)90623-3. [DOI] [PubMed] [Google Scholar]
- ROBINSON D. S., HARRIS P. M. The production of lipolytic activity in the circulation of the hind limb in response to heparin. Q J Exp Physiol Cogn Med Sci. 1959 Jan;44(1):80–90. doi: 10.1113/expphysiol.1959.sp001378. [DOI] [PubMed] [Google Scholar]
- TROUT D. L., ESTES E. H., Jr, FRIEDBERG S. J. Titration of free fatty acids of plasma: a study of current methods and a new modification. J Lipid Res. 1960 Apr;1:199–202. [PubMed] [Google Scholar]
- YOSHITOSHI Y., NAITO C., OKANIWA H., USUI M., MOGAMI T., TOMONO T. Kinetic studies on metabolism of lipoprotein lipase. J Clin Invest. 1963 May;42:707–713. doi: 10.1172/JCI104762. [DOI] [PMC free article] [PubMed] [Google Scholar]


