The paper in PNAS by Martineau et al. (1) raises a key public health issue: how much vitamin D do you need to fight tuberculosis (TB)? Martineau et al. (1) found that there is a significant association of vitamin D deficiency with susceptibility to TB and that the impact is greater in HIV-infected than noninfected individuals. In addition, Martineau et al. (1) discover a striking temporal relationship between vitamin D deficiency and TB. The reporting of new TB cases in Cape Town, South Africa, was lowest in the months after the seasonal increase in serum 25-hydroxyvitamin D (25D) levels, whereas the reporting of new TB cases was highest in the months following the season with the lowest serum 25D levels.
The major source of vitamin D for humans derives from sun exposure; in the skin, UVB induces conversion of 7-dehydrocholesterol to previtamin D3 and then vitamin D3 (cholecalciferol). In the liver, vitamin D3 is 25-hydroxylated to form 25D. 25D is then converted in the kidney by the 1-α-hydroxylase, CYP27b1, to 1,25-dihydroxyvitamin D (1,25D), the bioactive, hormonal form of vitamin D that is bound with high affinity and specificity by the vitamin D receptor (VDR). Serum 1,25D levels are maintained in a constant range by parathyroid hormone regulation of the CYP27b1 gene. Therefore, circulating levels of 25D are the best clinical assessment of adequate vitamin D status. Some of the factors that determine 25D levels include latitude (25D levels can be maintained year round in the equatorial regions between the Tropic of Cancer and Tropic of Capricorn), skin color (10 times as much UVB is required to produce the same amount of vitamin D in dark- vs. light-skinned individuals), outdoor activity, body surface exposed, oral supplementation, and SNPs in several vitamin D metabolism genes.
Martineau et al. (1) also report that vitamin D deficiency is present in greater than 60% of a population of active and latent TB patients in Cape Town, South Africa. The frequency of vitamin D deficiency is higher than anticipated but may be, in part, expected because of at least three risk factors in the study population: (i) they are black; (ii) they reside in Capetown (33°S latitude), which is well south of the Tropic of Capricorn; and (iii) they are poorly nourished in terms of oral vitamin D supplementation. Although vitamin D status has been previously suggested as a contributing factor to the incidence of TB, a relationship between seasonal vitamin D status and TB case notification has not previously been evaluated in the same population.
A number of investigations have linked serum 25D levels to both TB disease susceptibility and progression. Meta-analysis of observational studies suggested a 70% probability that a healthy individual would have higher 25D serum levels than an individual with untreated TB. Nnoaham and Clarke (2) concluded that “it is more likely that low body vitamin D levels increase the risk of active tuberculosis.”
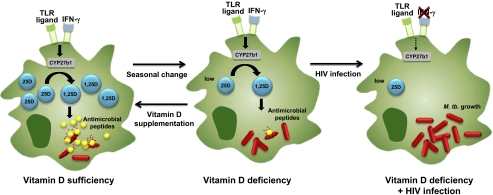
The association of low 25D levels with TB raises two questions. (i) If 1,25D is the bioactive form of vitamin D and its levels are kept constant, how do 25D levels contribute to disease pathogenesis? (ii) What is the mechanism by which 25D or 1,25D facilitates killing of this formidable pathogen? It has been known for 25 y (3, 4) that 1,25D, albeit at distinctly supraphysiological concentrations, can activate human macrophages to kill intracellular Mycobacterium tuberculosis, the causative agent of TB. The biologic relevance of the vitamin D antimicrobial pathway was demonstrated by the discovery that activation of monocytes and macrophages either by the innate immune system [Toll-like receptors (TLRs)] (5–8) or the acquired immune response (IFN-γ) (9) up-regulated CYP27b1 and VDR. Although both TLR ligands and IFN-γ activate different receptors and signaling pathways, they converge on a common effector pathway through the induction of IL-15, which by itself can induce CYP27b1 and the VDR. The up-regulation of CYP27b1 results in the intracellular conversion of 25D to 1,25D to sufficient levels to activate VDR signaling, triggering an antimicrobial response that includes vitamin D-dependent induction of autophagy (7–9), phagolysosomal fusion, and up-regulation of cathelicidin and DEFB4, which are antimicrobial peptides that kill M. tuberculosis (5, 8, 9) (Fig. 1). The antimicrobial response was dependent on serum 25D levels, with the inability of 25D-deficienct serum to support an antimicrobial response, which was restored by supplementation of the sera with 25D (Fig. 1). It is noteworthy that the human vitamin D-dependent antimicrobial pathway is distinct from mouse antimicrobial pathways, because only the human cathelicidin gene has vitamin D response elements in its promoter region.
Fig. 1.
Vitamin D and the macrophage antimicrobial response to TB. In the presence of sufficient levels of 25D, activation by TLRs or IFN-γ up-regulates CYP27b1, resulting in conversion to 1,25D followed by induction of antimicrobial peptides that kill M. tuberculosis. This antimicrobial pathway is blocked when levels of 25D are deficient, but can be restored in vitro by 25D supplementation. In HIV-infected individuals that are vitamin D-deficient, the lack of IFN-γ production provides an additional immune deficiency.
One aspect of the work by Martineau et al. (1) is the finding that low 25D levels are more strongly associated with TB infection in HIV-infected individuals. Studies measuring 25D levels in HIV-infected populations have also shown that serum 25D levels are inversely correlated with susceptibility to HIV infection (10). Antiretroviral therapy may reduce vitamin D metabolite levels by blocking CYP27b1 activity (protease inhibitors) (11) and increasing enzymatic catabolism of 25D, thereby preventing conversion to 1,25D (nonnucleoside reverse transcriptase inhibitors) (12). We postulate that, in HIV-infected individuals, the immune deficit from vitamin D deficiency would be exacerbated by the diminished number of CD4+ T cells producing IFN-γ (Fig. 1).
There is a long history of using vitamin D to treat TB with some apparent success. Finsen discovered that UVB successfully treated the cutaneous form of TB, a discovery for which he was awarded the Nobel Prize in 1903. Dowling and Prosser Thomas (13) reported the treatment of this disease with oral vitamin D in 1946 (13). Vitamin D supplementation has been shown to provide a useful adjuvant to chemotherapy in pulmonary TB, enhancing antimicrobial responses (14) and accelerating clearance of bacilli in some patients (15).
Do you have enough vitamin D to fight TB? One author (R.L.M.) was recently told that his vitamin D was slightly low. The doctor knew this from the lab report: “the reference range for vitamin D (25D) here at the University of California at Los Angeles (UCLA) was determined based on the scientific literature, recommendations that are provided by the test manufacturer, and correlation data....” The Endocrine Society and the Institute of Medicine (IOM) have interpreted the scientific literature differently. The Endocrine Society's clinical practice guidelines define vitamin D deficiency as 25D < 20 ng/mL (50 nmol/L) and vitamin D insufficiency as 25D levels of 21–29 ng/mL (52.5–72.5 nmol/L) (16). The IOM, in their vitamin D supplementation guidelines, agreed that the critical level for deficiency was 20 ng/mL (50 nmol/L) but did not define vitamin D insufficiency as a health problem (17). The IOM supplementation guidelines are designed to achieve serum levels of 25D of 50 nmol/L, but not 70–75 nmol/L. The difference between the guidelines is partly because of the IOM's recalculation of existing data on vitamin D levels and bone disease (18). There is agreement that the efficacy of vitamin D supplementation in infectious, autoimmune, and cardiovascular disease needs further evaluation.
We do not yet know how much vitamin D is needed to fight TB. However, the paper by Martineau et al. (1) powerfully strengthens the case for clinical trials to determine whether vitamin D supplementation helps to prevent and/or treat TB as well as other diseases associated with vitamin D deficiency and insufficiency. As the need for additional clinical studies is becoming clear, serum vitamin D levels in the US population have been declining (19, 20). This may be related to a concomitant decrease in the use of vitamin D-supplemented products and a decrease in outdoor activity. However, sun exposure is not recommended as a means to increase serum 25D levels because of an increased risk for skin cancer. It should also be noted that the study by Martineau et al. (1) reports low levels of 25D in African blacks with TB. These individuals are living south of the equatorial region at the same latitude as the Southern United States. There is an increasingly high incidence of vitamin D deficiency among African Americans (20) and an increased susceptibility to TB. In planning clinical trials, it will be important to determine whether higher levels of vitamin D supplementation are needed for people with darker skin and others at risk for vitamin D deficiency. It is a tragic irony that vitamin D, which costs a penny a day and may enhance innate and acquired immunity to TB, is not considered a worthy investment for critical clinical trials that could determine whether supplementation can prevent and/or treat disease.
Footnotes
The authors declare no conflict of interest.
See companion article on page 19013.
References
- 1.Martineau AR, et al. Reciprocal seasonal variation in vitamin D status and tuberculosis notifications in Cape Town, South Africa. Proc Natl Acad Sci USA. 2011;108:19013–19017. doi: 10.1073/pnas.1111825108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: A systematic review and meta-analysis. Int J Epidemiol. 2008;37:113–119. doi: 10.1093/ije/dym247. [DOI] [PubMed] [Google Scholar]
- 3.Rook GAW, et al. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986;57:159–163. [PMC free article] [PubMed] [Google Scholar]
- 4.Crowle AJ, Ross EJ, May MH. Inhibition by 1,25(OH)2-vitamin D3 of the multiplication of virulent tubercle bacilli in cultured human macrophages. Infect Immun. 1987;55:2945–2950. doi: 10.1128/iai.55.12.2945-2950.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu PT, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 6.Martineau AR, et al. IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: The role of cathelicidin LL-37. J Immunol. 2007;178:7190–7198. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- 7.Yuk JM, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6:231–243. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Shin DM, et al. Mycobacterial lipoprotein activates autophagy via TLR2/1/CD14 and a functional vitamin D receptor signaling. Cell Microbiol. 2010;12:1648–1665. doi: 10.1111/j.1462-5822.2010.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabri M, et al. Vitamin D is required for IFN-{gamma}-mediated antimicrobial activity of human macrophages. Sci Transl Med. 2011;3:104ra102. doi: 10.1126/scitranslmed.3003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta S, et al. Vitamin D status of HIV-infected women and its association with HIV disease progression, anemia, and mortality. PLoS One. 2010;5:e8770. doi: 10.1371/journal.pone.0008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cozzolino M, et al. HIV-protease inhibitors impair vitamin D bioactivation to 1,25-dihydroxyvitamin D. AIDS. 2003;17:513–520. doi: 10.1097/00002030-200303070-00006. [DOI] [PubMed] [Google Scholar]
- 12.Dao CN, et al. Low vitamin D among HIV-infected adults: Prevalence of and risk factors for low vitamin D Levels in a cohort of HIV-infected adults and comparison to prevalence among adults in the US general population. Clin Infect Dis. 2011;52:396–405. doi: 10.1093/cid/ciq158. [DOI] [PubMed] [Google Scholar]
- 13.Dowling GB, Prosser Thomas EW. Treatment of lupus vulgaris with calciferol. Lancet. 1946;1:919–922. doi: 10.1016/s0140-6736(46)90616-2. [DOI] [PubMed] [Google Scholar]
- 14.Martineau AR, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176:208–213. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 15.Martineau AR, et al. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: A double-blind randomised controlled trial. Lancet. 2011;377:242–250. doi: 10.1016/S0140-6736(10)61889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holick MF, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 17.Ross AC, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maxmen A. Nutrition advice: The vitamin D-lemma. Nature. 2011;475:23–25. doi: 10.1038/475023a. [DOI] [PubMed] [Google Scholar]
- 19.Looker AC, et al. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr. 2008;88:1519–1527. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169:626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]