Abstract
In lower vertebrates, locomotor burst generators for axial muscles generally produce unitary bursts that alternate between the two sides of the body. In lamprey, a lower vertebrate, locomotor activity in the axial ventral roots of the isolated spinal cord can exhibit flexibility in the timings of bursts to dorsally-located myotomal muscle fibers versus ventrally-located myotomal muscle fibers. These episodes of decreased synchrony can occur spontaneously, especially in the rostral spinal cord where the propagating body waves of swimming originate. Application of serotonin, an endogenous spinal neurotransmitter known to presynaptically inhibit excitatory synapses in lamprey, can promote decreased synchrony of dorsal–ventral bursting. These observations suggest the possible existence of dorsal and ventral locomotor networks with modifiable coupling strength between them. Intracellular recordings of motoneurons during locomotor activity provide some support for this model. Pairs of motoneurons innervating myotomal muscle fibers of similar ipsilateral dorsoventral location tend to have higher correlations of fast synaptic activity during fictive locomotion than do pairs of motoneurons innervating myotomes of different ipsilateral dorsoventral locations, suggesting their control by different populations of premotor interneurons. Further, these different motoneuron pools receive different patterns of excitatory and inhibitory inputs from individual reticulospinal neurons, conveyed in part by different sets of premotor interneurons. Perhaps, then, the locomotor network of the lamprey is not simply a unitary burst generator on each side of the spinal cord that activates all ipsilateral body muscles simultaneously. Instead, the burst generator on each side may comprise at least two coupled burst generators, one controlling motoneurons innervating dorsal body muscles and one controlling motoneurons innervating ventral body muscles. The coupling strength between these two ipsilateral burst generators may be modifiable and weakening when greater swimming maneuverability is required. Variable coupling of intrasegmental burst generators in the lamprey may be a precursor to the variable coupling of burst generators observed in the control of locomotion in the joints of limbed vertebrates.
Introduction
The locomotor network of the lamprey is usually conceived as a chain of coupled segmental oscillators (Mullins et al. 2011). Each segmental oscillator generates motoneuron bursting that alternates between the left and right sides due to reciprocal inhibitory connections. The individual segmental oscillators along the spinal cord are coupled via intersegmental neurons to produce the head-to-tail propagation of the bursts for forward swimming. In this model, there is flexibility in the intersegmental coupling of the swim oscillators that not only allows forward swimming, but also backward swimming by a reversal of the propagation of the bursts to a tail-to-head direction. This review will consider the possibility of another site for flexibility in the lamprey’s locomotor network: the control of dorsally-located myotomal muscles versus ventrally-located myotomal muscles. It is proposed that the segmental locomotor network is subdivided into separate, but coupled, networks for the control of dorsal and ventral myotomes and that the coupling between these intrasegmental networks is modifiable, allowing for a variable degree of independent control of dorsal and ventral myotomal muscles during swimming.
The lamprey’s locomotor network
The lamprey is a lower vertebrate that has been used to investigate the neural origins and control of locomotion. This work has been facilitated by the demonstration that the isolated spinal cord produces rhythmic ventral-root bursts when exposed to an excitatory amino acid such as glutamate (Cohen and Wallén 1980), and these rhythmic bursts were further shown to share many features in common with the electromyographic activity of myotomal muscles in the intact swimming lamprey (Wallén and Williams 1984). This rhythmic activity in the isolated spinal cord is referred to as fictive swimming and is considered to reflect the activity of the spinal locomotor networks (Cohen and Wallén 1980; Wallén and Williams 1984). The cellular and synaptic mechanisms of the spinal locomotor networks have been explored using intracellular microelectrodes, and these studies have revealed several classes of nerve cells that are active during fictive swimming. Details of the electrical properties, pharmacology, morphology, and synaptic interactions of these neurons have been reported (Buchanan 1982; Buchanan and Cohen 1982; Buchanan and Grillner 1987; Buchanan et al. 1989; Viana Di Prisco et al. 1990; Buchanan 1993; Parker 2006; Mahmood et al. 2009). A proposed model for the segmental locomotor network of the lamprey has been simulated with varying degrees of detail and has been shown to reproduce various aspects of the swimming of lamprey (Ekeberg et al. 1991; Buchanan 1992; Grillner et al. 2007). The lamprey’s swimming and its underlying networks have features in common with the swimming of leeches (Mullins et al. 2011) and Xenopus tadpoles (Roberts et al. 2008).
When the spinal cord is cut down its midline, bursting of ventral roots in each hemicord can still be observed (Cangiano and Grillner 2003). This has led to the view that the lamprey’s segmental network consists of a burst generator on each side of the spinal cord coupled with reciprocal inhibition (Kotaleski et al. 1999; Cangiano and Grillner 2005; but see Hoffman and Parker 2010). The individual segmental locomotor networks are coupled with other segments via intersegmental neurons and these connections help to coordinate the networks into the head-to-tail propagation of the bursts. While the details of this intersegmental coupling are not known, simulation studies reveal that simple spread of the segmental connectivity to adjacent segments is sufficient to account for head-to-tail propagation (Williams 1992). The head-to-tail phase relation among the segmental oscillators can be reversed to produce backwards swimming (Islam et al. 2006). Whether this involves a modulation of the coupling synaptic strengths or a change in the excitability gradient of the segmental oscillators is not known (Sigvardt and Williams 1996).
Musculature used by the lamprey for swimming
The propulsive muscles for the lamprey’s swimming are the myotomal muscles surrounding the body. Unlike higher fish, the lamprey lacks lateral fins and has only small dorsal midline fins in the caudal half of its body. Lateral fins in higher fish play an important role during swimming not only in propulsion, but in steering and compensatory movements (Drucker and Lauder 2001, 2003). Lacking lateral fins, the lamprey must rely on differential activation of the myotomal muscles at different dorsoventral levels to produce turning movements and compensatory movements that maintain proper orientation of the body.
The myotomal muscles are organized segmentally into stacks of muscle fibers oriented longitudinally along the body (Hardisty and Rovainen 1982). The muscle fibers of one segment on one side of the body are innervated by about 80–100 motoneurons on the ipsilateral side of the spinal cord (Rovainen et al. 1973), and an individual myotomal motoneuron innervates ipsilateral myotomal muscle fibers located at a single dorsoventral level of the body (Teräväinen and Rovainen 1971). After the ventral root leaves the spinal canal, it immediately branches into a dorsal branch carrying axons of motoneurons innervating dorsally-located muscle fibers and a ventral branch carrying axons of motoneurons innervating ventrally-located muscle fibers (Hardisty and Rovainen 1982).
The myotomal muscles of the most rostral body have a similar organization to the myotomes of the rest of the body, but with some differences (Hardisty and Rovainen 1982). This most rostral region is often referred to as the “gill region” because on each side of the body it contains the seven gills that are innervated by visceral vagal motoneurons of the brainstem. The myotomal muscles in the gill region are clearly divided into those located dorsal to the gills (epibranchial segmental muscles) and those ventral to the gills (hypobranchial segmental muscles). Each segmental ventral root innervates a corresponding epibranchial muscle segment. However, the hypobranchial muscle segments are divided into only about half as many segments as the epibranchial muscle segments. In addition, the axons of the hypobranchial motoneurons take a rather indirect route to the muscle; the axons project caudally to the last gill, curving ventrally around the gill and projecting rostrally to innervate the appropriate rostrocaudally-situated hypobranchial muscle.
Descending control of motoneurons innervating dorsal versus ventral myotomes
One mechanism for the differential activation of motoneurons innervating body muscles of different dorsoventral levels has been demonstrated in the descending control systems in the lamprey. Descending control of the spinal motor networks is mediated by the two main descending systems in the lamprey: the reticulospinal system and the vestibulospinal system (Ronan 1989; Swain et al. 1993). It is known that the reticulospinal neurons make monosynaptic dual electrical/chemical excitatory synapses onto spinal myotomal motoneurons (Rovainen 1967; 1974), but little is known about the patterns of these direct connections with respect to motoneurons innervating dorsally-located myotomal muscles (dorMNs) versus motoneurons innervating ventrally-located myotomal muscles (venMNs). However, several studies have examined the effects of individual reticulospinal neurons and vestibulospinal neurons (Zelenin et al. 2003, 2007) on the locomotor bursting in dorsal and ventral branches of the ventral root.
The experiments of Zelenin et al. (2003; 2007) were carried out on the isolated spinal cord and brainstem preparation of the lamprey, in which fictive locomotion was induced by bath perfusion of an excitatory amino acid, d-glutamate. Extracellular recordings were made from the dorsal and ventral branches of the ventral root on both sides of a single spinal segment, while an individual reticulospinal neuron was stimulated repetitively with an intracellular microelectrode to produce several thousand action potentials. Post-stimulus histograms of the locomotor bursting in the four ventral root branches, representing the four body quadrants, revealed that most reticulospinal and vestibulospinal neurons had excitatory or inhibitory effects on one or more of the four ventral root branches. Thus, during locomotor activity, an individual descending neuron can affect the firing of motoneurons on both sides of the spinal cord and can, in many cases, exhibit differential effects on the dorsal and ventral branches on one side. Different descending cells elicited different patterns of activity, and some 20 different patterns of descending outputs from single reticulospinal cells to motoneurons innervating the four muscle quadrants were found (Zelenin et al. 2007). As an example of one of these patterns, individual reticulospinal neurons were found that inhibit the firing of dorMNs bilaterally, while exciting venMNs bilaterally. These reticulospinal neurons would be recruited to produce downward body movements. These results clearly demonstrate that descending neurons control dorMNs and venMNs differentially. Presumably, then, commands for turning and compensatory movements are accomplished in part by activating subsets of descending cells with the appropriate spinal effects on the myotomal quadrants to produce the required movement.
Activities of dorsal and ventral motoneurons during fictive swimming
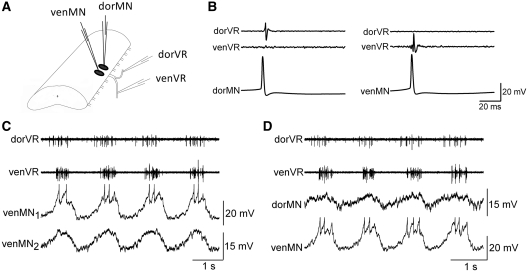
Recordings of dorsal and ventral branches of the ventral root, during fictive locomotion in the isolated spinal cord, reveal that the motoneurons innervating dorsal versus ventral portion of the myotome generally burst in near synchrony (Fig. 1). Slight differences in the phase relationship can be observed as shown by the example in Fig. 1C and D in which the dorsal branch (dorVR) is slightly phase-advanced compared to the ventral branch (venVR). Simultaneous intracellular recording of dorMNs and venMNs (identified by their projections in branches of the ventral root as in Fig. 1B), allow direct comparisons of the underlying waveforms during fictive locomotion. Even during nearly synchronous bursting of dorVR and venVR, these paired recordings reveal differences in the shapes of the locomotor oscillations for the dorMNs and venMNs. Indications of such differences in waveform between pairs of motoneurons were first reported by Wallén et al. (1985). They proposed that the differences were due to differences in inputs to dorMN and venMN, although in their study the dorsal/ventral projections of the motoneurons were not confirmed. In general, the observed differences in waveform could be either due to differences in synaptic inputs or to differences in the morphologies of the cells. Wallén et al. (1985), however, found no major differences in cell size or extent of their overall dendritic trees between dorMNs and venMNs. Therefore, the differences in the waveforms during fictive swimming likely indicate that dorMNs and venMNs receive inputs from different sets of premotor interneurons.
Fig. 1.
Fictive swimming activity in dorsal and ventral branches of the ventral root, and in motoneurons projecting out those branches. (A) Schematic showing the dorsal and ventral branches of the ventral roots (dorVR and venVR), the recordings of their spiking activity with suction electrodes, and recordings of intracellular membrane potential from motoneurons with sharp microelectrodes (dorMN and venMN). (B) Individual motoneurons can be identified according to their projection within the branches of the ventral root. (C) Intracellular recording of two motoneurons, both venMNs, in the same spinal segment during fictive swimming induced with d-glutamate. The two venMNs have similar oscillatory waveforms. (D) A pair of intracellular recordings of a dorMN and a venMN. These two motoneurons have somewhat different oscillatory waveforms.
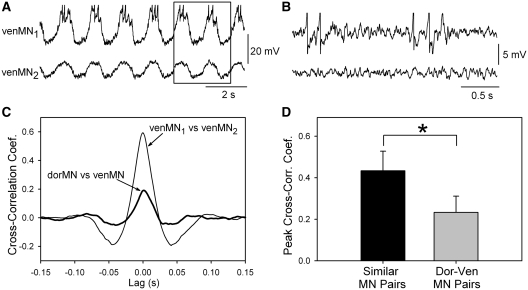
To test whether dorMNs and venMNs receive different synaptic inputs during fictive swimming, a previously used cross-correlation technique (Buchanan and Kasicki 1999) was employed to compare the synaptic inputs to pairs of motoneurons. In this technique, two motoneurons in the same spinal segment are recorded simultaneously, each with an intracellular microelectrode (Fig. 2A). The membrane potential oscillations of swimming in the motoneurons are removed by applying a digital notch filter to the recordings, leaving only the fast synaptic activity that underlies the oscillations (Fig. 2B). Cross-correlation of the fast synaptic activity is then performed, and the peak amplitude of the cross-correlation coefficient (CCF) is a measure of the degree of common synaptic inputs. When one or both of the motoneurons is firing action potentials, the cross-correlation is done in the region of traces without action potentials, which is the time when the motoneurons are receiving mainly inhibitory inputs. As shown previously, this restriction of the region for the cross-correlation does not significantly affect the results (Buchanan and Kasicki 1999). When the cross-correlation was performed on the venMN pair in Fig. 2A, the peak CCF was 0.6 (Fig. 2C) (within the range from 0 to 1.0, from no correlation to a perfect correlation). In contrast, when two motoneurons innervating different dorsoventral levels of the body were recorded simultaneously, the peak CCF was lower, 0.2 (Fig. 2C). Figure 2D shows the means of the peak CCFs of similar motoneuron pairs versus different motoneuron pairs. The similar motoneuron pairs had a significantly higher peak CCF than did the different motoneuron pairs (P < 0.001, t-test). The distances between the motoneuron pairs was not a contributing factor to this difference because all of the pairs were located in the same segment, and there was no significant difference in the means of the distances between motoneurons in the two groups (P = 0.15, t-test). The finding of a lower peak CCF between different pairs of motoneurons compared to similar pairs is consistent with the existence of separate populations of premotor interneurons conveying locomotor signals to the dorMNs and venMNs.
Fig. 2.
Comparison of underlying synaptic activity in motoneurons during fictive swimming using cross-correlation of waveforms. (A) Intracellular recording of membrane potentials in two motoneurons projecting out the same ventral branch of the ventral root during fictive swimming (venMNs). The slow swim oscillations in each recording were removed with a digital filter to allow cross-correlation of the underlying fast synaptic activity. Cross-correlations of the two waveforms, excluding regions of spiking, were performed (see Buchanan and Kasicki 1999 for details of method). The boxed region is shown with greater amplification and after filtering in panel B. (B) An amplified view of the boxed region of panel A showing the membrane potential of the two motoneurons after filtering. Similarities in synaptic inputs are apparent. (C) The cross-correlogram of the two venMNs is shown along with a cross-correlogram of two motoneurons projecting out different ventral root branches (thick line). The two similarly-projecting motoneurons had a higher correlation than did the pair of motoneurons projecting in different ventral root branches. (D) Means of the peak cross-correlation coefficients (CCFs) for the 8 pairs of similar motoneurons (projecting out same ventral-root branch) and the 11 dorsal–ventral pairs of motoneurons. The means were significantly different (*P < 0.001; t-test) suggesting that there are separate populations of premotor interneurons for dorMNs and venMNs.
The existence of different premotor inputs to dorMNs versus venMNs does not necessarily indicate that there are separate locomotor networks. However, it is known in lamprey that interneurons that synapse upon motoneurons are not specialized for a premotor role as shown by their demonstrated synaptic interactions with other spinal interneurons (Buchanan 1982). The model of the lamprey locomotor network (Buchanan and Grillner 1987) reflects these findings that the same interneurons that are thought to generate the locomotor activity also convey the locomotor signals to the motoneurons. Thus, the cross-correlation study shown in Fig. 2 indicates the existence of separate premotor interneurons for dorMNs and venMNs, and this finding suggests the existence of separate locomotor networks.
Slow modulations of locomotor activity
A further indication that there may exist separate, though coupled, segmental locomotor networks for the dorsal and ventral portions of the myotome is the phenomenon of slow modulation of the fast-swimming rhythm. It has been reported for many years that during fictive locomotion in the lamprey spinal cord, there can be a rhythmic waxing and waning of the strength of ventral root bursts, with a periodicity of 20 s or longer. These slow modulations were first reported in fin motoneurons (Buchanan and Cohen 1982) in the isolated spinal cord preparation. Myotomal ventral root bursting could be induced to show a slow rhythmic modulation of the fast-swimming rhythm when exposed to strychnine, an antagonist of the main inhibitory neurotransmitter of the spinal cord (glycine) at a concentration too low to disrupt the expression of the fast rhythm (0.2 µM strychnine) (McPherson et al. 1994). Aoki et al. (2001) observed that these slow modulations could also be induced by low concentrations of bicuculline, an antagonist of gamma-aminobutyric acid (GABA). Aoki et al. (2001) further showed that the slow modulations can have different timings between the dorsal and ventral branches of the ventral root of the same spinal segment. The most common phase relationship that they observed was one in which the dorVR and venVR on the same side of the spinal cord were modulated in antiphase, such that while the activity of dorVR was increasing in strength, that of venVR was weakening. In this pattern, the slow modulations of the dorsal branches on the two sides of the cord were in synchrony with one another (Aoki et al. 2001).
How neurotransmitter antagonists induce these slow modulations is not known, but the antiphasic pattern of the dorsal and ventral branches of the ventral root indicates the existence of a mechanism for dissociating the normally synchronous dorsal–ventral activity and suggests the existence of separate, but coupled, dorsal, and ventral locomotor networks. Perhaps the normal synchrony is a consequence of strong coupling between dorsal–ventral locomotor networks, and with the addition of a neurotransmitter antagonist, the coupling is weakened, allowing a less synchronized pattern, reminiscent of a beating phenomenon of weakly-coupled oscillators with different intrinsic frequencies (Rand et al. 1988).
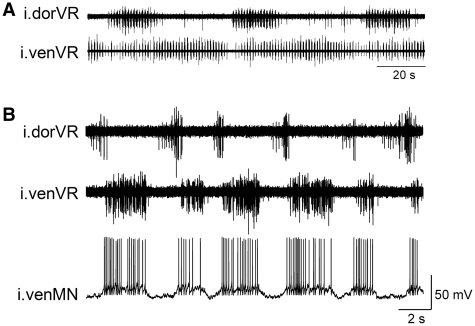
Slow modulation of fictive swimming without the addition of a neurotransmitter blocker is rarely observed in the midbody region of the lamprey’s spinal cord (segments #15–45 of about 100 total segments). In contrast, the most rostral region of the spinal cord (i.e., the gill region) exhibits spontaneous, robust slow modulation of the fictive swimming rhythm with the same antiphasic pattern in dorVR and venVR as reported by Aoki et al. (2001). As shown in Fig. 3A, these slow modulatory rhythms can be extremely powerful, such that the dorVR has silent periods of many seconds. Therefore, spontaneous slow modulations that have very different patterns of activation of the dorVR versus the venVR, in the most rostral spinal cord, is strongly suggestive that there are separate, but coupled, networks controlling the dorsal and ventral myotomal motoneurons. The rostral region of spinal cord can also spontaneously exhibit other forms of de-synchronized activity between dorsal and ventral branches of the ventral root during fictive swimming. An example is shown in Fig. 3B in which the dorVR swim-bursts are short, while the venVR swim-bursts are long, and the dorVR bursts occur during the silent period between the venVR bursts.
Fig. 3.
Examples of spontaneous non-synchronous bursting of the dorsal and ventral branches of one ventral root in the rostral spinal cord in the presence of d-glutamate. (A) Slow modulation of the fast swim rhythm with alternating activity in the ipsilateral dorsal and ventral branches (i.dorVR and i.venVR) of the same spinal segment. (B) Ipsilateral dorsal and ventral branches of the ventral root exhibiting bursting of different durations and phasing. Intracellular recording of an ipsilateral venMN (i.venMN) shows that the activity of the membrane potential matches the bursting of the venVR, and the venMN shows little synaptic input related to the dorVR.
Serotonin as an endogenous modulator of dorsal and ventral locomotor networks
The spontaneous de-synchronizing of dorVR and venVR bursting during fictive swimming suggests that there may be an endogenous process to alter the coupling between the networks. Why would this be an advantage in motor control? During normal swimming, the dorsal and ventral networks presumably are strongly coupled, and are therefore synchronously active. However, during more demanding motor-control tasks, there may be a need for more independent control of dorsal and ventral myotomes, since the lamprey lacks lateral fins and must rely on differential activation of myotomal muscles at different dorsoventral levels. Thus, a weakening of the coupling between dorsal and ventral locomotor networks may provide an advantage in lamprey motor control to allow more flexibility in the amplitude, duration, and timing of the locomotor signals to different dorsoventral levels.
Is there an endogenous modulator of the coupling between dorsal and ventral locomotor networks? The occurrence of spontaneous dissociation of locomotor bursting that can occur in the dorsal and ventral branches of the ventral root in the rostral spinal cord of the lamprey suggests that the coupling may be modifiable, perhaps by the presence of an endogenous neuromodulator. There are several reasons to suggest serotonin as a candidate. First, serotonin can produce presynaptic inhibition of neurotransmission in the lamprey’s spinal cord (Buchanan and Grillner 1991; Schwartz et al. 2005), which would be consistent with the demonstrated ability of applied neurotransmitter antagonists to induce slow modulations of swimming activity. Second, serotonin has powerful effects on the locomotor networks, producing a slowing of the rhythm and an increase in intensity of ventral-root bursting. In addition, serotonin changes the phase lag as the bursts propagate down the spinal cord (Harris-Warrick and Cohen 1985). Thus, serotonin appears to be capable of altering the coupling of segmental oscillators. Finally, serotonin is present in the spinal cord of lamprey, both from descending serotonergic cells of the brainstem (Brodin et al. 1986) and in spinal serotonergic cells located in the ventral midline that form a dense plexus in the ventromedial region of the spinal cord (Van Dongen et al. 1985).
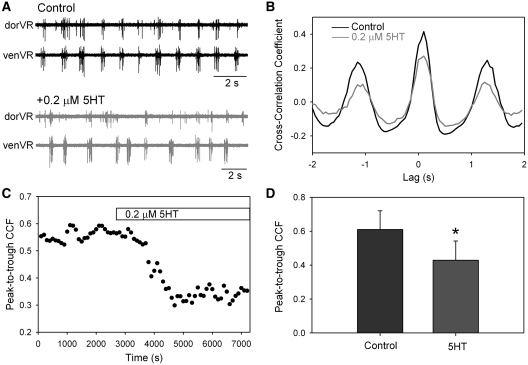
To test the possible involvement of serotonin in modulating the coupling of dorsal and ventral locomotor networks, a low concentration of serotonin (0.2 µM) was applied to the isolated spinal cord during fictive swimming, while recording the bursting in dorsal and ventral branches of the ventral root (Fig. 4). To assess the degree of synchronous bursting over long sequences of fictive swimming, cross-correlation techniques were used. For this, the motor bursts recorded from dorVR and venVR were converted into rectified and smoothed waveforms. Cross-correlations of these waveforms from dorVR and venVR were performed in 100 s sequences of bursting, and the peak-to-trough amplitude of the CCF was used as a measure of the degree of synchronous bursting. An example of a decrease in synchrony induced by application of 0.2 µM serotonin is shown in Fig. 4A and B, and the time course of the effect of serotonin on the CCF in one preparation is shown in Fig. 4C. In all seven preparations tested, there was a decrease in the CCFs after the application of the serotonin (Fig. 4D). However, these effects were usually transient, often showing recovery in the continued presence of the serotonin after several tens of minutes. A decrease in cross-correlation occurs when there is a change either in the timing of the two signals with respect to one another and/or a change in the relative amplitudes of the two signals. Inspection of the seven preparations revealed clear changes in the relative timing, but not in the amplitude, of the bursts in four preparations; clear changes in relative amplitude, but not in timing, in one preparation; and no obvious change in two preparations in spite of a decrease in the cross-correlation. Overall, the results are consistent with a change in the coupling between two oscillators.
Fig. 4.
The effect of serotonin on the synchrony of bursting in the dorsal and ventral branches of the ventral root during fictive swimming. (A) Example of bursting before and after addition of serotonin to the bath. After serotonin, the dorsal and ventral branches were not as well synchronized as in the control. (B) After rectifying and smoothing the bursts of the ventral root, the waveforms of the dorsal and ventral branches were used to create a cross-correlogram. An epoch of 100 s was used for the correlogram. The peak-to-trough CCF was lower after adding the serotonin. (C) A plot of the time course of the fall in the peak-to-trough CCF (i.e., the degree of burst synchrony) after adding serotonin to the bath. (D) In seven preparations, the mean peak-to-trough CCF decreased significantly after adding serotonin (5-HT). (*P < 0.001; paired t-test).
Higher concentrations of serotonin (>1 µM) induced more dramatic changes in the swim rhythm, characterized by slow and intense ventral-root bursting as previously reported (Harris-Warrick and Cohen 1985). Under these conditions of intense bursting, the dorsal/ventral bursting became tightly synchronized with high CCFs.
Conclusion
The locomotor system of the lamprey is organized to allow different degrees of muscle fiber activation at different dorsoventral positions within the myotome during swimming. At the level of the motor units, this organization is accomplished by the restriction of the muscle fibers of a given motoneuron to a particular dorsoventral location within the myotome. At the level of the descending control of motoneurons via reticulospinal neurons, the motoneurons innervating different dorsoventral levels receive different patterns of synaptic inputs from the reticulospinal neurons. At the level of the spinal locomotor networks, it was shown that motoneurons innervating different dorsoventral levels receive synaptic inputs from different premotor interneurons during fictive swimming. The ability of the motor system to differentially control the contractions of the myotomes at different dorsoventral levels is clearly important for steering and for maintenance of equilibrium in the lamprey, which swims without the benefit of lateral fins.
It has generally been assumed that the locomotor network in lamprey is a unitary burst generator on each side of the spinal cord, which provides a uniform output signal to all the myotomal motoneurons on one side of the cord. In this conception, the descending control system would modify the output strengths of the motoneurons by direct synaptic inputs at the level of the motoneurons. This model seems adequate to provide control for steering and compensatory movements. However, the assumption of a unitary burst generator for the locomotor network is challenged by the observation in the isolated spinal cord of locomotor bursting that is not uniform between dorsally-innervating motoneurons and ventrally-innervating motoneurons. These observations indicate that even in the absence of descending inputs, the locomotor network of the spinal cord does not always generate a simple unitary activation of all ipsilateral motoneurons of a myotome.
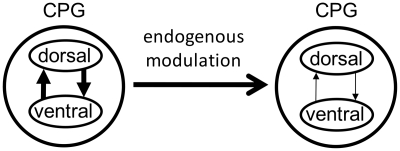
This finding of variability in swimming burst timing and amplitude at different dorsoventral levels, and the finding that motoneurons innervating different dorsoventral levels receive different premotor inputs during fictive swimming suggest that the segmental locomotor networks may be subdivided into two subnetworks: one specialized for the control of the dorsal portion of the myotome and another specialized for control of the ventral portion of the myotome (Fig. 5). These two subnetworks are conceived as having modifiable coupling strength such that under normal conditions, the networks are strongly coupled and burst in synchrony, providing a uniform output signal to the myotomes at all dorsoventral levels. However, there may be swimming conditions during which descending control of a simple unitary burst generator is inadequate to meet the demands of those conditions. If greater maneuverability is required, there may be an advantage to weakening the coupling between dorsal and ventral locomotor networks (Fig. 5). For example, during predatory attacks or during swimming in a strong and variable current, weakening of the coupling may allow for greater cycle-by-cycle variability in the relative activation of the dorsal and ventral networks to cope with quickly changing demands and perturbations.
Fig. 5.
Summary model of the dorsal/ventral locomotor networks. It is proposed that the locomotor central pattern generator (CPG) comprises dorsal and ventral components, respectively, serving dorsal and ventral myotomal muscles of the lamprey body. Normally, these two components are tightly coupled, but it is proposed that release of an endogenous modulator, perhaps serotonin, can weaken the coupling of the two oscillators, allowing greater flexibility in the activation patterns, perhaps under demands for greater maneuverability during swimming.
In mammals, a conceptual model for the locomotor CPG (Grillner 1985) proposes that there are two unit burst generators at each joint, one for flexor muscles and one for extensor muscles, and these two burst generators are coupled with reciprocal inhibition to provide the alternating activity of flexors and extensors underlying rhythmic movements of the limbs at each joint. This would be analogous to the reciprocal inhibition of the burst generators on the two sides of the lamprey’s spinal cord. The mammalian model further proposes that the unit burst generators of flexors at the hip, knee, and ankle joints are coupled with excitatory connections to provide near synchronous activation during forward walking, and there is similar coupling among the extensor burst generators. Burst generators in opposite limbs, especially those of the hip, are also coupled for interlimb coordination. The coupling among the various burst generators within the limb and between limbs is envisioned to be modifiable to allow for changes in timing of muscle activation among joints and between limbs with changes in the speed and gait of stepping, or for the expression of other forms of rhythmic limb movements, such as backward walking or scratching (Orlovsky et al. 1999). Similarly for lamprey, the proposed dorsal and ventral locomotor networks would be coupled to produce near synchrony under normal conditions, but the coupling can be modified to produce subtle or larger shifts in relative timing or amplitude of the dorsal and ventral portions of the myotomal contractions during swimming, depending upon the conditions.
Although variability in dorsal versus ventral motoneuron activity occurs during fictive swimming in the isolated spinal cord, it is not known whether similar flexibility occurs in the intact swimming lamprey. If this flexibility is demonstrated in behaving lamprey, it may serve as a model system for examining the mechanisms underlying modification of coupling of burst generators that is observed in burst generators in the control of locomotion in the joints of limbed vertebrates.
Funding
National Institutes of Health (NS40755 to J.T.B.).
Acknowledgments
I thank Mr Manuel Vega for his contribution to some of the experiments.
References
- Aoki F, Wannier T, Grillner S. Slow dorsal-ventral rhythm generator in the lamprey spinal cord. J Neurophysiol. 2001;85:211–8. doi: 10.1152/jn.2001.85.1.211. [DOI] [PubMed] [Google Scholar]
- Brodin L, Buchanan JT, Hökfelt T, Grillner S, Verhofstad AAJ. A spinal projection of 5-hydroxytryptamine neurons in the lamprey brainstem; evidence from combined retrograde tracing and immunohistochemistry. Neurosci Lett. 1986;67:53–7. doi: 10.1016/0304-3940(86)90207-7. [DOI] [PubMed] [Google Scholar]
- Buchanan JT. Identification of interneurons with contralateral, caudal axons in the lamprey spinal cord: synaptic interactions and morphology. J Neurophysiol. 1982;47:961–75. doi: 10.1152/jn.1982.47.5.961. [DOI] [PubMed] [Google Scholar]
- Buchanan JT. Neural network simulations of coupled locomotor oscillators in the lamprey spinal cord. Biol Cybern. 1992;66:367–74. doi: 10.1007/BF00203673. [DOI] [PubMed] [Google Scholar]
- Buchanan JT. Electrophysiological properties of identified classes of lamprey spinal neurons. J Neurophysiol. 1993;70:2313–25. doi: 10.1152/jn.1993.70.6.2313. [DOI] [PubMed] [Google Scholar]
- Buchanan JT, Cohen AH. Activities of identified interneurons, motoneurons, and muscle fibers during fictive swimming in the lamprey and effects of reticulospinal and dorsal cell stimulation. J Neurophysiol. 1982;47:948–60. doi: 10.1152/jn.1982.47.5.948. [DOI] [PubMed] [Google Scholar]
- Buchanan JT, Grillner S. Newly identified “glutamate” interneurons and their role in locomotion in the lamprey spinal cord. Science. 1987;236:312–4. doi: 10.1126/science.3563512. [DOI] [PubMed] [Google Scholar]
- Buchanan JT, Grillner S. 5-hydroxytryptamine depresses reticulospinal excitatory postsynaptic potentials in motoneurons of the lamprey. Neurosci Lett. 1991;112:71–4. doi: 10.1016/0304-3940(91)90196-z. [DOI] [PubMed] [Google Scholar]
- Buchanan JT, Grillner S, Cullheim S, Risling M. Identification of excitatory interneurons contributing to the generation of locomotion in the lamprey: structure, pharmacology, and function. J Neurophysiol. 1989;62:59–69. doi: 10.1152/jn.1989.62.1.59. [DOI] [PubMed] [Google Scholar]
- Buchanan JT, Kasicki S. Segmental distribution of common synaptic inputs to spinal motoneurons during fictive swimming in the lamprey. J Neurophysiol. 1999;82:1156–63. doi: 10.1152/jn.1999.82.3.1156. [DOI] [PubMed] [Google Scholar]
- Cangiano L, Grillner S. Fast and slow locomotor burst generation in the hemispinal cord of the lamprey. J Neurophysiol. 2003;89:2931–42. doi: 10.1152/jn.01100.2002. [DOI] [PubMed] [Google Scholar]
- Cangiano L, Grillner S. Mechanisms of rhythm generation in a spinal locomotor network deprived of crossed connections: the lamprey hemicord. J Neurosci. 2005;25:923–35. doi: 10.1523/JNEUROSCI.2301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AH, Wallén P. The neuronal correlate of locomotion in fish. “Fictive swimming” induced in an in vitro preparation of the lamprey spinal cord. Exp Brain Res. 1980;41:11–8. doi: 10.1007/BF00236674. [DOI] [PubMed] [Google Scholar]
- Drucker EG, Lauder GV. Wake dynamics and fluid forces of turning maneuvers in sunfish. J Exp Biol. 2001;204:431–42. doi: 10.1242/jeb.204.3.431. [DOI] [PubMed] [Google Scholar]
- Drucker EG, Lauder GV. Function of pectoral fins in rainbow trout: behavioral repertoire and hydrodynamic forces. J Exp Biol. 2003;206:813–26. doi: 10.1242/jeb.00139. [DOI] [PubMed] [Google Scholar]
- Ekeberg O, Wallén P, Lansner A, Traven H, Brodin L, Grillner S. A computer based model for realistic simulations of neural networks I. The single neuron and synaptic interaction. Biol Cybern. 1991;65:81–90. doi: 10.1007/BF00202382. [DOI] [PubMed] [Google Scholar]
- Grillner S. Neurobiological bases of rhythmic motor acts in vertebrates. Science. 1985;228:143–9. doi: 10.1126/science.3975635. [DOI] [PubMed] [Google Scholar]
- Grillner S, Kozlov A, Dario P, Stefanini C, Menciassi A, Lansner A, Hellgren Kotaleski J. Modeling a vertebrate motor system: pattern generation, steering, and control of body orientation. Prog Brain Res. 2007;165:221–34. doi: 10.1016/S0079-6123(06)65014-0. [DOI] [PubMed] [Google Scholar]
- Hardisty MW, Rovainen CM. Morphological and functional aspects of the muscular system. In: Hardisty MW, Potter IC, editors. The biology of lampreys. Vol. 4 A. London: Academic Press; 1982. pp. 137–231. [Google Scholar]
- Harris-Warrick RM, Cohen AH. Serotonin modulates the central pattern generator for locomotion in the isolated lamprey spinal cord. J Exp Biol. 1985;116:27–46. doi: 10.1242/jeb.116.1.27. [DOI] [PubMed] [Google Scholar]
- Hoffman N, Parker D. Lesioning alters functional properties in isolated spinal cord hemisegmental networks. Neuroscience. 2010;168:732–43. doi: 10.1016/j.neuroscience.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Islam SS, Zelenin PV, Orlovsky GN, Grillner S, Deliagina TG. Pattern of motor coordination underlying backward swimming in the lamprey. J Neurophysiol. 2006;96:451–60. doi: 10.1152/jn.01277.2005. [DOI] [PubMed] [Google Scholar]
- Kotaleski JH, Lansner A, Grillner S. Neural mechanisms potentially contributing to the intersegmental phase lag in lamprey.II. Hemisegmental oscillations produced by mutually coupled excitatory neurons. Biol Cybern. 1999;81:299–315. doi: 10.1007/s004220050564. [DOI] [PubMed] [Google Scholar]
- Mahmood R, Restrepo CE, El Manira A. Transmitter phenotypes of commissural interneurons in the lamprey spinal cord. Neuroscience. 2009;164:1057–67. doi: 10.1016/j.neuroscience.2009.08.069. [DOI] [PubMed] [Google Scholar]
- McPherson DR, Buchanan JT, Kasicki K. Effects of strychnine on fictive swimming in the lamprey: evidence for glycinergic inhibition, discrepancies with model predictions, and novel modulatory rhythms. J Comp Physiol A. 1994;175:311–21. doi: 10.1007/BF00192990. [DOI] [PubMed] [Google Scholar]
- Mullins OJ, Hackett JT, Buchanan JT, Friesen WO. Neuronal control of swimming behavior: comparison of vertebrate and invertebrate model systems. Prog Neurobiol. 2011;93:244–69. doi: 10.1016/j.pneurobio.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlovsky GN, Deliagina TG, Grillner S. Neuronal control of locomotion from Mollusc to man. Oxford: Oxford University Press; 1999. pp. 157–204. [Google Scholar]
- Parker D. Complexities and uncertainties of neuronal network function. Phil Trans Roy Soc Lond B. 2006;361:81–99. doi: 10.1098/rstb.2005.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand RH, Cohen AH, Holmes PJ. Systems of coupled oscillators as models of central pattern generators. In: Cohen AH, Rossignol S, Grillner S, editors. Neural control of rhythmic movements in vertebrates. New York: John Wiley and Sons; 1988. pp. 333–68. [Google Scholar]
- Roberts A, Li W-C, Soffe SR. Roles for inhibition: studies on networks controlling swimming in young frog tadpoles. J Comp Physiol A. 2008;194:185–93. doi: 10.1007/s00359-007-0273-3. [DOI] [PubMed] [Google Scholar]
- Ronan M. Origins of the descending spinal projections in Petromyzontid and Myxinoid agnathans. J Comp Neurol. 1989;281:54–68. doi: 10.1002/cne.902810106. [DOI] [PubMed] [Google Scholar]
- Rovainen CM. Physiological and anatomical studies on large neurons of the central nervous system of the sea lamprey (Petromyzon marinus). 1. Müller and Mauthner cells. J Neurophysiol. 1967;30:1000–23. doi: 10.1152/jn.1967.30.5.1000. [DOI] [PubMed] [Google Scholar]
- Rovainen CM. Synaptic interactions of reticulospinal neurons and nerve cells in the spinal cord of the sea lamprey. J Comp Neurol. 1974;154:207–44. doi: 10.1002/cne.901540207. [DOI] [PubMed] [Google Scholar]
- Rovainen CM, Johnson PA, Roach EA, Mankovsky JA. Projections of individual axons in lamprey spinal cord determined by tracings through serial sections. J Comp Neurol. 1973;149:193–202. doi: 10.1002/cne.901490205. [DOI] [PubMed] [Google Scholar]
- Schwartz EJ, Gerachshenko T, Alford S. 5-HT prolongs ventral root bursting via presynaptic inhibition of synaptic activity during fictive locomotion in lamprey. J Neurophysiol. 2005;93:980–8. doi: 10.1152/jn.00669.2004. [DOI] [PubMed] [Google Scholar]
- Sigvardt KA, Williams TL. Effects of local oscillator frequency on intersegmental coordination in the lamprey locomotor CPG: theory and experiment. J Neurophysiol. 1996;76:4094–103. doi: 10.1152/jn.1996.76.6.4094. [DOI] [PubMed] [Google Scholar]
- Swain GP, Snedeker JA, Ayers J, Selzer ME. Cytoarchitecture of spinal-projecting neurons in the brain of the larval sea lamprey. J Comp Neurol. 1993;336:194–210. doi: 10.1002/cne.903360204. [DOI] [PubMed] [Google Scholar]
- Teräväinen H, Rovainen CM. Fast and slow motoneurons to body muscle of the sea lamprey. J Neurophysiol. 1971;34:990–8. doi: 10.1152/jn.1971.34.6.990. [DOI] [PubMed] [Google Scholar]
- Van Dongen PAM, Hökfelt T, Grillner S, Verhofstad AAJ, Steinbusch HWM, Cuello AC, Terenius L. Immunohistochemical demonstration of some putative neurotransmitters in the lamprey spinal cord and spinal ganglia: 5-hydroxytryptamine-, tachykinin-, and neuropeptide Y-immunoreactive neurons and fibers. J Comp Neurol. 1985;234:501–22. doi: 10.1002/cne.902340408. [DOI] [PubMed] [Google Scholar]
- Viana Di Prisco G, Wallén P, Grillner S. Synaptic effects of intraspinal stretch receptor neurons mediating movement-related feedback during locomotion. Brain Res. 1990;530:161–6. doi: 10.1016/0006-8993(90)90675-2. [DOI] [PubMed] [Google Scholar]
- Wallén P, Grillner S, Feldman JL, Bergelt S. Dorsal and ventral myotome motoneurons and their input during fictive locomotion in lamprey. J Neurosci. 1985;5:654–61. doi: 10.1523/JNEUROSCI.05-03-00654.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallén P, Williams TL. Fictive locomotion in the lamprey spinal cord in vitro compared with swimming in the intact and spinal animal. J Physiol. 1984;347:225–39. doi: 10.1113/jphysiol.1984.sp015063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TL. Phase coupling by synaptic spread in chains of coupled neuronal oscillators. Science. 1992;258:662–5. doi: 10.1126/science.1411575. [DOI] [PubMed] [Google Scholar]
- Zelenin PV, Orlovsky GN, Deliagina TG. Sensory-motor transformation by individual command neurons. J Neurosci. 2007;27:1024–32. doi: 10.1523/JNEUROSCI.4925-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenin PV, Pavlova EL, Grillner S, Orlovsky GN, Deliagina TG. Comparison of the motor effects of individual vestibulo- and reticulospinal neurons on dorsal and ventral myotomes in lamprey. J Neurophysiol. 2003;90:3161–7. doi: 10.1152/jn.00555.2003. [DOI] [PubMed] [Google Scholar]