Abstract
Background and Purpose
Greater gait variability has been observed in persons with Down syndrome (DS). An understanding of baseline patterns of variability, how these patterns relate to adaptive control of gait, and whether increasing or decreasing variability is better, is necessary for physical therapists to determine if and when to intervene. Our aim was to describe patterns of gait variability across the lifespan in persons with DS.
Methods
We examined differences in patterns of gait variability in new walkers, preadolescents, and adults with DS and typical development (TD). We collected kinematic data while participants walked on a treadmill, and analyzed the data using the nonlinear measures of Lyapunov Exponent (LyE) and Approximate Entropy (ApEn).
Results/Discussion
Beyond the greater gait variability demonstrated across the lifespan in persons with DS compared to their peers with TD, we report herein significant differences in nonlinear measures of patterns of variability. Preadolescents demonstrated higher LyE and ApEn values compared to new walkers and adults, suggesting they are more adaptive in their use of variability during gait.
Clinical Interpretation/Conclusion
From a clinical perspective, our results suggest that it may be of value to focus interventions on increasing adaptive use of variability during gait in new walkers and adults with DS. Experience with increased variability through practice under variable conditions or with perturbations may improve adaptive use of variability during gait.
INTRODUCTION
People often demonstrate increased amount of variability in movement trajectories with aging.1–7 Consequently, a decrease in the amount of variability, whether it be gait variability, finger force variability, or finger movement variability, are all cited as positive outcomes of rehabilitation interventions for older adults.8–11 Although decreasing variability toward levels similar to younger persons can have a positive effect, a full understanding of how variability relates to the control of movement is still being discovered. Recently, scientists and clinicians have recognized that both the amount and pattern of variability observed within movement trajectories over time can affect control of movement. Impaired movement patterns can contain too much or too little variability; in addition to the amount of variability, the patterns of variability may contribute to increased or decreased adaptive control of movement.12–15 Thus, both amount and pattern of variability should be considered when attempting to understand adaptive control of movement.
Down syndrome (DS) is one example of a population often described as demonstrating greater variability. Across the lifespan, persons with DS demonstrate more variability in movement trajectories compared to their peers with typical development (TD).16–19 Persons with DS differ from persons with TD in some neurophysiologic and musculoskeletal characteristics, including hypotonia, high ligamentous laxity, and reduced capacity to produce muscle force. We believe these conditions increase the challenge of dynamic upright posture, especially in the earlier and later stages of life, leading to the emergence of not only more variable but also unique gait patterns.16–18,20 Although persons with DS demonstrate higher amounts of variability in movements like walking and gripping compared to their peers with TD, some research suggested that they use this variability functionally, to compensate for their biomechanical instability, thus this variability optimizes control of their movement.21,22 If this is true, then it may be inadvisable for physical therapists to intervene with the intention of decreasing the amount of variability in functional behaviors. One way to investigate this is to study the patterns of variability within the higher amount of variability, and identify the relationship between the patterns and adaptive control of movement.
Current literature suggests that physical therapists should have their patients practice with increased or decreased amounts of movement variability, as needed, to help them learn adaptive use of variability.13–15 Choices about training strategies are difficult without an understanding of the baseline amount and pattern of variability within a particular population, and how these relate to functional control of movement. Adults with DS demonstrate higher amounts of variability in gait and are more likely to have a history of falls than their peers with TD.23 Although greater gait variability is related to increased likelihood of falls and mobility disability in older adults with TD,24–27 it is not clear whether a causal link exists. Although adults with DS demonstrate greater gait variability, younger persons with DS also have high amounts of variability. It is possible that adults have amounts and patterns of gait variability similar to preadolescents, and that other factors contribute to the greater falls in adults. Alternatively, it is possible that adults have experienced changes in their ability to adaptively use variability during gait, and these changes negatively affect their gait patterns and perhaps link to an increased likelihood of falls.
Our study aimed to expand our knowledge of increased amounts of gait variability in persons with DS by describing patterns of gait variability across the lifespan. Further, we interpreted our findings in relation to the decisions physical therapists must make regarding efforts to affect gait variability in this population. We examined changes in patterns of walking variability across the lifespan in persons with and without DS using the nonlinear analyses. We used Lyapunov Exponent (LyE) to quantify the local stability (overlap or dispersion) of trajectories of knee movement from one stride to the next. We used Approximate Entropy (ApEn) to quantify the regularity of the patterns observed in size of successive step widths and step lengths. Previous work has shown that 8- to 10-year-old children with DS had higher LyE and ApEn values indicating less local stability and less regularity in their patterns of lower extremity segmental angles during walking compared to their peers with TD.19 We hypothesized that because preadolescents are at their performance peak in terms of skill and efficiency, new walkers and adults with DS would show less locally stable, less regular trajectories of movement (larger LyE and ApEn values) than preadolescents with DS. Further, due to the inherent group differences in body structure and function, we predicted that persons with DS would demonstrate less locally stable, less regular trajectories of movement (larger LyE and ApEn values) across the lifespan, compared to their peers with TD.
METHODS
Data Collection
Participants with DS and TD, representing three developmental levels: new walkers, preadolescence, and adulthood, came to the Developmental Neuromotor Control Laboratory at the University of Michigan (total n= 58; Table 1). Participants were recruited through various community activity and support groups in Michigan and Northern Ohio; all participated in adequately-powered studies with similar protocols in which gait measures (but not nonlinear measures) were the primary dependent variables. The University of Michigan Institutional Review Board approved all procedures. Prior to participation, we explained our study to participants and caregivers. Participants signed an assent or consent form as appropriate, with consent for assenting adults and children provided by legal guardians. Toddlers wore diapers covered by black tights. Preadolescents and adults wore bathing suits or close-fitting shorts and tank tops. We attached markers (2.5 cm diameter) bilaterally at the temporomandibular joint, acromion process, lateral humeral epicondyle, styloid process, greater trochanter, femoral condyle, 10 cm above lateral malleolus, heel bony prominence and third metatarsophalangeal joint. We used a 6-camera Vicon Peak Motus (Vicon Peak Performance, Centennial, CO, USA) real-time system to collect 3-dimensional reflective marker position data at a sampling rate of 60 Hz.
Table 1.
Age ranges and number of participants per group
| Group | Age Range | N (total = 58) |
|---|---|---|
| New walkers (3 mo. experience) | DS =17.5–46.5 mo. | 9 |
| TD = 13–22.5 mo. | 9 | |
| Preadolescents | DS = 8–10 yrs. | 8 |
| TD = 8–10 yrs. | 8 | |
| Adults | DS = 35–62 yrs. | 12 |
| TD = 35–54 yrs. | 12 |
DS = Down syndrome, TD = typical development.
Participants walked barefoot over a 5.3-m GAITRite mat (CIR Systems Inc, Havertown, PA, USA) 4–6 times at their preferred speed. We used GAITRite software to calculate average walking speed for each participant and subsequently determine belt speeds for trials on a treadmill (Parker Treadmill Company, Auburn, AL, USA) Based on previous work in our lab,16,17 we operationally defined comfortable treadmill speed for all participants as 75% of their self-selected overground speed. Comfortable walking speeds on a treadmill are slower than overground walking speeds,28 and participants with DS are often cognitively unable to select their most comfortable speed in the novel context of the treadmill. Participants performed two 30-second trials each at 45%, 75%, and 110% of their overground walking speed; trials progressed from slow to fast speeds. All participants walked without touching the handrail and were guarded closely as they walked. Here we present results from the 75% speed only.
We used a Healthometer scale (Precision Weighing Balances, Bradford, MA, USA) to obtain body weight and an anthropometer (Siber Hegner and Co., Zurich, Switzerland) to record height and body segment lengths. To assess motor task performance and developmental levels, we used age-appropriate instruments: the motor component of the Bayley Scales of Infant Development (The Psychological Corporation, San Antonio, TX; new walkers); the 8-item balance subtest of the Bruininks-Oseretsky Test of Motor Proficiency (American Guidance Service, Circle Pines, MN; preadolescents) and Berg Balance Scale29,30 (adults).
Data Analysis: Theory and Definitions
Stability, regularity, and adaptability of gait can be defined in multiple ways. Herein, we use the term stability in reference to the LyE values, which quantify the local stability (overlap or dispersion) of trajectories of movement from one repetition to the next. We use the term regularity in reference to the ApEn values, which quantify patterns observed in size of successive step widths and step lengths. We define the most adaptable gait patterns as those that are mid-range (although not necessarily the middle) on the continuum of LyE and ApEn values.13,14,31 Mid-range LyE values are considered adaptive as they represent patterns of variability that are neither too stable (i.e., rigid) or too unstable, while mid-range ApEn values are considered adaptive as they represent patterns of variability that are neither too regular (i.e., rigid) or too irregular.13,14
Previous publications have described nonlinear measures in detail, and discussed their associated clinical applications.13,32,33 Briefly, LyE measures the divergence within the trajectories of entire movement cycles, such as walking strides, by quantifying their exponential separation in state space. We used LyE to measure the divergence in the trajectory of the knee joint marker from one stride to the next. An example of how LyE was calculated from data related to knee marker displacement in the vertical direction is given in Figure 1. Larger values (closer to 0.5) indicate more dispersion, possible randomness, and less similarity between the trajectories of successive walking strides. Smaller values (closer to 0) indicate less divergence, possible rigidity, and more similarity between the trajectories of successive walking strides.34 ApEn quantifies the regularity of the pattern within a time series. ApEn values exist on a continuum of 0 (completely regular pattern) to 2 (completely irregular, absence of pattern).34 A long stride alternating consistently with a short stride represents a more regular pattern than a random series of unique stride lengths, although both behaviors would be recognizable as a cyclic pattern of walking with similar values for mean and range of stride length as calculated using linear measures.
Figure 1.
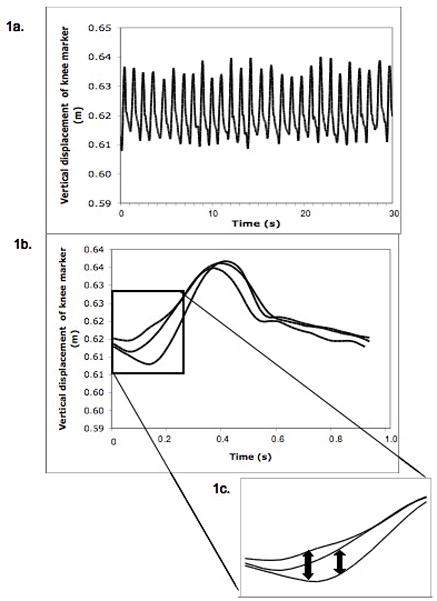
Lyapunov Exponent (LyE) calculation visual analogy, using data from consecutive stride cycles of the knee marker of an adult participant with Down syndrome (DS). Knee marker vertical position time series (1a). Three strides extracted from the time series and overlaid (1b). Magnified version of an isolated segment of the state space illustrating divergence between neighboring trajectories (1c).
It is important to note that LyE calculations are based on continuous kinematic data (eg, the knee marker trajectory throughout the stride), while ApEn calculations are based on discrete spatial-temporal variables (eg, step length and step width). We selected these nonlinear analyses deliberately; LyE allowed us to assess the stability of the knee trajectory across continuous successive strides, while ApEn calculations allowed us to assess the regularity in step length and width from one step to the next. These are specific gait characteristics often reported for typical and atypical populations.
Data Analysis: Procedures
For the LyE analyses, we identified a marker to represent the cyclical motion of each stride through space. Our pilot analyses showed that the knee marker provided cleaner and more clearly cyclic data than the hip, ankle, heel, or toe markers. We analyzed only the anterior-posterior and vertical direction time series of the left knee data because lateral motion of the knee is not a significant contributor to stride dynamics during walking. We analyzed displacement of the marker, as opposed to joint angles or acceleration or other calculated variables because we measured displacement directly. We feel that using a direct measurement as the basis for nonlinear calculations is particularly important to minimize error due to the nature of the calculation.
Time series lengths for LyE calculation were 276 points for new walkers and 1800 points for preadolescents and adults. For toddlers, these points reflect 7 or 8 strides, the maximum number they can produce continuously on a treadmill at this stage of motor development. For preadolescents and adults, the points represent approximately 24–39 strides (see Smith and colleagues35 for examples of time series and toe, knee, and hip time series and discussion of application of LyE to short new walker data sets). Time series lengths for ApEn calculation were 48–78 steps for preadolescents and adults, and 14–16 continuous steps for new walkers. Once all data sets were cropped (as necessary) to the correct length, we extracted knee and heel marker data and calculated step width, step length and stride length. Group means of the gait parameters for the walking strides used to calculate LyE and ApEn are provided in Table 2.
Table 2.
Gait parameters during treadmill walking group means (standard deviations)
| New Walker | Preadolescent | Adult | ||||
|---|---|---|---|---|---|---|
| DS | TD | DS | TD | DS | TD | |
| Stride length (m) | 0.38(0.04) | 0.35(0.05) | 0.68(0.08) | 0.91(0.04) | 0.62(0.04) | 1.03(0.03) |
| Step width (m) | 0.18(0.03) | 0.13(0.03) | 0.14(0.03) | 0.08(0.03) | 0.14(0.02) | 0.09(0.02) |
| Treadmill Speed (m/s) | 0.52(0.09) | 0.52(0.10) | 0.75(0.06) | 0.82(0.10) | 0.54(0.17) | 0.75(0.16) |
DS = Down syndrome, TD = typical development.
We subsequently determined the parameters and tested assumptions necessary for LyE and ApEn calculations. We calculated the necessary parameters, time delay and embedding dimension, using Tools for Dynamics software (Applied Nonlinear Sciences, LLC. Del Mar, CA, USA). Briefly, time delay is an estimation of the first minimum of mutual average information and embedding dimension is the minimum number of variables required to form a valid state space from a given time series.34 We found an average time delay of 3 for all data and embedding dimensions of 8 for toddlers’ knee time series, and 5 for preadolescents and adults. The larger value for toddlers reflects the greater ‘noise’ present in their movements.35 We tested our data for deterministic structure (mathematically defined as a non-random structure) using a surrogate data comparison method and Chaos Data Analyzer (CDA) software Professional Version (Physics Academic Software, American Institute of Physics. New York, New York, USA). We found no significant differences between LyE values for the original and surrogate anterior-posterior direction new walker LyE data. This indicated that the data, while collected during walking, were not mathematically definable as having a periodic structure, again reflecting the increased ‘noise’ present in toddlers’ movements.35 For this reason, these data were excluded from further analysis. Finally, we calculated LyE and ApEn. We used CDA software to calculate LyE data and custom MATLab (Mathworks Inc., Natick, MA, USA) programs for ApEn values. We calculated ApEn for the successive step lengths or widths using ApEn input parameters of m = 2 and r = 0.2.34
Statistical Methods
Statistics were calculated using SPSS software (Version 17.0; SPSS Inc., Chicago, IL, USA). Significance was accepted at α < 0.05. In most cases, we used 2 (group) by 3 (age) ANOVA full factorial models with Bonferroni corrections and follow-up tests for linear and quadratic trends. For the anterior-posterior LyE data, because new walker data could not be included, we used a 2 (group) by 2 (age) ANOVA with Bonferroni corrections and follow-up tests for linear trends only, as a quadratic trend is not possible with only two data points. We used linear and quadratic trend tests within the ANOVA to assess the shape of change across the lifespan, using one test to examine the trend across the groups with DS and another to examine the groups with TD. A linear trend would indicate increase or decrease in the measure across the lifespan, while a quadratic trend would indicate a “U” or inverted “U” shape across the lifespan. It is also possible to have significant linear and quadratic trends simultaneously. In this case, it means the data increase or decrease greatly and then flatten out, so that the “U” or inverted “U” (quadratic trend) is significant and the data also show a significant overall increase or decrease over time (linear trend).
RESULTS
LyE: Local Stability of Limb Trajectories Across Successive Strides
To test for differences in stability of knee trajectories in the vertical direction across strides, we used a 2 (group) by 3 (age) ANOVA with vertical direction LyE values as the dependent variable. The age effect was significant (F[2, 46] = 17.53, p < 0.01), while the group effect and group-by-age interaction were not (see Figure 2a).
Figure 2.
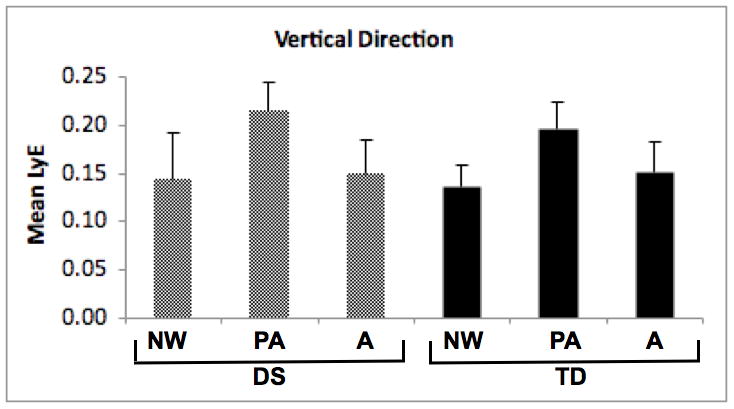
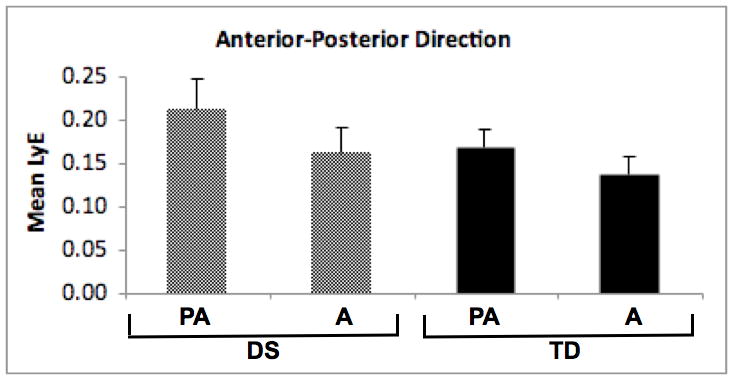
Vertical (2a) and anterior-posterior (2b) direction Lyapunov Exponent (LyE) values for participants with Down syndrome (DS) and typical development (TD). NW = new walkers, PA = preadolescents, A = adults.
For follow-up analysis, we tested for linear and quadratic trends across the three age groups within the DS and TD groups. For the DS group, the quadratic trend test was significant (p < 0.01) while the linear trend test was not. Pairwise comparisons revealed that preadolescents had higher vertical direction LyE values than new walkers or adults (p < 0.01 for all). For the TD group, the quadratic trend test was again significant (p < 0.01) while the linear trend test was not. Follow-up pairwise comparisons revealed that preadolescents had higher vertical direction LyE values than new walkers and adults (p < 0.01 for all).
We used a similar a 2 (group) by 2 (age) ANOVA for differences in LyE values in the anterior-posterior direction. This analysis did not include the new walkers, whose anterior-posterior direction data failed surrogation analysis. The group effect was significant (F[1, 34] = 14.44, p < 0.01), as was the age effect (F[1, 34] = 18.92, p < 0.01). The group-by-age interaction was not significant (see Figure 2b). Inspection of means for the group effect revealed higher LyE values in the anterior-posterior direction for the group with DS, while the age effect showed higher LyE values in preadolescents compared to adults.
For follow-up analysis, we tested for a linear trend across the preadolescent and adult groups within the DS and TD groups. We found a significant linear trend (p = 0.01) for the DS group, reflecting lower anterior-posterior LyE values in adults than preadolescents. Follow-up in the TD group also revealed a significant linear trend (p = 0.04), again reflecting lower anterior-posterior LyE values in adults than preadolescents.
ApEn: Regularity of Pattern of Successive Step Lengths and Widths
To test for differences in step length ApEn values, we used a 2 (group) by 3 (age) ANOVA. The age effect was significant (F[2, 49] = 9.37, p < 0.01), while the group effect and group-by-age interaction were not (see Figure 3a). Inspection of means revealed an inverted “U” shape with highest values in preadolescents.
Figure 3.
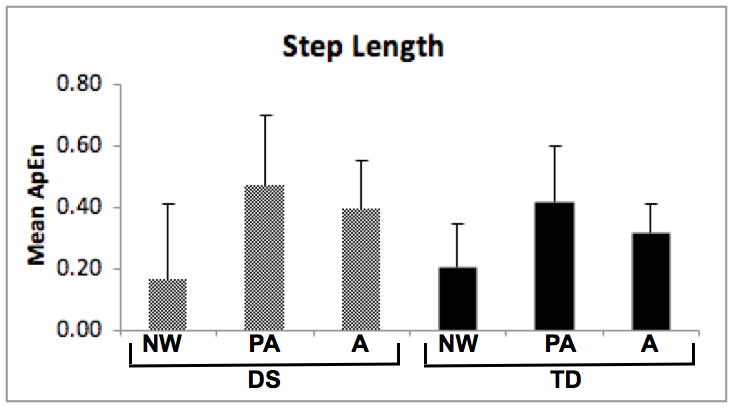
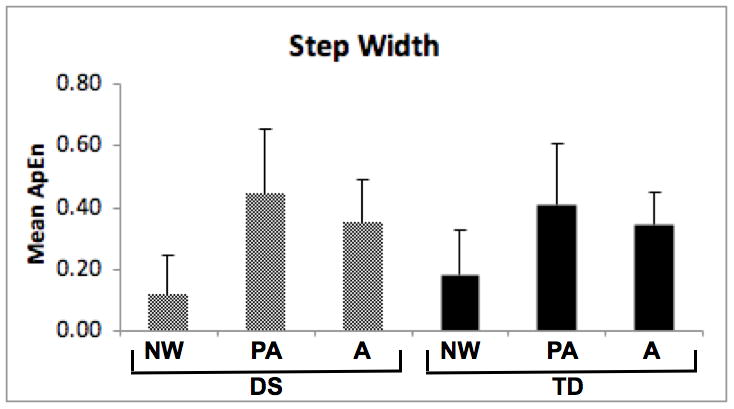
Approximate Entropy (ApEn) of step length (3a) and step width (3b) for participants with Down syndrome (DS) and typical development (TD). NW = new walkers, PA = preadolescents, A = adults.
For follow-up analysis of step length data, we tested for linear and quadratic trends across the three age groups within the DS and TD groups. The DS group showed significant linear (p = 0.03) and quadratic (p = 0.05) trends. Pairwise comparisons revealed that the DS new walkers had significantly smaller ApEn step length values than the DS preadolescent (p = 0.01) or adults (p = 0.01) while the quadratic trend indicated higher values in the preadolescents compared to both younger and older participants. For the TD group, the quadratic trend was significant (p = 0.02) and the linear trend was not, indicating higher values in preadolescents and lower values in older and younger participants.
To test for differences in ApEn of step width values, we used a 2 (group) by 3 (age) ANOVA. The age effect was significant (F[2, 49] = 15.23, p < 0.01), while the group effect and group-by-age interaction were not (see Figure 3b). Inspection of the means revealed an inverted “U” shape with highest values in preadolescents.
For follow-up analysis of step width data, we tested for linear and quadratic trends across the three age groups within the DS and TD groups. For the DS group, follow-up analyses showed significant linear and quadratic trends (p < 0.01 for both) indicating higher ApEn values for preadolescents, lower values for adults and lowest values for new walkers. We obtained a significant quadratic trend (p = 0.01) but not linear trend for the TD group, demonstrating higher ApEn values for preadolescents and lower values for adults and new walkers.
DISCUSSION
Beyond the greater gait variability demonstrated across the lifespan in persons with DS compared to their peers with TD, we show here that persons with DS also experience an inverted “U”-shaped developmental trajectory in their patterns of variability across age groups. Overall, our results suggest that preadolescents may be more adaptable in their gait patterns compared to new walkers or adults (aged 35 years and beyond), as indicated by higher LyE values (ie, less stability) and higher ApEn values (ie, less regularity). That is, at either end of their years of walking experience their patterns of variability are relatively more stable and regular, potentially rendering their gait less adaptable to changes in task or environmental conditions.
Preadolescents with DS demonstrated closer to optimal walking performance compared to their younger and older peers with DS; they were able to produce more continuous walking strides than new walkers and preferred to walk faster than adults (see Table 2). Previous researchers have described preadolescents with DS as being in one of the most consistent periods in their lives as they have had at least 6 years of walking practice accompanied by steady physical growth.17 Because preadolescents demonstrate closer to optimal walking performance than their younger and older peers, our interpretation of the values obtained is that their LyE and ApEn values are also at their peak and that they have learned to use their variability to adapt as well as possible during locomotion.
From a clinical perspective, these results suggest that physical therapists may be able to intervene to improve gait performance, specifically patterns of variability related to stability and regularity, in new walkers and adults with DS. The strategy might be to provide practice with less stable and less regular patterns of variability using perturbations of speed or terrain to promote adaptive use of gait variability. This strategy assumes that practice will lead to improved performance despite the presence of hypotonia, high ligamentous laxity and reduced capacity to produce muscle force. Our findings hint improved performance may be possible, though, as these factors are present across the lifespan yet preadolescents with DS demonstrate different patterns of variability compared to their younger and older peers. Additionally, although amount of gait variability may not change with intervention, LyE and ApEn values could be used to quantify changes in patterns of gait variability in response to intervention. We provide here a baseline description of mean LyE and ApEn values for patterns of stability and regularity of gait variability observed across the lifespan in persons with DS.
For future research and application, preadolescents’ LyE and ApEn values may represent the best possible values, and their younger and older peers’ lower values may represent walking patterns that are less adaptable as a result of too much stability and regularity in the pattern. This is in contrast to our hypotheses that their younger and older peers would have higher values representative of walking patterns that are less adaptable as a result of too little stability and regularity in the pattern. Theoretically, extreme values may be on either side of the ideal value, and this understanding should begin to influence physical therapy interventions as we discover more. For example, a treadmill walking intervention at a constant speed may decrease the amount of gait variability and promote more stable and more regular patterns of variability while walking at different and changing speeds may increase amount of gait variability and promote less stable and less regular patterns of variability. Interventions designed to promote practice with increased or decreased amounts of variability, and more or less stable and regular patterns of movement variability should help patients learn better adaptive use of variability.
It should be noted that LyE and ApEn values can only be interpreted in a relative way, that is, on a continuum in comparison to similar data collected and analyzed in the same manner. With the algorithms used in the software we applied to our data, the LyE values lie on a continuum from 0 to 0.5. A periodic sine wave, with no divergence from one trajectory to the next, produces a LyE value of 0. A random signal, with maximal divergence, produces a LyE value of 0.5. Our results showed LyE values ranging from approximately 0.15 to 0.20, indicating that divergence in participants’ knee trajectories was closer to the periodic end of the continuum. Occasions where participants with DS had higher LyE values than their peers with TD reflected more divergence in their movement trajectories. In our data, because both young and older groups were lower, ideal values for LyE in persons with DS appeared to be around 0.20, indicating a balance between stability and adaptability of performance. This value, however, would not necessarily be the ideal for a different population or for any other gait parameter of interest, such as ankle or center of mass motion.
ApEn values exist on a continuum of 0 to 2. Complete regularity of pattern produces an ApEn value of 0, while complete irregularity and lack of a pattern is represented by an ApEn value of 2. Our participants’ ApEn values ranged from approximately 0.12 to 0.48, indicating that step lengths and widths were closer to the end of the continuum associated with a regular pattern. This was not unexpected, as consecutive walking steps represent a more periodic and regular pattern as compared to variables such as center of pressure sway. For step length and width, preadolescents demonstrated higher ApEn values than their older and younger peers, indicating less regularity of pattern across successive steps and more adaptability of behavior. In our data, ideal values for ApEn appeared to be around 0.48, a balance between regularity and adaptability of performance. As with our LyE results, this is not an ideal value that would necessarily apply in a different population or to any other variable of interest.
Our results show that patterns of gait variability are different across the lifespan within the group of persons with DS as well as the group with TD, and suggest that preadolescents in both groups are able to use higher amounts of variability in an adaptive way compared to their younger and older peers. The nonlinear measures, for the most part, reflected lifespan differences and did not reflect overall differences between the DS and TD groups. The lack of group differences in ApEn and vertical direction LyE values may be related to statistical analysis characteristics. The power to statistically demonstrate differences between groups decreased when the much larger age effects were included in the same analysis. In a study with similar dependent variables and only one age group (8- to10-year olds), group differences in LyE and ApEn of lower extremity segmental angles were observed. Results indicated statistically higher LyE and ApEn values indicating less stability and less regularity in patterns of lower extremity segmental angles for children with DS when compared to their peers with TD.19 Group means for vertical direction LyE and ApEn step length in our study are consistent in direction with results from this previous study.19 It is, however, difficult to compare the actual LyE and ApEn values we obtained to those of other studies, as the use of different gait parameters leads to different values of LyE and ApEn. Buzzi and Ulrich19 obtained LyE values ranging from 0.12 to 0.2 and ApEn values of 0.22 to 0.52 for lower extremity segmental angles of children with DS and TD. Jordan and colleagues36 calculated LyE values of around 0.1 at the ankle near the walk-run transition speed in healthy adult females. Stergiou and colleagues37,38 obtained LyE values around 0.10–0.12 and ApEn near 0.20 to 0.26 from the knee flexion/extension angle from participants with and ACL-deficient and contralaterally-intact knee. Despite slightly different analysis techniques and/or dependent variables, our data do appear to be in a similar range as results from other studies of treadmill walking in various populations.
LIMITATIONS
We are limited in our ability to make claims about how gait variability patterns change for a person across the lifespan as our data are cross-sectional as opposed to longitudinal. Our interpretation of gait as more adaptable is also limited as we used a treadmill to collect data and did not test adaptability of gait to external manipulations. We do, however, believe that our context is an appropriate paradigm to lay a foundation of data. Nonlinear analyses are better applied to longer bouts of continuous walking and using a treadmill allowed us to obtain long, continuous walking trials from preadolescents and adults. Toddlers, however, are only able to produce 7 or 8 continuous walking strides on a treadmill at this point in developmental time, leading to shorter data sets than are typically used when performing nonlinear analyses. For these reasons, our ability to apply nonlinear analyses to new walker data was limited (see Smith, Stergiou and Ulrich35 for discussion of potential confounding effect of application of LyE to short new walker data sets). We also appreciate the need for software development to allow clinicians to collect and analyze data without using research laboratory resources and for correlation of nonlinear measures with clinical rating scales of body structure and function, activity and participation.
CONCLUSIONS
Overall our work suggests that, given their inherent neurophysiological constraints, persons with DS control their gait in a way that is functional for them. The quality of this solution, however, varies with age, as the stability and regularity of their patterns of gait variability are different across the lifespan. Preadolescents are typically at a performance peak for their lifespan, with the nonlinear measures suggesting that they are more adaptable in their walking patterns than younger and older age groups. New walkers may have difficulty adapting because they lack experience with this skill. By 35 years of age and beyond, adaptability of adults’ gait may diminish due to a decline in their amount of walking or a hesitation to challenge themselves during locomotion. These results may suggest that physical therapists can intervene to improve gait performance, specifically patterns of variability related to stability and regularity which may reflect adaptive control of gait, in new walkers and adults with DS.
Acknowledgments
We would like to thank the participants and their families, as well as our funding sources. This work was supported by University of Michigan Rackham Graduate School and Blue Cross Blue Shield of Michigan Foundation (B. A. Smith), NIH K25HD047194 (N. Stergiou), and NIH R01 HD42728-01 (B. D. Ulrich). B. A. Smith was supported by grant H424C010067 from the U.S. Office of Special Education and Rehabilitative Services to D. Ulrich (PI). N. Stergiou was supported by grants K25HD047194 from the NICHD/NIH and H133G040118 from NIDRR/US Dept. of Education. Preliminary results were presented at the North American Society for the Psychology of Sport and Physical Activity 2007 and 2008 Annual Conferences. Results were presented at the International Society of Motor Control conference in Marseilles, France, in July 2009. This work was completed in partial fulfillment of B. A. Smith’s Ph.D. in Kinesiology at the University of Michigan.
References
- 1.Darling WG, Cooke JD, Brown SH. Control of simple arm movements in elderly humans. Neurobiol Aging. 1989;10:149–57. doi: 10.1016/0197-4580(89)90024-9. [DOI] [PubMed] [Google Scholar]
- 2.Panzer VP, Bandinelli S, Hallett M. Biomechanical assessment of quiet standing and changes associated with aging. Arch Phys Med Rehabil. 1995;76:151–7. doi: 10.1016/s0003-9993(95)80024-7. [DOI] [PubMed] [Google Scholar]
- 3.Callisaya ML, Blizzard L, Schmidt MD, McGinley JL, Srikanth VK. Ageing and gait variability--a population-based study of older people. Age Ageing. 39:191–7. doi: 10.1093/ageing/afp250. [DOI] [PubMed] [Google Scholar]
- 4.Owings TM, Grabiner MD. Variability of step kinematics in young and older adults. Gait Posture. 2004;20:26–9. doi: 10.1016/S0966-6362(03)00088-2. [DOI] [PubMed] [Google Scholar]
- 5.Grabiner PC, Biswas ST, Grabiner MD. Age-related changes in spatial and temporal gait variables. Arch Phys Med Rehabil. 2001;82:31–5. doi: 10.1053/apmr.2001.18219. [DOI] [PubMed] [Google Scholar]
- 6.Menz HB, Lord SR, Fitzpatrick RC. Age-related differences in walking stability. Age Ageing. 2003;32:137–42. doi: 10.1093/ageing/32.2.137. [DOI] [PubMed] [Google Scholar]
- 7.Buzzi UH, Stergiou N, Kurz MJ, Hageman PA, Heidel J. Nonlinear dynamics indicates aging affects variability during gait. Clin Biomech. 2003;18:435–43. doi: 10.1016/s0268-0033(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 8.Galica AM, Kang HG, Priplata AA, et al. Subsensory vibrations to the feet reduce gait variability in elderly fallers. Gait Posture. 2009;30:383–7. doi: 10.1016/j.gaitpost.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sosnoff JJ, Voudrie SJ. Practice and age-related loss of adaptability in sensorimotor performance. J Mot Behav. 2009;41:137–46. doi: 10.3200/JMBR.41.2.137-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kornatz KW, Christou EA, Enoka RM. Practice reduces motor unit discharge variability in a hand muscle and improves manual dexterity in old adults. J Appl Physiol. 2005;98:2072–80. doi: 10.1152/japplphysiol.01149.2004. [DOI] [PubMed] [Google Scholar]
- 11.Hausdorff JM, Nelson ME, Kaliton D, et al. Etiology and modification of gait instability in older adults: A randomized controlled trial of exercise. J Appl Physiol. 2001;90:2117–29. doi: 10.1152/jappl.2001.90.6.2117. [DOI] [PubMed] [Google Scholar]
- 12.Brach JS, Berlin JE, VanSwearingen JM, Newman AB, Studenski SA. Too much or too little step width variability is associated with a fall history in older persons who walk at or near normal gait speed. J Neuroeng Rehab. 2005;2:21. doi: 10.1186/1743-0003-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harbourne RT, Stergiou N. Movement variability and the use of nonlinear tools: Principles to guide physical therapist practice. Phys Ther. 2009;89:267–82. doi: 10.2522/ptj.20080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stergiou N, Harbourne R, Cavanaugh J. Optimal movement variability: A new theoretical perspective for neurologic physical therapy. J Neurol Phys Ther. 2006;30:120–9. doi: 10.1097/01.npt.0000281949.48193.d9. [DOI] [PubMed] [Google Scholar]
- 15.Fetters L. Perspective on variability in the development of human action. Phys Ther. 2010;90:1860–7. doi: 10.2522/ptj.2010090. [DOI] [PubMed] [Google Scholar]
- 16.Smith BA, Kubo M, Black DP, Holt KG, Ulrich BD. Effect of practice on a novel task--walking on a treadmill: Preadolescents with and without Down syndrome. Phys Ther. 2007;87:766–77. doi: 10.2522/ptj.20060289. [DOI] [PubMed] [Google Scholar]
- 17.Ulrich BD, Haehl V, Buzzi UH, Kubo M, Holt KG. Modeling dynamic resource utilization in populations with unique constraints: Preadolescents with and without Down syndrome. Hum Mov Sci. 2004;23:133–56. doi: 10.1016/j.humov.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Smith BA, Ulrich BD. Early onset of stabilizing strategies for gait and obstacles: Older adults with Down syndrome. Gait Posture. 2008;28:448–55. doi: 10.1016/j.gaitpost.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buzzi UH, Ulrich BD. Dynamic stability of gait cycles as a function of speed and system constraints. Motor Control. 2004;8:241–54. doi: 10.1123/mcj.8.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Black DP, Chang CL, Kubo M, Holt KG, Ulrich BD. Developmental trajectory of dynamic resource utilization during walking: Toddlers with and without Down syndrome. Hum Mov Sci. 2009;28:141–54. doi: 10.1016/j.humov.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latash ML, Anson GJ. Synergies in health and disease: Relations to adaptive changes in motor coordination. Phys Ther. 2006;86:1151–60. [PubMed] [Google Scholar]
- 22.Black DP, Smith BA, Wu J, Ulrich BD. Uncontrolled manifold analysis of segmental angle variability during walking: Preadolescents with and without Down syndrome. Exp Brain Res. 2007;183:511–21. doi: 10.1007/s00221-007-1066-1. [DOI] [PubMed] [Google Scholar]
- 23.Smith BA, Ashton-Miller JA, Ulrich BD. Gait adaptations in response to perturbations in adults with Down syndrome. Gait Posture. 2010;32:6. doi: 10.1016/j.gaitpost.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brach JS, Studenski SA, Perera S, VanSwearingen JM, Newman AB. Gait variability and the risk of incident mobility disability in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2007;62:983–8. doi: 10.1093/gerona/62.9.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: A 1-year prospective study. Arch Phys Med Rehab. 2001;82:1050–6. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- 26.Barak Y, Wagenaar RC, Holt KG. Gait characteristics of elderly people with a history of falls: A dynamic approach. Phys Ther. 2006;86:1501–10. doi: 10.2522/ptj.20050387. [DOI] [PubMed] [Google Scholar]
- 27.Maki BE. Gait changes in older adults: Predictors of falls or indicators of fear. J Am Geriatr Soc. 1997;45:313–20. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 28.Alton F, Baldey L, Caplan S, Morrissey LC. A kinematic comparison of overground and treadmill walking. Clin Biomech. 1998;13:434–40. doi: 10.1016/s0268-0033(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 29.Berg K, Wood-Dauphinee S, Williams JI, Gayton D. Measuring balance in the elderly: Preliminary development of an instrument. Physiother Can. 1989;41:304–11. [Google Scholar]
- 30.Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: Validation of an instrument. Can J Public Health. 1992;83 (Suppl 2):S7–11. [PubMed] [Google Scholar]
- 31.Hausdorff JM. Gait dynamics, fractals and falls: Finding meaning in the stride-to-stride fluctuations of human walking. Hum Mov Sci. 2007;26:555–89. doi: 10.1016/j.humov.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohgi S, Morita S, Loo KK, Mizuike C. Time series analysis of spontaneous upper-extremity movements of premature infants with brain injuries. Phys Ther. 2008;88:1022–33. doi: 10.2522/ptj.20070171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dusing SC, Kyvelidou A, Mercer VS, Stergiou N. Infants born preterm exhibit different patterns of center-of-pressure movement than infants born at full term. Phys Ther. 2009;89:1354–62. doi: 10.2522/ptj.20080361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stergiou N. Innovative analyses of human movement. Champaign, IL: Human Kinetics; 2004. [Google Scholar]
- 35.Smith BA, Stergiou N, Ulrich BD. Lyapunov exponent and surrogation analysis of patterns of variability: Profiles in new walkers with and without Down syndrome. Motor Control. 2010;14:16. doi: 10.1123/mcj.14.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jordan K, Challis JH, Cusumano JP, Newell KM. Stability and the time-dependent structure of gait variability in walking and running. Hum Mov Sci. 2009;28:113–28. doi: 10.1016/j.humov.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Stergiou N, Moraiti C, Giakas G, Ristanis S, Georgoulis AD. The effect of the walking speed on the stability of the anterior cruciate ligament deficient knee. Clin Biomech. 2004;19:957–63. doi: 10.1016/j.clinbiomech.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Georgoulis AD, Moraiti C, Ristanis S, Stergiou N. A novel approach to measure variability in the anterior cruciate ligament deficient knee during walking: The use of the approximate entropy in orthopaedics. J Clin Monit Comput. 2006;20:11–8. doi: 10.1007/s10877-006-1032-7. [DOI] [PubMed] [Google Scholar]


