Abstract
The tremendous ability of the skin epidermis to regenerate is due to the presence of epidermal stem cells that continuously produce keratinocytes which undergo terminal differentiation to a keratinized layer that provides the skin’s barrier properties. The ability to control this process in vitro has made it possible to develop various types of tissue engineered skin grafts, some of which being among the first tissue engineered products to ever reach the marketplace. In the past 30 years these products have been applied with some success to the treatment of chronic skin wounds, such as diabetic and venous ulcers, as well as deep acute wounds, such as burns. Current technologies remain partially effective in their ability to restore other skin structures, for example the dermis, which is critical to the overall long-term appearance and function of the skin. Furthermore, to this day none of these approaches regenerate skin appendages (e.g. hair follicles, sweat glands). The use of earlier progenitor and stem cells, including embryonic stem cells is gaining interest to overcome such limitations. Furthermore, recent evidence suggests that “adult” stem cells, which are present in the circulation of the patient, home into areas of injury and likely participate in the wound healing process. In this paper, we start with an overview of the wound healing process and current methods used for wound treatment, both conventional and tissue engineered-based. We then review current research on the various types of stem cells used for skin tissue engineering and wound healing, and provide future directions.
Keywords: stem cells, wound healing, skin tissue engineering, skin appendages
I. INTRODUCTION TO WOUND HEALING
I.A. Cost and Prevalence of Wounds
A wound is defined as a disruption of normal anatomic structure and function.1 Skin wound treatment is a very diverse part of the health care system, encompassing surgical and accidental lacerations, burns, pressure ulcers, diabetic and venous ulcers. The treatment of wounds and associated complications exceeds $20 billion annually in the US.2 Chronic and nonhealing wounds are especially costly because they require repetitive treatments; for example, a diabetic foot ulcer typically costs $50,000 to treat.3 Chronic wounds affect 1% of the population at any given time.4
I.B. Anatomy of Skin
The largest organ in the body, skin consists of three layers: epidermis, dermis, and hypodermis. The epidermis is in fact a multi-layered epithelium extending from the basement membrane that separates it from the dermis to the air. Except for the basement membrane, it is virtually devoid of extracellular matrix (ECM). Progenitor cells are located on the basement membrane and undergo continuous self-renewal and differentiation to keratinocytes. The keratinocytes migrate towards the surface of the skin where they eventually get sloughed off. While this process occurs, keratinocytes undergo terminal differentiation and maturation.5 As they approach the skin surface, they form a keratinized layer of dead cells which confers the main barrier properties of the skin.6
Below the epidermis is the dermis, the thickest of the three layers of skin which accounts for most of the skin’s mechanical properties and resilience. The dermis is a connective tissue comprised of ECM, fibroblasts, vascular endothelial cells, and skin appendages (hair follicles, sweat glands, etc.).6 Fibroblasts secrete collagen and elastin, providing mechanical strength and elasticity to the skin, respectively.
The hypodermis underneath the dermis is composed of adipose tissue, which functions as insulation and cushioning between the skin and other skeletal structures, like bone and muscle.6
I.C. Acute and Chronic Wounds
Cutaneous wound healing requires a well-orchestrated integration of the complex biological and molecular events of cell migration and proliferation, as well as ECM deposition, angiogenesis, and remodeling.7 Acute skin wounds result from some form of trauma, and undergo a repair process that, when it is orderly and timely, lead to a benign scar.1 Failure of this process, because the wound area and/or depth exceed the patient’s ability to heal, may lead to an undesirable scar or a chronic or nonhealing wound. The ability to heal seems to diminish with age, for various reasons, including age-related decreased strength and elasticity of skin,6 decreased blood flow to the extremities due to sedentary lifestyle, smoking, etc. Several studies, both in humans and in experimental animals, also suggest that psychological stress negatively impacts on wound healing.8, 9 In many instances, patients with chronic wounds (most notably diabetic foot ulcers) have other underlying conditions, such as diabetes, that impair wound healing.
II. PHYSIOLOGY OF SKIN WOUND HEALING
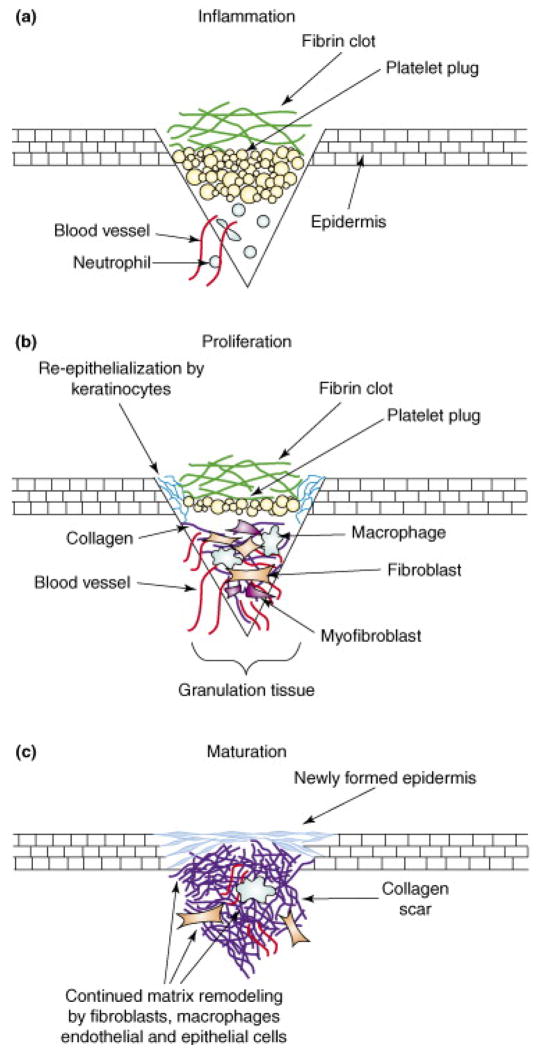
The skin wound healing process can be divided into 3 phases (shown in Figure 1):
Figure 1.
Phases of Wound Healing. Reproduced by permission from ref 10.
II.A. Inflammatory Phase
The earliest phase of wound healing starts with blood coagulation leading to the formation of a blood clot covering the wound, which acts as a temporary shield against pathogens as well as fluid loss. Although blood flow within the wound is often impaired due to destruction of the blood vessels, it is elevated in the areas immediately adjacent to the wound, and local inflammatory agents (activated complement, histamine, etc.) increase vascular permeability leading to plasma extravasation and the generation of a fibrin matrix, also causing swelling and redness.6 This matrix is rapidly invaded by neutrophils, followed by monocytes and other immunocompetent cells to remove dead tissue and control infection.5 This inflammatory phase typically lasts for the first 4 days.10
II.B. Proliferation Phase
Inflammatory cells secrete a host of factors which promote the recruitment and proliferation of vascular endothelial cells and fibroblasts. Fibroblasts begin to secrete collagen, gradually replacing the fibrin matrix. As more collagen is deposited and undergoes cross-linking, the mechanical strength of the wound increases. Fibroblasts may also differentiate into myofibroblasts that express α-smooth muscle actin, which causes the wound to contract, thus reducing the wound area that needs to be closed by cell proliferation. Vascular endothelial cells and capillaries invade through a process of angiogenesis extending from nearby healthy tissue, as well as from the recruitment of endothelial progenitors, which are present in low levels in the circulation. This “granulation tissue” is observed between days 5–20.6 Keratinocytes also start to migrate from the wound edges and proliferate on the surface of the granulation tissue, below the blood clot.10 The base of hair follicles (not shown on figure), which is located fairly deep into the dermis, is also an important source of keratinocytes for large area wounds. If these structures are destroyed (as is the case in deep second degree and third degree burns), reepithelialization is very slow and medical interventions, such as skin grafting (described further below) become necessary.
II.C. Maturation Phase
In this last phase, the wound has reepithelialized and the dermis has regained most of its tensile strength, although it is no longer as elastic as normal skin, and may be susceptible to re-opening. The scar will continue to undergo further remodeling over a time scale of months to years.6
III. CURRENT TREATMENT STRATEGIES FOR WOUND HEALING
III.A. Traditional Strategies
1. Skin Grafting with Autografts
Wounds that extend deep into the dermis tend to heal very poorly and slowly because no keratinocytes remain to reform the epithelium. For such wounds, skin grafting with an “autograft” is the treatment of choice; since the patient donates its own tissue, there is no risk of rejection.5 The technique is extremely well established and evolved from use in the back alleys of India in pre-Christian times.11 A dermatome, which is a surgical instrument that holds a razor-sharp blade parallel to the skin surface, is used to “shave” a thin layer of skin from the donor site (most commonly a conspicuous area such as inner thighs and buttocks) that includes the full epidermis and portion of the dermis, or what is commonly known a split-thickness graft.12 The skin graft is then placed on the wound site. If large areas need to be covered, such as in cases of extensive burns, the graft is meshed to enable stretching it over the larger area (typically using an expansion ratio of 1:3). The appearance of the healed wound is best if the graft is thicker (thus including more dermis) and unmeshed, and those factors are taken into consideration depending on the site of grafting. Conversely, healing of the donor site will be more compromised if a thicker graft is harvested. In general, the thicker the underlying dermis, the better the graft take, the faster the healing, and the better the long-term appearance of the healed wound. Donor sites will heal and can be reharvested, albeit a limited number of times because the dermis does not regenerate and becomes thinner each time.
2. Skin Allografts and Xenografts
Skin grafting with an autograft may not be immediately possible because of limited availability of donor tissue. In this instance, wounds may be covered with allografts, which will serve as temporary covering since they typically get rejected by the host’s immune system after a week.5 Allografts are harvested from consenting donors after death and stored frozen in skin banks where they can be used whenever needed.13 Allografts provide a barrier function and it is thought that growth factors released from these grafts have a positive effect on wound healing until an autograft can be placed onto the wound. Xenografts made of pig skin have also been used for the same purpose.14
III.B. Tissue Engineered Skin Substitutes
Skin was the first tissue application to be successfully engineered in the laboratory, first with the development of biodegradable matrix materials that can emulate the dermis, and subsequently the development of keratinocyte culture techniques leading to live cultured skin products.
1. Matrix-based products
The first engineered skin substitutes, which are still in use today, consist of porous matrices which function as templates for dermal regeneration. The matrices are placed on the wound bed and allowed to integrate and vascularize. After sufficient revascularization of the matrix, these products must be covered with autografts.15 Integra® is the first commercially available engineered skin substitute and consists of a matrix of cross-linked collagen and chondroitin-6-sulfate copolymer are mixed together to form the dermal matrix. A silicon sheet is attached to one side that functions as a temporary epidermal layer.13 Integra® is primarily used for the treatment of deep burn wounds, which are prone to forming undesirable scars. The matrix undergoes degradation while the host’s cells invade and proliferate within it, thus promoting dermal regeneration while inhibiting wound contraction, leading to a better function and appearance of the healed wound.16 Another skin substitute, Alloderm®, is made from decellularized donor skin. Removing all the cells and keeping only the protein component, prevents an allogeneic immunological response and also reduces the risk of disease transmission. 13, 17 It is used both for wound repair and reconstructive surgery. As is the case with Integra, an autograft must be eventually applied to reepithelialize the wound.
2. Cell-based products
Cultured skin began when methods for harvesting keratinocytes from patients and proliferating the cells in vitro became available. This pioneering work led to Epicel™, a cultured autologous epidermis which was first produced in 1988. It takes several weeks for a skin biopsy to be expanded into sufficient cultured epidermis that can be applied onto the patient, and the product is very costly, on the order of $800 per a 50 cm2 area.13 The product does not have a dermis, and is only a few cells thick, therefore it is very fragile and difficult to use. For these reasons, its use is limited to catastrophic burns where very little autologous viable skin remains.
Another tissue engineered skin product consists of allogeneic neonatal dermal fibroblasts cultured on a polyglactin mesh. The cells produce ECM matrix proteins as the mesh degrades, producing a matrix usable on the wound.13 This product is a dermal analog called Dermagraft®, which has been used to cover diabetic foot ulcers. Although this product is eventually rejected, it appears to help restore the dermis and promote keratinocyte migration to close the wound.18
Another allogeneic skin product is Apligraf®, a bilayered construct using fibroblasts and keratinocytes to create a dermis and epidermis, respectively.13 Both sets of cells are taken from neonatal foreskin, and the fibroblasts are mixed with type 1 collagen to form a strong network of cells and matrix proteins. The keratinocytes are then seeded onto the construct and stratified into layers. One potential negative consequence to using this product is that some wounds have contracted more than using skin grafts.5 As any other allogeneic skin, Apligraf® ultimately get rejected.19
A new approach is to distribute a “minced micrograft” over the wound area. This technique excises a small area (~ 2 cm2) of full thickness skin from the patient and minces it. The mixture, which contains both the dermal and epidermal components of skin, is mixed with a hydrogel and applied onto the wound. The distributed cells proliferate and participate to the wound healing. This may be a future alternative to traditional skin grafts, since only a small area is needed at the donor site, and it is also cheaper and simpler than products that attempt to emulate the skin geometry.20
IV. STEM CELLS & WOUND HEALING
While engineered skin substitutes represent significant advances in wound care, their use is not routine because of their high cost, limited effectiveness, and their inability to reconstitute skin appendages.21 Stem cells, defined based on the findings of Ernest A.McCulloch and James E. Till,22, 23 are characterized by (1) a prolonged self-renewal capacity and (2) the ability to differentiate into mature stages and different tissue types by asymmetric replication.24, 25 Stem cells, due to their ability to differentiate into various tissue types by asymmetric replication, may help create those skin components that are not found in the tissue engineered skin substitutes. Stem cells have two distinguishing properties: i) They are undifferentiated cells that renew themselves for the entire life span of an organism through cell division and ii) they have a remarkable capacity to develop from a common precursor into multiple cell types with specialized functions. Among the main sources of cells that might be used for repair and regeneration of injured skin are adult stem cells, embryonic stem cells (ESCs), and induced pluripotent stem cells (iPS) cells.
IV.A. The Involvement of Stem Cells in the Wound Healing Process
While it is well-known that during the inflammatory phase of wound healing, blood-borne immunocompetent cells invade the wound area, recent evidence suggests that bone marrow-derived stem cells are also recruited into the wound site.26, 27 This is not completely surprising, since a small number of hematopoeitic and mesenchymal stem cells is always present in peripheral blood. Furthermore, severe injury has been shown to increase the number of circulating stem cells.28 Evidence of this phenomenon has been shown in various models of tissue injury. For example, Badiavas et al. used a skin wound model in mice that had been transplanted with green fluorescent protein (GFP) tagged bone marrow. They found GFP-labeled cells in the wound site that had differentiated into various lineages.29 In similar experiments, Fathke et al. reported that distant bone marrow-derived stem cells contribute to the reconstitution of the dermal fibroblast population in cutaneous wounds.30 These findings suggest a potential important contribution of stem cell homing to the wound healing process, which is currently not well understood and warrants further study.
IV.B. Adult stem cells from bone marrow
The development of therapies using stem cells in the context on injury and wound healing has primarily relied on adult stem cells, and especially mesenchymal stromal cells, also known as mesenchymal stem cells (MSCs). MSCs are self-renewing and capable of differentiating into various tissues and cells, including skin cells.31, 32 MSCs can be isolated from the patient’s bone marrow and other tissues such as adipose tissue, nerve tissue, umbilical cord blood, and dermis.33–37 The other important benefit of MSCs is that even allogeneic MSCs induce little immunoreactivity in the host after local transplantation or systemic administration.38, 39 Hence, MSCs have received considerable attention for modulating wound repair.40 MSCs have been examined in skin repair and regeneration after various acute and chronic skin injuries, such as acute incisional and excisional wounds, diabetic skin ulcers, radiation burns, and thermal burns.7, 41, 42
Bone marrow-derived MSCs appear to synthesize higher amounts of collagen and several growth and angiogenic factors, when compared to native dermal fibroblasts, indicating a potential use in accelerating wound healing.43 The effects of bone marrow impregnated collagen on wound healing were studied in a microcirculatory mouse model, showing significant increases in angiogenesis.44 Patients with chronic leg ulcers demonstrated successful wound closure after a treatment with these impregnated collagen matrices. A variation of this approach described by Falaga et al.45 utilized a fibrin polymer spray to apply cultured autologous MSCs obtained from bone marrow aspirates to accelerate the rate of healing of acute and non-healing cutaneous wounds in both humans and mice. This approach may represent a feasible method for introducing cells into wounds. Badiavas and Falanga published clinical results using autologous bone marrow cells directly applied on chronic cutaneous ulcerations from 3 patients with wounds resistant to standard conventional treatment for more than 1 year.46 All patients showed improvement of their wounds within days following administration, characterized by a steady overall decrease in wound size and an increase in the vascularity of the dermis and the dermal thickness of the wound bed. Another study of chronic diabetic foot ulcers involved a 29-day treatment with an autologous graft composed of autologous skin fibroblasts on biodegradable collagen membranes combined with autologous MSCs applied directly to the wound and injected into the edges of the wound on days 1, 7, and 17; the wound size decreased, and the vascularity of the dermis and dermal thickness of the wound increased.42 A case report of a severe buttock radiation burn has described treatment combining physical techniques, surgery, and autologous bone marrow-MSCs therapy; clinical evolution (radiation pain and healing progression) during the 11-month follow-up was favorable, with no recurrence of radiation inflammatory waves seen on radiography.41
While these results with MSCs are impressive, the treatment approach generally requires that the MSCs must be cultured in sufficient numbers for topical application; this may not be a major issue for small chronic wounds, but may become very impractical when treating large wounds. Relevant to the last point is that severe burns and trauma tend to cause bone marrow suppression with concomitant decrease in MSCs either as a result of silver sulphadiazine toxicity47 or sepsis.48, 49 Another consideration is that bone marrow MSCs significantly decrease with age, which may reduce the applicability of using autologous MSCs for chronic wounds.50
IV.C. Embryonic stem cells and induced pluripotent stem cells
The embryo with its developmental plasticity and high proliferative capacity is thought to be the ultimate source for pluripotent stem cells.51, 52 Aside from ethical concerns regarding the use of human embryos for cell harvesting which can now be circumvented to a large extent,53 a major limitation of using embryonic stem cells (ESCs) for skin therapies is that ESC-derived skin is allogeneic and therefore cannot be used as a permanent wound coverage. Since allogeneic and xenogeneic skin are already available at reasonable cost, there is no clear advantage of using ESC-derived skin from the clinical standpoint. However, reports have shown successful differentiation of ESC-derived skin in vitro,54 and such studies may yield useful and important information about skin development.
Induced pluripotent stem cells (iPS cells) are a type of pluripotent stem cell artificially derived from a non-pluripotent cell, typically an adult somatic cell, by inducing a “forced” expression of certain genes. iPS cells were first produced in 2006 from mouse cells55 and in 2007 from human cells.56 This was a potentially important advancement in stem cell research, as it may overcome two important obstacles associated with human ESCs: immune rejection after transplantation and ethical concerns regarding the use of human embryos.
The technique was first described by Yamanaka,57 who, in 2007, showed that the introduction of four genes (Oct-3/4, Sox2, c- Myc, and KLF4) into an adult human skin cell could reprogram the cell back to an embryonic state. These induced pluripotent stem cells were shown to be remarkably similar to ESCs in morphology, proliferation potential, gene expression pattern, pluripotency, and telomerase activity. Like ESCs, iPS cells could be coaxed into becoming other types of cells—from skin to nerve to muscle. Since one of the four genes used (namely, c-Myc) is oncogenic, and 20% of the chimeric mice developed cancer, safety issues were a concern. Subsequently, Takahashi et al. successfully reprogrammed dermal fibroblasts into iPS cells without using the c-Myc retrovirus.56 Finally, Dimo et al. derived iPS cells from two octogenarians with Lou Gehrig’s disease, aged 82 and 89, suggesting that iPS cells can be successfully derived from sick and/or older patients, who are more likely to need iPS cell-based therapies than young healthy individuals.58 Further developments with iPS cell technology have focused on switching from viruses to using plasmids to deliver the time-reversing genes into adult cells in order to eliminate safety concerns associated with viral vectors.59 Recent studies also suggest that the generation of iPS cells may be possible without any genetic alterations as described by Zhou et al., who used recombinant proteins channeled into the cells via poly-arginine anchors.60
IV.D. Inducing skin lineage commitment in stem cells
1. Keratinocytes
There are two approaches to commit ES cells and adult stem cells (of non-epidermal origin) to the keratinocyte lineage in vitro. One approach would be to expose the cells to a cocktail of exogenous cytokines, growth factors, chemicals, and extracellular matrix (ECM) substrata over a prolonged duration of in vitro culture. Only a fraction of the stem cells would be expected to undergo commitment to the keratinocyte lineage, because many of these cytokines, growth factors, chemicals, and ECM substrata would exert non-specific pleitropic effects on stem cell differentiation into multiple lineages. At best, the cocktail combination of various cytokines, growth factors, chemicals, and ECM substrata can be ‘optimized’ by trial and error, to maximize the proportion of stem cells committing to the keratinocyte lineage, while at the same time yielding a large number of other undesired lineages. Hence, extensive selection/purification and proliferation of the commited keratinocyte progenitors is likely to be required.
By using such an approach, Coraux et al.54 managed to achieve commitment and subsequent differentiation of murine ES cells into the keratinocyte lineage, in the presence of a cocktail combination of bone morphogenetic protein-4 (BMP-4), ascorbate, and ECM derived from human normal fibroblasts (HNFs) and murine NIH-3T3 fibroblasts. Nevertheless, it must be noted that the study of Coraux et al.54 also reported a high degree (approximately 80%) of non-specific differentiation into multiple uncharacterized lineages, and no attempt was made to purify differentiated keratinocytes or keratinocyte progenitors from the mixture of lineages derived from murine ES cells. Bagutti et al.61 reported that coculture with human dermal fibroblasts (HDFs) as well as HDF-conditioned media could induce beta integrin- deficient murine ES cells to commit and differentiate into the keratinocyte lineage. However, as with the study of Coraux et al.,54 the keratinocytes were interspersed with differentiated cells of other lineages. Recently, differentiation of human ES cells into the keratinocyte lineage was also reported by Green et al.62 However, this study was based on in vivo teratoma formation within a SCID mouse model, and to date, there are no parallel in vitro studies that have been reported.
With adult stem cells of non-epidermal origin, there are also few studies 63, 64 which have successfully achieved re-commitment and trans-differentiation to the keratinocyte lineage. Even so, these studies were based primarily on the transplantation of undifferentiated stem cells in vivo, with the observed trans-differentiation occurring sporadically and at extremely low frequencies. Moreover, the validity of the experimental data may be clouded by controversy over the artifact of stem cell fusion in vivo.65 To date, there are no parallel in vitro studies that have achieved recommitment and trans-differentiation of non-epidermal adult stem cells to the keratinocyte lineage. It can therefore be surmised that the use of exogenous cytokines, growth factors, chemicals, and ECM substrata to induce ES cell and nonepidermal adult stem cell commitment to the keratinocyte lineage is a relatively inefficient, time-consuming, and labor-intensive process that would require extensive selection and purification of the committed keratinocyte progenitors. Hence, it would be technically challenging to apply this to the clinical situation.
The other approach for inducing ES cell and non-epidermal adult stem cell commitment to the keratinocyte lineage is through genetic modulation. This may be achieved by transfecting stem cells with recombinant DNA constructs encoding for the expression of signaling proteins that promote commitment to the keratinocyte lineage. Of particular interest are the Lef-1/Tcf family of Wnt regulated transcription factors that act in concert with b-catenin,66, 67 c-myc which is a downstream target of the Wnt-signaling pathway,68, 69 and the transactivation domain containing isoform of transcription factor p63 (Tap63).70, 71 Interestingly, the transcription factor GATA-3, which is well known to be a key regulator of T-cell lineage determination, has also been shown to be essential for stem cell lineage determination in skin, where it is expressed at the onset of epidermal stratification and Inner Root Sheath (IRS) specification in follicles.72 Recombinant overexpression of p6373 and c-Myc74 has been reported to promote commitment and differentiation to the keratinocyte lineage.
The disadvantage of directing differentiation through genetic modulation is the potential risks associated with utilizing recombinant DNA technology in human clinical therapy. For example, the overexpression of any one particular protein within transfected stem cells would certainly have unpredictable physiological effects upon transplantation in vivo. This problem may be overcome by placing the recombinant expression of the particular protein under the control of ‘switchable’ promoters, several of which have been developed for expression in eukaryotic systems. Such ‘switchable’ promoters could be responsive to exogenous chemicals,75 heat shock,76 or even light.77 Genetically modified stem cells may also run the risk of becoming malignant within the transplanted recipient. Moreover, there are overriding safety concerns with regard to the use of recombinant viral based vectors in the genetic manipulation of stem cells.78 It remains uncertain as to whether legislation would ultimately permit the use of genetically modified stem cells for human clinical therapy. At present, the potential detrimental effects of transplanting genetically modified stem cells in vivo are not well studied. More research needs to be carried out on animal models to address the safety aspects of such an approach.
More recently, there is emerging evidence that some transcription factors (which are commonly thought of as cytosolic proteins) have the ability to function as paracrine cell to cell signaling molecules.79 This is based on intercellular transfer of transcription factors through atypical secretion and internalization pathways.79 Hence, there is an exciting possibility that transcription factors implicated in commitment to the keratinocyte lineage may in the future be genetically engineered to incorporate domains that enable them to participate in novel paracrine signaling mechanisms. This in turn would have tremendous potential for inducing the commitment of ES cells and non-epidermal adult stem cells to the keratinocyte lineage.
2. Skin appendages
Skin appendages, including hair follicles, sebaceous glands and sweat glands, are linked to the epidermis but project deep into the dermal layer. The skin epidermis and its appendages provide a protective barrier that is impermeable to harmful microbes and also prevents dehydration. To perform their functions while being confronted with the physicochemical traumas of the environment, these tissues undergo continual rejuvenation through homeostasis, and, in addition, they must be primed to undergo wound repair in response to injury. The skin’s elixir for maintaining tissue homeostasis, regenerating hair, and repairing the epidermis after injury is its stem cells.
The hair follicle is composed of an outer root sheath that is contiguous with the epidermis, an inner root sheath and the hair shaft. The matrix surrounding the dermal papilla, in the hair root, contains actively dividing, relatively undifferentiated cells and is therefore a pocket of MSCs that are essential for follicle formation. The lower segment of each hair follicle cycles through periods of active growth (anagen), destruction (catagen) and quiescence (telogen).80 A specialized region of the outer root sheath of the hair follicle, known as the bulge, is located below the sebaceous gland, which is also the attachment site of the arrector pili muscle, receiving inputs from sensory nerve endings and blood vessels. Furthermore, the hair follicle bulge is a reservoir of slow-cycling multipotent stem cells.81, 82 Subsets of these follicle-derived multipotent stem cells can be activated and migrate out of hair follicles to the site of a wound to repair the damaged epithelium; however, they contribute little to the intact epidermis. These hair follicle stem cells can also contribute to the growth of follicles themselves and the sebaceous gland. For example, in the absence of hair follicle stem cells, hair follicle and sebaceous gland morphogenesis is blocked, and epidermal wound repair is compromised.83 In addition to containing follicle epidermal stem cells, the bulge contains melanocyte stem cells.84 Recent studies show that nestin, a marker for neural progenitor cells, is selectively expressed in cells of the hair follicle bulge and that these stem cells can differentiate into neurons,85 glia, keratinocytes, smooth muscle cells, melanocytes and even blood vessels.86, 87 Examination of close developmental and anatomical parallels between epithelial tissue and dermal tissue in skin and hair follicles has revealed dermal tissue to have stem cells. Paus et al. indicated that hair follicle dermal sheath cells might represent a source of dermal stem cells that not only incorporate into the hair-supporting papilla, low down in the follicle, but also move up and out from the follicle dermal sheath into the dermis of adjoining skin.88 Hair follicle dermal sheath cells taken from the human scalp can form new dermal papilla, induce the formation of hair follicles, and produce hair shafts when transplanted onto skin.89 There is also a clear transition from dermal sheath to dermal papilla cells.90 When the follicle dermal cells are implanted into skin wounds, they can be incorporated into the new dermis in a manner similar to that of skin wound-healing fibroblasts.91 However, these cell populations still lack specific markers for purifying and distinguishing the stem cells from their progeny. Furthermore, of prime importance is improving our understanding of the relation between bulge cells and interfollicular epidermal stem cells and between bulge cells and other stem cells inhabiting the skin and the mechanisms of hair growth.
Recently, cell replacement therapy has offered a novel and powerful medical technology for skin repair and regeneration: a new population of stem cell, called a neural crest stem cell, from adult hair follicles, was discovered to have the ability to differentiate in vitro to keratinocytes, neurons, cartilage/bone cells, smooth muscle cells, melanocytes, glial cells, and adipocytes.92–96 In mammalian skin, skin-derived neural progenitors were isolated and expanded from the dermis of rodent skin and adult human scalp and could differentiate into both neural and mesodermal progeny.97, 98 Skin-derived neural progenitor cells were isolated based on the sphere formation of floating cells after 3–7 days of culture in uncoated flasks with epidermal growth factor and fibroblast growth factor, and characterized by the production of nestin and fibronectin, markers of neural precursors. In addition, skin-derived neural progenitor cells were identified as neural crest derived by the use of Wnt1 promoter driving LacZ expression in the mouse. Some of the LacZ-positive cells were found in the skin of the face, as well as in the dermis and dermal papilla of murine whisker.99 These skin derived neural crest cells have already shown promising results in regenerative medicine such as the promotion of regenerative axonal growth after transplantation into injured adult mouse sciatic nerves 95 or spinal cord repair,100 resulting in the recovery of peripheral nerve function. This new study marks an important first step in the development of real stem-cell-based therapies and skin tissue regeneration.
V. SUMMARY AND FUTURE DIRECTIONS
Skin tissue engineering technologies have been available for the past 3 decades, and provide a number of alternatives to traditional skin grafting. There is nevertheless much room for improvement given the many practical and therapeutic limitations of tissue engineered skin. The “holy grail” of skin tissue engineering and skin wound regeneration remains the inability to reliably reconstitute skin appendages, most notably hair follicles and sweat glands. The availability of adult stem cells and iPS cells from the patient provide opportunities for eventually generating these structures without the risk of immune rejection. Recent studies with skin-derived progenitors provide clues as to the mechanisms that generate and maintain skin appendages, and this information will eventually form the basis of new therapies that address these limitations.
Acknowledgments
Financial support from the Shriners Hospitals for Children, and the National Institutes of Health (1R21AR056446) is gratefully acknowledged.
ABBREVATIONS
- ECM
extracellular matrix
- ESC
embryonic stem cell
- iPS
induced pluripotent stem cells
- GFP
green fluorescent protein
- MSCs
mesenchymal stem cells
- BMP-4
bone morphogenetic protein-4
- HNFs
human normal fibroblasts
- HDFs
human dermal fibroblasts
- IRS
inner root sheath
References
- 1.Atiyeh BS, Ioannovich J, Al-Amm CA, El-Musa KA. Management of acute and chronic open wounds: the importance of moist environment in optimal wound healing. Curr Pharm Biotechnol. 2002 Sep;3(3):179–95. doi: 10.2174/1389201023378283. [DOI] [PubMed] [Google Scholar]
- 2.Braddock M, Campbell CJ, Zuder D. Current therapies for wound healing: electrical stimulation, biological therapeutics, and the potential for gene therapy. Int J Dermatol. 1999 Nov;38(11):808–17. doi: 10.1046/j.1365-4362.1999.00832.x. [DOI] [PubMed] [Google Scholar]
- 3.Beckrich K, Aronovitch SA. Hospital-acquired pressure ulcers: a comparison of costs in medical vs. surgical patients. Nurs Econ. 1999 Sep-Oct;17(5):263–71. [PubMed] [Google Scholar]
- 4.Crovetti G, Martinelli G, Issi M, Barone M, Guizzardi M, Campanati B, et al. Platelet gel for healing cutaneous chronic wounds. Transfus Apher Sci. 2004 Apr;30(2):145–51. doi: 10.1016/j.transci.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Blitterswijk CAv, Thomsen P. Tissue engineering. 1. Amsterdam; Boston: Elsevier/Academic Press; 2008. [Google Scholar]
- 6.Harvey C. Wound healing. Orthop Nurs. 2005 Mar-Apr;24(2):143–57. doi: 10.1097/00006416-200503000-00012. quiz 58–9. [DOI] [PubMed] [Google Scholar]
- 7.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007 Oct;25(10):2648–59. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 8.Walburn J, Vedhara K, Hankins M, Rixon L, Weinman J. Psychological stress and wound healing in humans: a systematic review and meta-analysis. J Psychosom Res. 2009 Sep;67(3):253–71. doi: 10.1016/j.jpsychores.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Cole-King A, Harding KG. Psychological factors and delayed healing in chronic wounds. Psychosom Med. 2001 Mar-Apr;63(2):216–20. doi: 10.1097/00006842-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Rhett JM, Ghatnekar GS, Palatinus JA, O’Quinn M, Yost MJ, Gourdie RG. Novel therapies for scar reduction and regenerative healing of skin wounds. Trends Biotechnol. 2008 Apr;26(4):173–80. doi: 10.1016/j.tibtech.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Davis JS. Story of plastic surgery. Ann Surg. 1941;113:651–6. doi: 10.1097/00000658-194105000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akan M, Yildirim S, Misirlioglu A, Ulusoy G, Akoz T, Avci G. An alternative method to minimize pain in the split-thickness skin graft donor site. Plast Reconstr Surg. 2003 Jun;111(7):2243–9. doi: 10.1097/01.PRS.0000060087.93322.2F. [DOI] [PubMed] [Google Scholar]
- 13.Bello YM, Falabella AF, Eaglstein WH. Tissue-engineered skin. Current status in wound healing. Am J Clin Dermatol. 2001;2(5):305–13. doi: 10.2165/00128071-200102050-00005. [DOI] [PubMed] [Google Scholar]
- 14.Solovey P, Kyryk O, Barchuk V. “Xenografts--liophilized pig skin” as a burn wound cover. Burns. 2007;33(1, Supplement 1):S85–S6. [Google Scholar]
- 15.Supp DM, Boyce ST. Engineered skin substitutes: practices and potentials. Clin Dermatol. 2005 Jul-Aug;23(4):403–12. doi: 10.1016/j.clindermatol.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 16.Stiefel D, Schiestl C, Meuli M. Integra Artificial Skin((R)) for burn scar revision in adolescents and children. Burns. 2010 Feb;36(1):114–20. doi: 10.1016/j.burns.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 17.Pushpoth S, Tambe K, Sandramouli S. The use of AlloDerm in the reconstruction of full-thickness eyelid defects. Orbit. 2008;27(5):337–40. doi: 10.1080/01676830802319054. [DOI] [PubMed] [Google Scholar]
- 18.Marston WA, Hanft J, Norwood P, Pollak R. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003 Jun;26(6):1701–5. doi: 10.2337/diacare.26.6.1701. [DOI] [PubMed] [Google Scholar]
- 19.Hu S, Kirsner RS, Falanga V, Phillips T, Eaglstein WH. Evaluation of Apligraf persistence and basement membrane restoration in donor site wounds: a pilot study. Wound Repair Regen. 2006 Jul-Aug;14(4):427–33. doi: 10.1111/j.1743-6109.2006.00148.x. [DOI] [PubMed] [Google Scholar]
- 20.Boggio P, Tiberio R, Gattoni M, Colombo E, Leigheb G. Is there an easier way to autograft skin in chronic leg ulcers? ‘Minced micrografts’, a new technique. J Eur Acad Dermatol Venereol. 2008 Nov;22(10):1168–72. doi: 10.1111/j.1468-3083.2008.02737.x. [DOI] [PubMed] [Google Scholar]
- 21.Burd A, Ahmed K, Lam S, Ayyappan T, Huang L. Stem cell strategies in burns care. Burns. 2007 May;33(3):282–91. doi: 10.1016/j.burns.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 22.Becker AJ, Mc CE, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963 Feb 2;197:452–4. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- 23.Siminovitch L, McCulloch EA, Till JE. The Distribution of Colony-Forming Cells among Spleen Colonies. J Cell Physiol. 1963 Dec;62:327–36. doi: 10.1002/jcp.1030620313. [DOI] [PubMed] [Google Scholar]
- 24.Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000 Jan 7;100(1):157–68. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 25.Cha J, Falanga V. Stem cells in cutaneous wound healing. Clin Dermatol. 2007 Jan-Feb;25(1):73–8. doi: 10.1016/j.clindermatol.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Cottler-Fox MH, Lapidot T, Petit I, Kollet O, DiPersio JF, Link D, et al. Stem cell mobilization. Hematology Am Soc Hematol Educ Program. 2003:419–37. doi: 10.1182/asheducation-2003.1.419. [DOI] [PubMed] [Google Scholar]
- 27.Fu S, Liesveld J. Mobilization of hematopoietic stem cells. Blood Rev. 2000 Dec;14(4):205–18. doi: 10.1054/blre.2000.0138. [DOI] [PubMed] [Google Scholar]
- 28.Kucia M, Ratajczak J, Reca R, Janowska-Wieczorek A, Ratajczak MZ. Tissue-specific muscle, neural and liver stem/progenitor cells reside in the bone marrow, respond to an SDF-1 gradient and are mobilized into peripheral blood during stress and tissue injury. Blood Cells Mol Dis. 2004 Jan-Feb;32(1):52–7. doi: 10.1016/j.bcmd.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 29.Badiavas EV, Abedi M, Butmarc J, Falanga V, Quesenberry P. Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol. 2003 Aug;196(2):245–50. doi: 10.1002/jcp.10260. [DOI] [PubMed] [Google Scholar]
- 30.Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, et al. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells. 2004;22(5):812–22. doi: 10.1634/stemcells.22-5-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakagawa H, Akita S, Fukui M, Fujii T, Akino K. Human mesenchymal stem cells successfully improve skin-substitute wound healing. Br J Dermatol. 2005 Jul;153(1):29–36. doi: 10.1111/j.1365-2133.2005.06554.x. [DOI] [PubMed] [Google Scholar]
- 32.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001 May 4;105(3):369–77. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 33.Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007 Oct;48(1):15–24. doi: 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 34.Dai Y, Li J, Li J, Dai G, Mu H, Wu Q, et al. Skin epithelial cells in mice from umbilical cord blood mesenchymal stem cells. Burns. 2007 Jun;33(4):418–28. doi: 10.1016/j.burns.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 35.Perng CK, Ku HH, Chiou SH, Chen IL, Tsai FT, Yang YP, et al. Evaluation of wound healing effect on skin-defect nude mice by using human dermis-derived mesenchymal stem cells. Transplant Proc. 2006 Nov;38(9):3086–7. doi: 10.1016/j.transproceed.2006.08.146. [DOI] [PubMed] [Google Scholar]
- 36.Shih DT, Lee DC, Chen SC, Tsai RY, Huang CT, Tsai CC, et al. Isolation and characterization of neurogenic mesenchymal stem cells in human scalp tissue. Stem Cells. 2005 Aug;23(7):1012–20. doi: 10.1634/stemcells.2004-0125. [DOI] [PubMed] [Google Scholar]
- 37.Jones EA, Kinsey SE, English A, Jones RA, Straszynski L, Meredith DM, et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002 Dec;46(12):3349–60. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- 38.Mansilla E, Marin GH, Sturla F, Drago HE, Gil MA, Salas E, et al. Human mesenchymal stem cells are tolerized by mice and improve skin and spinal cord injuries. Transplant Proc. 2005 Jan-Feb;37(1):292–4. doi: 10.1016/j.transproceed.2005.01.070. [DOI] [PubMed] [Google Scholar]
- 39.Liu H, Kemeny DM, Heng BC, Ouyang HW, Melendez AJ, Cao T. The immunogenicity and immunomodulatory function of osteogenic cells differentiated from mesenchymal stem cells. J Immunol. 2006 Mar 1;176(5):2864–71. doi: 10.4049/jimmunol.176.5.2864. [DOI] [PubMed] [Google Scholar]
- 40.Bajada S, Mazakova I, Richardson JB, Ashammakhi N. Updates on stem cells and their applications in regenerative medicine. J Tissue Eng Regen Med. 2008 Jun;2(4):169–83. doi: 10.1002/term.83. [DOI] [PubMed] [Google Scholar]
- 41.Lataillade JJ, Doucet C, Bey E, Carsin H, Huet C, Clairand I, et al. New approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapy. Regen Med. 2007 Sep;2(5):785–94. doi: 10.2217/17460751.2.5.785. [DOI] [PubMed] [Google Scholar]
- 42.Vojtassak J, Danisovic L, Kubes M, Bakos D, Jarabek L, Ulicna M, et al. Autologous biograft and mesenchymal stem cells in treatment of the diabetic foot. Neuro Endocrinol Lett. 2006 Dec;27( Suppl 2):134–7. [PubMed] [Google Scholar]
- 43.Han SK, Yoon TH, Lee DG, Lee MA, Kim WK. Potential of human bone marrow stromal cells to accelerate wound healing in vitro. Ann Plast Surg. 2005 Oct;55(4):414–9. doi: 10.1097/01.sap.0000178809.01289.10. [DOI] [PubMed] [Google Scholar]
- 44.Ichioka S, Kouraba S, Sekiya N, Ohura N, Nakatsuka T. Bone marrow-impregnated collagen matrix for wound healing: experimental evaluation in a microcirculatory model of angiogenesis, and clinical experience. Br J Plast Surg. 2005 Dec;58(8):1124–30. doi: 10.1016/j.bjps.2005.04.054. [DOI] [PubMed] [Google Scholar]
- 45.Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007 Jun;13(6):1299–312. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 46.Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003 Apr;139(4):510–6. doi: 10.1001/archderm.139.4.510. [DOI] [PubMed] [Google Scholar]
- 47.Gamelli RL, Paxton TP, O’Reilly M. Bone marrow toxicity by silver sulfadiazine. Surg Gynecol Obstet. 1993 Aug;177(2):115–20. [PubMed] [Google Scholar]
- 48.Gamelli RL, He LK, Liu H. Recombinant human granulocyte colony-stimulating factor treatment improves macrophage suppression of granulocyte and macrophage growth after burn and burn wound infection. J Trauma. 1995 Dec;39(6):1141–6. doi: 10.1097/00005373-199512000-00023. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 49.Shoup M, Weisenberger JM, Wang JL, Pyle JM, Gamelli RL, Shankar R. Mechanisms of neutropenia involving myeloid maturation arrest in burn sepsis. Ann Surg. 1998 Jul;228(1):112–22. doi: 10.1097/00000658-199807000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rao MS, Mattson MP. Stem cells and aging: expanding the possibilities. Mech Ageing Dev. 2001 May 31;122(7):713–34. doi: 10.1016/s0047-6374(01)00224-x. [DOI] [PubMed] [Google Scholar]
- 51.Hadjantonakis A, Papaioannou V. The stem cells of early embryos. Differentiation. 2001 Oct;68(4–5):159–66. doi: 10.1046/j.1432-0436.2001.680403.x. [DOI] [PubMed] [Google Scholar]
- 52.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998 Nov 6;282(5391):1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 53.Klimanskaya I, Chung Y, Becker S, Lu SJ, Lanza R. Human embryonic stem cell lines derived from single blastomeres. Nature. 2006 Nov 23;444(7118):481–5. doi: 10.1038/nature05142. [DOI] [PubMed] [Google Scholar]
- 54.Coraux C, Hilmi C, Rouleau M, Spadafora A, Hinnrasky J, Ortonne JP, et al. Reconstituted skin from murine embryonic stem cells. Curr Biol. 2003 May 13;13(10):849–53. doi: 10.1016/s0960-9822(03)00296-3. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006 Aug 25;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 56.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007 Nov 30;131(5):861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 57.Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007 Jun 7;1(1):39–49. doi: 10.1016/j.stem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 58.Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008 Aug 29;321(5893):1218–21. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 59.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008 Nov 7;322(5903):949–53. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 60.Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009 May 8;4(5):381–4. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bagutti C, Hutter C, Chiquet-Ehrismann R, Fassler R, Watt FM. Dermal fibroblast-derived growth factors restore the ability of beta(1) integrin-deficient embryonal stem cells to differentiate into keratinocytes. Dev Biol. 2001 Mar 15;231(2):321–33. doi: 10.1006/dbio.2000.0149. [DOI] [PubMed] [Google Scholar]
- 62.Green H, Easley K, Iuchi S. Marker succession during the development of keratinocytes from cultured human embryonic stem cells. Proc Natl Acad Sci U S A. 2003 Dec 23;100(26):15625–30. doi: 10.1073/pnas.0307226100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kataoka K, Medina RJ, Kageyama T, Miyazaki M, Yoshino T, Makino T, et al. Participation of adult mouse bone marrow cells in reconstitution of skin. Am J Pathol. 2003 Oct;163(4):1227–31. doi: 10.1016/S0002-9440(10)63482-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Korbling M, Katz RL, Khanna A, Ruifrok AC, Rondon G, Albitar M, et al. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med. 2002 Mar 7;346(10):738–46. doi: 10.1056/NEJMoa3461002. [DOI] [PubMed] [Google Scholar]
- 65.Alison MR, Poulsom R, Otto WR, Vig P, Brittan M, Direkze NC, et al. Recipes for adult stem cell plasticity: fusion cuisine or readymade? J Clin Pathol. 2004 Feb;57(2):113–20. doi: 10.1136/jcp.2003.010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999 Oct;126(20):4557–68. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 67.Merrill BJ, Gat U, DasGupta R, Fuchs E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 2001 Jul 1;15(13):1688–705. doi: 10.1101/gad.891401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waikel RL, Kawachi Y, Waikel PA, Wang XJ, Roop DR. Deregulated expression of c-Myc depletes epidermal stem cells. Nat Genet. 2001 Jun;28(2):165–8. doi: 10.1038/88889. [DOI] [PubMed] [Google Scholar]
- 69.Honeycutt KA, Roop DR. c-Myc and epidermal stem cell fate determination. J Dermatol. 2004 May;31(5):368–75. doi: 10.1111/j.1346-8138.2004.tb00687.x. [DOI] [PubMed] [Google Scholar]
- 70.Koster MI, Kim S, Mills AA, DeMayo FJ, Roop DR. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev. 2004 Jan 15;18(2):126–31. doi: 10.1101/gad.1165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koster MI, Roop DR. Transgenic mouse models provide new insights into the role of p63 in epidermal development. Cell Cycle. 2004 Apr;3(4):411–3. [PubMed] [Google Scholar]
- 72.Kaufman CK, Zhou P, Pasolli HA, Rendl M, Bolotin D, Lim KC, et al. GATA-3: an unexpected regulator of cell lineage determination in skin. Genes Dev. 2003 Sep 1;17(17):2108–22. doi: 10.1101/gad.1115203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Laurenzi V, Rossi A, Terrinoni A, Barcaroli D, Levrero M, Costanzo A, et al. p63 and p73 transactivate differentiation gene promoters in human keratinocytes. Biochem Biophys Res Commun. 2000 Jun 24;273(1):342–6. doi: 10.1006/bbrc.2000.2932. [DOI] [PubMed] [Google Scholar]
- 74.Gandarillas A, Watt FM. c-Myc promotes differentiation of human epidermal stem cells. Genes Dev. 1997 Nov 1;11(21):2869–82. doi: 10.1101/gad.11.21.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rudolph B, Hueber AO, Evan GI. Reversible activation of c-Myc in thymocytes enhances positive selection and induces proliferation and apoptosis in vitro. Oncogene. 2000 Apr 6;19(15):1891–900. doi: 10.1038/sj.onc.1203508. [DOI] [PubMed] [Google Scholar]
- 76.Schmidt M, Heimberger T, Gruensfelder P, Schler G, Hoppe F. Inducible promoters for gene therapy of head and neck cancer: an in vitro study. Eur Arch Otorhinolaryngol. 2004 Apr;261(4):208–15. doi: 10.1007/s00405-003-0621-z. [DOI] [PubMed] [Google Scholar]
- 77.Shimizu-Sato S, Huq E, Tepperman JM, Quail PH. A light-switchable gene promoter system. Nat Biotechnol. 2002 Oct;20(10):1041–4. doi: 10.1038/nbt734. [DOI] [PubMed] [Google Scholar]
- 78.Conget PA, Minguell JJ. Adenoviral-mediated gene transfer into ex vivo expanded human bone marrow mesenchymal progenitor cells. Exp Hematol. 2000 Apr;28(4):382–90. doi: 10.1016/s0301-472x(00)00134-x. [DOI] [PubMed] [Google Scholar]
- 79.Prochiantz A, Joliot A. Can transcription factors function as cell-cell signalling molecules? Nat Rev Mol Cell Biol. 2003 Oct;4(10):814–9. doi: 10.1038/nrm1227. [DOI] [PubMed] [Google Scholar]
- 80.Fuchs E, Nowak JA. Building epithelial tissues from skin stem cells. Cold Spring Harb Symp Quant Biol. 2008;73:333–50. doi: 10.1101/sqb.2008.73.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Christiano AM. Epithelial stem cells: stepping out of their niche. Cell. 2004 Sep 3;118(5):530–2. doi: 10.1016/j.cell.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 82.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, et al. Defining the epithelial stem cell niche in skin. Science. 2004 Jan 16;303(5656):359–63. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008 Jul 3;3(1):33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Steingrimsson E, Copeland NG, Jenkins NA. Melanocyte stem cell maintenance and hair graying. Cell. 2005 Apr 8;121(1):9–12. doi: 10.1016/j.cell.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 85.Amoh Y, Li L, Katsuoka K, Hoffman RM. Multipotent hair follicle stem cells promote repair of spinal cord injury and recovery of walking function. Cell Cycle. 2008 Jun 15;7(12):1865–9. doi: 10.4161/cc.7.12.6056. [DOI] [PubMed] [Google Scholar]
- 86.Amoh Y, Li L, Yang M, Jiang P, Moossa AR, Katsuoka K, et al. Hair follicle-derived blood vessels vascularize tumors in skin and are inhibited by Doxorubicin. Cancer Res. 2005 Mar 15;65(6):2337–43. doi: 10.1158/0008-5472.CAN-04-3857. [DOI] [PubMed] [Google Scholar]
- 87.Amoh Y, Yang M, Li L, Reynoso J, Bouvet M, Moossa AR, et al. Nestin-linked green fluorescent protein transgenic nude mouse for imaging human tumor angiogenesis. Cancer Res. 2005 Jun 15;65(12):5352–7. doi: 10.1158/0008-5472.CAN-05-0821. [DOI] [PubMed] [Google Scholar]
- 88.Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999 Aug 12;341(7):491–7. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 89.Reynolds AJ, Lawrence C, Cserhalmi-Friedman PB, Christiano AM, Jahoda CA. Trans-gender induction of hair follicles. Nature. 1999 Nov 4;402(6757):33–4. doi: 10.1038/46938. [DOI] [PubMed] [Google Scholar]
- 90.Reynolds AJ, Jahoda CA. Hair matrix germinative epidermal cells confer follicle-inducing capabilities on dermal sheath and high passage papilla cells. Development. 1996 Oct;122(10):3085–94. doi: 10.1242/dev.122.10.3085. [DOI] [PubMed] [Google Scholar]
- 91.Gharzi A, Reynolds AJ, Jahoda CA. Plasticity of hair follicle dermal cells in wound healing and induction. Exp Dermatol. 2003 Apr;12(2):126–36. doi: 10.1034/j.1600-0625.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 92.Hunt DP, Morris PN, Sterling J, Anderson JA, Joannides A, Jahoda C, et al. A highly enriched niche of precursor cells with neuronal and glial potential within the hair follicle dermal papilla of adult skin. Stem Cells. 2008 Jan;26(1):163–72. doi: 10.1634/stemcells.2007-0281. [DOI] [PubMed] [Google Scholar]
- 93.Amoh Y, Li L, Yang M, Moossa AR, Katsuoka K, Penman S, et al. Nascent blood vessels in the skin arise from nestin-expressing hair-follicle cells. Proc Natl Acad Sci U S A. 2004 Sep 7;101(36):13291–5. doi: 10.1073/pnas.0405250101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Amoh Y, Li L, Campillo R, Kawahara K, Katsuoka K, Penman S, et al. Implanted hair follicle stem cells form Schwann cells that support repair of severed peripheral nerves. Proc Natl Acad Sci U S A. 2005 Dec 6;102(49):17734–8. doi: 10.1073/pnas.0508440102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Amoh Y, Li L, Katsuoka K, Penman S, Hoffman RM. Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. Proc Natl Acad Sci U S A. 2005 Apr 12;102(15):5530–4. doi: 10.1073/pnas.0501263102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hoffman RM. The pluripotency of hair follicle stem cells. Cell Cycle. 2006 Feb;5(3):232–3. doi: 10.4161/cc.5.3.2397. [DOI] [PubMed] [Google Scholar]
- 97.Toma JG, Akhavan M, Fernandes KJ, Barnabe-Heider F, Sadikot A, Kaplan DR, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001 Sep;3(9):778–84. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 98.Toma JG, McKenzie IA, Bagli D, Miller FD. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005 Jun-Jul;23(6):727–37. doi: 10.1634/stemcells.2004-0134. [DOI] [PubMed] [Google Scholar]
- 99.Sieber-Blum M, Grim M. The adult hair follicle: cradle for pluripotent neural crest stem cells. Birth Defects Res C Embryo Today. 2004 Jun;72(2):162–72. doi: 10.1002/bdrc.20008. [DOI] [PubMed] [Google Scholar]
- 100.McKenzie IA, Biernaskie J, Toma JG, Midha R, Miller FD. Skin-derived precursors generate myelinating Schwann cells for the injured and dysmyelinated nervous system. J Neurosci. 2006 Jun 14;26(24):6651–60. doi: 10.1523/JNEUROSCI.1007-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]