Graphical abstract
The genes encoding the enzymes required for UDP-xylose and UDP-galactose synthesis in Trichomonas vaginalis have been identified and the products of the recombinant enzymes analysed.
Highlights
► Xylose and galactose are components of Trichomonas vaginalis glycans. ► T. vaginalis UDP-xylose synthase and UDP-galactose epimerase genes identified. ► Enzymes were expressed in recombinant form, purified and assayed.
Abbreviations: GalE, UDP-galactose-4′-epimerase; UDP-GlcA, UDP-glucuronic acid; UXS, UDP-xylose synthase
Keywords: UDP-xylose, UDP-galactose, Trichomonas vaginalis
Abstract
The presence of xylose and galactose residues in the structure of trichomonad lipoglycans was indicated by previous studies and the modification of any glycoconjugate with either monosaccharide requires the respective presence of the nucleotide sugars, UDP-xylose and UDP-galactose. Biosynthesis of UDP-xylose de novo is mediated by UDP-xylose synthase (UXS; UDP-glucuronic acid decarboxylase), which converts UDP-glucuronic acid to UDP-xylose, whereas UDP-galactose can be generated from UDP-glucose by UDP-galactose epimerases (GalE). Trichomonas vaginalis cDNAs, encoding proteins with homology to these enzymes from other eukaryotes, were isolated. The recombinant T. vaginalis UDP-xylose synthase and UDP-galactose epimerase were expressed in Escherichia coli and tested via high pressure liquid chromatography to demonstrate their enzymatic activities. Thereby, in this first report on enzymes involved in glycoconjugate biosynthesis in this organism, we demonstrate the existence of xylose and galactose synthesising pathways in T. vaginalis.
Trichomonas vaginalis is a parasitic flagellated protozoan which causes human trichomoniasis, one of the most common sexually transmitted diseases in humans. Despite its wide spread and high prevalence, with more than 200 million affected people and at least three million new cases per year in the USA [1,2], it has proven to be an underestimated disease. Indeed, an infection with T. vaginalis causes not only vaginitis, exocervicitis and urethritis, it is also implicated in miscarriages and occurrence of human immunodeficiency virus. Cytopathogenicity starts with the adhesion of the protozoan to the host cell and indeed glycoconjugates such as a lipoglycan covering its cell surface are important for the parasite's interaction with its host [3]. The structure of this lipoglycan [4,5], as well as of its protein-linked N-glycans [20], have been recently determined to contain monosaccharides such as xylose and galactose. In order to perform the relevant xylosylation and galactosylation reactions necessary for the biosynthesis of these glycan structures in vivo, the organism requires the relevant nucleotide sugars, UDP-xylose and UDP-galactose.
UDP-xylose is the product of a two-step conversion from UDP-glucose: first, dehydrogenation of UDP-glucose is catalyzed by UDP-glucose dehydrogenase (UGD, EC 1.1.1.22) thus forming UDP-glucuronic acid (UDP-GlcA). Then, UDP-glucuronic acid decarboxylase (UDP-xylose synthase; UXS, EC 4.1.1.35) acts on UDP-GlcA to form UDP-xylose [6]. Depending on the organism, UXS may be cytosolically or lumenally located. In plants, the biosynthesis of UDP-xylose by different UXS isoforms occurs both in the cytosol and in membrane-bound compartments [7,8]. Mammals and nematodes on the other hand express only one UXS, which is located in the golgi apparatus [9,10], whereas the fungus Cryptococcus expresses only one, probably cytosolic, form [11]. In bacteria such as Micromonospora echinospora and Sinorhizoboium meliloti UDP-xylose is also synthesised from UDP-glucuronic acid [12,13]. On the other hand, the de novo biosynthesis of UDP-galactose from UDP-glucose is mediated by the cytosolic UDP-galactose epimerase (GalE; EC 5.1.3.2); the relevant GalE genes have been identified from a number of organisms and in T. brucei GalE is essential for growth [14].
Considering that xylose and galactose are components of several Trichomonas glycoconjugates we expected that this organism possesses at least one UXS and one GalE gene. Homology searching of the T. vaginalis genome from the G3 strain [15] was performed and the annotation suggested the presence of two putative homologues of UDP-galactose epimerase (GalE1, TVAG_186740 and TVAG_101620) and one putative homologue of UDP-xylose synthase (TVAG_178290). In the case of the UDP-xylose synthase the relevant reading frame is predicted to encode a protein of 313 residues lacking a transmembrane domain; therefore, the T. vaginalis enzyme is proposed to be a cytosolic protein as is the case with the fungal and some plant isoforms. Using RNA extracted from T. vaginalis (C1 strain; ATCC 30001), the UXS and one of the GalE reading frames were isolated by two-step RT-PCR using the primer pairs Tv-UXS-for-NcoI (catgccatggtgagtacacctaccaagagtac) and Tv-UXS-rev-SacI (ccgagctctagtaacatttagaaaatgtttta) or Tv-GalE1-for-BamHI (cgcggatccatgtctatcctcatacaggc) and Tv-GalE1-rev-HindIII (cccaagcttttaagctctgtagccatttgg) prior to ligation into the pET30a vector. The recombinant proteins were expressed in Escherichia coli (DE3) pLysS Gold cells upon induction with isopropyl-β-d-thiogalactopyranoside at 25 or 37 °C for 3 h; cells were lysed and the recombinant proteins isolated by purification on Ni/nitrilotriacetate resin and elution with 250 mM imidazole. The purified forms of the recombinant proteins were analysed by SDS–PAGE and Western blotting and displayed molecular masses of ∼40 kDa (data not shown) in agreement with the size predicted from the amino acid sequences.
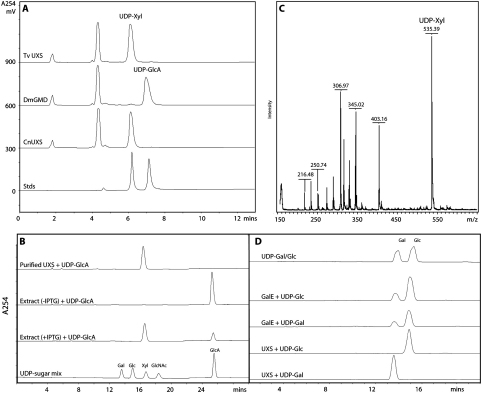
In the UXS sequence, two ‘silent’ nucleotide alterations were observed in the UXS cDNA cloned from the C1 strain; the amino acid sequence is thereby identical with that predicted from the genomic sequence available from the G3 strain (data not shown; Genbank/EBI accession number HE575670). The trichomonad UXS protein is 57% identical to the human sequence over 307 amino acids and 50% identical to predicted, but uncharacterised, proteins from Trypanosoma cruzi (XP_820252 and XP_806161). UDP-xylose synthase activity of UXS was verified by assaying with UDP-glucuronic acid (UDP-GlcA) as substrate and NAD+ as a cofactor. The negative control (with Drosophila melanogaster GDP-mannose dehydratase) displayed no conversion of UDP-GlcA to UDP-Xyl, whereas incubations with the positive control (UXS from Cryptococcus neoformans [11]) and the UXS from T. vaginalis showed the generation of a clear UDP-xylose peak as judged by either SAX (Fig. 1A) or RP-HPLC (Fig. 1B). Substrate conversion was dependent on incubation time and the amount of protein, whereas no product was formed after heat inactivation of the protein (data not shown). The UDP-xylose produced by the purified T. vaginalis enzyme was collected from a number of HPLC runs prior to analysis by MALDI-TOF MS and NMR. The m/z value of 535.4 for the [M−H]− molecular ion (Fig. 1C) is as expected, whereas the in-source fragment of 403.1 corresponds to loss of a pentose to yield UDP. Key chemical shifts in the 1H and 31P NMR spectra, as compared to literature data [16], confirm the identity of the UXS enzymatic product as UDP-xylose (Table 1).
Fig. 1.
Test of UDP-xylose synthase and UDP-galactose epimerase activities. UDP-xylose synthase (UXS) and UDP-galactose/glucose epimerase (GalE1) activities were assayed by incubating the enzyme (1 μl purified enzyme or 5 μl E. coli crude extract) in the presence of the relevant 3 mM UDP-sugar and 3 mM NAD+ in 80 mM Tris–Cl, pH 7.7 at 30 or 37 °C (final volume 50 μL). (A) SAX-HPLC of 4 h assays performed using UDP-glucuronic acid as substrate and lysates of bacteria expressing T. vaginalis UXS, Drosophila GMD (negative control) or Cryptococcus UXS (positive control). After injection onto a Hypersil column (0.5 × 25 cm), the column was washed for 10 min with buffer A (2 mM ammonium formate, pH 3.2; the flow rate was 1.5 ml/min) prior to elution with a linear gradient from 0 to 40% buffer B (600 mM ammonium formate, pH 3.2) as described [7]; absorbance at 254 nm was recorded. Standard UDP-xylose elutes at around 6 min as compared to the UDP-glucuronic acid at 7 min (Stds). The peak at 4.5 min corresponds to imidazole present in the lysis buffer. (B) Ion-pair RP-HPLC of assays of recombinant UXS in either purified form or in an E. coli extract (+IPTG) showing conversion of UDP-GlcA to UDP-Xyl. A control extract of E. coli transformed with the UXS plasmid but not induced (-IPTG) showed no such activity. Analysis was performed using a Cosmosil C18-AR-II column (250 mm × 4.6 mm; Nacalai Tesque, Kyoto, Japan): buffer A was 20 mM triethylamine-acetate (pH 7) and buffer B was 20 mM triethylamine-acetate (pH 7) containing 10% acetonitrile [19]. After isocratic elution with 100% buffer A, a gradient of 1% per minute (buffer B) was applied after 15 min. (C) Negative-mode MALDI-TOF MS of pooled UXS products; the m/z of UDP-xylose [M-H]− of 535.3 compares to the calculated molecular mass of 536.2. For analysis, 1 μl of an aliquot of UDP-Xyl was spotted onto a MALDI plate, vacuum dried prior to application of 1 μl 2,5-dihydroxybenzoic acid (DHB; 2% in 30% acetonitrile/70% 50 mM (NH4)2SO4); the dried and crystallized sample was analysed by MALDI-TOF MS using a Bruker Ultraflex instrument. (D) Ion-pair RP-HPLC of GalE1 assays; purified recombinant T. vaginalis GalE converted UDP-Gal partly into UDP-Glc and also UDP-Glc partly into UDP-Gal, whereas purified recombinant T. vaginalis UXS displayed no such activity.
Table 1.
NMR analysis of the UXS assay product. Chemical shifts (1H and 31P NMR) are expressed as ppm. Approximately 300 μg of HPLC-purified reaction product was lyophilised twice and taken up in 0.6 ml D2O. Spectra were recorded at 300 K at 600.18 MHz for 1H and at 242.96 MHz for 31P with a Bruker AV III 600 spectrometer. Data acquisition and processing were performed with the standard Bruker software. 1H spectra were referenced internally to sodium 2,2-dimethyl-2-silapentane-5-sulphonate and 31P spectra to phosphoric acid (δ = 0).
| H1 | H2 | H3 | H4 | H5, 5′ | H6 | α | β | |
|---|---|---|---|---|---|---|---|---|
| Xylose | 5.59 | 3.55 | 3.69 | 3.61 | 3.71 | |||
| Ribose | 6.01 | 4.41 | 4.44 | 4.27 | 4.24 | |||
| Uracil | 6.02 | 7.99 | ||||||
| Pyrophosphate | −11.28 (19.5 Hz) | −12.98 (21.4 Hz) |
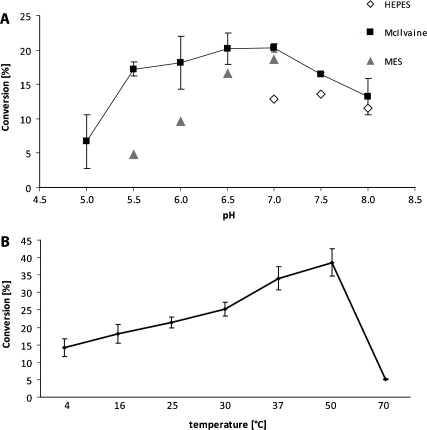
The purified UXS was examined further in terms of its pH and temperature optima. The enzyme exhibited a good activity over a broad pH range (5.5–8.0) with an optimum of around pH 7.0 (Fig. 2A). This optimum compares well to that for Cryptococcus UXS [6,11], Sinorhizobium UXS [12] and endomembrane-associated Arabidopsis UXS2 [17], but is higher than that for the plant UXS3 [7]. The activity of the enzyme was not significantly affected by either MgCl2 or MnCl2 (data not shown). The expressed protein was active at temperatures from 4 to 50 °C whereas its activity was nearly abolished at 70 °C (Fig. 2B) and the highest activity could be achieved at 37 to 50 °C, consistent with efficient function within a mammalian host; a similar temperature optimum has been observed for Arabidopsis UXS2 [17]. Incubating UDP-xylose synthase for 1 h, in the absence of substrate or cofactor, at various temperatures indicated that the enzyme is quite stable between 4 and 30 °C, whereas its stability decreases with higher temperatures (data not shown).
Fig. 2.
Enzymatic characteristics of purified recombinant T. vaginalis UXS. A. The variation of UDP-xylose yield with pH was examined by incubations in the presence of McIlvaine buffer (black squares, pH 5.0–8.0; n = 2), HEPES buffer (open rhombi, 7.0–8.0) and MES buffer (grey triangles, 5.5–7.0) for 2 h at 30 °C. (B) The dependency of UXS reaction on temperature was based on assays (n = 3) performed at pH 7.4 for 2 h; the error bars correspond to the standard deviation.
In the case of UDP-galactose epimerase, the two identified (TVAG_186740 and TVAG_101620) open reading frames predicted protein sequences of 340 residues with 92% identity. Therefore we decided to clone only one of these cDNAs and designated the reading frame TVAG_186740 as GalE1. One consistent nucleotide alteration in three cDNA clones suggests that the GalE1 from the C1 strain differs in one non-conserved amino acid as compared to the G3 strain (residue 98 is Glu rather than Lys). The trichomonad GalE1 is 57% identical to the 335 amino acids long human homologue and 38% to the characterised T. cruzi enzyme (AJ577814). Incubations to test epimerase activity were analysed using the same ion-pair RP-HPLC system as for the UDP-xylose synthase assays; it was found that T. vaginalis GalE1 could convert UDP-glucose into UDP-galactose and vice versa with final ratios of either 22:78 or 24:76 UDP-Gal:UDP-Glc (Fig. 2D), suggesting that the equilibrium favours the formation of UDP-Glc. A similar ratio of 1:3 UDP-Gal:UDP-Glc was found when using Saccharomyces fragilis as a source of epimerase activity [18]. A control experiment with the purified T. vaginalis UXS indicated no such conversion, verifying that no UDP-galactose epimerase activity from the E. coli host was present in fractions eluted from the Ni(II)-chelation column.
In conclusion, we show that recombinant forms of T. vaginalis UDP-xylose synthetase (UXS) and UDP-galactose epimerase (GalE1) are indeed enzymatically active and so are the first glycosylation-related enzymes from this organism to be characterised; furthermore, this UXS is the first to be studied from a unicellular parasite.
Acknowledgements
We thank Julia Walochnik (Medizinische Universität Wien) for providing T. vaginalis RNA, Ebrahim Razzazi-Fazeli (Veterinärmedizinische Universität Wien) for access to the mass spectrometer, Katharina Paschinger for aiding revision of the text and Tamara Doering (Washington University, St Louis) for the kind gift of the C. neoformans UXS plasmid. This work was funded by the Austrian Fonds zur Förderung der wissenschaftlichen Forschung [P20565 to I.B.H.W.].
References
- 1.Fiori P.L., Rappelli P., Addis M.F. The flagellated parasite Trichomonas vaginalis: new insights into cytopathogenicity mechanisms. Microbes Infect. 1999;1:149–156. doi: 10.1016/s1286-4579(99)80006-9. [DOI] [PubMed] [Google Scholar]
- 2.Schwebke J.R., Burgess D. Trichomoniasis. Clin Microbiol Rev. 2004;17:794–803. doi: 10.1128/CMR.17.4.794-803.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastida-Corcuera F.D., Okumura C.Y., Colocoussi A. Trichomonas vaginalis lipophosphoglycan mutants have reduced adherence and cytotoxicity to human ectocervical cells. Eukaryot Cell. 2005;4:1951–1958. doi: 10.1128/EC.4.11.1951-1958.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh B.N., Hayes G.R., Lucas J.J. Structural details and composition of Trichomonas vaginalis lipophosphoglycan in relevance to the epithelial immune function. Glycoconj J. 2009;26:3–17. doi: 10.1007/s10719-008-9157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan C.M., Mehlert A., Richardson J.M. Chemical structure of Trichomonas vaginalis surface lipoglycan: a role for short galactose (β1-4/3) N-acetylglucosamine repeats in host cell interaction. J Biol Chem. 2011 doi: 10.1074/jbc.M111.280578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ankel H., Feingold D.S. Biosynthesis of uridine diphosphate d-xylose. II. Uridine diphosphate d-glucuronate carboxy-lyase of Cryptococcus laurentii. Biochemistry. 1966;5:182–189. doi: 10.1021/bi00865a024. [DOI] [PubMed] [Google Scholar]
- 7.Harper A.D., Bar-Peled M. Biosynthesis of UDP-Xylose. Cloning and characterization of a novel Arabidopsis gene family, UXS, encoding soluble and putative membrane-bound UDP-glucuronic acid decarboxylase isoforms. Plant Physiol. 2002;130:2188–2198. doi: 10.1104/pp.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki K., Watanabe K., Masumura T. Characterization of soluble and putative membrane-bound UDP-glucuronic acid decarboxylase (OsUXS) isoforms in rice. Arch Biochem Biophys. 2004;431:169–177. doi: 10.1016/j.abb.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 9.Moriarity J.L., Hurt K.J., Resnick A.C. UDP-glucuronate decarboxylase, a key enzyme in proteoglycan synthesis. Cloning, characterisation and localisation. J Biol Chem. 2002;277:16968–16975. doi: 10.1074/jbc.M109316200. [DOI] [PubMed] [Google Scholar]
- 10.Hwang H.-Y., Horvitz H.R. The SQV-1 UDP-glucuronic acid decarboxylase and the SQV-7 nucleotide-sugar transporter may act in the golgi apparatus to affect Caenorhabditis elegans vulval morphogenesis and embryonic development. Proc Natl Acad Sci USA. 2002;99:14218–14223. doi: 10.1073/pnas.172522199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bar-Peled M., Griffith C.L., Doering T.L. Functional cloning and characterisation of a UDP-glucuronic acid decarboxylase: the pathogenic fungus Cryptococcus neoformans elucidates UDP-xylose synthesis. Proc Natl Acad Sci USA. 2001;98:12003–12008. doi: 10.1073/pnas.211229198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu X., Lee S.G., Bar-Peled M. Biosynthesis of UDP-xylose and UDP-arabinose in Sinorhizobium meliloti 1021: first characterization of a bacterial UDP-xylose synthase, and UDP-xylose 4-epimerase. Microbiology. 2011;157:260–269. doi: 10.1099/mic.0.040758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simkhada D., Oh T.J., Pageni B.B. Characterization of CalS9 in the biosynthesis of UDP-xylose and the production of xylosyl-attached hybrid compound. Appl Microbiol Biotechnol. 2009;83:885–895. doi: 10.1007/s00253-009-1941-8. [DOI] [PubMed] [Google Scholar]
- 14.Roper J.R., Guther M.L., Macrae J.I. The suppression of galactose metabolism in procylic form Trypanosoma brucei causes cessation of cell growth and alters procyclin glycoprotein structure and copy number. J Biol Chem. 2005;280:19728–19736. doi: 10.1074/jbc.M502370200. [DOI] [PubMed] [Google Scholar]
- 15.Carlton J.M., Hirt R.P., Silva J.C. Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science. 2007;315:207–212. doi: 10.1126/science.1132894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ernst C., Klaffke W. Chemical synthesis of uridine diphospho-d-xylose and UDP-l-arabinose. J Org Chem. 2003;68:5780–5783. doi: 10.1021/jo034379u. [DOI] [PubMed] [Google Scholar]
- 17.Pattathil S., Harper A.D., Bar-Peled M. Biosynthesis of UDP-xylose: characterization of membrane-bound AtUxs2. Planta. 2005;221:538–548. doi: 10.1007/s00425-004-1471-7. [DOI] [PubMed] [Google Scholar]
- 18.Leloir L.F. The enzymatic transformation of uridine diphosphate glucose into a galactose derivative. Arch Biochem Biophys. 1951;33:186–190. doi: 10.1016/0003-9861(51)90096-3. [DOI] [PubMed] [Google Scholar]
- 19.Oka T., Jigami Y. Reconstruction of de novo pathway for synthesis of UDP-glucuronic acid and UDP-xylose from intrinsic UDP-glucose in Saccharomyces cerevisiae. FEBS J. 2006;273:2645–2657. doi: 10.1111/j.1742-4658.2006.05281.x. [DOI] [PubMed] [Google Scholar]
- 20.Paschinger K, Hykollari A, Razzazi-Fazeli E, Greenwell P, Leitsch D, Walochnik J, Wilson IBH. The N-glycans of Trichomonas vaginalis contain variable core and antennal modifications. Glycobiology 2011, in press, doi:10.1093/glycob/cwr149. [DOI] [PMC free article] [PubMed]