Abstract
Hypoxic-ischemic (H-I) injury to the developing brain is a significant cause of morbidity and mortality in humans. Other than hypothermia, there is no effective treatment to prevent or lessen the consequences of neonatal H-I. Increased expression of the NAD synthesizing enzyme nicotinamide mononucleotide adenylyl transferase 1 (Nmnat1) has been shown to be neuroprotective against axonal injury in the peripheral nervous system. To investigate the neuroprotective role of Nmnat1 against acute neurodegeneration in the developing CNS, we exposed wild-type mice and mice overexpressing Nmnat1 in the cytoplasm (cytNmnat1-Tg mice) to a well-characterized model of neonatal H-I brain injury. As early as 6 h after H-I, cytNmnat1-Tg mice had strikingly less injury detected by MRI. CytNmnat1-Tg mice had markedly less injury in hippocampus, cortex, and striatum than wild-type mice as assessed by loss of tissue volume 7 d days after H-I. The dramatic protection mediated by cytNmnat1 is not mediated through modulating caspase3-dependent cell death in cytNmnat1-Tg brains. CytNmnat1 protected neuronal cell bodies and processes against NMDA-induced excitotoxicity, whereas caspase inhibition or B-cell lymphoma-extra large (Bcl-XL) protein overexpression had no protective effects in cultured cortical neurons. These results suggest that cytNmnat1 protects against neonatal HI-induced CNS injury by inhibiting excitotoxicity-induced, caspase-independent injury to neuronal processes and cell bodies. As such, the Nmnat1 protective pathway could be a useful therapeutic target for acute and chronic neurodegenerative insults mediated by excitotoxicity.
Keywords: magnetic resonance imaging, transgenic, apoptosis, necrosis
Injury to the developing brain in newborns caused by hypoxia-ischemia (H-I) is a major cause of chronic disability and mortality often resulting in cognitive impairment, seizures, and motor abnormalities in the survivors (1, 2). Despite advances in the quality of perinatal medical care, functional abnormalities among the survivors of H-I remain common. Understanding the molecular mechanisms of H-I brain injury may provide new insights into pathogenesis and treatment. Our laboratory and those of others have used a well-characterized animal model of neonatal H-I in rodents (modified Levine model) to study the molecular mechanisms of neonatal brain injury and potential treatment strategies (3–6). In rats and mice, the H-I insult results in histological brain injury to the hemisphere ipsilateral to carotid ligation that is similar in many ways to injuries in the developing human brain after H-I (7–9). Studies have shown that this H-I insult to the neonatal brain results in several morphological forms of cell death: (i) early necrotic cell death; (ii) delayed cell death that has many features of apoptosis; and (iii) a form of cell death with a continuum of necrotic and apoptotic features (10–12). Blocking NMDA-type glutamate receptors can prevent most of the brain injury in this model (13), indicating that excitotoxicity can initiate all these cell-death pathways.
Nmnat1 is an NAD biosynthetic enzyme present in all living organisms (14). Because of its significant role in maintaining the equilibrium of reductive equivalents in cells, Nmnat1 is considered a potential target for the development of novel therapeutics for several pathological conditions (14). The Wlds mutant mouse that overexpresses a slow Wallerian degeneration protein (WldS), a fusion protein composed of the N-terminal 70 amino acids of ubiquitination factor E4 linked to full-length Nmnat1 exhibits slow axonal Wallerian degeneration in response to nerve injury in the peripheral nervous system (PNS). This WldS fusion protein protects axons from degeneration initiated by a variety of insults both in vitro and in vivo (15–17). Further, it has been shown in multiple axonal injury paradigms that expression of Nmnat1 robustly protects axons in vitro and in vivo in the PNS (18–20).
Here, we hypothesized that overexpression of Nmnat1 in the brain could be neuroprotective against acute neurodegeneration in the developing brain. We tested this notion using mice overexpressing the NAD synthesizing enzyme nicotinamide mononucleotide adenylyl transferase 1 (cytNmnat1-Tg) and a neonatal H-I paradigm; further, we sought to identify possible cell-death pathways influenced by Nmnat1 expression. We found that Nmnat1 overexpression was markedly neuroprotective in this model of brain injury. However, unlike previous studies with Nmnat1, we found that in this model Nmnat1 prevented neuronal cell death as well as axonal degeneration. Although Nmnat1 blocked excitotoxic, NMDA-dependent axonal degeneration and neuronal cell death, it did not block the activation of caspase-3 induced by the ischemic injury. These findings suggest that targeting pathways modulated by Nmnat1 specifically to block excitotoxicity-induced brain injury could be useful therapeutically.
Results
CytNmnat1 Protects the CNS of Neonatal Mice Against H-I–Induced Tissue Injury.
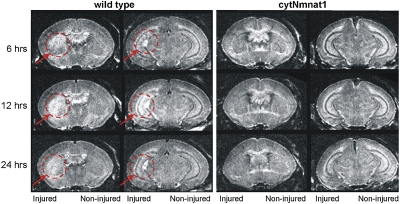
Studies have shown that changes observed with MRI 3–6 h after H-I predict the histopathological and behavioral defects present several weeks later in mice and rats (21, 22). This observation suggests that blocking the early changes seen on MRI can be used as a biomarker for protection against later tissue loss or neurodevelopmental disability after H-I. To determine the pattern of early injury in animals at postnatal (P) day 7, we performed MRI on cytNmnat1-Tg (n = 6) and wild-type (n = 7) littermate mice 6, 12, and 24 h after H-I. T2-weighted (T2W) images were obtained with the following parameters: repetition time (TR) 4 s, echo time (TE) 80 ms, and resolution of 59 × 59 × 250 μm. As early as 6 h after H-I, marked differences were found between T2W images from cytNmnat1-Tg and wild-type animals. T2W hyperintensity was clearly evident in the striatum and hippocampus in the wild-type mice ipsilateral to carotid ligation, but no corresponding changes were found in cytNmnat1 Tg mice (Fig. 1). Similarly, changes on MRI were increased 12 and 24 h after H-I in the wild-type mice, but no MRI changes were noted at these time points in the cytNmnat-Tg mice (Fig. 1).
Fig. 1.
Acute H-I damage in hippocampus, cortex, and striatum is inhibited in cytNmnat1-Tg mice. P7 mice underwent unilateral carotid artery ligation and exposure to hypoxia for 45 min. T2W MRI images were acquired 6, 12, and 24 h after H-I. Represent examples of T2W MRI images from wild-type (n = 7) and cytNmnat1-Tg (n = 6) mice are shown. Circles with arrows demarcate areas of early tissue injury observed only in wild-type animals.
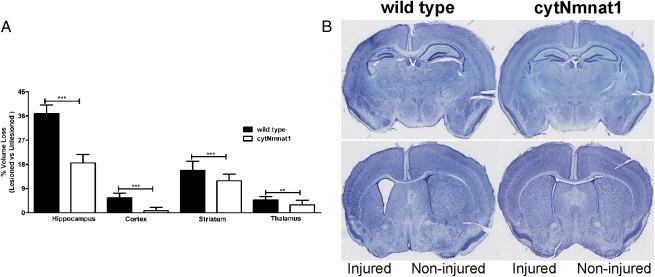
Growth rates did not differ in wild-type and cytNmnat1-Tg animals with or without H-I, as measured by their weights at P7 and P46 (Fig. S1). We demonstrated previously that tissue loss 7 d after injury is a useful histological measure of long-term outcome of neonatal H-I (8, 23, 24). At P14, 7 d after H-I, mice were killed, and their brains were processed for histological analysis. The volume of the hippocampus, cortex, striatum, and thalamus was assessed as described previously (9). CytNmnat1-Tg mice are markedly protected against neonatal H-I injury in all four brain regions examined (Fig. 2). The difference in tissue volume loss in wild-type versus cytNmnat1-Tg mice was notable in hippocampus (36.84 vs. 18.55%, n = 19, P < 0.0001), cortex (5.53 vs. 0.75%, n = 19, P < 0.0001), striatum (15.83 vs. 11.86%, n = 19, P < 0.0002), and thalamus (4.69 vs. 2.93, n = 15, P < 0.008) and represented ∼50, 86, 25, and 37% less injury, respectively (Fig. 2). Findings on MRI corresponded closely with the tissue loss analysis 7 d after H-I; however, although there was virtually complete protection against MRI changes, the protection was not complete at the tissue level as assessed after 7 d. That Nmnat1 protection was more robust against early changes after H-I than against later effects on tissue loss suggests that Nmnat1 selectively affects an earlier event or pathway in the H-I–induced damage to a greater extent than a more delayed pathway.
Fig. 2.
Decreased tissue loss in hippocampus, cortex, striatum, and thalamus of cytNmnat1-Tg mice after H-I. (A) P7 mice underwent unilateral carotid artery ligation and exposure to hypoxia for 45 min. At P14, animals were killed, and brain tissues were assessed for regional size analysis in the ipsilateral (lesioned) hemisphere vs. the contralateral (unlesioned) hemisphere. (B) Representative examples of brain injury in wild-type (n = 19) and cytNmnat1-Tg (n = 19) animals. Error bars show SE; **P < 0.001 and ***P < 0.0001.
H-I causes severe metabolic perturbation in the brain (25). To investigate the metabolic status of the brain after H-I, we measured NAD and ATP levels in wild-type and cytNmnat1-Tg littermates 24 h after H-I. Consistent with the previous reports, the steady-state NAD level in the cortex or hippocampus in the absence of HI did not differ between wild-type and cytNmnat1-Tg mice (19). The steady-state levels of ATP in the hippocampus of P7 wild-type and cytNmnat1-Tg animals that had not undergone H-I also did not differ significantly (Fig. S2). Hence, cytNmnat1 does not up-regulate the steady-state levels of ATP and NAD in transgenic animals.
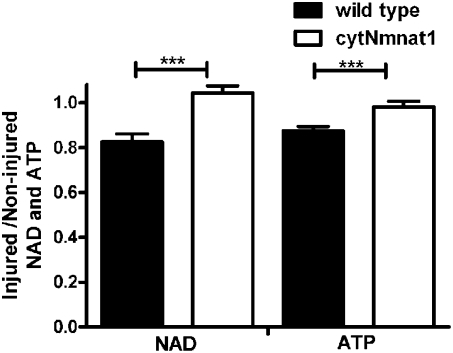
At 24 h after H-I, NAD and ATP levels in the injured hippocampus of wild-type mice were ∼20% lower than those observed in the hippocampus of the uninjured hemisphere (ratio of injured/uninjured = 0.82 and 0.84 respectively, n = 5, P < 0.0001) but were maintained in cytNmnat1-Tg animals (ratio of injured/uninjured = 1.0 and 1.0 respectively, n = 5, P < 0.0001) (Fig. 3). Further, to estimate other adenine nucleotides at 24 h after H-I, we measured ADP and AMP levels in the injured hippocampus of wild-type mice. ADP and AMP levels were ∼20% lower than those observed in the hippocampus of the uninjured hemisphere (ratio of injured/uninjured = 0.82, n = 5, P < 0.002 and 0.82, n = 5, P < 0.0005, respectively), but the levels were maintained in cytNmnat1-Tg animals (ratio of injured/uninjured = 0.95, n = 5, P < 0.002 and 1.0, respectively, n = 5, P < 0.0005) (Fig. S3).These results further confirm that cytNmnat1 overexpression protects the developing brain both histologically and metabolically.
Fig. 3.
H-I–induced changes in metabolism are blocked in cytNmnat1-Tg mice. NAD and ATP levels in injured and noninjured hippocampus tissue lysates of wild-type (n = 5) and cytNmnat1-Tg (n = 5) mice were assayed 24 h after H-I using HPLC. Error bars show SE; ***P < 0.0001.
Inflammatory markers in the developing brain increase in response to H-I (26–28). To assess the level of neuroinflammation, we determined the levels of cytokines in the hippocampus of injured and noninjured hemispheres by multianalyte profiling and markers of microglia and astrocytes by immunohistochemistry in tissues of wild-type and cytNmnat1-Tg animals at P9 (2 d after H-I) and P14 (7 d after H-I), respectively. Cytokine levels were significantly higher in the injured hemispheres of wild-type and cytNmnat1-Tg animals than in the noninjured hemisphere (Table S1). CytNmnat1-Tg animals had significantly reduced levels of cytokines in the injured hemisphere compared with wild-type animals in 17 of the 20 cytokines tested, whereas the majority of cytokine levels were not significantly different in the noninjured hemisphere (Table S1). The levels of microglial activation were assessed by the area of the hippocampus covered by ionized calcium-binding adaptor molecule 1 (IBA1)-positive microglia. IBA1 staining was increased significantly in the injured hemisphere of wild-type animals compared with cytNmnat1-Tg animals (Fig. S4). The uninjured hemisphere of wild-type and ctyNmnat1-Tg animals had significantly lower levels of microglial activation compared with the injured hemisphere. Interestingly, in the uninjured hippocampus the transgenic animals had higher levels of IBA1 staining than the wild-type mice (Fig. S4A). The astrocyte marker GFAP was present at high levels in the injured and uninjured hemispheres (injured > uninjured) of the animals (Fig S4B). Although there clearly was some increase in GFAP staining in the injured hemisphere of both wild-type and ctyNmnat1-Tg mice, the extent of staining of GFAP in the wild-type and cytNmnat1-Tg animal brains made it difficult to quantify the amount of GFAP staining reliably. Qualitatively, it appears that the GFAP staining is similar in cytNmnat1-Tg and wild-type animals after HI. Overall, compared with wild-type animals, cytNmnat1-Tg animals exhibited reduced cytokine levels and microglial activation in the injured hemisphere.
CytNmnat1 Does Not Influence Caspase-3 Activation After H-I in Neonatal Mice.
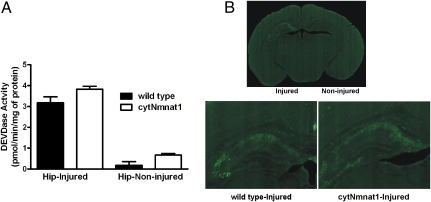
Caspase-3 activation plays an important role in H-I–induced injury in neonatal mice and occurs later than excitotoxicity-induced cell death (6, 23, 29, 30). Activation of caspase-3 is robust in many neurons after neonatal H-I, and both pan-caspase and caspase-3–specific inhibitors have been reported to protect against neonatal H-I in rats and mice (6, 23). To assess whether cytNmnat1 is neuroprotective through a caspase-3–dependent mechanism, we examined the levels of caspase-3 activation (measured by DEVDase activity) in the hippocampus 24 h after H-I (Fig. 4A). DEVDase activity was ∼15–20 fold higher in the injured hemispheres than in the uninjured hemispheres of both wild-type and cytNmnat1-Tg mice. Interestingly, DEVDase activity in the injured tissue of cytNmnat1-Tg mice was not significantly different from that in the injured wild-type tissue (3.2 vs. 3.8 pmol ⋅ min−1 ⋅ mg−1 protein, n = 9 and n = 12, respectively) (Fig. 4A). Immunohistochemical examination of brain tissue further confirmed that activated caspase-3 staining of neurons in the hippocampus, striatum, and cortex is similar in the injured hemispheres of wild-type and cytNmnat1 animals (Fig. 4B). Our results suggest that the robust CNS protection against H-I–induced damage observed in cytNmnat1-Tg mice does not result from modulation of the caspase-3–dependent cell-death pathway.
Fig. 4.
Caspase-3 activation after H-I is similar in wild-type and cytNmnat1-Tg mice. (A) Asp-Glu-Val-Asp-7-amino-4-methylcoumarin (DEVD-AMC) cleavage activity was assayed 24 h after H-I in hippocampal lysates from both injured and noninjured wild-type (n = 9) and cytNmnat1-Tg mice (n = 12). There were no quantitative differences in DEVD-AMC cleavage activity between wild-type and cytNmnat1-Tg animals. (B) The presence of cleaved (activated) caspase-3 in the hippocampus of wild-type and cytNmnat1-Tg mice was assessed 24 h after neonatal H–I by immunostaining using a cleaved caspase-3–specific antibody in wild-type (n = 5) and cytNmnat1-Tg (n = 5) mice.
CytNmnat1 Protects Neuronal Processes and Cell Bodies Against NMDA-Mediated Excitotoxicity in Cortical Neurons.
Both necrosis and apoptosis are prominent forms of cell death in neonatal animals that undergo H-I, with necrotic cell death beginning earlier in the course of neurodegeneration and apoptotic pathways invoked at a later stage (10, 11, 23). However, most of the H-I–induced brain injury in P7 rats can be blocked by pretreatment with the NMDA receptor (NMDAR) antagonist, MK801 (13, 31). This finding suggests that glutamate-mediated toxicity via NMDARs in the setting of neonatal H-I initiates cell death with both necrotic and apoptotic features.
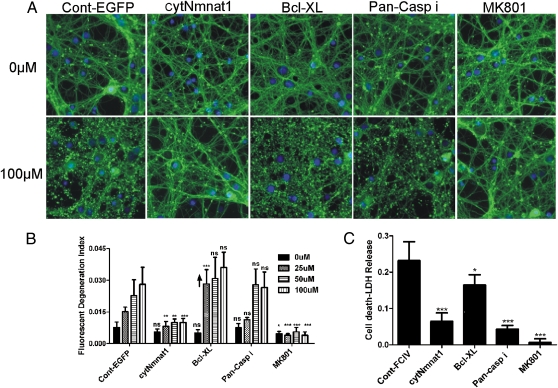
To test whether cytNmnat1 protects against NMDA-mediated excitotoxicity, we treated cultured embryonic (E) day 14.5 cortical neurons with NMDA. We examined neurons infected with lentivirus expressing EGFP (as a control) or with cytNmnat1. Seven days after infection [day in vitro (DIV) 10], the neurons were treated with 0, 25, 50, or 100 μM of NMDA for 12 or 24 h. Neuronal processes were stained with α-tubulin antibody to enable quantification of axonal degeneration through automated image analysis (32). NMDA induced dose- and time-dependent neurite degeneration in control EGFP-expressing neurons. The degeneration was inhibited completely by treatment of the cells with the NMDAR antagonist MK801 at 5 μM. CytNmnat1 expression strongly protected the neuronal processes against all doses of NMDA studied both 12 and 24 h after exposure (Fig. 5 A and B and Fig. S5). B cell lymphoma-extra large (Bcl-XL) protein is highly expressed in mammalian brain and inhibits cytochrome c-mediated caspase activation in neurons (33). To assess the role of caspase-dependent cell death in our current excitotoxicity paradigm, cortical neurons expressing Bcl-XL and neurons treated with pan-caspase inhibitor [N-Benzyloxycarbonyl-Val-Ala-Asp(O-Me) fluoromethyl ketone (Z-VAD-FMK), 50 μM] were treated for 12 or 24 h with 0, 25, 50, or 100 μM of NMDA. Unlike the effects of Nmnat1, Bcl-XL expression or treatment with pan-caspase inhibitor did not protect neuronal processes against NMDA-mediated excitotoxicity (Fig. 5 A and B).
Fig. 5.
CytNmnat1 protects neuronal processes and cell bodies against NMDA-mediated excitotoxicity. Primary cortical neurons infected with EGFP (control), cytNmnat1, or Bcl-XL lentiviruses were treated with 0, 25, 50, or 100 μM of NMDA on DIV10 for 24 h. In cells treated with the pan-caspase inhibitor Z-VAD-FMK (Pan-Casp i) or the NMDA antagonist MK801, primary cortical neurons were incubated on DIV10 before NMDA treatment with 0, 25, 50, and 100 μM for 24 h. The cells then were stained with α-tubulin (green, neuronal process) and with bisbenzimide (blue, nucleus). (A) Representative images of DIV10 primary cortical neurons treated with 0 or 100 μM NMDA. (B) The fluorescent axonal degeneration index was quantified 24 h after NMDA treatments as described in SI Materials and Methods. P values were calculated by one-way ANOVA comparing control with other treatments. The upward arrow on the Bcl-XL graph indicates that the degeneration is significantly higher than with EGFP. (C) Conditioned media from cortical cultures were collected 12 h after exposure to NMDA and assayed for LDH activity using a cytotoxicity detection kit. P values were calculated by one-way ANOVA comparing EGFP (control) with other treatments. Error bars show SE; *P < 0.05, **P < 0.001, ***P < 0.0001; ns, not significant.
When we examined these cultures, Nmnat1 also appeared to protect cortical neuronal cell bodies from dying. Most other studies of WldS or Nmnat1 consistently have observed axonal protection with minimal or no effects on the cell body. To explore this potentially unique effect of Nmnat1, we used a lactate dehydrogenase (LDH) release assay. Degenerating neurites do not release LDH (34, 35); thus prevention of LDH release suggests that cell bodies likely are being protected. Conditioned media from the cortical neuron cultures were collected 12 h after NMDA treatment, and LDH was measured to determine the extent of neuronal cell death. CytNmnat1 and MK801 significantly protected against neuronal LDH release (Fig. 5C). Thus, cytNmnat1 robustly blocks NMDA-mediated damage to neuronal processes and likely to cell bodies. Interestingly, Bcl-XL and the pan-caspase inhibitor provided protection against NMDA-mediated neuronal LDH release but did not protect neuronal processes, further distinguishing the effects of Nmnat1 and processes affecting apoptotic death. Overall, these results suggest that cytNmnat1, unlike antiapoptotic agents, can block NMDA-mediated damage both to neuronal processes and to cell bodies.
Given the key role of NMDARs in excitotoxic death, we tested the possibility that cytNmnat1 neuroprotection might arise from depression of NMDAR function or expression. We measured NMDA-mediated currents in cultured hippocampal neurons in response to the application of 10 μM NMDA. There was no difference between cytNmnat1- and EGFP-expressing neuronal cells in the peak current or in the amount of receptor desensitization (Fig. S6). This result suggests that the protective effect of Nmnat1 affects a pathway downstream of the NMDAR.
Discussion
Axonal degeneration is considered a key predictor of the outcome after CNS damage in many disorders such as head and spinal cord trauma, metabolic encephalopathies, multiple sclerosis, and white-matter diseases. In other neurological diseases such as Alzheimer's disease, Huntington disease, and Parkinson disease, axonal degeneration is thought to precede neuronal cell death and to be an important cause of brain dysfunction (36). In this study we tested whether overexpression of cytNmnat1 in the CNS delays or protects neuronal process and/or cell body degeneration in a model of neonatal H-I brain injury. We used a well-described H-I animal model and primary neuronal culture to show that cytNmnat1 markedly protects neuronal processes as well as cell bodies against H-I–induced cellular injury. The protective effect of cytNmnat1 is strikingly visible within 6 h of the insult in vivo. The protective effect is independent of caspase-3–dependent cell death, and primary neuronal culture studies suggest that Nmnat1 exerts its effect by inhibiting pathways downstream of the NMDA excitotoxicity necrosis-mediated pathways.
The cortex, hippocampus, dorsolateral basal ganglia, thalamus, and periventricular white matter are major areas of damage in the human infant brain after significant H-I, and a large number of surviving patients suffer from motor disturbances, intellectual impairment, and seizures (1, 2). MRI findings within hours or days of injury in neonatal rats and mice identify the extent of injury and predict tissue loss and neurodevelopmental impairments later in life (21, 22, 37). Hence, interventions that block MRI-detectable injury after H-I are likely also to be associated with improved outcome in neonates. We found that Nmnat1 provides robust protection against these early MRI changes and against subsequent tissue loss as well as maintenance of cellular metabolic integrity and reduced levels of cytokines and microglial activation in H-I neonatal mice. Transduction of cytNmnat1 protein locally to axons after mechanical insult markedly protects against degeneration (38), suggesting that targeting either Nmnat1 or its targets in the early phase of H-I injury may provide new therapeutic opportunities.
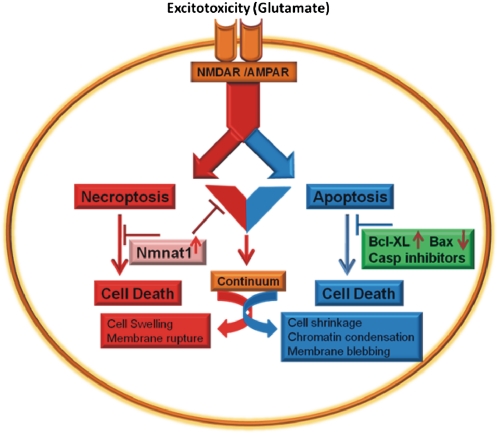
Infants with H-I–induced encephalopathy (HIE) have elevated levels of glutamate and aspartate in cerebrospinal fluid, and the increase in these excitatory amino acid levels correlates with the severity of HIE (39). The increase in excitatory amino acids in the brain might produce abnormalities in the function of excitatory synapses, resulting in abnormal activity and injury to neurons after H-I in infants (40, 41). Apoptosis and necrosis are the two distinctive cell-death pathways with distinct pathological hallmarks and molecular mechanisms prominent in neonatal animals that undergo H-I (Fig. 6) (3, 10, 23, 42, 43). Of all of the identified caspases, caspase-3 is a key player in neuronal apoptosis elicited by diverse stimuli (33). Pan-caspase and caspase-3–specific inhibitors as well as Bcl-XL overexpression and Bcl-2–associated X protein (Bax) knockout have been shown in some studies to protect significantly against up to 40–50% of tissue loss after neonatal H-I in rats and mice (Fig. 6) (6, 23, 44, 45). Hence, caspase-dependent apoptotic cell-death pathways appear to play some role in the cell death after neonatal H-I. However, most of the H-I–induced brain injury in P7 rats can be blocked by pretreatment with the NMDAR antagonist, MK801 (13). This result suggests that glutamate toxicity in the setting of neonatal H-I initiates both necrotic and apoptotic cell-death pathways (13, 31). Further, it has been shown that necrosis is the cell-death pathway that is used immediately after the H-I in neonates and after glutamate challenges in cortical cultures (10, 43, 46).
Fig. 6.
Model of neuronal cell death in neonatal H-I initiated by NMDA-mediated excitotoxicity. Excitotoxicity after neonatal H-I initiates necrosis and apoptosis as well as cell death that has features of both necrosis and apoptosis (3, 10, 21, 46, 47). Pan-caspase and caspase-3–specific inhibitors as well as overexpression of Bcl-XL and knockout of Bax significantly protect against neonatal H-I in rats and mice by blocking caspase-dependent cell-death pathways (6, 23, 41, 42). In the present study, cytNmnat1 protects against H-I–mediated damage largely by influencing the necrosis/necroptosis pathway but not the apoptosis pathway.
Our current study shows that cytNmnat1 does not modulate or block the caspase-3 activation in neonatal mice after H-I, strongly suggesting that cytNmnat1 prevents damage by influencing the necrotic rather than apoptotic contribution to the damage provoked by this insult. That Nmnat1 blocks against both MRI changes but not against all tissue loss or caspase-3 activation in vivo suggests that it blocks a pathway downstream of NMDAR activation. This notion is supported by the observation that we see no effect of Nmnat1 on NMDAR-mediated currents. A recent study found that Nmnat1 mediates its axonal protective effect in the presence of activated caspase-6 after tropic factor deprivation in dorsal root ganglion neurons (18). Interestingly, necrostatin, a small-molecule inhibitor of programmed necrosis (necroptosis), also fails to inhibit the activation of the caspase-3 pathways in animal models of neonatal brain injury (47) even though it provides significant protection in neonatal H-I and other mice models of acute injury (47–49). Whether the pathways activated by Nmnat1 and necrostatin overlap is unclear and will be an important area for future study. Our findings further emphasize that H-I–mediated excitotoxicity initiates pathways that can be modulated and targeted independently in the neonatal brain.
We also found that cytNmnat1 protects both cortical neuronal cell bodies and their processes in response to NMDA-mediated excitotoxicity. This finding is contrary to those of most other studies, in which Nmnat-mediated protection is restricted to axons (18, 50–52). Our results suggest that Nmnat1 modulates a common pathway for cell body death and neuronal process degeneration after an excitotoxic insult or that it can influence multiple pathways of degeneration simultaneously. Furthermore, in this paradigm, antiapoptotic factors such as Bcl-XL overexpression and treatment with caspase inhibitors showed no protection of neuronal processes even though they provided cell body protection. Together these findings accentuate the unique features of excitotoxicity-mediated damage and raise several questions regarding the mechanism of neurodegeneration in this context. For instance, does neuronal process degeneration precede cell body death in neonatal H-I damage? Are self-destructive neuronal processes and cell body processes regulated similarly or independently in this paradigm of acute neuronal damage?
A recent study reports that nicotinamide phosphoribosyltransferase (Nampt), the rate-limiting enzyme in mammalian NAD biosynthesis, protects against ischemic stroke in rodents (53). Given that Nampt and Nmnat1 are in the NAD-synthesis pathway, they may share similar mechanistic pathways in protection against ischemic stroke. Our study shows that cyNmnat1 is strongly protective against excitotoxic damage, apparently through modulation of nonapoptotic pathways (Fig. 6). By blocking specific aspects of excitotoxicity-mediated events, cytNmnat1 markedly protects against tissue loss after brain H-I in neonatal mice. Given the critical roles of NMDA-induced excitotoxicity in a wide range of brain-injury paradigms (stroke/ischemia, epilepsy) and probably in neurodegenerative disorders (Alzheimer's disease, motor neuron disease, Huntington disease, and Parkinson disease), it is probable that Nmnat1 protects against neuronal cell death by a common mechanism. If so, the findings described in this study have potential broad relevance, because the multiple types of cell death mediated by NMDARs could offer additional therapeutic targets for diseases involving excitotoxicity. It has been shown that axonal degeneration can be blocked by the transduction of cytNmnat1 protein into transected axons (38). This result suggests that Nmnat1's action on axons is mediated through local mediators, and effective treatments for neuronal degeneration potentially could be delivered after an acute insult. Recent advances in drug delivery to the CNS by agents such as nanoparticles open an intriguing opportunity to translate the in vitro observation that axonal degeneration is blocked by cytNmnat1 protein transduction to in vivo animal models and clinical applications. Further, the identification of the Nmnat-regulated components and signaling pathways responsible for blocking excitotoxic cell death by methods such as high-throughput assays may enable development of therapeutic agents. In this regard, an understanding of the Nmnat1-mediated neuroprotective pathway in the CNS may provide an avenue for novel therapeutic strategies in acute and chronic disorders of the CNS.
Materials and Methods
Animals.
Mice overexpressing the cytNmnat1 transgene under the control of the mouse prion promoter on a C57BL6/CBA (B6/CBA) background were produced as described (19). To produce cytNmnat1 and wild-type littermate pups for the H-I experiments, cytNmnat mice on a B6/CBA background were bred to B6/CBA F1 nontransgenic, wild-type mice. Care was provided to animals in compliance with National Institute of Health guidelines on the use of laboratory animals.
MRI.
MR images were acquired on a Varian Unity INOVA MRI system with an 11.74-Tesla, 26-cm-diameter clear-bore horizontal magnet (Agilent/Varian/Magnex). The system was equipped with an 8-cm inner diameter and an actively shielded gradient and shim coil assembly driven by Copley high-performance gradient amplifiers (Copley Controls), providing a gradient of 120 G/cm with a rise time of 298 μs. A 15-mm-diameter quadrature rf Litzcage coil (Doty Scientific) was used for rf transmission and reception. P7 mice were immobilized in the supine position in a custom-built, MRI-compatible head holder and were kept anesthetized using 0.4 ∼1.0% isoflurane (balance O2). The pup's respiratory rate was monitored and used to adjust the anesthetic level. The body temperature, monitored by a temperature probe taped to the abdomen, was kept at 37 °C by blowing heated air through the magnet bore. T2W images were acquired using a multislice, spin-echo sequence which incorporated a binomial-series water/fat frequency-selective excitation 3°–15°–30°–30°–15°–3° plus spoiling gradients at the beginning of each slice-selection loop. The time delay between the adjacent pulses was set to 298.7 μs, thereby generating a 180° phase shift between water and fat transverse magnetizations after each pulse. The pups were imaged 6, 12, and 24 h after H-I with the following parameters: TR 4 s, TE 80 ms, four signal averages, and spatial resolution of 59 × 59 × 250 μm.
Additional materials and methods are available in SI Methods and Materials.
Supplementary Material
Acknowledgments
We thank Ann Benz for assistance with rat hippocampal cultures. This work was supported by National Institutes of Health Grant NS35902 (to D.M.H. and J.J.N.). P.B.V. is supported by American Health Assistance postdoctoral fellowship AHAF 3857–4328.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107325108/-/DCSupplemental.
References
- 1.Shankaran S, Woldt E, Koepke T, Bedard MP, Nandyal R. Acute neonatal morbidity and long-term central nervous system sequelae of perinatal asphyxia in term infants. Early Hum Dev. 1991;25(2):135–148. doi: 10.1016/0378-3782(91)90191-5. [DOI] [PubMed] [Google Scholar]
- 2.Volpe JJ. Neurology of the Newborn. 5th Ed. Philadelphia: Saunders/Elsevier; 2008. [Google Scholar]
- 3.Vannucci RC. Experimental biology of cerebral hypoxia-ischemia: Relation to perinatal brain damage. Pediatr Res. 1990;27(4 pt 1):317–326. doi: 10.1203/00006450-199004000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Levine S. Anoxic-ischemic encephalopathy in rats. Am J Pathol. 1960;36:1–17. [PMC free article] [PubMed] [Google Scholar]
- 5.Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9(2):131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 6.Han BH, et al. Selective, reversible caspase-3 inhibitor is neuroprotective and reveals distinct pathways of cell death after neonatal hypoxic-ischemic brain injury. J Biol Chem. 2002;277:30128–30136. doi: 10.1074/jbc.M202931200. [DOI] [PubMed] [Google Scholar]
- 7.Sheldon RA, Chuai J, Ferriero DM. A rat model for hypoxic-ischemic brain damage in very premature infants. Biol Neonate. 1996;69:327–341. doi: 10.1159/000244327. [DOI] [PubMed] [Google Scholar]
- 8.Han BH, et al. Clusterin contributes to caspase-3-independent brain injury following neonatal hypoxia-ischemia. Nat Med. 2001;7(3):338–343. doi: 10.1038/85487. [DOI] [PubMed] [Google Scholar]
- 9.West T, Atzeva M, Holtzman DM. Caspase-3 deficiency during development increases vulnerability to hypoxic-ischemic injury through caspase-3-independent pathways. Neurobiol Dis. 2006;22:523–537. doi: 10.1016/j.nbd.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Northington FJ, Ferriero DM, Graham EM, Traystman RJ, Martin LJ. Early neurodegeneration after hypoxia-ischemia in neonatal rat is necrosis while delayed neuronal death is apoptosis. Neurobiol Dis. 2001;8(10):207–219. doi: 10.1006/nbdi.2000.0371. [DOI] [PubMed] [Google Scholar]
- 11.Portera-Cailliau C, Price DL, Martin LJ. Excitotoxic neuronal death in the immature brain is an apoptosis-necrosis morphological continuum. J Comp Neurol. 1997;378(1):70–87. [PubMed] [Google Scholar]
- 12.Ikonomidou C, Mosinger JL, Olney JW. Hypothermia enhances protective effect of MK-801 against hypoxic/ischemic brain damage in infant rats. Brain Res. 1989;487(1):184–187. doi: 10.1016/0006-8993(89)90956-6. [DOI] [PubMed] [Google Scholar]
- 13.McDonald JW, Silverstein FS, Johnston MV. MK-801 protects the neonatal brain from hypoxic-ischemic damage. Eur J Pharmacol. 1987;140:359–361. doi: 10.1016/0014-2999(87)90295-0. [DOI] [PubMed] [Google Scholar]
- 14.Zhai RG, Rizzi M, Garavaglia S. Nicotinamide/nicotinic acid mononucleotide adenylyltransferase, new insights into an ancient enzyme. Cell Mol Life Sci. 2009;66:2805–2818. doi: 10.1007/s00018-009-0047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conforti L, et al. NAD(+) and axon degeneration revisited: Nmnat1 cannot substitute for Wld(S) to delay Wallerian degeneration. Cell Death Differ. 2007;14(1):116–127. doi: 10.1038/sj.cdd.4401944. [DOI] [PubMed] [Google Scholar]
- 16.Mack TG, et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci. 2001;4:1199–1206. doi: 10.1038/nn770. [DOI] [PubMed] [Google Scholar]
- 17.Perry VH, Brown MC, Lunn ER. Very slow retrograde and wallerian degeneration in the CNS of C57BL/Ola mice. Eur J Neurosci. 1991;3(1):102–105. doi: 10.1111/j.1460-9568.1991.tb00815.x. [DOI] [PubMed] [Google Scholar]
- 18.Vohra BP, et al. Amyloid precursor protein cleavage-dependent and -independent axonal degeneration programs share a common nicotinamide mononucleotide adenylyltransferase 1-sensitive pathway. J Neurosci. 2010;30:13729–13738. doi: 10.1523/JNEUROSCI.2939-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasaki Y, Vohra BP, Baloh RH, Milbrandt J. Transgenic mice expressing the Nmnat1 protein manifest robust delay in axonal degeneration in vivo. J Neurosci. 2009;29:6526–6534. doi: 10.1523/JNEUROSCI.1429-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- 21.Adén U, et al. MRI evaluation and functional assessment of brain injury after hypoxic ischemia in neonatal mice. Stroke. 2002;33:1405–1410. doi: 10.1161/01.str.0000014608.78503.db. [DOI] [PubMed] [Google Scholar]
- 22.Neil JJ. Diffusion imaging concepts for clinicians. J Magn Reson Imaging. 2008;27(1):1–7. doi: 10.1002/jmri.21087. [DOI] [PubMed] [Google Scholar]
- 23.Cheng Y, et al. Caspase inhibitor affords neuroprotection with delayed administration in a rat model of neonatal hypoxic-ischemic brain injury. J Clin Invest. 1998;101:1992–1999. doi: 10.1172/JCI2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almli CR, et al. BDNF protects against spatial memory deficits following neonatal hypoxia-ischemia. Exp Neurol. 2000;166(1):99–114. doi: 10.1006/exnr.2000.7492. [DOI] [PubMed] [Google Scholar]
- 25.Palmer C, Brucklacher RM, Christensen MA, Vannucci RC. Carbohydrate and energy metabolism during the evolution of hypoxic-ischemic brain damage in the immature rat. J Cereb Blood Flow Metab. 1990;10(2):227–235. doi: 10.1038/jcbfm.1990.39. [DOI] [PubMed] [Google Scholar]
- 26.Silverstein FS, et al. Cytokines and perinatal brain injury. Neurochem Int. 1997;30:375–383. doi: 10.1016/s0197-0186(96)00072-1. [DOI] [PubMed] [Google Scholar]
- 27.Bartha AI, et al. Neonatal encephalopathy: Association of cytokines with MR spectroscopy and outcome. Pediatr Res. 2004;56:960–966. doi: 10.1203/01.PDR.0000144819.45689.BB. [DOI] [PubMed] [Google Scholar]
- 28.Lodygensky GA, et al. Diffusion characteristics associated with neuronal injury and glial activation following hypoxia-ischemia in the immature brain. Magn Reson Med. 2011;66:839–845. doi: 10.1002/mrm.22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shibata M, et al. Caspases determine the vulnerability of oligodendrocytes in the ischemic brain. J Clin Invest. 2000;106:643–653. doi: 10.1172/JCI10203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han BH, et al. BDNF blocks caspase-3 activation in neonatal hypoxia-ischemia. Neurobiol Dis. 2000;7(1):38–53. doi: 10.1006/nbdi.1999.0275. [DOI] [PubMed] [Google Scholar]
- 31.Hattori H, Morin AM, Schwartz PH, Fujikawa DG, Wasterlain CG. Posthypoxic treatment with MK-801 reduces hypoxic-ischemic damage in the neonatal rat. Neurology. 1989;39:713–718. doi: 10.1212/wnl.39.5.713. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki Y, Vohra BP, Lund FE, Milbrandt J. Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide. J Neurosci. 2009;29:5525–5535. doi: 10.1523/JNEUROSCI.5469-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yakovlev AG, Faden AI. Caspase-dependent apoptotic pathways in CNS injury. Mol Neurobiol. 2001;24(1-3):131–144. doi: 10.1385/MN:24:1-3:131. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka T, Ueno M, Yamashita T. Engulfment of axon debris by microglia requires p38 MAPK activity. J Biol Chem. 2009;284:21626–21636. doi: 10.1074/jbc.M109.005603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin LJ, et al. Hypoxia-ischemia causes abnormalities in glutamate transporters and death of astroglia and neurons in newborn striatum. Ann Neurol. 1997;42:335–348. doi: 10.1002/ana.410420310. [DOI] [PubMed] [Google Scholar]
- 36.Medana IM, Esiri MM. Axonal damage: A key predictor of outcome in human CNS diseases. Brain. 2003;126:515–530. doi: 10.1093/brain/awg061. [DOI] [PubMed] [Google Scholar]
- 37.Derugin N, et al. Magnetic resonance imaging as a surrogate measure for histological sub-chronic endpoint in a neonatal rat stroke model. Brain Res. 2005;1066:49–56. doi: 10.1016/j.brainres.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 38.Sasaki Y, Milbrandt J. Axonal degeneration is blocked by nicotinamide mononucleotide adenylyltransferase (Nmnat) protein transduction into transected axons. J Biol Chem. 2010;285:41211–41215. doi: 10.1074/jbc.C110.193904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riikonen RS, Kero PO, Simell OG. Excitatory amino acids in cerebrospinal fluid in neonatal asphyxia. Pediatr Neurol. 1992;8(1):37–40. doi: 10.1016/0887-8994(92)90050-9. [DOI] [PubMed] [Google Scholar]
- 40.Johnston MV, Ferriero DM, Vannucci SJ, Hagberg H. Models of cerebral palsy: Which ones are best? J Child Neurol. 2005;20:984–987. doi: 10.1177/08830738050200121001. [DOI] [PubMed] [Google Scholar]
- 41.Johnston SC, Zhao S, Dudley RA, Berman MF, Gress DR. Treatment of unruptured cerebral aneurysms in California. Stroke. 2001;32:597–605. doi: 10.1161/01.str.32.3.597. [DOI] [PubMed] [Google Scholar]
- 42.Pulera MR, et al. Apoptosis in a neonatal rat model of cerebral hypoxia-ischemia. Stroke. 1998;29:2622–2630. doi: 10.1161/01.str.29.12.2622. [DOI] [PubMed] [Google Scholar]
- 43.Young C, Tenkova T, Dikranian K, Olney JW. Excitotoxic versus apoptotic mechanisms of neuronal cell death in perinatal hypoxia/ischemia. Curr Mol Med. 2004;4(2):77–85. doi: 10.2174/1566524043479158. [DOI] [PubMed] [Google Scholar]
- 44.Parsadanian AS, Cheng Y, Keller-Peck CR, Holtzman DM, Snider WD. Bcl-xL is an antiapoptotic regulator for postnatal CNS neurons. J Neurosci. 1998;18:1009–1019. doi: 10.1523/JNEUROSCI.18-03-01009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gibson ME, et al. BAX contributes to apoptotic-like death following neonatal hypoxia-ischemia: Evidence for distinct apoptosis pathways. Mol Med. 2001;7:644–655. [PMC free article] [PubMed] [Google Scholar]
- 46.Ankarcrona M, et al. Glutamate-induced neuronal death: A succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 47.Northington FJ, et al. Necrostatin decreases oxidative damage, inflammation, and injury after neonatal HI. J Cereb Blood Flow Metab. 2011;31(1):178–189. doi: 10.1038/jcbfm.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Degterev A, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1(2):112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 49.You Z, et al. Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J Cereb Blood Flow Metab. 2008;28:1564–1573. doi: 10.1038/jcbfm.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deckwerth TL, Johnson EM., Jr Neurites can remain viable after destruction of the neuronal soma by programmed cell death (apoptosis) Dev Biol. 1994;165(1):63–72. doi: 10.1006/dbio.1994.1234. [DOI] [PubMed] [Google Scholar]
- 51.Beirowski B, Babetto E, Coleman MP, Martin KR. The WldS gene delays axonal but not somatic degeneration in a rat glaucoma model. Eur J Neurosci. 2008;28:1166–1179. doi: 10.1111/j.1460-9568.2008.06426.x. [DOI] [PubMed] [Google Scholar]
- 52.Wang AL, Yuan M, Neufeld AH. Degeneration of neuronal cell bodies following axonal injury in Wld(S) mice. J Neurosci Res. 2006;84:1799–1807. doi: 10.1002/jnr.21075. [DOI] [PubMed] [Google Scholar]
- 53.Wang P, et al. Nicotinamide phosphoribosyltransferase protects against ischemic stroke through SIRT1-dependent adenosine monophosphate-activated kinase pathway. Ann Neurol. 2011;69(2):360–374. doi: 10.1002/ana.22236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.