Abstract
Turgor regulation at reduced water contents was closely associated with changes in the elastic quality of the cell walls of individual needles and shoots of naturally drought-resistant seedlings of white spruce (Picea glauca [Moench] Voss.) and of seedlings of intermediate resistance that had been pretreated with paclobutrazol, a stress-protecting, synthetic plant-growth regulator. Paclobutrazol-treated seedlings showed marked increases in drought resistance, and pressure-volume analysis combined with Chardakov measurements confirmed observations that water stress was ameliorated during prolonged drought. Turgor was maintained in the paclobutrazol-treated and in the naturally resistant drought-stressed seedlings despite water contents near or below the turgor-loss volumes of well-watered controls. The maintenance of turgor in these seedlings was in large part a function of the dynamic process of cell wall adjustment, as reflected by marked reductions in both the saturated and turgor-loss volumes and by large increases in the elastic coefficients of the tissues. Shear and Young's moduli, calculated from pressure-volume curves and the radii and wall thicknesses of mesophyll cells, also confirmed observed changes in the elastic qualities of the cell walls. Elastic coefficients of well-watered, paclobutrazol-treated seedlings were consistently larger than those in well-watered controls and several times larger than the values in untreated plants, which succumbed rapidly to drought. In contrast, untreated seedlings that withstood prolonged drought without wilting displayed elastic coefficients similar to those in seedlings that had been treated with paclobutrazol but that had not been exposed to drought.
In theory, plants can regulate turgor by solute accumulation, i.e. by osmotic adjustment, and possibly by elastic adjustment of their cell walls (Dainty, 1976). Osmotic adjustment, like stomatal closure, allows plants to avoid desiccation and turgor loss by the maintenance of water content. Data also indicate that plants subjected to dehydration may avoid reduced water potential and maintain turgor by reduction of their TLV via tissue shrinkage associated with elastic adjustment of their cell walls (Buxton et al., 1985; Eze et al., 1986; Levitt, 1986; Fan et al., 1994). Although osmotic adjustment is well documented in some species, there has been no conclusive evidence that plant tissues can maintain turgor at reduced water volumes by physiological adjustment of their cell walls (Weisz et al., 1989; Chazen and Neumann, 1994; Nabril and Coudret, 1995). The contractions in tissue volume observed in higher plants (Kozlowski, 1972) have usually not been viewed as mechanisms of drought resistance (Bray, 1993; Bohnert et al., 1995).
Broyer (1952) examined the relationship between osmotic work and volumetric expansion of plant tissues, and Phillip (1958) used the term “bulk-volumetric-elastic modulus” to describe the elastic potential of the cell wall. Cosgrove (1988) suggested that although the cell-volumetric elastic coefficient takes the same mathematical form as the bulk-volumetric elastic modulus used in physics, plant physiologists use it to describe the elasticity of thin-walled plant cells, in which mass is not necessarily conserved during changes in turgor, so it should be referred to as the cell-volumetric modulus. Young's modulus of the cell walls of giant algae was effectively measured by pressure-probe analysis (Kamiya et al., 1963), but higher plants are more complex, with small and variable cells, and are not as amenable to direct measurement of Young's modulus with a pressure probe. Nevertheless, methods have been developed to estimate cell wall elasticity in higher plants using the pressure probe without measurement of cellular dimensions (Murphy and Ortega, 1995). The strong agreement between the pressure-probe and pressure-chamber techniques (Murphy and Smith, 1994), combined with equations for the moduli of Young (Tyree and Jarvis, 1982) and Shear (Wu et al., 1985), allow for the estimation of cell wall elasticity via bulk pressure-volume analysis alone and in combination with microscopic sampling of cell size.
For reasons that are still poorly understood, the relationship between turgor and cell volume may be exponential or linear, depending on the plant species and on changes in the elastic quality of the cell walls (Cosgrove, 1988). Results from the present study using white spruce (Picea glauca) indicate that plants may actively govern turgor-volume relationships during drought by induction of marked changes in the elastic properties of their cell walls and by attendant reductions in the saturated volume and TLV of their cells. White spruce seedlings were used as the model system in our studies because of their low apoplastic volumes, their small intercellular air spaces, and their moderately low coefficient of nonlinearity during pressure-volume analysis over most physiological water contents (Tyree and Hammel, 1972). Pressure-chamber analyses of well-watered and drought-stressed seedlings, with and without the drought-protecting effects of paclobutrazol, a triazole derivative and synthetic plant-growth regulator (Marshall et al., 1991, 1992), suggested that the potential to induce large changes in wall elasticity does exist within plant species and that it may play a key role in the drought response.
MATERIALS AND METHODS
Plant Material and Growth-Regulator Treatment
Seeds of white spruce (Picea glauca [Moench] Voss.) were disinfected for 15 min in 3% (v/v) hydrogen peroxide, thoroughly rinsed in distilled water, and then stratified at 4°C for 2 weeks before sowing three seeds per tube in plastic forms (RIGI-POT model 67–50, IPL Products, Brampton, Ontario) lightly filled with peat, perlite, and vermiculite (3:2:2). After emergence the seedlings were thinned to one per tube and grown in a growth chamber (Conviron, Asheville, NC) with day/night (16 h/8 h) temperatures of 23°/18°C, 80% RH, and 212 μmol m−2 s−1 PAR from a mix of very high output fluorescent (F96T12-CW-1500, General Electric), incandescent (I-line 130, General Electric), and red (75R30 P1, General Electric) lights. Ten-centimeter plastic pots were filled with 30 g of dry peat, perlite, and vermiculite (3:2:2), and saturated in trays of water for several days.
Three 16-week-old seedlings were planted in each saturated pot and allowed to recover from transplanting for 2 weeks prior to treatment with paclobutrazol to increase drought resistance. Treated pots received a total of 200 μmol (60 mg) of paclobutrazol (Zeneca Agro, Stoney Creek, Ontario) applied in two equal root drenches 3 d apart, each with 100 μmol of paclobutrazol in 50 mL of water (Marshall et al., 1991). All plants were kept well watered with 150 mg L−1 of 20–20–20 N/P/K commercial fertilizer.
Experimental Design
One month after the initial treatment with paclobutrazol, pots of paclobutrazol-treated and untreated seedlings were placed in separate trays of tap water and covered loosely with plastic wrap for 12 h before initiating drought by withholding water. The saturated seedlings were randomly assigned to drought treatment and sampling groups before starting experiments using a randomized-block design with four or more replications per test. In addition, 100 randomly selected seedlings not treated with paclobutrazol were drought-stressed in a growth chamber under the conditions described above. Seedlings that wilted early in the drought period (≤9 d) were judged to be drought-sensitive and were harvested for measurement of water status. However, only seedlings that remained turgid and showed no signs of damage after prolonged drought were classified as drought-tolerant and harvested for measurement of water status after 12 d. The percent survival was assessed by rewatering pots for a minimum of 3 weeks after cessation of drought. Seedlings that remained wilted, turned brown, and became brittle were judged to be dead. Experimental results were analyzed statistically by two-way analysis of variance followed by Tukey's test for multiple comparisons using the SAS package (SAS Institute, Cary, NC).
Measurement of Plant Water Status and Cell Wall Elasticity
Shoot Water Relations
For tests requiring saturated seedlings, pots were watered to soil capacity, covered loosely in plastic wrap, and placed in the dark for 24 or 48 h prior to harvest and measurements. Pots from the drought time-course experiments were not saturated before harvest but were removed from the growth chamber and placed in a dark laboratory cupboard for 4 h before measurements were begun. The FW of cut shoots were recorded and components of water potential were determined using a pressure chamber (model 600, PMS Instruments, Corvallis, OR) as described by Tyree and Hammel (1972) and modified by Buxton et al. (1985). Extruded sap was collected in a series of preweighed microtubes plugged with absorbent paper, and the sap weight was recorded to four decimal places. The components of water potential and turgor-loss points were determined by reciprocal pressure-volume curves.
Elasticity
Cross-sectional diameters and wall thicknesses of mesophyll cells, the most numerous and elastic cells, were used to calculate Shear and Young's moduli (Eqs. 1 and 2) from tissue averages of pressure and volume, based on the assumption of an isotropic, thin-shelled sphere with a Poisson ratio of one-half, and using the equations from Tyree and Jarvis (1982) and Wu et al. (1985). The cross-sections were cut at the midpoints of fresh needles obtained from nine treated and nine untreated water-saturated seedlings. The sections were stained and saturated with water for 1 h in 0.1% cellufluor, rinsed in water, and photographed under UV light using a microscope (Zeiss). Diameters and wall thicknesses were measured in every mesophyll cell intersected by a random transect though the cortex and vascular cylinder.
Since the osmotic work required for elastic expansion is stored in the elastic cell walls, this total recoverable energy was calculated per unit cell wall volume (Eq. 3). The average density of woody cell wall materials (1.5 g mL−1) was used to convert DW to the volume of solid wall material (Forbes, 1956).
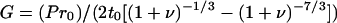 |
1 |
where G is the Shear modulus, P is pressure, r0 is the initial cell radius, t0 is the initial cell wall thickness, V is total water volume, and ν is equal to V/TLV − 1.
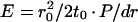 |
2 |
where E is Young's modulus and dr is the change in cell radius.
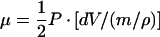 |
3 |
where μ is the total recoverable energy from osmotic work, dV is the change in water volume, m is the mass of the cell wall, and ρ is the cell wall density.
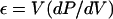 |
4 |
where ε is the cell-volumetric-elastic coefficient, calculated according to the methods of Dainty (1976), Steudle et al. (1982), and Cosgrove (1988), and dP is the change in pressure.
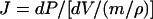 |
5 |
where J is an elastic coefficient per unit of cell wall material used to evaluate the treatment effects on cell wall elasticity irrespective of treatment-induced increases in cell wall mass.
Needle Water Relations
FW of excised needles were recorded and saturated moisture contents were subsequently determined by placing 25 preweighed needles on the bottom of a vial, adding 20 mL of water, covering the vial, and then weighing the needles after 24 h. Tissue was dried in a forced-draft oven at 70°C for 24 h and then reweighed for calculation of water contents as: % FW = (FW − DW/FW) × 100 and % SW = (SW − DW/SW) × 100. Relative water content was calculated as (FW − DW/SW − DW) × 100. Water content at TLV was calculated as % FWTLV = (FWTLV − DWTLV/FWTLV) × 100. FWTLV was calculated by subtracting the total weight of extruded sap (see above) from the initial FW of a shoot. Water potentials of freshly excised needles were also assessed by the Chardakov method using Suc standards (Slavik, 1974).
RESULTS
Paclobutrazol treatment caused significant reductions in the FW and SW water contents of needles from well-watered seedlings, but relative water contents of the treated and untreated needles were not significantly affected (Table I). Differences were also not observed in cross-sectional radii of water-saturated mesophyll cells (92 versus 93 μm) or in cell wall thicknesses (5.6 versus 5.2 μm) when sections from untreated control or paclobutrazol-treated needles were sectioned in water. However, Shear and Young's moduli (Eqs. 1 and 2), calculated from the bulk-pressure-volume curves and the average dimensions of mesophyll cells, were larger in cell walls from paclobutrazol-treated seedlings than in the untreated controls. Similar trends were obtained for the elastic coefficients, with large increases associated with reduced saturated volumes and lower TLV in the treated tissues (Table II).
Table I.
The effects of paclobutrazol, 4 weeks after treatment, on the water contents of fresh and artificially saturated needles from intermediately drought-tolerant white spruce seedlings growing in moist soil or after 12 d of drought
| Treatment | Water Content | ||
|---|---|---|---|
| % FW | % SW | % RWCa | |
| Untreated, watered | 73.6a | 75.8a | 89.1a |
| Untreated, 12 d of drought | 57.4c | 69.5b | 60.4b |
| Paclobutrazol, watered | 66.6b | 68.5b | 91.9a |
| Paclobutrazol, 12 d of drought | 67.2b | 68.9b | 92.5a |
Means followed by different letters within columns are significantly different at P ≤ 0.05.
RWC, Relative water content.
Table II.
Effects of paclobutrazol on the elasticity of saturated shoots from intermediately drought-tolerant white spruce seedlings 6 weeks after treatment
| Treatment | Water Content | ε | J | G | E | |
|---|---|---|---|---|---|---|
| % SW | % TLV | MPa | ||||
| Untreated | 74.1a | 66.0a | 14.5b | 3.3b | 80.7b | 440b |
| Paclobutrazol | 66.4b | 60.2b | 19.7a | 7.5a | 106.5a | 575a |
Seedlings were saturated for 24 h before measurement of the water content of the SW and TLV of the shoots. ε, Cell-volumetric-elastic coefficient; J, elastic coefficient calculated per unit cell wall material; G, Shear modulus; E, Young's modulus. Means followed by different letters within columns are significantly different at P ≤ 0.05.
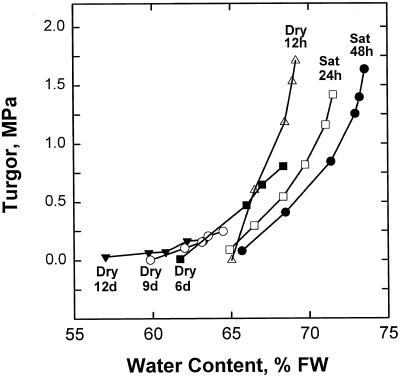
The positions and slopes of the pressure-volume curves showed marked changes as seedlings made the transition from saturation to severe water deficit (Fig. 1). After saturation for 24 or 48 h, the shoots reached higher water contents without concomitant increases in turgor compared with still-turgid plants that had not been watered for 12 h. Plants drought-stressed for 9 or 12 d approached zero turgor and TLV was sharply reduced (Fig. 1).
Figure 1.
Representative pressure-volume relations of intermediately drought-tolerant white spruce seedlings saturated for 24 h (□) or 48 h (•), turgid plants not watered for 12 h (▵), and plants not watered for 6 d (▪), 9 d (○), or 12 d (▾).
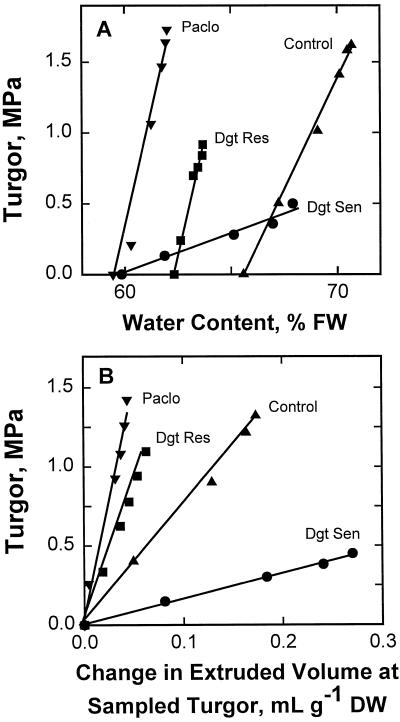
Relationships between turgor and water contents and turgor related to changes in volume of water extruded from drought-resistant versus drought-sensitive seedlings and from paclobutrazol-treated versus untreated control seedlings were also plotted (Fig. 2). Water contents ranged from those at plasmolysis to those at turgor points at the times of sampling. Paclobutrazol-treated and naturally drought-resistant seedlings had lower water contents at high turgor values and steeper pressure-volume curves than well-watered or drought-sensitive seedlings (Fig. 2). In contrast, the slopes and turgor values obtained from drought-sensitive seedlings were notably low, but their water contents were above those of the drought-resistant and paclobutrazol-treated plants (Fig. 2A; Table III). Moreover, the stressed, paclobutrazol-treated, and naturally resistant seedlings had large elastic coefficients and maintained high turgor values at water contents near or below those of the stressed, untreated and the stressed, sensitive seedlings, which had low turgor and showed visible wilt and sharply reduced elastic coefficient values (Table III).
Figure 2.
Relationships between turgor and water content (A) and between turgor and the change in extruded volumes (B) in intermediately drought-tolerant, well-watered, untreated controls (▴); intermediately drought-tolerant, well-watered, paclobutrazol-treated seedlings (▾); drought-stressed (12 d), naturally drought-resistant seedlings (▪); and drought-stressed (9 d), naturally drought-sensitive seedlings (•).
Table III.
Comparisons of intermediately tolerant and paclobutrazol-treated seedlings and drought-sensitive and drought-resistant seedlings of white spruce
| Treatment | Water Content | Ψw | Ψp | Ψs | ε | J |
|---|---|---|---|---|---|---|
| % FW | MPa | |||||
| Untreated, intermediately tolerant, watered | 70.8a | −0.79a | 1.71a | −2.50a | 19.7b | 6.0b |
| Paclobutrazol, intermediately tolerant, watered | 65.1c | −0.69a | 1.66a | −2.35a | 30.8a | 11.8a |
| Untreated, sensitive, 9 d of drought | 67.9b | −1.91c | 0.58c | −2.49a | 4.2c | 1.5c |
| Untreated, resistant, 12 d of drought | 65.7c | −1.30b | 0.98b | −2.28a | 28.0a | 10.1a |
| Untreated, intermediately tolerant, 15 d of drought | 64.5c | −2.26c | 0.22d | −2.48a | 2.9c | 1.1c |
| Paclobutrazol, intermediately tolerant, 15 d of drought | 64.4c | −1.98c | 0.94bc | −2.92b | 11.7bc | 15.2a |
Shoots from treated and untreated seedlings were not saturated with water before measurement of water content, water potential (Ψw), solute potential (Ψs), and turgor (Ψp), cell-volumetric-elastic coefficients (ε), and elastic coefficients per unit cell wall volume (J). Means followed by different letters within columns are significantly different at P ≤ 0.05.
The amounts of volumetric expansion and osmotic work required to attain a given level of turgor were sharply reduced in the paclobutrazol-treated and naturally resistant seedlings (Table IV). Well-watered, paclobutrazol-treated plants required only 59% as much water (in milliliters per 100 g of DW) to reach 1.0 MPa of turgor as the well-watered, untreated, randomly selected controls. However, after 15 d of drought the treated seedlings only required 21% as much water as the untreated plants, and the drought-stressed, naturally resistant seedlings required only 15% as much water as the drought-sensitive seedlings to reach 1.0 MPa of turgor. These differences in volumetric expansion far exceeded the small differences in TLV recorded for the drought-resistant versus the drought-sensitive seedlings or for the paclobutrazol-treated versus the untreated stressed or the well-watered seedlings (Table IV).
Table IV.
Comparisons of intermediately tolerant control and paclobutrazol-treated seedlings and drought-sensitive and drought-resistant seedlings of white spruce
| Treatment | Water Content at TLV | Volumetric Expansion from TLV to 1.0 MPa Turgor | Osmotic Work for 1.0 MPa Turgor | ||
|---|---|---|---|---|---|
| % FW | mL 100 g−1 DW | % FW | mL 100 g−1 | Nma100 mL−1 | |
| Untreated, intermediately tolerant, watered | 65.3a | 1.86a | 3.4c | 9.8c | 7.3c |
| Paclobutrazol, intermediately tolerant, watered | 61.4b | 1.60b | 2.1d | 5.8d | 4.3d |
| Untreated, sensitive, 9 d of drought | 59.3c | 1.46c | 18.6a | 45.3a | 34.0a |
| Untreated, resistant, 12 d of drought | 63.1b | 1.71b | 2.5cd | 6.9d | 5.1d |
| Untreated, intermediately tolerant, 15 d of drought | 58.4c | 1.41c | 23.3a | 64.4a | 48.3a |
| Paclobutrazol, intermediately tolerant, 15 d of drought | 59.5c | 1.50c | 5.3b | 13.7b | 10.3b |
Shoots from treated and untreated seedlings were not saturated with water before measurement of TLV and volumetric expansion as expressed by changes in water volume and osmotic work required to establish equal turgor. Means followed by different letters within columns are significantly different at P ≤ 0.05.
Newton-meters (Joules).
In a separate experiment Chardakov analysis for water potentials helped to confirm the reduction in water stress in paclobutrazol-treated tissues. Isolated needles from untreated, wilted versus treated, still-turgid seedlings had higher water contents (66.3% versus 64% FW, respectively), but the treated needles had less negative water potentials (−3.8 MPa versus −2.2 MPa, respectively). These paclobutrazol-induced changes in the water relations of the needles were closely associated with increased drought resistance and survival, as measured by rewatering 15 treated and 15 untreated drought-stressed seedlings. All paclobutrazol-treated seedlings survived 15 d of drought, in contrast to the untreated seedlings, of which only 13% survived.
DISCUSSION
Our results indicate that physiological adjustments in cell wall elasticity constitute an important component in the drought-resistance mechanism of white spruce seedlings. This conclusion is supported by several observations. Normal turgor values were maintained in paclobutrazol-treated seedlings despite water contents that were at or below the TLV of both saturated and well-watered untreated seedlings (Fig. 2A; Tables II and III). Drought tolerance in the naturally resistant and paclobutrazol-treated plants was characterized by the maintenance of turgor during water loss, by significant reductions in the amounts of osmotic work required to attain a given level of turgor in drought-tolerant plants (Tables III and IV), and by volumetric-elastic coefficients (Table III) that were consistently larger than values obtained from well-watered, untreated controls, and several times larger than values from untreated plants sensitive to drought (Table III).
Shear and Young's moduli obtained from the needles of water-saturated seedlings also indicated that the observed differences in bulk elasticities of the treated and untreated tissues were a function of the elastic quality of the cell walls and did not result from changes in cellular dimensions. It should be noted, however, that relaxation of tissues immersed in water for 24 h before pressure-volume analysis reduced the volumetric-elastic coeffient values, indicating that cell wall resilience was reduced and that adaptation to drought was partially reversed when water was available (Tables II and III). This observation indicates that the drought response, at least in white spruce, cannot be evaluated in resaturated seedlings without the introduction of artifacts.
The maintenance of turgor, increased elastic coefficient values, and reductions in TLV in drought-resistant seedlings were apparently not a function of stomatal control of water loss, osmotic acquisition of water via osmotic adjustment, or of large changes in water potential gradients within the shoots (Nonami and Boyer, 1989; Weisz et al., 1989). In previous work we showed that differences in initial transpiration rates between coniferous seedlings pretreated with paclobutrazol and untreated controls were less than 2% of maximum recorded values (Marshall et al., 1991). Moreover, the results in Table III show that, with the exception of the paclobutrazol-treated but severely stressed seedlings in which osmotic adjustment apparently occurred, solute potentials did not change significantly during the study. Turgor in treated and naturally resistant seedlings also remained high, despite water contents below those of untreated and drought-sensitive seedlings. In addition, stressed, drought-resistant seedlings also maintained significantly less negative water potentials than untreated, stressed seedlings as measured by the pressure chamber and Chardakov techniques. Nevertheless, our results do not impinge on the physiological significance of transpirational control of water loss during the onset of drought (Buxton et al., 1985; Marshall et al., 1991); rather, they simply describe another probable facet of the drought-response process.
During severe drought plants can moderate turgor loss by gas cavitation and the bulk transfer of water from the xylem vessels to the symplasm (Millburn and Johnson, 1966). In the current study, however, the reduction in water volumes of turgid shoots and excised needles of paclobutrazol-treated seedlings before any imposition of stress (Tables I and II), in addition to a similar reduction in untreated tissues during moderate water stress, which should not cause cavitation (Tyree et al., 1984), indicate that the prevention of wilt and the maintenance of turgor did not result from transfer of water from the xylem to the needles (Tyree and Dixon, 1986).
Reductions of about 7% in the SW and FW water volumes (Table I) in nonstressed, treated needles with cellular dimensions similar to untreated control needles might have resulted in part from an increase in cellular starch induced by paclobutrazol treatment (Upadhyaya et al., 1990). However, increased plastidial starch does not readily explain the exceptionally large differences (paclobutrazol-induced and natural) in the elastic moduli (Table III), in osmotic work, and in the amounts of volumetric expansion (Table IV) needed to achieve 1 MPa of turgor in the stress-resistant versus the more stress-sensitive seedlings. We conclude, therefore, that the induction of large elastic moduli in cell walls before and during the onset of drought may facilitate the transition to lower TLV and the maintenance of turgor, even as water content declines.
Under these conditions and in accordance with the classical cell-water-relations formula, water potentials would either stabilize or become less negative (Table III). During studies with Senecio spp., however, Salleo (1983) noted that even small losses of water from leaves that had naturally thick, rigid cell walls with inherently high cell-volumetric-elastic coefficient values caused large reductions in turgor and more negative leaf water potentials, thereby increasing the osmotic flow of water into the roots. By contrast, in Ziziphus mauritiana the drought response was characterized by osmotic adjustment, large increases in the elastic moduli of leaves, and significant amounts of cell shrinkage, as evidenced by a 20% increase in the ratio of dry to turgid leaf weights (Clifford et al., 1998). Drought also induced osmotic adjustment in three cultivars of sugarcane, but also decreased cell-volumetric elastic coefficient values and wall resilience, yielding nearly constant symplast volumes but only partial maintenance of turgor (Saliendra and Meinzer, 1991). Fan et al. (1994) noted a decline in cell-volumetric elastic coefficient values and cell wall resilience in two of three woody species during tests of the drought response.
Can these conflicting reports regarding the opposite effects of drought on the cell-volumetric-elastic coefficients of plant tissue be resolved? Data in Table III show that elastic coefficient values fell sharply in sensitive seedlings exposed to mild drought and in seedlings of intermediate tolerance exposed to severe drought. However, in seedlings with natural or paclobutrazol-induced drought resistance, much larger elastic coefficient values were recorded, and yet FW water contents either declined or remained stable. Some of the data from Fan et al. (1994) are consistent with these trends. They recorded a 12% increase in the cell-volumetric elastic coefficient in drought-resistant jack pine, but an 18% to 20% decrease in the cell-volumetric elastic coefficient in the less-resistant black spruce and flooded gum. Although Saliendra and Meinzer (1991) consistently noted decreases in elastic moduli during drought and mild stress of relatively desiccation-intolerant sugarcane (Ashton, 1956; Levitt, 1972), the largest decrease in wall resilience occurred in the least drought resistant of the three cultivars.
All of these observations suggest that large increases in the elastic moduli of cell walls accompanied by tightening of the walls around the protoplasts to maintain turgor may provide an effective and dynamic mechanism of desiccation tolerance. The mechanism we visualize would provide a tenable explanation for the rise in pressure potentials previously observed in carnation (Eze et al., 1986), cabbage (Levitt, 1986), and pine and spruce (Buxton et al., 1985) during periods of water loss. Although the factors that control the mechanism of cell wall adjustment are far from clear, Passioura's (1994) enzyme-mediated model of cell expansion, in which changes in turgor alter the distribution of slack and taut populations of microfibril-binding hemicelluloses, could be invoked to help explain our observed reductions in TLV and the maintenance of turgor during drought. Moreover, our evidence to date implicates the involvement of osmotically induced, tightly bound cell wall proteins (Marshall et al., 1992, 1993; Marshall, 1996), although loosely bound wall proteins may also function in the stress-adjustment process (Bozarth et al., 1987; Showalter, 1993).
ACKNOWLEDGMENTS
The authors would like to thank Dr. E. Blumwald for the generous use of his laboratory facilities at the University of Toronto to repeat and confirm some of these measurements. We are also grateful to Dr. J. Dainty for consultations and helpful comments and to Patricia Dumbroff for typing and editing the manuscript.
Abbreviations:
- DW
dry weight
- FW
fresh weight
- SW
saturated weight
- TLV
turgor-loss volume
Footnotes
J.G.M. was supported by graduate fellowships from Forestry Canada and the Natural Sciences and Engineering Research Council of Canada (NSERC). A research operating grant from NSERC to E.B.D. is also gratefully acknowledged.
Present address: Hebrew University Faculty of Agriculture, Rehovot, 76100 Israel.
LITERATURE CITED
- Ashton FM. Effects of a series of cycles of low and high soil water on the rate of apparent photosynthesis in sugarcane. Plant Physiol. 1956;31:266–274. doi: 10.1104/pp.31.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert HJ, Nelson DE, Jenses RG. Adaptions to environmental stresses. Plant Cell. 1995;7:1099–1111. doi: 10.1105/tpc.7.7.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth CS, Mullet JE, Boyer JS. Cell wall proteins at low water potentials. Plant Physiol. 1987;85:261–267. doi: 10.1104/pp.85.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray EA. Molecular responses to water deficit. Plant Physiol. 1993;103:1035–1040. doi: 10.1104/pp.103.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyer TC. On volumetric enlargement and work expenditure by an osmotic system in plants. Physiol Plant. 1952;5:459–469. [Google Scholar]
- Buxton GF, Cyr DR, Dumbroff EB, Webb DP. Physiological responses of three northern conifers to rapid and slow induction of moisture stress. Can J Bot. 1985;63:1171–1176. [Google Scholar]
- Chazen O, Neumann PM. Hydraulic signals from the roots and rapid cell-wall hardening in growing maize (Zea mays L.) leaves are primary responses to polyethylene glycol-induced water deficits. Plant Physiol. 1994;104:1385–1392. doi: 10.1104/pp.104.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford SC, Arndt SK, Corlett JE, Joshi S, Sankhla N, Popp M, Jones HG. The role of solute accumulation, osmotic adjustment and changes in cell wall elasticity in drought tolerance in Ziziphus mauritiana (Lamk.) J Exp Bot. 1998;49:967–977. [Google Scholar]
- Cosgrove DJ. In defense of the cell volumetric elastic modulus. Plant Cell Environ. 1988;11:67–69. [PubMed] [Google Scholar]
- Dainty J. Water relations of plant cells. In: Gottingen AP, Zimmermann MH, editors. Encyclopedia of Plant Physiology, Vol 2. New Series, Part A. Berlin: Springer-Verlag; 1976. pp. 12–35. [Google Scholar]
- Eze JMO, Mayak S, Thompson JE, Dumbroff EB. Senescence in cut carnation flowers: temporal and physiological relationships among water status, ethylene, abscisic acid and membrane permeability. Physiol Plant. 1986;68:323–328. [Google Scholar]
- Fan S, Blake TJ, Blumwald E. The relative contribution of elastic and osmotic adjustments to turgor maintenance of woody species. Physiol Plant. 1994;90:408–413. [Google Scholar]
- Forbes RD (1956) Forestry Handbook. Ronald Press, New York, section 21, p 10
- Kamiya N, Tazawa M, Takata T. The relation of turgor pressure to cell volume in Nitella with special reference to mechanical properties of the cell wall. Protoplasma. 1963;57:501–521. [Google Scholar]
- Kozlowski TT. Shrinking and swelling of plant tissues. In: Kozlowski TJ, editor. Water Deficits and Plant Growth, Vol III. Toronto, Canada: Academic Press; 1972. pp. 1–64. [Google Scholar]
- Levitt J (1972) Responses of Plants to Environmental Stresses. Academic Press, New York, pp 357, 419
- Levitt J. Recovery of turgor by wilted, excised cabbage leaves in the absence of water uptake. Plant Physiol. 1986;82:147–153. doi: 10.1104/pp.82.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JG (1996) Biophysical and biochemical mechanisms of dehydration resistance in three conifers. PhD thesis. University of Waterloo, Waterloo, Ontario, Canada
- Marshall JG, Ghosh S, Dumbroff EB. Turgor regulation in response to osmotic stress is mediated by cell-wall adjustment in the roots of jack pine (abstract no. 762) Plant Physiol. 1992;99:S-128. [Google Scholar]
- Marshall JG, Scarratt JB, Dumbroff EB. Induction of drought resistance by abscisic acid and paclobutrazol in jack pine. Tree Physiol. 1991;8:415–422. [Google Scholar]
- Marshall JG, Thatcher BJ, Rutledge RG, Dumbroff EB. Isolation and partial characterization of an osmotically-induced, low-molecular-weight cell-wall polypeptide (abstract no. 888) Plant Physiol. 1993;102:S-155. [Google Scholar]
- Milburn JA, Johnson RPC. The conduction of sap. II. Detection of vibrations produced by sap cavitation in Ricinus xylem. Planta. 1966;69:43–52. doi: 10.1007/BF00380209. [DOI] [PubMed] [Google Scholar]
- Murphy R, Ortega KE. A new pressure probe method to determine the average volumetric elastic modulus of cells in plant tissue. Plant Physiol. 1995;107:995–1005. doi: 10.1104/pp.107.3.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R, Smith JAC. Plant Cell Environ. 1994;17:15–29. [Google Scholar]
- Nabril M, Coudret A. Effects of sodium chloride on growth, tissue elasticity, and solute adjustment in two Acacia nilotica subspecies. Physiol Plant. 1995;93:217–224. [Google Scholar]
- Nonami H, Boyer JS. Turgor and growth at low water potentials. Plant Physiol. 1989;89:798–804. doi: 10.1104/pp.89.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passioura JB. The physical chemistry of the primary cell wall: implications for control of expansion rate. J Exp Bot. 1994;45:1675–1682. [Google Scholar]
- Phillip JR. Propagation of turgor and other properties through cell aggregations. Plant Physiol. 1958;33:271–274. doi: 10.1104/pp.33.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliendra NZ, Meinzer FC. Symplast volume, turgor, stomatal conductance and growth in relation to osmotic and elastic adjustment in droughted sugarcane. J Exp Bot. 1991;42:1251–1259. [Google Scholar]
- Salleo S. Water relations parameters of two Sicilian species of Senecio (groundsel) measured by the pressure bomb technique. New Phytol. 1983;95:179–188. [Google Scholar]
- Showalter AM. Structure and function of plant cell wall proteins. Plant Cell. 1993;5:9–23. doi: 10.1105/tpc.5.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavik B (1974) Ecological Studies, Analysis and Synthesis. Methods of Studying Plant Water Relations, Vol 9. Springer-Verlag, New York, pp 25–29
- Steudle E, Ferrier JM, Dainty J. Measurement of volumetric and transverse elastic extensibilities of Chara corallina internodes by combining the external force and pressure probe techniques. Can J Bot. 1982;60:1503–1511. [Google Scholar]
- Tyree MT, Dixon MA. Water stress induced cavitation and embolism in some woody plants. Physiol Plant. 1986;66:397–405. [Google Scholar]
- Tyree MT, Dixon MA, Tyree EL, Johnson R. Ultrasonic acoustic emissions from the sapwood of cedar and hemlock. An examination of three hypotheses concerning cavitation. Plant Physiol. 1984;75:988–992. doi: 10.1104/pp.75.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Hammel HT. The measurement of the turgor pressure and the water relations of plants by the pressure-bomb technique. J Exp Bot. 1972;23:267–282. [Google Scholar]
- Tyree MT, Jarvis PG. Water in tissues and cells. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, editors. Physiological Plant Ecology. II. Water Relations and Carbon Assimilation, Encyclopedia of Plant Physiology, New Series, Vol 12B. New York: Springer-Verlag; 1982. pp. 36–77. [Google Scholar]
- Upadhyaya A, Davis TD, Larsen MH, Walser RH, Sankhla N. Uniconizole-induced thermotolerance in soybean seedling root tissue. Physiol Plant. 1990;79:78–84. [Google Scholar]
- Weisz PR, Randall HC, Sinclair TR. Water relations of turgor recovery and restiffening of wilted cabbage leaves in the absence of water uptake. Plant Physiol. 1989;91:433–439. doi: 10.1104/pp.91.1.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HI, Spence RD, Sharpe PJH, Goeschl JD. Cell wall elasticity: A critique of the bulk elastic modulus approach and an analysis using polymer elastic principles. Plant Cell Environ. 1985;8:563–570. doi: 10.1111/j.1365-3040.1985.tb01694.x. [DOI] [PubMed] [Google Scholar]