Abstract
Clinical studies and mathematical models predict that, to achieve malaria elimination, combination therapies will need to incorporate drugs that block the transmission of Plasmodium falciparum sexual stage parasites to mosquito vectors. Efforts to measure the activity of existing antimalarials on intraerythrocytic sexual stage gametocytes and identify transmission-blocking agents have, until now, been hindered by a lack of quantitative assays. Here, we report an experimental system using P. falciparum lines that stably express gametocyte-specific GFP-luciferase reporters, which enable the assessment of dose- and time-dependent drug action on gametocyte maturation and transmission. These studies reveal activity of the first-line antimalarial dihydroartemisinin and the partner drugs lumefantrine and pyronaridine against early gametocyte stages, along with moderate inhibition of mature gametocyte transmission to Anopheles mosquitoes. The other partner agents monodesethyl-amodiaquine and piperaquine showed activity only against immature gametocytes. Our data also identify methylene blue as a potent inhibitor of gametocyte development across all stages. This thiazine dye almost fully abolishes P. falciparum transmission to mosquitoes at concentrations readily achievable in humans, highlighting the potential of this chemical class to reduce the spread of malaria.
Keywords: artemisinin-based combination therapies, transfection
With ∼225 million individuals infected and an estimated 780,000 deaths annually—largely among African children—Plasmodium falciparum malaria remains one of the world's most devastating diseases. Encouragingly, in recent years, the global adoption of highly effective artemisinin-based combination therapies (ACTs) as first-line treatments and the distribution of long-lasting insecticide-treated bednets have helped reduce the prevalence of malaria in many endemic settings (1). This recent success contributed to the launch in 2007 of a new campaign of malaria eradication (2). Clinical reports and mathematical models show that additional reductions in the incidence of disease can be achieved with interventions combining vector control approaches and treatments that not only cure patients but also decrease transmission (3).
Current antimalarials reduce P. falciparum malaria-associated morbidity and mortality by targeting the pathogenic asexual blood stages within infected erythrocytes. This activity indirectly impacts parasite transmission to its Anopheles mosquito vector by limiting the number of asexual forms that can differentiate into mature intraerythrocytic gametocytes. Nevertheless, patients cleared of asexual parasites can still transmit surviving gametocytes. Ideal combination therapies would, thus, target both the rapidly replicating asexual stages and the less metabolically active, nonreplicating mature gametocytes. This ideal combination is of particular relevance in highly endemic regions, where P. falciparum infections are often asymptomatic and thus, go untreated and where clinical symptoms tend to develop late in the course of infection, allowing gametocytes to mature and continue the transmission cycle (4, 5).
Most of the available information on the impact of existing antimalarials on gametocyte development has been gathered from field-based clinical trials, and in vitro studies remain sparse (6). Activity against immature gametocytes has been observed with a number of drugs that target metabolic pathways in asexual blood stage parasites. These drugs include chloroquine and quinine, which interfere with heme detoxification, and atovaquone, which targets mitochondrial electron transport (6, 7). Artemisinins have also been observed to inhibit immature sexual stages and reduce gametocyte carriage in infected human hosts (8, 9). The experimental design of many of the studies, however, has often made it difficult to distinguish between direct gametocytocidal activity and the antiasexual properties that reduce the pool of parasites available to initiate gametocytogenesis. Earlier in vitro evaluations of drug gametocytocidal activity typically relied on laborious, subjective microscopic observations of parasite morphology to assess cell viability (6). Recent advances include the use of hydroethidine or alamarBlue as fluorescent markers of metabolic activity (10, 11) or GFP reporter lines to assess commitment to gametocytogenesis (12, 13), notably after antimalarial drug treatment of asexual forms (10). These methods, however, have yet to be exploited to assess dose-dependent drug effects over time or define rates of action with purified, mature gametocyte populations. Also, these studies have not measured drug-mediated inhibition of P. falciparum transmission to Anopheles, a technically challenging endeavor.
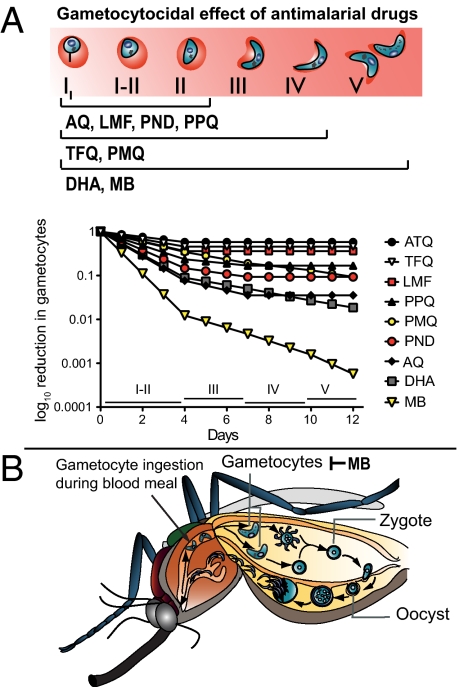
Here, we have engineered a set of P. falciparum lines expressing stably integrated gametocyte-specific GFP-luciferase reporters, and we have developed assays to specifically monitor parasite sexual development throughout the five stages of gametocytogenesis and determine the kinetics of gametocytocidal action of antimalarial agents. Luciferase-based assays permitted quantitative assessment of the effects of key primary and partner components of ACTs on P. falciparum gametocyte maturation. Select compounds were then further investigated for their ability to inhibit gametocyte transmission to Anopheles vectors. Our results show that ACT compounds in clinical use are generally active against immature gametocytes. We also observed partial activity of dihydroartemisinin (DHA) against late stage gametocytes, consistent with a reduction in the number of oocysts in mosquitoes fed on DHA-treated gametocytes. Exquisite potency was observed against early and mature gametocytes with the synthetic dye methylene blue (MB), which also almost fully blocked transmission. These results provide the foundation to identify transmission-blocking agents that can be integrated into curative combination therapies.
Results
Engineering of Unmarked NF54attB Recombinant Parasites Amenable to Site-Specific Recombination.
To produce P. falciparum reporter lines expressing GFP-luciferase under the control of gametocyte-specific promoters, we used Bxb1 mycobacteriophage integrase-mediated recombination to deliver transgenes into the P. falciparum genome (14). Prior strategies have used transgenes on episomally replicating plasmids (13, 15), where the absence of selection during gametocyte development can lead to the loss of plasmids and signal heterogeneity between parasites. Here, transgene integration was applied to the NF54 strain, which produces highly infectious gametocytes and is being used in human malaria vaccine trials (16). Our integration strategy was designed to eliminate the selectable marker that might otherwise interfere with antimalarial modes of action.
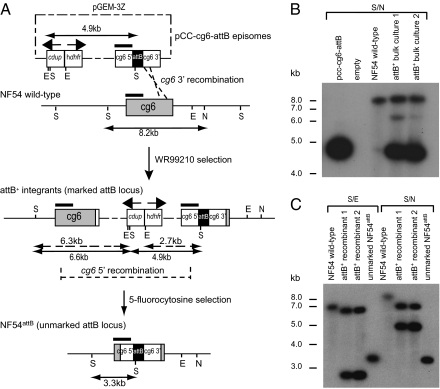
Bxb1 integrase catalyzes homology-directed recombination between a short attP site and a target attB site. NF54 parasites containing an attB site were generated by transforming asexual blood stages with the pCC-cg6-attB plasmid, which contains an attB site cloned between sequences homologous to the cg6 gene along with the human dhfr-positive selectable marker and the cdup-negative marker (Fig. 1A). After selection for human dhfr (using the antifolate WR99210), PCR was used to detect attB+ integrants that resulted from single cross-over recombination on the 3′ side of cg6. Integration was confirmed by Southern blot hybridization (Fig. 1B), and clones were obtained by limiting dilution. After removal of WR99210, the negative selection agent 5-fluorocytosine was applied to select for intralocus recombination between duplicated cg6 5′ sequences, leading to excision of cdup and its flanking human dhfr marker (Fig. 1A). The genomic organization of an unmarked parasite clone, named NF54attB, was confirmed by Southern blot hybridization (Fig. 1C).
Fig. 1.
Integration of an unmarked attB site for site-specific recombination in P. falciparum NF54 parasites. (A) Integration of the plasmid pCC-cg6-attB into the cg6 gene and subsequent intralocus recombination leading to removal of the selectable markers from the recombinant locus. This plasmid expresses human dihydrofolate reductase (hdhfr) and Saccharomyces cerevisiae cytosine deaminase–uracyl phosphotransferase (cdup) for positive and negative selection, respectively (58). cg6 5′ and 3′ coding sequences were placed on either side of an attB site to permit single cross-over homologous recombination between the pCC-cg6-attB plasmid and the endogenous cg6 gene. Single cross-overs were identified between the cg6 3′ homologous regions, leading to plasmid integration into the genome. Selection with 5-fluorocytosine, which is activated into a toxic compound by CDUP, was used to obtain parasites that have lost the hdhfr and cdup selectable markers. This selection was achieved through intralocus recombination between the duplicated cg6 5′ sequences, leading to the formation of a single unmarked attB locus. The black rectangle illustrates the 0.4-kb cg6 probe used for Southern blot analysis, and restriction digest fragment sizes are indicated. (B) Southern blot analysis confirming pCC-cg6-attB plasmid integration into NF54 parasites. DNAs were digested with SalI/NruI (S/N) and hybridized with the cg6 probe. Bulk cultures contained a mixture of integrants that yielded band sizes of 6.6 and 4.9 kb (characteristic of plasmid integration in the 3′ region of cg6) as well as episomal transfectants that showed the 4.9-kb plasmid band and the 8.2-kb nonrecombinant cg6 locus. (C) Southern blot analysis confirming unmarking of attB+ parasites. DNAs were digested with SalI/EcoRV (S/E) or S/N and hybridized with the cg6 probe. This process produced bands of 6.8 and 8.2 kb, respectively, in NF54 wild-type parasites. In comparison, band sizes of 6.3 and 2.7 or 6.6 and 4.9 kb were detected in marked attB+ recombinant parasites digested with S/E or S/N, respectively. Unmarked NF54attB parasites yielded a single band of 3.3-kb on digestion with either restriction enzyme pair, consistent with loop-out recombination and excision of the selectable markers.
Generation of P. falciparum Recombinant Parasites Expressing GFP-Luciferase Under the Control of Gametocyte-Specific Promoters.
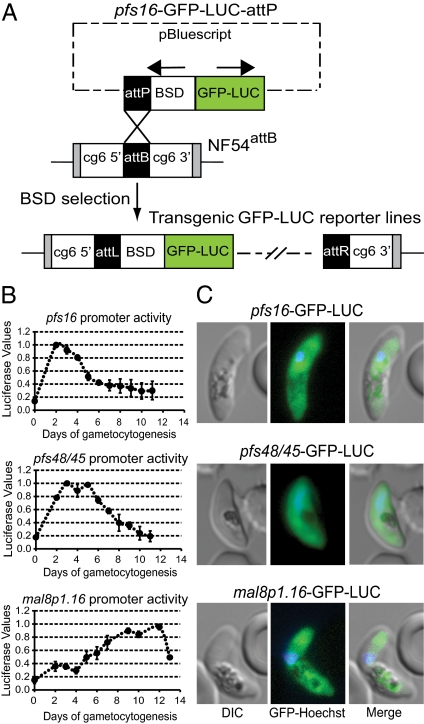
Gametocyte-specific promoters were chosen from genome-wide expression profiles of the developmentally distinct asexual and sexual blood stages (17, 18). We selected pfs16, which is expressed throughout the five stages of gametocytogenesis and is one of the earliest markers of gametocyte development, pfs48/45, which is principally active in early and intermediate gametocyte stages, and mal8p1.16, which is specific for late stage gametocytes (19). Although both pfs48/45 and mal8p1.16 are minimally expressed in asexual parasites, pfs16 transcripts can be detected postgametocyte induction in asexual parasites that have committed to sexual differentiation in the next cycle of intraerythrocytic development (20). These promoters were cloned upstream of a GFP-firefly luciferase fusion, and the resulting attP-containing gametocyte reporter plasmids were cotransfected into NF54attB parasites along with the pINT plasmid that expresses Bxb1 mycobacteriophage integrase (Fig. 2A). Integrase-mediated attB × attP recombination yielded NF54pfs16, NF54pfs48/45, and NF54mal8p1.16 reporter lines, which were confirmed by PCR and subsequently cloned.
Fig. 2.
Engineering and characterization of gametocytogenesis-specific GFP-luciferase reporter lines. (A) Schematic of the Bxb1 integrase (INT) -mediated genomic insertion of attP-containing plasmids that used gametocyte-specific promoters (pfs16, pfs48/45, or mal8p1.16) to express the GFP-luciferase (GFP-LUC) fusion. Cloned unmarked NF54attB parasites were cotransformed with the integrase-expressing plasmid pINT (14) and GFP-LUC-attP plasmids. After integration, the attB site was destroyed, leaving left (attL) and right (attR) flanking regions. (B) Assessment of pfs16, pfs48/45, and mal8p1.16 promoter-driven luciferase expression throughout gametocytogenesis. Luciferase signals were measured daily from triplicate wells harvested from the parasite lines NF54pfs16, NF54pfs48/45, and NF54mal8p1.16. Values were normalized to gametocyte numbers and plotted as a proportion of peak promoter activity (normalized to 1.0; mean ± SEM peak luciferase values were 152,398 ± 1,722, 52,052 ± 897, and 2,081 ± 506 for NF54pfs16, NF54pfs48/45, and NF54mal8p1.16). Days of gametocytogenesis are represented on the x axis. (C) Assessment of gametocyte promoter-driven GFP expression by fluorescence microscopy. NF54pfs16, NF54pfs48/45, and NF54mal8p1.16 parasites were imaged as gametocyte stages V, III, and V, respectively.
To profile sexual stage reporter activity, we triggered gametocytogenesis by starvation-induced stress (21). Luciferase activity was measured in gametocytes harvested daily for 12–14 consecutive days (Fig. 2B). Expression of the reporter driven by the pfs16 promoter began, as anticipated, in asexual parasites after gametocyte induction and increased ∼10-fold to peak around day 2 of gametocytogenesis (stages I and II). Promoter activity progressively decreased but reached a plateau at a signal level ∼threefold above background in mature gametocytes (days 10–12), suggesting that the NF54pfs16 reporter line can be exploited to monitor both early stage and mature gametocytes.
Expression of luciferase driven by the pfs48/45 promoter peaked, as previously reported (18), between days 3 and 5 of gametocytogenesis, which makes the NF54pfs48/45 reporter line well suited to monitor stages II and III development. Comparison of luciferase values between NF54pfs16 and NF54pfs48/45 revealed threefold stronger maximal promoter activity for the pfs16 5′ UTR (Fig. 2). Reporter expression under the control of the mal8p1.16 promoter was minimal in the early stages of gametocytogenesis and only increased 6–8 d after commitment to sexual differentiation. Promoter activity peaked on days 10–12 of gametocytogenesis, corresponding to expression in late stage (stages IV and V) gametocytes (Fig. 2B). Fluorescence microscopy confirmed the expression of the GFP-luciferase fusion in the gametocyte cytosol for all reporter lines (Fig. 2C).
Gametocytocidal Activity of Common Antimalarial Drugs.
We assessed the gametocytocidal activity of a panel of nine antimalarial drugs, including DHA, the active metabolite of artemisinins and itself a first-line agent, and the ACT partner drugs lumefantrine, monodesethyl-amodiaquine (mdAQ; the active metabolite of amodiaquine), piperaquine, and pyronaridine. Additionally, we tested the Malarone component atovaquone, the 8-aminoquinolines primaquine and tafenoquine, and the thiazine dye MB (structures listed in Fig. S1). Given our emphasis on drugs that are used in first-line therapy or are candidate transmission-blocking agents, we did not include the former gold standard antimalarial drug, chloroquine. Other studies have clearly established that chloroquine exerts activity on early stage gametocytes (6, 10, 13, 22).
Gametocytes from the NF54pfs16, NF54pfs48/45, and NF54mal8p1.16 reporter lines were exposed to compounds at concentrations corresponding to 0.5×, 1×, and 5× the mean IC50 value obtained against asexual blood stage parasites. IC50 values (Table 1) were <70 nM for all compounds tested, with the exception of the prophylactic antiliver stage agents primaquine and tafenoquine, which were far less active against asexual stages (IC50 values of 1.3 and 4.4 μM, respectively). Treatment was initiated on day 2 (corresponding to stages I and II), 5 (stage III), 8 (stage IV), or 11 (stage V) after induction of gametocytogenesis from synchronized parasite cultures (Fig. 3A). Drug treatment was maintained for a total of 3 d followed by the removal of drug. Luciferase activity was measured daily for 4–12 d after treatment onset.
Table 1.
Activity of antimalarials on P. falciparum gametocyte maturation and transmission
| Gametocyte maturation assays |
Gametocyte transmission assays |
||||||||
| Antimalarial drug | Asexual blood stage mean 1× IC50 (nM) | Fold IC50 | I and II | III | IV | V | Fold IC50 | Reduction in oocysts | Block in transmission |
| Dihydroartemisinin | 24 | 0.5× | +++ | − | − | − | |||
| Dihydroartemisinin | 24 | 1× | +++ | + | + | + | 1× | + | − |
| Dihydroartemisinin | 24 | 5× | +++ | +++ | ++ | + | 5× | ++ | − |
| Lumefantrine | 66 | 0.5× | − | − | − | − | |||
| Lumefantrine | 66 | 1× | − | − | − | − | 1× | ++ | − |
| Lumefantrine | 66 | 5× | +++ | − | − | − | 5× | +++ | − |
| Monodesethyl-amodiaquine | 61 | 0.5× | +++ | − | − | − | |||
| Monodesethyl-amodiaquine | 61 | 1× | +++ | − | − | − | 0.5× | − | − |
| Monodesethyl-amodiaquine | 61 | 5× | +++ | + | − | − | 2.5× | − | − |
| Piperaquine | 35 | 0.5× | + | − | − | − | |||
| Piperaquine | 35 | 1× | + | − | − | − | 1× | − | − |
| Piperaquine | 35 | 5× | +++ | − | − | − | 5× | − | − |
| Pyronaridine | 17 | 0.5× | − | − | − | − | |||
| Pyronaridine | 17 | 1× | + | − | − | − | 1× | − | − |
| Pyronaridine | 17 | 5× | +++ | − | − | − | 5× | +++ | − |
| Atovaquone | 1.5 | 0.5× | − | − | − | − | |||
| Atovaquone | 1.5 | 1× | − | − | − | − | |||
| Atovaquone | 1.5 | 5× | + | − | − | + | |||
| Primaquine | 1,320 | 0.5× | + | − | − | − | |||
| Atovaquone | 1,320 | 1× | ++ | − | − | − | |||
| Primaquine | 1,320 | 5× | +++ | ++ | + | + | |||
| Tafenoquine | 4,400 | 0.5× | + | − | − | − | |||
| Tafenoquine | 4,400 | 1× | ++ | − | − | − | |||
| Tafenoquine | 4,400 | 5× | ++ | + | − | − | |||
| Methylene blue | 30 | 0.5× | +++ | + | + | − | |||
| Methylene blue | 30 | 1× | +++ | ++ | + | + | 0.25× | + | − |
| Methylene blue | 30 | 5× | +++ | +++ | +++ | ++ | 1.25× | +++ | +++ |
Mean inhibition relative to no drug control: −, <25%; +, 25–50%; ++, 50–75%; +++, >75%. Gametocyte inhibition data for stages I–V were collated from two to five independent experiments and are displayed in Figs. 3 and 4. Oocyst data were from two experiments and are summarized in Fig. 5 and Tables S1–S3. Atovaquone, primaquine, and tafenoquine were not tested in transmission assays.
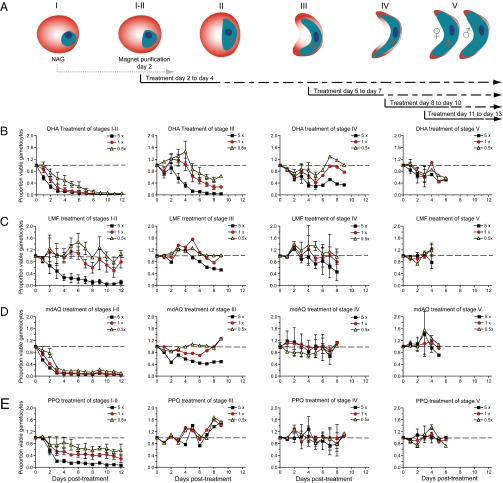
Fig. 3.
Activity of the active artemisinin metabolite DHA and ACT partner drugs on gametocyte maturation in vitro. (A) Experimental scheme to assess impact of antimalarials at different stages of gametocyte maturation. After gametocyte induction by limiting nutrients, cultures were treated for 6 d with NAG to eliminate asexual parasites, and gametocytes were magnet-purified on day 2 postinduction. Drug treatments of 3-d duration were initiated on days 2, 5, 8, or 11 (corresponding to stages I and II, III, IV, and V). Results are shown for (B) DHA, (C) lumefantrine (LMF), (D) mdAQ, and (E) piperaquine (PPQ). Drugs were tested at 0.5×, 1×, and 5× the IC50 concentration that produced a 50% inhibition of growth of asexual blood stage parasites (Table 1). Assays were performed in triplicate on two to five independent occasions. The gametocytocidal effect was measured relative to the luciferase signal emitted by untreated gametocyte controls cultured in parallel. Drug-specific effects (represented as means ± SEM) were calculated for up to 12 d after the beginning of treatment at day 0.
Each antimalarial produced some inhibition of gametocyte development during early stages (I and II), with the exception of atovaquone, which had little, if any, effect at any stage at the low nanomolar concentrations tested (Figs. 3 and 4 and Table 1). Drugs were generally less effective against stage III gametocytes, and most (with notable exceptions discussed below) displayed minimal activity against stages IV or V that are metabolically less active (6) (Table 1).
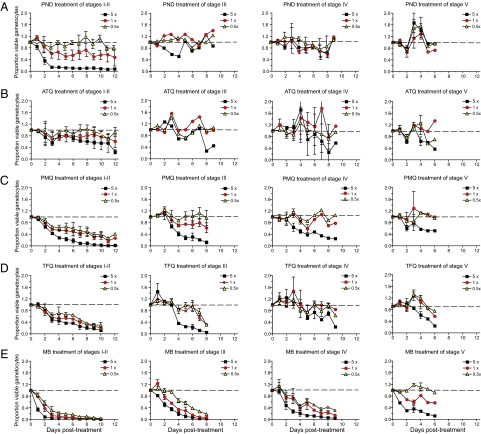
Fig. 4.
Identification of potent gametocytocidal activity of MB against P. falciparum gametocytes. Compounds were tested against gametocytes at different developmental stages, and results are illustrated as described in Fig. 3. Compounds were (A) pyronaridine (PND), (B) atovaquone (ATQ), (C) primaquine (PMQ), (D) tafenoquine (TFQ), and (E) MB. Drugs were tested at 5×, 1×, and 0.5× the IC50 concentration that produced a 50% inhibition of growth of asexual blood stage parasites (Table 1).
For DHA, we observed rapid and near-complete inhibition of luciferase expression by immature gametocytes (already evident 3 d after the onset of treatment) (Fig. 3B). A similar level of inhibition, albeit with slower kinetics, was observed in stage III gametocytes exposed to the highest drug concentration (5× IC50; i.e., 120 nM). DHA also exhibited activity against stage IV and V gametocytes (Fig. 3B), although the maximal inhibition only reached 60% in stage IV gametocytes and 35% in mature stage V gametocytes at the 5× concentration.
Among the ACT partner compounds, mdAQ showed a very rapid and potent gametocytocidal activity (>75% inhibition 3 d posttreatment) against the immature stage I and II sexual forms at all concentrations, including 0.5× (i.e., 30 nM) (Fig. 3D). For the other ACT partner drugs lumefantrine, piperaquine, and pyronaridine, only the 5× concentration (i.e., 330, 175, and 85 nM, respectively) produced comparable levels of inhibition against stage I and II parasites (Figs. 3 C and E and 4A). None of the ACT partner drugs were effective after parasites had reached stage III of gametocytogenesis.
We also tested the gametocytocidal activity of primaquine and tafenoquine. Both 8-aminoquinolines were moderately effective against immature gametocytes at all concentrations tested, but only the highest drug concentrations (5×; i.e., 6.6 and 22 μM, respectively) had an effect on late stages (Fig. 4 C and D). Importantly, these assays monitor drug effect over time, allowing us to observe delayed inhibition of mid- to late stage gametocytes in the presence of primaquine and tafenoquine (e.g., stages III and IV in Fig. 4 C and D). As discussed later, the lack of activity observed with primaquine in vitro, despite reports of its gametocytocidal properties in humans, may be related to the fact that this drug is rapidly metabolized in vivo and that its metabolites are thought to have the more potent transmission-blocking activity.
MB proved to be, by far, the most effective gametocytocidal agent tested. This compound inhibited stage I and II gametocytes by >90% at all concentrations (including 0.5×; i.e., 15 nM) (Fig. 4E). Furthermore, potent dose-dependent killing by MB was observed for all gametocytes up to stage IV. Mature stage V gametocytes were strongly affected by the highest concentration of the drug (5×; i.e., 150 nM), which produced >90% inhibition within 6 d of starting treatment.
Transmission-Blocking Activities of Antimalarial Compounds.
First-line ACT drugs in clinical use (DHA, lumefantrine, mdAQ, and piperaquine) or clinical development (pyronaridine) were subsequently evaluated for their impact on P. falciparum gametocyte infectivity to mosquitoes. Studies addressing this important feature of antimalarial action are rare (23), largely because of the difficulty of establishing the appropriate infrastructure to house P. falciparum-infected mosquitoes. Our experiments were designed to test whether ACT drug-treated late stage gametocytes would exhibit reduced infectivity. We also included MB given its potent activity profile against mature gametocytes (Fig. 4E). Parasite development within the mosquito was investigated by assessing the production of oocysts. These form under the insect midgut epithelium after exflagellation of male gametocytes to produce microgametes that fuse with female macrogametes and subsequent parasite traversal across the peritrophic matrix surrounding the blood meal (24). For the most frequently prescribed ACT (Coartem), which comprises lumefantrine plus an artemisinin derivative (25), we also examined exflagellation of stage V gametocytes as an indicator of maturity and infectivity (26).
After the production of mature gametocytes in vitro, cultures were exposed to drug on days 12–14. Gametocytes were formulated as artificial mosquito blood meals 3 or 4 d later and fed to A. stephensi mosquitoes. Mosquito midguts were dissected 6 or 7 d after gametocyte ingestion to ascertain the percentage of infected mosquitoes and quantify oocyst production. Control mosquitoes fed on untreated NF54 gametocytes had an average of 39 oocysts per midgut (range = 13–67) (Tables S1–S3). This average is considerably higher than oocyst numbers seen in Anopheles mosquitoes fed with gametocytes from patient isolates, which typically average one or two (27, 28).
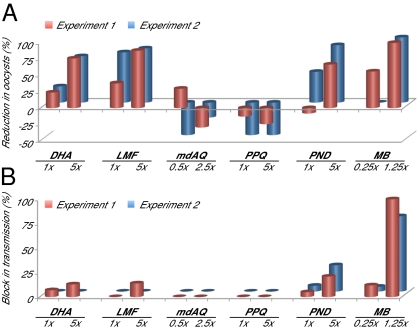
Experiments with the partner drugs mdAQ and piperaquine showed no detectable reduction in oocyst numbers or transmission blockade (Fig. 5), consistent with their lack of activity on late stage gametocytes (Fig. 3 D and E). More potent activity was observed on gametocyte treatment with DHA, which yielded oocyst numbers in infected mosquitoes that were reduced by ∼25% and 70% at 1× and 5× concentrations, respectively (Fig. 5A and Tables S1–S3). Initial studies showed that DHA inhibited the exflagellation of drug-treated stage V gametocytes by an average of 44% and 92% at 1× and 5× concentrations, respectively (data obtained in two separate experiments that were performed in duplicate). Nonetheless, DHA did not potently block transmission, because 13% of mosquitoes at most showed an absence of oocyst infection (Fig. 5B).
Fig. 5.
Transmission-blocking activity of selected antimalarial drugs. P. falciparum NF54 stage V gametocytes were cultured in the presence of drug for 3 d followed by culture in the absence of drug for 3–4 d before feeding to female A. stephensi mosquitoes. Midguts of blood-fed mosquitoes were dissected 6–7 d postfeeding, and oocyst numbers were scored. (A) Percent reduction in oocyst formation in mosquitoes fed on drug-treated gametocytes compared with solvent-treated controls (negative reduction equates to enhanced oocyst numbers). (B) Percent block in transmission in mosquitoes fed drug-treated gametocytes. This value denotes the percentage of mosquitoes in which no oocysts were observed after ingestion of drug-treated parasites; all mosquitoes fed on nontreated or mock-treated parasites had oocysts in their midguts. Transmission assays were performed in duplicate, with compounds evaluated at the concentrations indicated. Values were calculated from an average of 20 mosquitoes (range = 14–25) per drug treatment or untreated or DMSO mock-treated control (details provided in Tables S1–S3).
It is worth noting that gametocytocidal activity is not the only mechanism for transmission blockade. Lumefantrine has been observed in a rodent malaria model to moderately inhibit oocyst production directly within mosquitoes (29). This finding is consistent with our observations that lumefantrine reduced P. falciparum oocyst numbers >80% at the 5× concentration (Fig. 5A and Tables S1–S3), despite the lack of an appreciable effect on the viability of late stage gametocytes in vitro (Fig. 3C). Initial studies using exflagellation to monitor lumefantrine drug action on stage V gametocytes revealed an average inhibition of 41% and 53% at 1× and 5× concentrations, respectively. In the case of pyronaridine, this partner drug was ineffective against mature gametocytes (Fig. 4A), but it substantially inhibited oocyst production and partially blocked transmission (21–27% of mosquitoes fed on pyronaridine-treated gametocytes remained uninfected) (Fig. 5). Our findings with lumefantrine and pyronaridine suggest downstream effects on parasite development after the onset of gamete formation within the mosquito vector.
The most striking impact on transmission was observed in mosquitoes fed on MB-treated gametocytes. At a low concentration (1.25× the IC50, corresponding to 38 nM) (Table 1), 78–100% of the mosquitoes harbored no oocysts, and the remainder harbored a single oocyst, corresponding to a 98–100% reduction in oocyst density compared with controls (Fig. 5 A and B and Tables S1–S3). This effect is consistent with the substantial decrease in viable mature gametocytes in MB-treated cultures (Fig. 4E).
Discussion
Here, we report gametocyte stage-specific transgenic lines that allow P. falciparum maturation to be quantified throughout the five stages of gametocytogenesis. These reporter lines with stably integrated GFP-luciferase expression cassettes were used to quantitatively assess the activity of several key antimalarials on sexual stage development. In vitro results were complemented by direct observation of antimalarial drug action on gametocyte transmission to Anopheles mosquitoes.
Our data reveal highly potent gametocytocidal and transmission-blocking properties of the thiazine dye MB. Characterized by a short plasma half-life and a high bioavailability (18 h and 72%, respectively) (30), this compound was the first synthetic compound ever used in clinical therapy, dating back to the 1891 report of its antimalarial properties by the renowned chemist Paul Ehrlich (31, 32). Recently, there has been renewed interest in this affordable and already clinically registered compound, with a series of studies that have assessed the benefit of adding MB to antimalarial combinations (33–35). One recent field trial from Burkina Faso observed excellent clinical efficacy of a MB-amodiaquine combination when tested in children with uncomplicated falciparum malaria (34). Another study reported moderate curative activity with MB monotherapy, illustrating the need for this slow-acting drug to be combined with fast-acting agents (35). Our results show that MB interferes with gametocyte development at all stages and can block transmission through the potent clearance of stage V gametocytes. Furthermore, MB was the only compound that we tested that dramatically reduced the mosquito infection prevalence. These results agree with and extend preliminary clinical observations that MB-based treatment regimens reduced the gametocyte carriage rate in treated patients in addition to being effective in clearing asexual blood stage infections (33). Of note, we observed potent gametocytocidal activity at concentrations as low as 30 nM, corresponding to 10 ng/mL. This concentration is readily achieved in humans, for which the average Cmax was found to be 3,900 ng/mL after administration of a single 500-mg oral dose to adults (with a mean weight of 67 kg) (30). As a reference, combination trials of MB plus amodiaquine or artesunate in children recently used 10 mg/kg MB two times daily (34).
In humans, MB is used primarily to treat methemoglobinemia, a condition whereby the heme ferrous ion (Fe2+) is oxidized to the ferric state (Fe3+). The reduced form of MB, which acts as an electron donor, can reverse this oxidation. Against Plasmodium, the mode of action remains enigmatic (36, 37). The hypothesis that this highly selective, redox-cycling agent interfered with glutathione reductase was recently refuted by the demonstration that MB was equally active against P. falciparum parasites lacking this antioxidant enzyme (38). Other evidence suggests that MB can interfere with hemozoin formation, which occurs in the Plasmodium digestive vacuole and permits the detoxification of reactive iron heme moieties formed during hemoglobin degradation (37). Of note, MB is a weak base that could concentrate in this highly acidic organelle (with a pH of 5.2–5.5) (39). Such a mode of action would be similar to 4-aminoquinolines such as chloroquine or amodiaquine. However, mechanisms of resistance to those drugs, mediated by the multidrug transporters PfCRT and PfMDR1 (40, 41), do not impart cross-resistance to MB (36, 42), and a separate mode of action must underlie MB activity against mature gametocytes in which hemoglobin degradation no longer occurs. Given that these forms are often refractory to antimalarial drugs, our data suggest that MB or a derivative thereof could be a highly effective transmission-blocking adjunct to curative antimalarial combinations.
In response to the global dissemination of P. falciparum strains resistant to the former first-line drugs chloroquine and sulfadoxine-pyrimethamine, artemisinins have recently been adopted worldwide as the first-line antimalarial drugs, and they are used in combination with partner drugs that have longer plasma half-lives (25). Our luciferase data reveal that DHA, the active metabolite of artemisinins, has appreciable activity on early stage gametocytes and to a lesser extent, late stages. Our studies also reveal DHA-mediated impairment of both microgametocyte exflagellation and numbers of oocysts formed within the mosquito. A 70% decrease in oocyst numbers was achieved at concentrations (120 nM) that remain biologically relevant (mean maximal plasma concentration of DHA in a 3-d treatment regimen of DHA-lumefantrine is ∼250 nM) (43). Nevertheless, the block in transmission, measured by the percentage of infected mosquitoes, was minimal. This finding is consistent with field-based studies that document a rather modest effect of artemisinins on transmission of mature gametocytes (8, 9). A recent finding that artemisinins inhibit hemoglobin uptake and its subsequent hydrolysis may explain the marked activity of DHA against immature gametocytes (44).
Lumefantrine, the arylaminoalcohol partner drug of the most widely used ACT Coartem (25), exhibited minimal gametocytocidal potency on sexual forms beyond stage III of differentiation. However, substantial inhibition of infectivity was observed at concentrations (330 nM) far below those levels achieved pharmacologically (mean peak plasma concentration in malaria patients was ∼11 μM, with a half-life of 4 or 5 d) (1, 43). The reduction in oocyst numbers exceeded drug impact on exflagellation, suggesting that lumefantrine may impact the activation of female gametocytes, gamete formation, or possibly, oocyst development through an unknown mode of action, which was suggested by recent findings from rodent models (29).
Amodiaquine in combination with artesunate constitutes the second most widely used ACT, and the related bisquinoline piperaquine has recently become a first-line drug in some Southeast Asian countries. Pyronaridine, a Mannich base, has completed Phase III clinical trials in combination with artesunate, and this new ACT is under review for registration (1, 25). The chemical similarity of mdAQ, piperaquine, and pyronaridine to chloroquine, a 4-aminoquinoline, suggests a related mode of action involving inhibition of heme detoxification in the digestive vacuole (39). Consistent with this activity, mdAQ, piperaquine, and pyronaridine exert gametocytocidal activity only on immature gametocytes. Both mdAQ and piperaquine treatments failed to modulate gametocyte transmissibility when treating mature gametocytes. In contrast, pyronaridine (which is characterized by a relatively long terminal half-life of 7–9 d) treatment led to a significant reduction in oocyst numbers at concentrations still below the mean maximum plasma concentration in patients (160 nM) (45). Thus, although pyronaridine does not seem to affect gametocyte viability per se, as measured by expression of luciferase, our evidence suggests inhibition of subsequent parasite development within the mosquito.
Primaquine has a long history of use for the treatment of P. vivax and P. ovale malaria, where it is effective at preventing relapses through its activity against liver stage hypnozoites (7). Studies with P. falciparum infections indicate that a single dose of primaquine supplemented to ACTs can substantially shorten the period of gametocyte carriage and importantly, clear submicroscopic gametocytes (46, 47). In our assays, this 8-aminoquinoline drug displayed gametocytocidal activity against stage I–IV sexual forms, albeit at low micromolar concentrations compared with low to mid-nanomolar concentrations with DHA and MB. Primaquine also exhibited partial activity against mature (stage V) gametocytes, although this finding was only observed at the 5× concentration that corresponds to 6.6 μM. Pharmacokinetic studies in humans administered a standard oral dose of 45 mg primaquine showed Cmax levels of 0.3 and 3.1 μM of the parent compound and its primary metabolite carboxyprimaquine, respectively (48). Our data suggest that the rapidly metabolized parent compound is not a potent gametocytocidal agent and that its transmission-blocking activity observed clinically may be attributable to metabolites (49).
We also examined the gametocytocidal potency of the clinical candidate tafenoquine, a 5-phenoxy derivative of primaquine that is less toxic and has a longer plasma half-life (14 d vs. 5–6 h, respectively) (48, 50). In our assays, tafenoquine was comparable with primaquine in showing gametocytocidal activity (primarily against early stages), albeit at high concentrations (2.2–22 μM) (Fig. 4D). These concentrations are equivalent to or exceed the Cmax value of 4 μM observed in human volunteers administered a single oral dose of 400 mg tafenoquine (50). Our data are consistent with earlier reports of its limited gametocytocidal activity against rodent P. berghei or P. yoelii parasites, contrasting with the more potent in vivo transmission-blocking action of primaquine (51, 52). Studies with rat liver microsomes show that tafenoquine is slowly but extensively metabolized (53). However, we could find no reports of whether these metabolites are gametocytocidal. Tafenoquine has been reported to inhibit sporogony (i.e., the formation of sporozoites inside oocysts) in P. falciparum, P. vivax, and P. berghei (54, 55) and thus, has been described as transmission-blocking by virtue of this sporonticidal action (absent with the more potent gametocytocidal drug primaquine). Additional experiments with NF54 parasites are planned to investigate dose-dependent effects of tafenoquine and primaquine on gametocyte transmission as well as sporogony.
Whether these compounds act on gametocytes by inhibiting mitochondrial function and/or triggering oxidative stress remains to be elucidated (49). Primaquine, however, has known toxicity in G6PD-deficient patients, primarily as a result of hemolytic anemia (56). These safety concerns, combined with the elevated prevalence of this genetic disorder in malaria-endemic populations (57), emphasize the need to pursue alternative 8-aminoquinolines with reduced hematoxicity and also explore alternative chemical scaffolds that block the transmission of mature gametocytes.
Overall, our results suggest that, of the current ACTs, combinations pairing an artemisinin derivative with lumefantrine or pyronaridine might be the most effective at targeting both asexual and sexual stages. Conversely, the partner drugs piperaquine and mdAQ revealed no notable transmission-blocking activity, which was measured through oocyst density and infection prevalence in mosquitoes. Furthermore, none of the current ACT partner drugs were effective against mature stage V gametocytes, which was quantified in our luciferase assays, potentially reflecting the less metabolically active nature of late stage gametocytes that renders them insensitive to many antimalarials. Finally, we find that the broadly active compound MB might be an excellent gametocytocidal drug to add to the ACT armamentarium.
Our results with known antimalarials highlight the use of these GFP-luciferase reporter lines for deriving a quantitative readout of P. falciparum viability throughout gametocyte development. This system allows for the precise determination of both drug potency and kinetics of inhibition, revealing the dose-dependent susceptibility of each gametocyte stage. Both the luciferase and GFP stably integrated reporters are amenable to high-throughput approaches to screen for gametocytocidal compounds. Furthermore, NF54 parasites are efficiently transmitted to mosquitoes, allowing the measurement of drug susceptibilities during the various steps in the parasite transition from human to mosquito host. This dual approach of quantitatively assessing gametocyte maturation combined with mosquito transmission studies on active compounds can now be leveraged to identify gametocytocidal agents, with the ultimate objective of designing therapies that both cure malaria and prevent its transmission.
Materials and Methods
Plasmid Constructs and Parasite Transfections.
To generate the pCC-cg6-attB plasmid, 0.4-kb 5′ and 3′ fragments of cg6 (PlasmoDB identifier PF07_0036) were amplified using primers p1513 + p1514 and p1515 + p1516, respectively (Table S4). These fragments introduced 5′ and 3′ AatII sites and an internal 44-bp attB sequence (14), and they were used for overlapping PCR using primers p1513 + p1516. The corresponding product was subcloned into pCR2.1 (Invitrogen), and sequence-verified inserts were excised by AatII and ligated into pCC (58). pCC-cg6-attB was electroporated into NF54 parasites propagated in malaria culture medium (14) supplemented with 10% human AB+ serum. Transformed parasites were selected using 2.5 nM WR99210, and 1 μM 5-fluorocytosine was used to select for excision of the selectable markers after integration.
Gametocyte stage-specific reporter vectors were constructed by amplifying the 5′ UTRs of the gametocyte genes pfs16 (PFD0310w; 1.3-kb), pfs48/45 (PF13_0247; 1.4-kb), and mal8p1.16 (MAL8P1.16; 1.7-kb) with primers (p2145 + p2146, p2147 + p2148, and p2151 + p2152, respectively) containing ApaI or AvrII restriction sites (Table S4). Promoter regions were inserted into ApaI/AvrII-digested pDC2-cam-mRFP-Vps4-attP derived from pLN (14). This plasmid also contains the Mycobacterium smegmatis attP site and a blasticidin S-deaminase selectable marker cassette. The GFPmut3/LucIAV fusion cassette was isolated from the pU.K.GFPM3lucIAVcl1 plasmid (provided by B. Franke-Fayard and C. Janse, Leiden, The Netherlands) after XbaI and XhoI digestion. This GFP-luciferase fusion was cloned downstream of the gametocyte promoters and upstream of an hsp86 3′ UTR using AvrII and XhoI sites. The resulting plasmids (pfs16-GFP-LUC-attP, pfs48/45-GFP-LUC-attP, and mal8p1.16-GFP-LUC-attP) were cotransfected with the Bxb1 mycobacteriophage integrase-expressing vector pINT into NF54attB parasites as described (14). Transfectants were selected with 2 μg/mL blasticidin (Invitrogen) and 250 μg/mL G418 (for the first 6 d only), and they were first detected 20 d postelectroporation. Plasmid integration was confirmed by PCR, and clones were obtained by limiting dilution.
DNA Analysis.
P. falciparum trophozoite-infected erythrocytes were harvested and saponin-lysed. Parasite genomic DNA was extracted and purified using DNeasy Tissue kits (Qiagen). Integration of pCC-cg6-attB into the cg6 locus of NF54 parasites was detected using primers p1655 + p838 (specific to the cg6 5′UTR and the PbDT 3′UTR, respectively) and primers p1656 + p1636 (specific to the cg6 3′ UTR and human dhfr, respectively). These primer pairs produced bands of 3.4 and 2.7 kb, respectively, in integration-positive parasites. Subsequent intralocus recombination, leading to excision of the selectable markers, was identified using primers p1969 + p1970, both specific to cg6 (Table S4). PCR amplification with p1969 + p1970 yielded a doublet band migrating around 0.2-kb corresponding to fragments containing or lacking an internal attB site (depending on whether recombination leading to loss of the human dhfr and cdup markers occurred between the cg6 5′ or 3′ sequences) (Fig. 1A). The presence of attB in the unmarked cg6 locus was confirmed using primers p1829 (specific to the attB site) and p1656, yielding a 0.8-kb band. Integrase-mediated attB × attP site-specific recombination was detected using primers located on each side of the integration: p1969 + p836 (specific for cg6 and PcDT 5′UTR, respectively) and p1970 + p984 (specific for cg6 and the firefly luciferase coding sequence, respectively).
For Southern blot analysis, 1 μg genomic DNA was digested with SalI/NruI or SalI/EcoRV enzymes, electrophoresed on a 0.7% agarose gel, and transferred onto a Nytran nylon membrane overnight. Hybridization was performed at 55 °C with a 0.4-kb [32P]-cg6 probe obtained as a SalI/AatII fragment from pCC-cg6-attB.
Gametocyte Induction and Purification.
To produce gametocytes for in vitro assays, asexual blood stage parasites were first synchronized by 5% d-sorbitol (wt/vol) treatment for 10 min at 37 °C for two or more successive growth cycles. Synchronous parasites at 3% hematocrit were cultured in 100-cm2 Petri dishes to 10% parasitemia (ring stage), at which point gametocytogenesis was induced by feeding cultures with 50% conditioned media (21). The next day, trophozoite cultures were diluted fourfold. N-acetyl glucosamine (NAG; Sigma-Aldrich) was then added to a final concentration of 50 mM after all schizonts had ruptured. NAG treatment was maintained for the next two to three cycles of reinvasion to eliminate all asexual forms from the culture. Immature gametocytes (stages I and II) were magnetically purified by passage through a MACS CS column (Miltenyi Biotech) and transferred into 96-well plates at 2% hematocrit such that each well contained 1–2% gametocytes in a final volume of 150 μL. Giemsa-stained smears were examined daily to monitor development.
Luciferase Assays.
Gametocytes were harvested daily for up to 12–16 d. Cultures were concentrated by centrifugation, and pellets were stored at −80 °C. Cells were lysed at room temperature in 3× Luciferin Lysis Buffer (15 mM DTT, 0.6 mM CoA, 0.45 mM ATP, 0.42 mg/mL luciferin, 10 mM MgCl2, 10 mM Tris Base, 10 mM Tris⋅HCl, 0.03% Triton X-100). Luciferase activity was measured at 560 nm in a Victor3 luminescence plate reader (Perkin-Elmer). GFP expression from the different gametocyte-specific promoters was confirmed by fluorescence microscopy using a Nikon TiE PFS inverted microscope equipped with a CoolSNAP HQ2 monochrome camera.
Drugs and in Vitro Susceptibility Assays.
Atovaquone (provided by A. Vaidya, Philadelphia, PA), DHA (Avachem Scientific), lumefantrine (a gift from Philip Rosenthal, San Francisco, CA), MB (American Regent), mdAQ (a gift from Pascal Ringwald, Geneva, Switzerland), piperaquine (Avachem Scientific), primaquine (Sigma), pyronaridine (Avachem Scientific), and tafenoquine (GlaxoSmithkline) were used at three different concentrations corresponding to 0.5×, 1×, and 5× the mean IC50 value obtained with asexual blood stage parasites (Table 1). In vitro IC50 values against asexual parasites were calculated by nonlinear regression analysis of 72-h [3H]hypoxanthine-based growth inhibition assays (59). All drugs were prepared as stock solutions in 100% DMSO except for MB, which was dissolved in water. Drugs were diluted in RPMI-1640 to 50× IC50 (DHA was prepared fresh because of its poor stability in aqueous solution).
Gametocytes were transferred into 96-well plates at 2% parasitemia and 2% hematocrit starting on day 2 postinduction and cultured in the presence of NAG. Drugs were added for 3 d, with treatment initiated on days 2 (stages I and II), 5 (stage III), 8 (stage IV), or 11 (stage V) of gametocytogenesis, with daily medium changes. Stage I and II, III and IV, and V gametocytes were assessed using the pfs16-GFP-LUC, pfs48/45-GFP-LUC, and both pfs16-GFP-LUC and mal8p1.16-GFP-LUC lines, respectively. Untreated gametocytes, maintained on the same culture plates and processed in parallel, were used as controls.
To quantify the inhibitory effects of drugs on gametocytes, luciferase counts were normalized based on a weighted average of the data. The first part of the weighting consisted of dividing the luciferase counts for each day of the assay for treated and control lines by their starting values (day 0 or 1). Values for drug-treated parasites were then divided by values obtained for nontreated controls. The second part of the weighting consisted of dividing the raw treated values by the raw control values for each day of treatment. The average of these two calculations was then plotted as a function of time (Figs. 3 and 4). This method standardized the assays to a common scale while separating drug action from background changes in promoter activity or gametocyte numbers over time.
Transmission-Blocking Studies and Oocyst Formation.
NF54 gametocytes were induced by allowing asexual blood stage parasites to proliferate without the addition of fresh erythrocytes (60). Gametocyte cultures in six-well plates were treated at 1× or 5× the mean IC50 value (obtained against asexual blood stages) for all drugs except mdAQ (0.5× and 2.5×) and MB (0.25× and 1.25×). All cultures were prepared in parallel with controls corresponding to cultures that were untreated or treated with 0.05–0.1% DMSO, except for MB (water control). Gametocytes were exposed to drug with daily media changes from days 12 to 14 after their induction. Parasites were subsequently maintained in the absence of drug until blood feeding on days 17 and 18. The percentages of mature gametocytes in cultures were assessed by microscopy of Giemsa-stained thin blood smears. Functional microgametocytes were assessed for exflagellation activity at 18 °C after drug treatment of stage V gametocytes on days 15–17 and were quantified as exflagellation centers per 106 human erythrocytes.
Treated and control cultures were formulated as artificial mosquito blood meals at 50% hematocrit, with final stage V gametocytemias of 0.01–0.1% (after dilution of the starting cultures in which gametocytemias typically reached 0.7–1.7%). Blood meals were fed in duplicate to A. stephensi using artificial membrane feeders. Engorged mosquitoes were maintained at 26 °C and 78% relative humidity with 12-h light/dark cycles. In each feeding experiment, between 15 and 25 mosquitoes with developed ovaries (indicating blood feeding) were sampled 6 or 7 d postfeeding to assess oocyst formation. Mosquito midguts were dissected in 0.2% Mercurochrome, and oocyst numbers were counted under a phase-contrast microscope. The infection prevalence (defined as the percentage of mosquitoes with one or more oocyst) was determined for each experiment. Routinely, this prevalence was 100% in mosquitoes fed untreated or mock-treated (DMSO) gametocytes (Tables S1–S3). The intensity of mosquito infection was also evaluated as the mean number of oocysts per midgut. A Student t test was performed to determine statistical significance.
SI Materials and Methods Details.
In SI Materials and Methods, Fig. S1 shows chemical structures, Tables S1–S3 summarize compound activity against P. falciparum gametocyte transmission to Anopheles mosquitoes, Table S4 lists oligonucleotide sequences, and Table S5 shows the raw data used to generate Fig. P1 in the Author Summary.
Supplementary Material
Acknowledgments
We thank Joerg-Peter Kleim and others at GlaxoSmithKline for providing tafenoquine and Neha Passi and Catie Brownback for assistance with the manuscript. G.L.J. received funding through the National Science Foundation Graduate Research Fellowship Program. Funding support for R.T.E. was provided by US National Institutes of Health Grant T90 NR010824. This work was supported in part by US National Institutes of Health Grant AI079709 (to D.A.F.) and the Medicines for Malaria Venture (D.A.F.). Sanaria Inc. provided funding for the transmission-blocking experiments.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
See Author Summary on page 18867.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1112037108/-/DCSupplemental.
References
- 1.Eastman RT, Fidock DA. Artemisinin-based combination therapies: A vital tool in efforts to eliminate malaria. Nat Rev Microbiol. 2009;7:864–874. doi: 10.1038/nrmicro2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feachem R, Sabot O. A new global malaria eradication strategy. Lancet. 2008;371:1633–1635. doi: 10.1016/S0140-6736(08)60424-9. [DOI] [PubMed] [Google Scholar]
- 3.Alonso PL, et al. A research agenda to underpin malaria eradication. PLoS Med. 2011;8:e1000406. doi: 10.1371/journal.pmed.1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenwood BM. Asymptomatic malaria infections—do they matter? Parasitol Today. 1987;3:206–214. doi: 10.1016/0169-4758(87)90061-5. [DOI] [PubMed] [Google Scholar]
- 5.Stepniewska K, et al. Plasmodium falciparum gametocyte dynamics in areas of different malaria endemicity. Malar J. 2008;7:249. doi: 10.1186/1475-2875-7-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butcher GA. Antimalarial drugs and the mosquito transmission of Plasmodium. Int J Parasitol. 1997;27:975–987. doi: 10.1016/s0020-7519(97)00079-9. [DOI] [PubMed] [Google Scholar]
- 7.Bousema T, Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev. 2011;24:377–410. doi: 10.1128/CMR.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price RN, et al. Effects of artemisinin derivatives on malaria transmissibility. Lancet. 1996;347:1654–1658. doi: 10.1016/s0140-6736(96)91488-9. [DOI] [PubMed] [Google Scholar]
- 9.Targett G, et al. Artesunate reduces but does not prevent posttreatment transmission of Plasmodium falciparum to Anopheles gambiae. J Infect Dis. 2001;183:1254–1259. doi: 10.1086/319689. [DOI] [PubMed] [Google Scholar]
- 10.Peatey CL, et al. Effect of antimalarial drugs on Plasmodium falciparum gametocytes. J Infect Dis. 2009;200:1518–1521. doi: 10.1086/644645. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka TQ, Williamson KC. A malaria gametocytocidal assay using oxidoreduction indicator, alamarBlue. Mol Biochem Parasitol. 2011;177:160–163. doi: 10.1016/j.molbiopara.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eksi S, Suri A, Williamson KC. Sex- and stage-specific reporter gene expression in Plasmodium falciparum. Mol Biochem Parasitol. 2008;160:148–151. doi: 10.1016/j.molbiopara.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchholz K, et al. A high-throughput screen targeting malaria transmission stages opens new avenues for drug development. J Infect Dis. 2011;203:1445–1453. doi: 10.1093/infdis/jir037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nkrumah LJ, et al. Efficient site-specific integration in Plasmodium falciparum chromosomes mediated by mycobacteriophage Bxb1 integrase. Nat Methods. 2006;3:615–621. doi: 10.1038/nmeth904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dixon MWA, Peatey CL, Gardiner DL, Trenholme KR. A green fluorescent protein-based assay for determining gametocyte production in Plasmodium falciparum. Mol Biochem Parasitol. 2009;163:123–126. doi: 10.1016/j.molbiopara.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman SL, et al. Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum Vaccin. 2010;6:97–106. doi: 10.4161/hv.6.1.10396. [DOI] [PubMed] [Google Scholar]
- 17.Bozdech Z, et al. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. 2003;1:E5. doi: 10.1371/journal.pbio.0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young JA, et al. The Plasmodium falciparum sexual development transcriptome: A microarray analysis using ontology-based pattern identification. Mol Biochem Parasitol. 2005;143:67–79. doi: 10.1016/j.molbiopara.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Pradel G. Proteins of the malaria parasite sexual stages: Expression, function and potential for transmission blocking strategies. Parasitology. 2007;134:1911–1929. doi: 10.1017/S0031182007003381. [DOI] [PubMed] [Google Scholar]
- 20.Bruce MC, Carter RN, Nakamura K, Aikawa M, Carter R. Cellular location and temporal expression of the Plasmodium falciparum sexual stage antigen Pfs16. Mol Biochem Parasitol. 1994;65:11–22. doi: 10.1016/0166-6851(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 21.Fivelman QL, et al. Improved synchronous production of Plasmodium falciparum gametocytes in vitro. Mol Biochem Parasitol. 2007;154:119–123. doi: 10.1016/j.molbiopara.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Ecker A, Lakshmanan V, Sinnis P, Coppens I, Fidock DA. Evidence that mutant PfCRT facilitates the transmission to mosquitoes of chloroquine-treated Plasmodium gametocytes. J Infect Dis. 2011;203:228–236. doi: 10.1093/infdis/jiq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chotivanich K, et al. Transmission-blocking activities of quinine, primaquine, and artesunate. Antimicrob Agents Chemother. 2006;50:1927–1930. doi: 10.1128/AAC.01472-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alano P. Plasmodium falciparum gametocytes: Still many secrets of a hidden life. Mol Microbiol. 2007;66:291–302. doi: 10.1111/j.1365-2958.2007.05904.x. [DOI] [PubMed] [Google Scholar]
- 25.Wells TN, Alonso PL, Gutteridge WE. New medicines to improve control and contribute to the eradication of malaria. Nat Rev Drug Discov. 2009;8:879–891. doi: 10.1038/nrd2972. [DOI] [PubMed] [Google Scholar]
- 26.Sinden RE, Talman A, Marques SR, Wass MN, Sternberg MJE. The flagellum in malarial parasites. Curr Opin Microbiol. 2010;13:491–500. doi: 10.1016/j.mib.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Beier JC, Copeland RS, Mtalib R, Vaughan JA. Ookinete rates in Afrotropical anopheline mosquitoes as a measure of human malaria infectiousness. Am J Trop Med Hyg. 1992;47:41–46. doi: 10.4269/ajtmh.1992.47.41. [DOI] [PubMed] [Google Scholar]
- 28.Drakeley CJ, et al. Parasite infectivity and immunity to Plasmodium falciparum gametocytes in Gambian children. Parasite Immunol. 2004;26:159–165. doi: 10.1111/j.0141-9838.2004.00696.x. [DOI] [PubMed] [Google Scholar]
- 29.Delves MJ, Sinden RE. A semi-automated method for counting fluorescent malaria oocysts increases the throughput of transmission blocking studies. Malar J. 2010;9:35. doi: 10.1186/1475-2875-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walter-Sack I, et al. High absolute bioavailability of methylene blue given as an aqueous oral formulation. Eur J Clin Pharmacol. 2009;65:179–189. doi: 10.1007/s00228-008-0563-x. [DOI] [PubMed] [Google Scholar]
- 31.Guttmann P, Ehrlich P. über die Wirkung des Methylenblau bei Malaria. Berlin Klin Wochenschr. 1891;28:953–956. [Google Scholar]
- 32.Schirmer RH, Adler H, Pickhardt M, Mandelkow E. “Lest we forget you—methylene blue…”. Neurobiol Aging. 2011;32:2325.e7–2325.e16. doi: 10.1016/j.neurobiolaging.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Coulibaly B, et al. Strong gametocytocidal effect of methylene blue-based combination therapy against falciparum malaria: A randomised controlled trial. PLoS One. 2009;4:e5318. doi: 10.1371/journal.pone.0005318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zoungrana A, et al. Safety and efficacy of methylene blue combined with artesunate or amodiaquine for uncomplicated falciparum malaria: A randomized controlled trial from Burkina Faso. PLoS One. 2008;3:e1630. doi: 10.1371/journal.pone.0001630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bountogo M, et al. Efficacy of methylene blue monotherapy in semi-immune adults with uncomplicated falciparum malaria: A controlled trial in Burkina Faso. Trop Med Int Health. 2010;15:713–717. doi: 10.1111/j.1365-3156.2010.02526.x. [DOI] [PubMed] [Google Scholar]
- 36.Vennerstrom JL, Makler MT, Angerhofer CK, Williams JA. Antimalarial dyes revisited: Xanthenes, azines, oxazines, and thiazines. Antimicrob Agents Chemother. 1995;39:2671–2677. doi: 10.1128/aac.39.12.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schirmer RH, et al. Methylene blue as an antimalarial agent. Redox Rep. 2003;8:272–275. doi: 10.1179/135100003225002899. [DOI] [PubMed] [Google Scholar]
- 38.Pastrana-Mena R, et al. Glutathione reductase-null malaria parasites have normal blood stage growth but arrest during development in the mosquito. J Biol Chem. 2010;285:27045–27056. doi: 10.1074/jbc.M110.122275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vinetz JM, Clain J, Bounkeua V, Eastman RT, Fidock DA. In: Goodman & Gilman's The Pharmacological Basis of Therapeutics. Brunton L, Chabner B, Knollman B, editors. New York: McGraw-Hill; 2011. pp. 1383–1418. [Google Scholar]
- 40.Sá JM, et al. Geographic patterns of Plasmodium falciparum drug resistance distinguished by differential responses to amodiaquine and chloroquine. Proc Natl Acad Sci USA. 2009;106:18883–18889. doi: 10.1073/pnas.0911317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valderramos SG, et al. Identification of a mutant PfCRT-mediated chloroquine tolerance phenotype in Plasmodium falciparum. PLoS Pathog. 2010;6:e1000887. doi: 10.1371/journal.ppat.1000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pascual A, et al. In vitro activity of Proveblue (methylene blue) on Plasmodium falciparum strains resistant to standard antimalarial drugs. Antimicrob Agents Chemother. 2011;55:2472–2474. doi: 10.1128/AAC.01466-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Djimdé A, Lefèvre G. Understanding the pharmacokinetics of Coartem. Malar J. 2009;8(Suppl 1):S4. doi: 10.1186/1475-2875-8-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klonis N, et al. Artemisinin activity against Plasmodium falciparum requires hemoglobin uptake and digestion. Proc Natl Acad Sci USA. 2011;108:11405–11410. doi: 10.1073/pnas.1104063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramharter M, et al. Fixed-dose pyronaridine-artesunate combination for treatment of uncomplicated falciparum malaria in pediatric patients in Gabon. J Infect Dis. 2008;198:911–919. doi: 10.1086/591096. [DOI] [PubMed] [Google Scholar]
- 46.Bousema T, et al. Revisiting the circulation time of Plasmodium falciparum gametocytes: Molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar J. 2010;9:136. doi: 10.1186/1475-2875-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smithuis F, et al. Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: An open-label randomised trial. Lancet Infect Dis. 2010;10:673–681. doi: 10.1016/S1473-3099(10)70187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mihaly GW, Ward SA, Edwards G, Orme ML, Breckenridge AM. Pharmacokinetics of primaquine in man: Identification of the carboxylic acid derivative as a major plasma metabolite. Br J Clin Pharmacol. 1984;17:441–446. doi: 10.1111/j.1365-2125.1984.tb02369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vale N, Moreira R, Gomes P. Primaquine revisited six decades after its discovery. Eur J Med Chem. 2009;44:937–953. doi: 10.1016/j.ejmech.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 50.Brueckner RP, Lasseter KC, Lin ET, Schuster BG. First-time-in-humans safety and pharmacokinetics of WR 238605, a new antimalarial. Am J Trop Med Hyg. 1998;58:645–649. doi: 10.4269/ajtmh.1998.58.645. [DOI] [PubMed] [Google Scholar]
- 51.Coleman RE, Clavin AM, Milhous WK. Gametocytocidal and sporontocidal activity of antimalarials against Plasmodium berghei ANKA in ICR Mice and Anopheles stephensi mosquitoes. Am J Trop Med Hyg. 1992;46:169–182. doi: 10.4269/ajtmh.1992.46.169. [DOI] [PubMed] [Google Scholar]
- 52.Peters W, Robinson BL, Milhous WK. The chemotherapy of rodent malaria. LI. Studies on a new 8-aminoquinoline, WR 238,605. Ann Trop Med Parasitol. 1993;87:547–552. doi: 10.1080/00034983.1993.11812809. [DOI] [PubMed] [Google Scholar]
- 53.Idowu OR, Peggins JO, Brewer TG, Kelley C. Metabolism of a candidate 8-aminoquinoline antimalarial agent, WR 238605, by rat liver microsomes. Drug Metab Dispos. 1995;23:1–17. [PubMed] [Google Scholar]
- 54.Ponsa N, Sattabongkot J, Kittayapong P, Eikarat N, Coleman RE. Transmission-blocking activity of tafenoquine (WR-238605) and artelinic acid against naturally circulating strains of Plasmodium vivax in Thailand. Am J Trop Med Hyg. 2003;69:542–547. [PubMed] [Google Scholar]
- 55.Coleman RE, et al. Prevention of sporogony of Plasmodium falciparum and P. berghei in Anopheles stephensi mosquitoes by transmission-blocking antimalarials. Am J Trop Med Hyg. 1994;50:646–653. doi: 10.4269/ajtmh.1994.50.646. [DOI] [PubMed] [Google Scholar]
- 56.Wells TN, Burrows JN, Baird JK. Targeting the hypnozoite reservoir of Plasmodium vivax: The hidden obstacle to malaria elimination. Trends Parasitol. 2010;26:145–151. doi: 10.1016/j.pt.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 57.Ruwende C, et al. Natural selection of hemi- and heterozygotes for G6PD deficiency in Africa by resistance to severe malaria. Nature. 1995;376:246–249. doi: 10.1038/376246a0. [DOI] [PubMed] [Google Scholar]
- 58.Maier AG, et al. Exported proteins required for virulence and rigidity of Plasmodium falciparum-infected human erythrocytes. Cell. 2008;134:48–61. doi: 10.1016/j.cell.2008.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fidock DA, Nomura T, Wellems TE. Cycloguanil and its parent compound proguanil demonstrate distinct activities against Plasmodium falciparum malaria parasites transformed with human dihydrofolate reductase. Mol Pharmacol. 1998;54:1140–1147. doi: 10.1124/mol.54.6.1140. [DOI] [PubMed] [Google Scholar]
- 60.Ponnudurai T, Meuwissen JH, Leeuwenberg AD, Verhave JP, Lensen AH. The production of mature gametocytes of Plasmodium falciparum in continuous cultures of different isolates infective to mosquitoes. Trans R Soc Trop Med Hyg. 1982;76:242–250. doi: 10.1016/0035-9203(82)90289-9. [DOI] [PubMed] [Google Scholar]