Abstract
Purpose
Administration of bisphosphonates has recently been associated with the development of osteonecrotic lesions of the jaw (ONJ). To elucidate the potential contributions of osteogenic cells to the development and regeneration of ONJ, we have isolated primary cells from human alveolar and long/iliac bones, and examined the effects of pamidronate on cell viability, proliferation, osteogenesis and wound healing.
Materials and Methods
Primary human osteoblasts and bone marrow stromal cells were isolated from alveolar and iliac/long bone and marrow tissue. Cellular proliferation, alkaline phosphatase activity, apoptosis (TUNEL, Caspase-3, and DAPI assays) and wound healing in an in vitro scratch assay were assessed after exposure to pamidronate at a range of clinically relevant doses.
Results
Primary alveolar osteoblasts proliferated at significantly higher rates than long/iliac bone osteoblasts in vitro. Upon exposure of alveolar osteoblasts and long/iliac bone marrow stromal cells to pamidronate for more than 72h, we have observed significantly decreased cell viability, proliferation, osteogenesis and in vitro wound healing at ≥6 × 10−5 M pamidronate, with the induction of apoptosis in ~20% of cell population.
Conclusions
The remodeling activity of alveolar bone, indicated by higher proliferation of alveolar osteoblasts, could be negatively affected by exposure to high concentrations of pamidronate over extended periods of time. The absence of anabolic effects of pamidronate on alveolar osteoblasts, and induction of apoptosis in osteogenic cells could negatively affect bone balance at this site, and contribute to osteonecrosis of the jaw.
Keywords: osteonecrosis of the jaw, pamidronate, alveolar osteoblasts, BMSCs, apoptosis
INTRODUCTION
Bisphosphonates (BPs), potent inhibitors of osteoclastic bone resorption, are widely used in the treatment of osteoporosis, Paget's disease, hypercalcemia of malignancy, multiple myeloma, and bone metastases associated with breast, prostate, lung, and other soft tissue tumors. Recently, the occurrence of exposed necrotic bone lesions in the jaw (osteonecrosis of the jaw, ONJ) has been associated with the use of nitrogen-containing BPs1, 2. Osteonecrosis of the jaw is defined by several professional societies as an area of exposed bone in the maxillofacial region that does not heal within 6–8 weeks after identification, in a patient who is receiving or has been exposed to a BP and has not had radiation therapy to the craniofacial region2–8.
At the present time the natural history and pathophysiology of ONJ development is not clear. In the majority of cases, ONJ has been diagnosed in the oncologic population, where patients are receiving high doses of BPs with concommitant chemotherapeutic agents and are often immunosuppressed. Oversuppression of bone turnover due to increased sequestration of BPs in the jaws, and compromised blood supply have been considered the initiating events in the development of the lesions1. In addition, inhibition of wound healing, surgically-induced trauma, specific anatomy of the oral cavity, the presence of a diverse microbial flora and on-going inflammatory processes have been proposed as potential contributing factors2, 9.
Differences in bone physiology between different sites in the skeleton could be implicated in the development of ONJ. Craniofacial bones differ from long bones in developmental lineage, arising from neural crest, and mechanism of formation by intramembranous ossification. Differences in matrix composition, bone morphogenic protein expression, osteoclastic bone degradation and bone marrow stromal cell (BMSC) compartment have been reported, and suggest functional differences in turn-over and mechanical properties of bone at different anatomic locations10–13. In a recent study, pamidronate treatment of primary human mandibular and iliac crest BMSCs isolated from the same donors indicated differences in survival, osteogenesis and osteoclast recruitment between the two sites, suggesting the possibility of dysregulation of mandibular bone homeostasis after exposure to BPs14. In previous studies, treatment of osteogenic cells isolated from the long/iliac bone with lower BPs concentrations (≤10−5 M) resulted in anabolic effects15–18. However exposure to higher concentrations (≥10−4 M) resulted in decreased cell proliferation, viability, protein synthesis and mineralizaton17, 19–21. Decreased cell viability and migration have also been reported for oral mucosal and endothelial cells after BP treatment, suggesting the possibility that ONJ is a multifactorial disease21–26.
Given the specific differences in cell responses to BPs, and physiological differences between bone at different locations, our aim was to assess the effects of BPs on primary human alveolar osteoblasts, which to our knowledge have not been reported previously. We have hypothesized that the osteogenic ability of residing osteoblasts, as well as transplanted cells, might be directly affected by BPs. We have chosen to study the effects of pamidronate, a common intravenously-administered bisphosphonate, linked to ONJ, that is frequently used as a model compound in studies of BPs actions3, 23, 27–31. We have tested survival, proliferation, osteogenesis and wound healing of human osteoblasts isolated from the alveolar bone. In addition, we have extended our experiments to primary human osteogenic populations from the long/iliac bone and marrow, in order to compare their response to populations derived from intamembranous bone. We have treated the cells with a range of pamidronate concentrations (10−10 − 10−4 M), and report for the first time concentration-dependent inhibitory effects of pamidronate on primary human alveolar bone osteoblasts including induction of apoptosis.
MATERIALS AND METHODS
Materials
Pamidronate, ascorbic acid 2-phosphate, dexamethasone, β-glycerophosphate, paraformaldehyde and formalin-free fixative (Accustain) were purchased from Sigma-Aldrich (St. Louis, MO). Fetal bovine serum (FBS), Dulbecco's modified Eagle medium (DMEM), basic fibroblast growth factor (bFGF), 4′, 6-diamidino-2-phenylindole dihydrochloride (DAPI), gentamicin, Fungizone®, Dulbecco's PBS (D-PBS), non-essential amino acids and trypsin/EDTA were obtained from Invitrogen (Carlsbad, CA). Dulbecco's modified Eagle's media: nutrient mixture F-12 (DMEM/F-12) was from Lonza (Basel, Switzerland). All other substances were of analytical or pharmaceutical grade and obtained from Sigma-Aldrich.
Isolation and Culture of Primary Human Cells
Primary human osteoblast-like cells (“osteoblasts”) were isolated from discarded alveolar (n=7) and long/iliac bone surgical procedures (n=5). The study was approved by the Columbia University IRB. The bone tissue was minced, thoroughly washed to remove any remaining soft tissue, and placed in 6-well plates to initiate explant cultures. The culture medium consisted of DMEM/F12 supplemented with 20% FBS, 50 μg/mL gentamicin and 0.08% Fungizone®, and was changed twice per week. Long/iliac BMSCs were isolated from the bone marrow aspirate samples obtained from Lonza (n=2) or by washing and collecting the marrow fraction from the long bone sample (n=1). Adherent cells were cultured in DMEM supplemented with 10% FBS, 1% nonessential amino acids, 1 ng/mL bFGF, 50 μg/ml gentamicin and 0.08% Fungizone® according to the previously established protocols32. All cultures were incubated at 37°C and 5% CO2. Upon confluence, cells were dissociated from the plates by trypsinization, counted and subcultured at a density of 5 000 cells/cm2 for three passages. Osteoblast proliferation was compared between different tissue sources at passage one. Third passage cells were used in all other experiments, and exposed to a range of pamidronate concentrations, as noted in specific assays.
Proliferation Assay
Two thousand cells were seeded per well of 96-well plates (6,000 cells/cm2) in 100 μL of DMEM/F12 supplemented with 10% FBS, 50 μg/mL gentamicin and 0.08% Fungizone®, which was changed every two days. 100 mM pamidronate stock solution was prepared in deionized water and further diluted to final concentrations in culture media. The effect of pamidronate (10−10 − 10−4 M) on cell proliferation was evaluated at 24h, 48h, 72h and 168h after the addition to the cultures. Proliferation was measured by CellTiter96®Assay according to the manufacturer's instructions (Promega, Madison, WI). Briefly, MTS/PMS reagent was added directly to the culture media, incubated at 37°C for 1.5h and the absorbance was measured at 490 nm.
Alkaline Phosphatase Activity Assay
Alkaline phosphatase (AP) activity of the cells was assessed as an early marker of osteogenesis33, 34 in control medium (DMEM/F12 supplemented with 10% FBS, 50 μg/mL gentamicin and 0.08% Fungizone®) and osteogenic medium (control medium supplemented with 10 mM β-glycerophosphate, 100 nM dexamethasone and 0.05 mM L-ascorbic acid 2-phosphate) 24h and 168h after the addition of pamidronate (10−10 − 10−4 M) to the media. For quantitative assays, cells were seeded and cultured in 96-well plates, as described above for the proliferation assay. The AP activity was determined by following the conversion of p-nitrophenyl phosphate to p-nitrophenol (Sigma-Aldrich)32. Cells were lysed by adding 100 μL of a buffer containing 2-amino-2-methylpropanol and pnitrophenylphosphate (Sigma-Aldrich) to each well, and incubated at 37°C until the development of color. Reaction time was noted for AP activity calculation. Reactions were stopped by adding 50 μL 0.2 M NaOH. Absorbance was measured at 405 nm, and the AP activity was calculated from the standard curve and normalized to the reaction time and number of cells per well in each group. Cultures were fixed with Accustain, stained with DAPI (4′, 6-diamidino-2-phenylindole dihydrochloride), and the number of cells in randomly sampled fields was counted using fluorescent microscope (IX81, Olympus, Center Valley, PA) and Metamorph imaging software (Molecular Devices, Sunnyvale, CA). For qualitative assessments, cells were seeded into 48-well plates (6,000 cells/cm2) and cultured in osteogenic and control media with pamidronate, as described above. AP activity was detected by histological staining according to the manufacturer's instructions (Fast Blue RR Salt staining, Sigma-Aldrich).
TUNEL Assay
TUNEL assay, the most commonly used in situ test for apoptosis, was selected to probe for apoptosis induction as in our previous study24. Cells were seeded into Permanox chamber slides (Nunc, Rochester, NY) as described above for the proliferation assay, and incubated with pamidronate (0 M, 10−5 M, 6 × 10−5 M and 10−4 M) for 24h and 72h. Positive controls were obtained by incubating cells with 1 μM staurosporine (Sigma-Aldrich) for 6h. TUNEL assay was performed according to the manufacturer's instructions (Roche, Indianapolis, IN). Slides were fixed with 4% paraformaldehyde in PBS for 1 hour at room temperature, washed and permeabilized with 0.1% sodium citrate, 0.1% Triton X-100 solution for 2 minutes on ice. After washing, slides were incubated with enzyme and labeling solution for 1 hour at 37°C in a humidified chamber. All slides were counterstained with DAPI to detect pyknotic nuclei in the cells that have undergone apoptosis, and mounted with Vectashield mounting solution (Vector Labs, Burlingame, CA). TUNEL positive cells, total number of cells and nuclear condensation were assessed under the fluorescent microscope (IX81, Olympus, Center Valley, PA).
Caspase-3 Assay
Induction of apoptosis at high concentrations of pamidronate was confirmed by measuring activity of Caspase-3, a key enzyme in the execution of the apoptotic cascade that mediates DNA condensation, DNA fragmentation, and cell blebbing35. Alveolar osteoblasts and BMSCs were grown to 70% confluence in 6-well plates and treated with pamidronate (10−4 M and 3 × 10−4 M) for 24h, 48h, 72h and 96h. Positive controls were generated by incubation of cells with 1 μM staurosporine for 6h. Cells were collected in the culture medium and centrifuged at 5,000 × g for 5 minutes. Caspase-3 activity was assayed according to the manufacturer's instructions (Sigma-Aldrich). Briefly, cell pellets were lysed in the buffer provided in the kit and stored at −80°C. Upon thawing, samples were centrifuged at 13,400 × g for 10 minutes at 4°C, and assayed in 96-well plates by adding the Caspase-3 substrate (Acetyl-Asp-Glu-Val-Asp p-nitroanilide) and incubating overnight at 37°C. Absorbance was read at 405 nm, and the caspase activity was calculated from the standard curve and normalized to the total protein content determined using a Bradford reagent based protein assay (Sigma-Aldrich). A standard curve was generated using known concentrations of bovine serum albumin (Bio-Rad, Hercules, CA).
Wounding Assay
The in vitro model of wound healing was used to examine the effects of pamidronate treatment on the ability of alveolar osteoblasts and BMSCs to migrate and proliferate in a monolayer culture24. Cells were seeded into 6-well plates, grown to confluence and preincubated with pamidronate (0M, 10−5 M, 3 × 10−5 M, 6 × 10−5 M and 10−4 M) for 24h. Wounds were created with a standardized protocol using a trimmed comb containing 7 tines spaced 2.5 mm apart (Goody Products Inc., Peachtree City, GA) that had been washed with 70% ethanol and dried prior to use. Wounded monolayers were briefly washed, and the culture media were returned to the cells. Migration of the cells into wounded areas of the monolayer (percentage of overgrown area) was assessed by microscopy over four days.
Statistical Analyses
Statistical analyses were done by one-way and two-way ANOVA, followed by Tukey-Kramer HSD post-hoc test using Statistica software (StatSoft, Tulsa, OK). Significance level was set at 0.05.
RESULTS
Effects of Pamidronate on Cell Proliferation
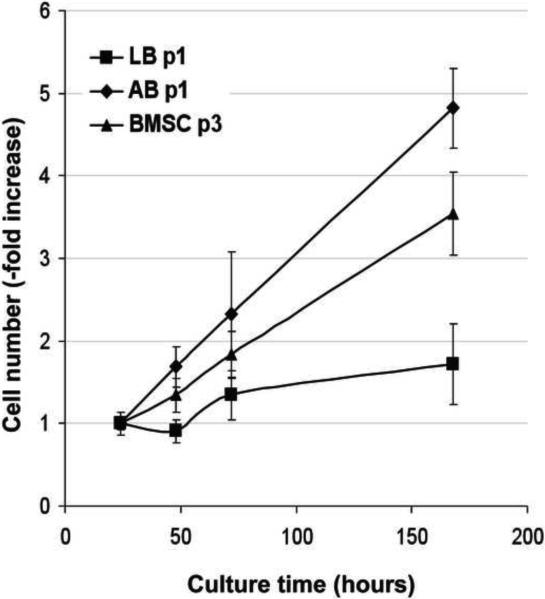
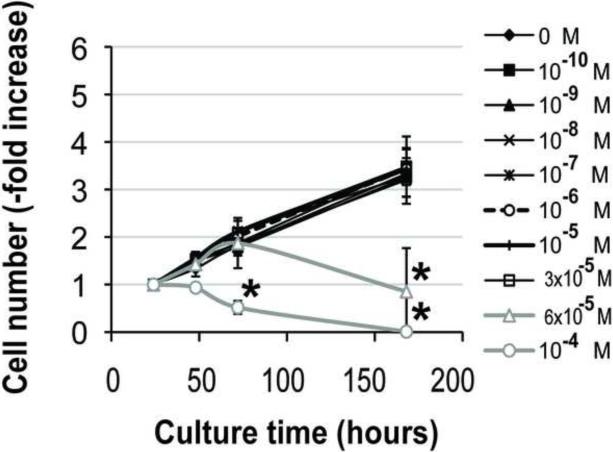
Primary human osteoblasts were isolated from samples of alveolar and long/iliac bone tissue by preparation of explant cultures. Proliferation of primary cells at passage 1 was markedly higher in alveolar osteoblasts (5-fold increase in cell number, n=4) than the long/iliac bone osteoblasts (<2-fold increase in cell number, n=3) (Fig. 1). Alveolar osteoblasts continued to proliferate over three passages, whereas osteoblasts from the long/iliac bones demonstrated only limited growth, which arrested before the second passage in all tested samples (n=5). Bone marrow stromal cells (n=3) were isolated from the marrow aspirates obtained from the long/iliac bones, and cultured in medium supplemented with 1 ng/ml bFGF. Under these conditions, BMSCs exhibited slightly lower growth rate than alveolar osteoblasts (Fig. 1), and were used in subsequent studies.
Figure 1. Primary alveolar bone osteoblasts, long bone osteoblasts and BMSCs proliferation.
Data represent averages ± SD of measurements (n=4) obtained from four alveolar bone (AB), three iliac/long bone (LB) and three BMSCs samples.
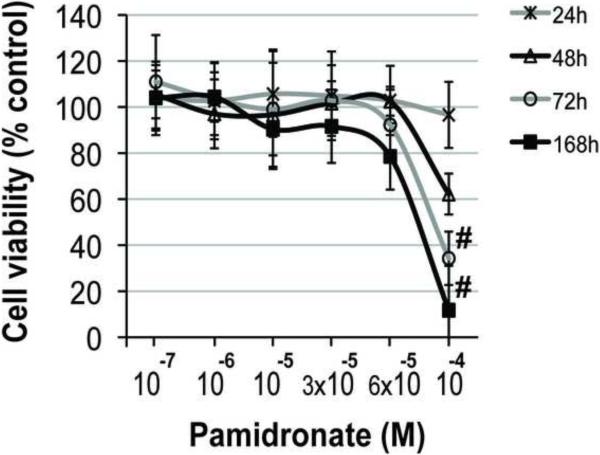
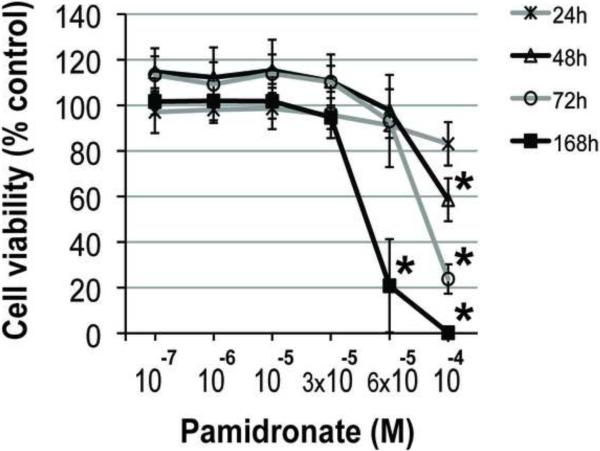
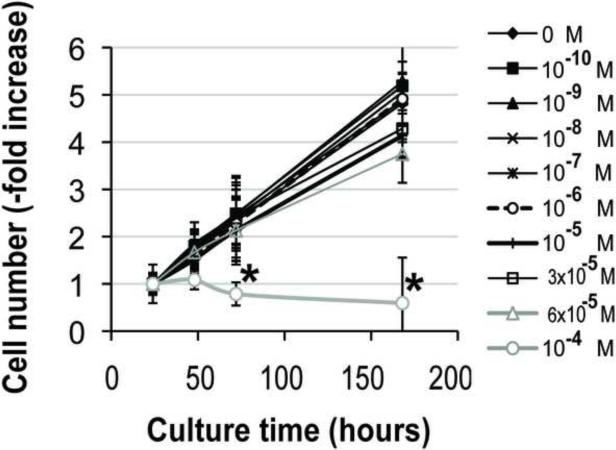
Addition of 10−4 M pamidronate to culture medium significantly decreased alveolar osteoblast viability after 72h and 168h (7 days) of treatment compared to all other concentrations (Fig. 2A, p<0.05), and induced cell detachment in 3 of 4 tested samples. Cell proliferation was significantly inhibited in this group after 72h and 168h compared to all other concentrations (Fig. 2C, p<0.001). Similarly, treatment of BMSCs with 10−4 M pamidronate for 48h, 72h and 168h, as well as treatment with 6 × 10−5 M pamidronate for 168h significantly decreased cell viability (Fig. 2B, p<0.001) compared to the lower concentrations. BMSCs proliferation was significantly inhibited with 10−4 M pamidronate after 72h and 168h, and with 6 × 10−5 M pamidronate after 168h (Fig. 2D, p<0.001) compared to the lower concentrations. We have not observed any significant anabolic effects at any of the pamidronate concentrations tested. Slightly increased BMSCs viability, noted in the lower pamidronate concentration groups (10−7 − 3 × 10−5 M) at 48h and 72h, was transient and declined by 168h of treatment.
Figure 2. Effects of pamidronate on osteogenic cell viability (A, B) and proliferation (C, D).
High concentrations (≥6 × 10−5 M) of pamidronate significantly affected survival and decreased cell proliferation of alveolar osteoblasts (A, C) and BMSCs (B, D) in comparison to lower concentration groups (#, p<0.05) (*, p<0.001). Data represent averages ± SD of measurements (n=4) obtained from four alveolar bone and three BMSCs samples.
Effects of Pamidronate on Alkaline Phosphatase Activity
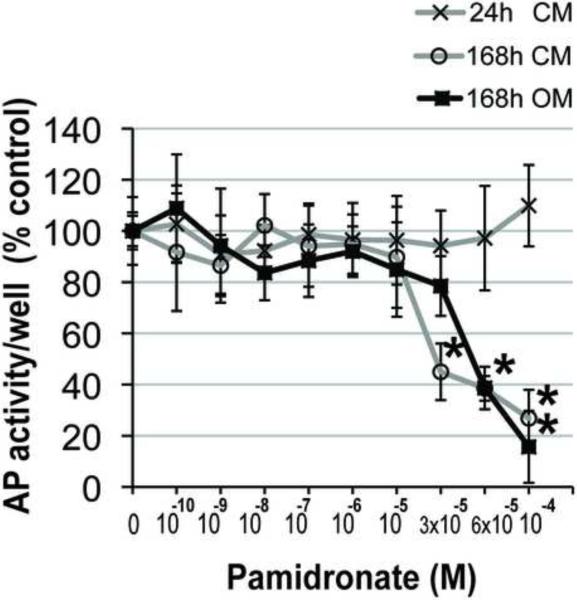
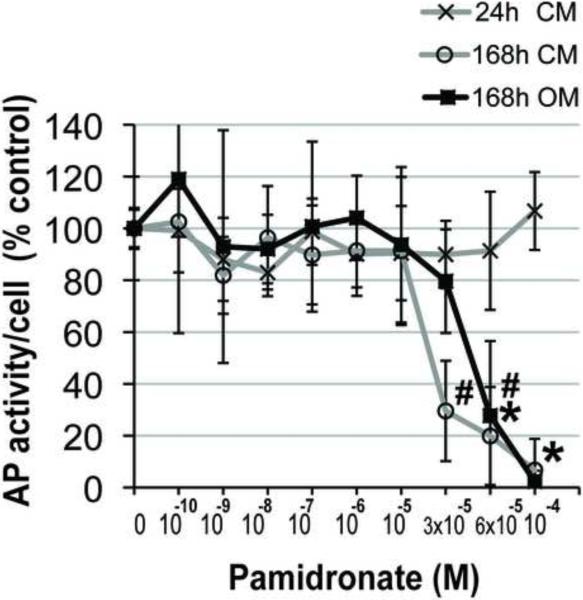
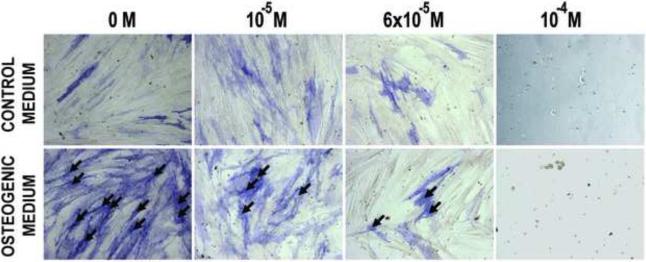
The effect of pamidronate supplementation on alkaline phosphatase (AP) activity, an early marker of osteogenic cell phenotype was assessed quantitatively at 24 and 168h. Alkaline phosphatase activity values of alveolar osteoblasts in control medium supplemented with 3 × 10−5 M, 6 × 10−5 M and 10−4 M pamidronate were 45%, 39% and 27% of the unsupplemented group, and differed significantly from all lower concentration groups after 168h (Fig. 3A, p<0.001). Alveolar osteoblasts cultured in osteogenic medium exhibited significantly lower AP activities with 6 × 10−5 M and 10−4 M pamidronate (39% and 16% of the unsupplemented group) as compared to all lower concentration groups after 168h (Fig. 3A, p<0.001). We have not detected anabolic effects of pamidronate supplementation on alveolar osteoblast AP activity in any of the concentration groups. AP activity per number of cells in the wells (Fig. 3B) and histological stains of AP activity (Fig. 3C) indicated that the total decrease per well resulted from the lower cell numbers, as well as from lower amounts of AP activity expressed per cell.
Figure 3. Effects of pamidronate on alveolar osteoblast osteogenesis.
(A, B) Quantitative assessments of AP activity indicated significant decrease at higher pamidronate concentrations (#, p<0.01) (*, p< 0.001). The cells were cultured 168h in unsupplemented medium (CM) and in medium with osteogenic supplements (OM). Data represent averages ± SD of measurements (n=4) obtained from two alveolar bone samples. (C) Histological stains (Fast Blue RR Salt) indicated a decrease in the number of cells strongly expressing AP activity (black arrows) at higher pamidronate concentrations. Magnification 100×.
Pamidronate and Cell Apoptosis
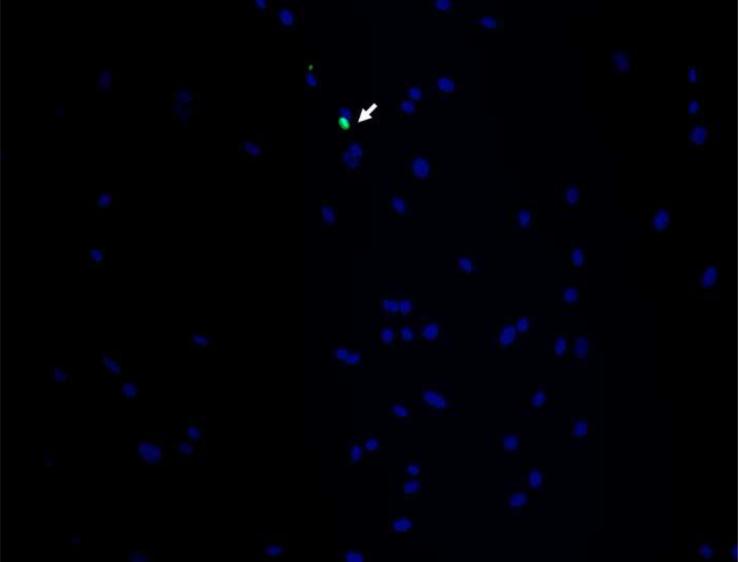
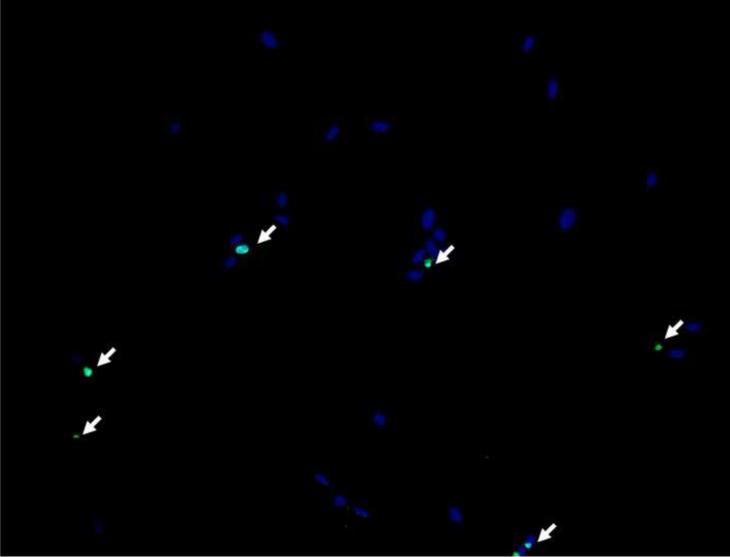
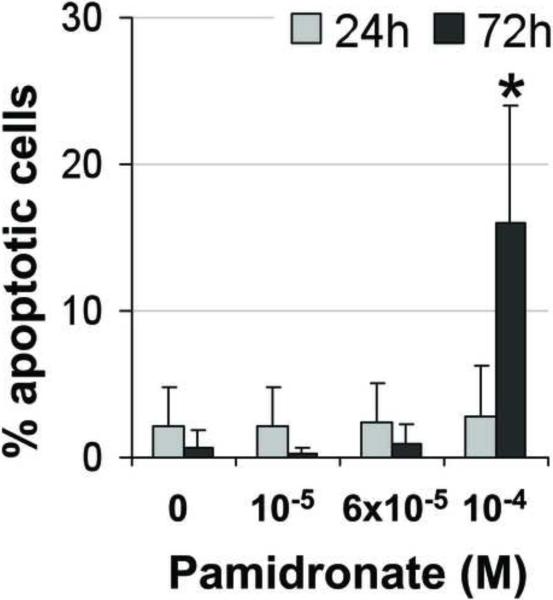
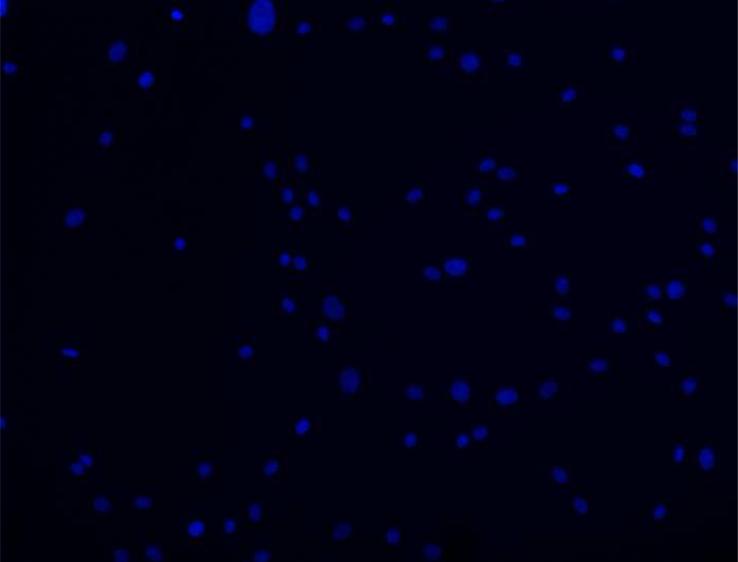
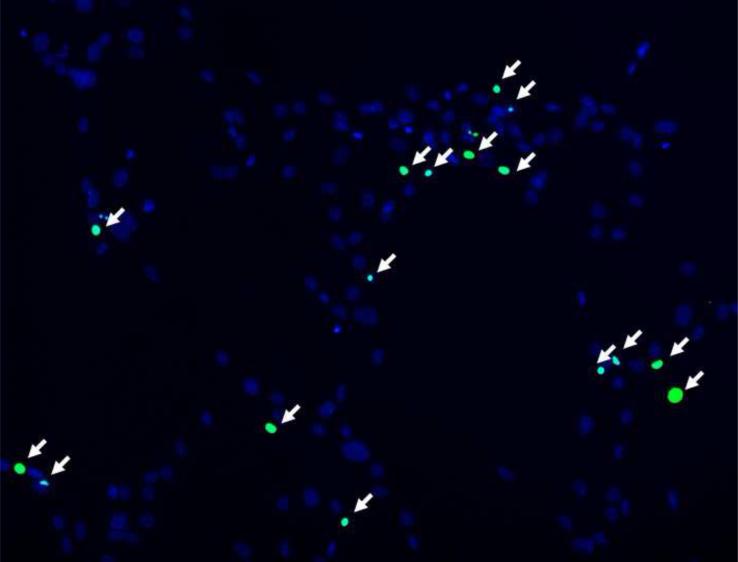
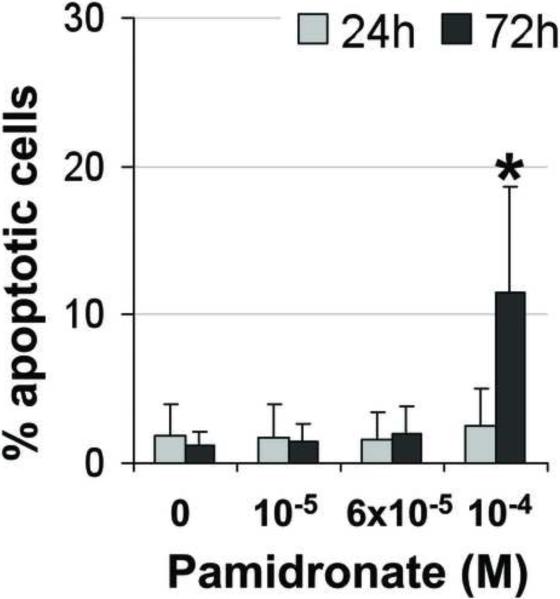
In order to examine pamidronate toxicity further, we have evaluated apoptosis by TUNEL assay, DAPI staining (Fig. 4A–E, G–K), and quantitation of caspase-3 activity (Fig. 4F, L). A significant increase in TUNEL positive apoptotic alveolar osteoblasts (16%) was seen in cultures supplemented with 10−4 M pamidronate after 72h when compared to other concentrations and incubation times (Fig. 4A, B, E, p<0.001). Similarly, BMSCs cultures exhibited a significant increase in apoptotic cells (12%) with 10−4 M pamidronate at 72h, compared to other concentrations and incubation times (Fig. 4G, H, K, p<0.001). In both cell types, counterstaining with DAPI showed nuclear condensation, the cells exhibited stressed morphology and started detaching from the culture surface at 72h (Fig. 4C, D, I, J).
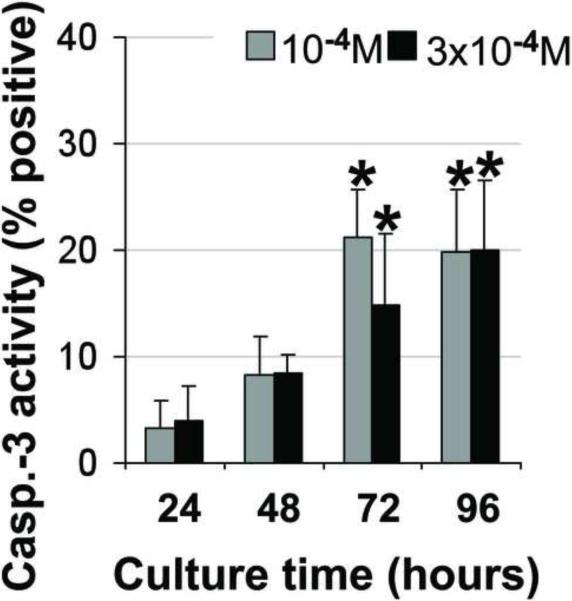
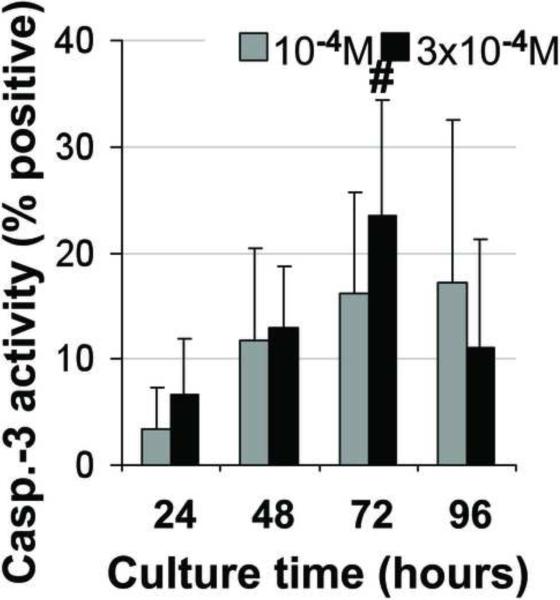
Figure 4. Induction of apoptosis in alveolar osteoblast (A, B, C, D, E, F) and BMSC (G, H, I, J, K, L) treated cultures.
Tunel assay (green stain) indicated the presence of apoptotic cells (white arrows) in the total cell population (blue stain - nuclei) treated with 10−4 M pamidronate (B, H) compared to untreated controls (A, G). Corresponding bright-field images are shown for pamidronate treated (D, J) and untreated cultures (C, I). Magnification 100×. Quantitative assessments of the Tunel slides (E, K) and measurements of caspase-3 activity (F, L) indicated a significant increase in the apoptotic cells after 72h of treatment with 10−4 M pamidronate compared to lower concentrations (*, p< 0.001). A similarly increasing trend with significant increases of caspase-3 activity after 72h was found with three-times higher pamidronate concentration (#, p<0.01) (*, p< 0.001). Data represent averages ± SD of measurements (n=3–4) obtained from three alveolar bone and three BMSCs samples.
Induction of apoptosis was further indicated in both cell types by significant increases in caspase-3 activity. Alveolar osteoblasts cultured with 10−4 M pamidronate exhibited significantly higher caspase-3 activity at 72h and 96h compared to 24h and 48h (Fig. 4F, p<0.001). Similarly, 3 × 10−4 M pamidronate induced a gradual increase in caspase-3 activity in alveolar osteoblasts, which was significantly higher at 72h and 96h compared to 24h (Fig. 4F, p<0.001). Caspase-3 activity levels measured in BMSCs were more variable, but exhibited a similar gradually-increasing trend over 72h as seen in the alveolar osteoblasts. Significantly increased caspase-3 activity was detected in BMSCs cultures treated with 3× 10−4 M pamidronate for 72h compared to 24h (Fig.4L, p<0.01).
Pamidronate and Wound Healing
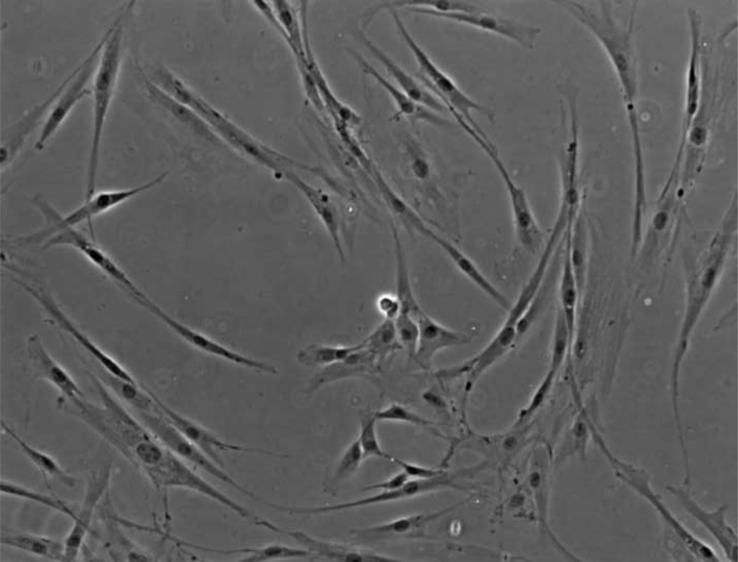
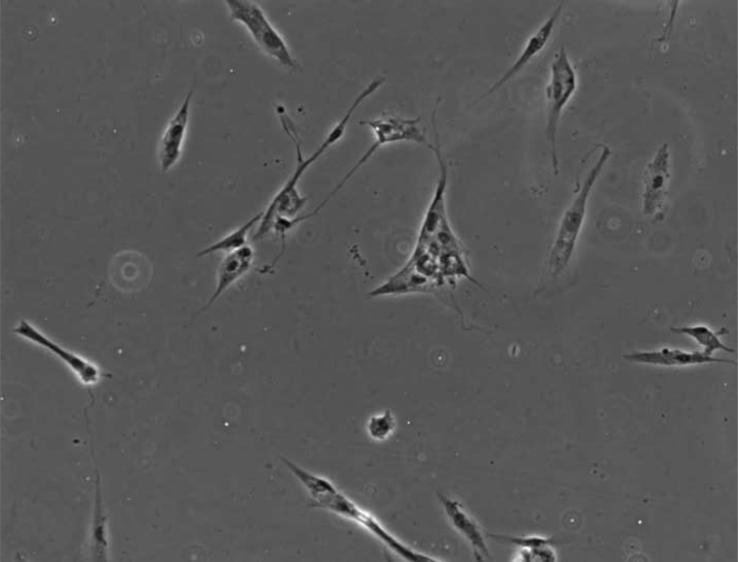
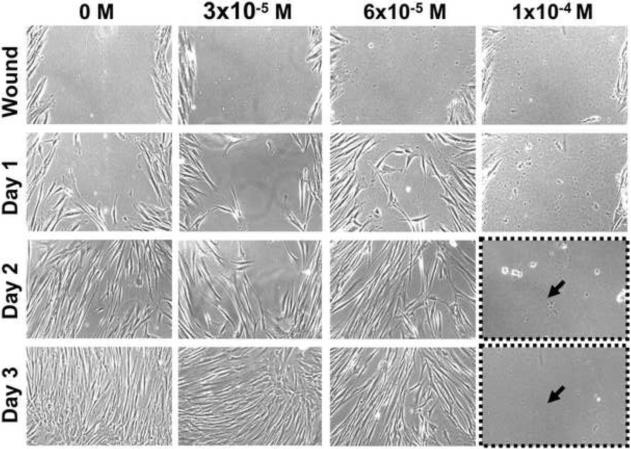
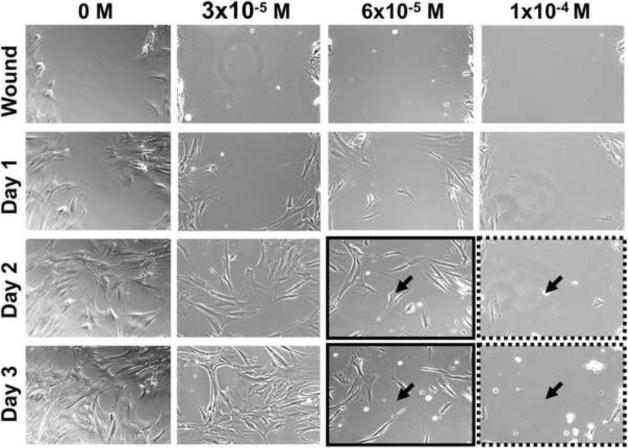
An in vitro model of wound healing was used to examine the ability of alveolar osteoblasts and BMSCs to migrate and proliferate during pamidronate treatment (Fig. 5). Confluent cell monolayers were preincubated with pamidronate (3 × 10−5, 6 × 10−5 and 10−4 M) for 24h, wounded by scratching, and the healing was observed daily. For both cell types, cell migration was noted 24h after wounding and continued until 72h, when the wound was closed in the control (unsupplemented) groups (Fig. 5) and the groups with 3 × 10−5M pamidronate. Alveolar osteoblasts exposed to 6 × 10−5 M pamidronate behaved similarly to control cultures (Fig. 5A), whereas BMSCs exhibited only ~20% wound closure at this concentration (maintained upon longer incubation) (Fig. 5B). Both cell types failed to heal the wound at 10−4 M pamidronate, and the monolayers completely detached 48h after wounding (72h days of pamidronate treatment, similar to our observations in proliferation studies) (Fig.5).
Figure 5. Wound healing assay.
Alveolar osteoblasts (A) and BMSCs (B) were pretreated with pamidronate for 24h, wounded and continuously monitored over several days. Pamidronate toxicity (black arrows) limited wound healing by BMSCs at 6 × 10−5 M pamidronate (solid brackets), and completely inhibited wound healing at 10−4 M in both cell types (dotted brackets). Representative images are shown from testing of two alveolar bone and two BMSC samples. Magnification 100×.
DISCUSSION
Our work was motivated by the increasing frequency of ONJ, a clinical condition associated with the use of nitrogen-containing BPs, previous traumatic injury, and several other risk factors. To date, the majority of knowledge regarding ONJ is based on case report studies1, 2, 36, and the true incidence, etiology, pathogenesis, and natural history of this condition have yet to be elucidated. In addition, restoration of oral tissues is challenging37, and in order to develop effective treatments, a fundamental understanding of cellular responses in the environment of diseased tissue is needed. Our group and others have previously reported the effects of BPs on oral mucosa cells, fibroblasts, vascular cells and bone cells isolated from non-oral sites21–26. Given the developmental and physiological differences between bone at different locations, the present study was aimed at assessing the specific effects of pamidronate on primary human alveolar and long/illiac bone osteoblasts. Our results show for the first time that pamidronate treatment inhibited cell viability, proliferation, osteogenesis and in vitro wound healing, and induced apoptosis in primary human alveolar osteoblasts. The exposure to ≥10−4 M threshold concentration for more than three days was shown to be neccessary for the inhibitory actions to take effect in vitro. Similar responses were found with primary human BMSCs from long/iliac bone marrow.
We have studied a wide range of pamidronate concentrations, which was based on reported maximum concentrations in the serum immediately after a 4h 90 mg pamidronate infusion (~10−5 M)38 and previous reports14, 17, 20, 21, 29, 39. As noted in previous work and supported by animal experiments24, 27, 40, 41, a range of 50 to 500 ng/mg (or 214 to 2 140 nmol/g) seems a reasonable estimate of the mean pamidronate concentration in bone tissue in humans that were treated for a period of 4 years with monthly infusions totaling 90 mg of the drug. Assuming a volume of 1 mL per g of bone, this amount corresponds to a concentration of 0.2 – 2 mM. Local concentrations of bisphosphonates in bone vary widely42, 43, and it has been speculated that due to the high rate of remodeling, alveolar bone could accumulate increased amounts of BPs. Recent studies indicate that cells in the bone microenvironment other than osteoclasts could be affected by bisphosphonates, when they are released from the mineralized surface39, 43, 44.
We have noted significant differences in the proliferative potential of alveolar and long/iliac bone osteoblasts (Fig.1), which corresponded with previous reports of rat calvarial osteoblasts45 and human maxillofacial BMSCs10 exhibiting higher proliferation than the same cell types isolated from the iliac/long bones. Even though our observations were limited by the relatively small number of samples originating from different donors, the observed differences were consistent. Due to the limited cell growth, we were unable to evaluate the effects of pamidronate on long bone osteoblasts. We have extended our studies to BMSCs from the long/iliac bone, a cell population commonly studied as a model for bone repair, in order to evaluate their healing potential in the presence of pamidronate.
Upon exposure of alveolar osteoblasts and BMSCs to pamidronate, we have noted considerable cell toxicity at ≥6 × 10−5 M pamidronate concentrations, which was accompanied by complete cell detachment in most cultures by 168h of treatment (Fig.2). The inhibitory effects on cell viability were highly consistent between different donors, and were in agreement with previous reports of human long bone17, 19, 21 and mouse calvarial osteoblasts29. Specific differences in cell sensitivity between the studies could possibly result from different subpopulations that are present in the primary cell preparations, including osteocytic cells. We have noted a somewhat higher sensitivity of BMSCs to pamidronate in comparison to alveolar osteoblasts, and a slight transitory increase in BMSC viability at 48h and 72h of treatment with lower pamidronate concentrations (Fig.2).
We have not been able to observe significant anabolic effects of pamidronate on either cell type during 168h treatment, in contrast to previous work reporting anabolic effects of BPs on long/iliac osteoblasts and BMSCs15, 16, 18. Our findings with long/iliac BMSCs are in agreement with a recent study, where a decrease in mandibular BMSCs viability was found with increasing pamidronate concentrations, whereas iliac bone BMSCs exhibited an increased cell viability at low concentrations, and a dose-dependent decrease at higher concentrations14. Interestingly, osteogenic populations tested in the current study were more sensitive than oral mucosa cells in our previous work, which did not proliferate but remained viable in the presence of 10−4 M pamidronate24.
In addition to decreased cell proliferation and viability, pamidronate treatment also decreased alkaline phosphatase activity of alveolar osteoblasts (Fig. 3). Alkaline phosphatase activity is an early marker of the osteogenic phenotype, which increases at the onset of bone matrix maturation phase during in vitro and in vivo osteogenesis. We have detected a slightly higher threshold of pamidronate inhibition (6 × 10−5 M) with the cells cultured in osteogenic medium as compared to the cells in control medium (3 − 10−5 M). This difference could possibly be attributed to L-ascorbic acid supplementation, which has been shown to promote bone matrix deposition and continuous proliferation of human osteoblasts during osteogenesis in vitro34, and might partially rescue the effects of pamidronate. The absence of anabolic effects on alkaline phosphatase activity with lower pamidronate concentrations corresponds to previous studies with mice calvarial osteoblasts29 and human mandibular BMSCs14. In order to understand the broader effects of pamidronate on osteogenesis of alveolar osteoblasts, further studies should be aimed at testing an array of early and late markers of osteoblastic phenotype, such as collagen type I synthesis, expression of bone matrix proteins (i.e. bone sialoprotein, osteocalcin) and potential for matrix mineralization.
One of the main mechanisms of BPs actions is the induction of osteoclast apoptosis46. Recent reports suggested apoptosis of mice calvarial osteoblasts and human long bone osteoblasts after treatment with pamidronate25, 29, and led us to explore if considerably decreased cell survival observed in our cultures could result from the induction of apoptosis. We used three complementary methods to evaluate the cells undergoing apoptosis: TUNEL assay to evaluate nuclear fragmentation, DAPI staining to visualize chromatin condensation, and caspase-3 assay to detect activation of apoptotic cascade. We observed significant increases in the number of apoptotic cells after 72h pamidronate treatment both in alveolar osteoblasts and in BMSCs (Fig.4). Notably, levels of apoptosis detected by TUNEL and caspase-3 assays were corresponding (15–20%) and suggested that only a part of cell population undergoes programmed cell death. In previous studies, 80% of mice calvarial osteoblasts and 40% of human long bone osteoblasts underwent apoptosis when exposed to pamidronate for 48h and 72h25, 29.
The large decrease in cell viability after 72h pamidronate treatment (Fig.2), suggests that apoptosis may not be the only mechanism of pamidronate toxicity in human alveolar osteoblasts and BMSCs. It has previously been suggested that pamidronate could affect cells by mechanisms other than mevalonate pathway inhibition, possibly by forming toxic complexes on the cell surface30, 31, 47. For example, treatment of cells with three times higher pamidronate concentration did not significantly increase or accelerate apoptosis induction in either alveolar osteoblasts or BMSCs (Fig.4 F,L). (deleted text)
Finally, we aimed to assess whether pamidronate treatment would affect wound healing responses of alveolar osteoblasts and iliac/long bone BMSCs in an in vitro model, since traumatic injury has been associated with the development of ONJ. We observed an absence of wound healing by alveolar osteoblasts and BMSCs at 10−4 M pamidronate, accompanied by complete cell detachment from the culture plates (Fig.5). Additionally, BMSCs exhibited incomplete wound closure at 6 × 10−5 M pamidronate, presumably due to limited cell proliferation at this concentration (Fig.2).
Notably, results from the current study differ significantly from our previous observations with mouse oral mucosa cells24, where apoptosis was not noted in oral mucosa cells after treatment with the same concentrations of pamidronate. Additionally, both intramembranous and endochondral derived osteogenic cells exhibited higher sensitivity to pamidronate than oral mucosa cells, which exhibited inhibited healing responses, but reached complete wound closure after 5 days of incubation24. In recent studies with human cells, treatment with 5 × 10−5 M pamidronate resulted in apoptosis of human oral fibroblasts; however the same concentration did not cause apoptosis but resulted in senescence of human oral keratinocytes22, 23.
At the present time it is unclear whether the ONJ lesion initiates in the bone or the soft tissue. Our data indicating a high proliferation activity and sensitivity of alveolar osteoblasts to pamidronate is in line with the previous work suggesting dysregulation of mandible bone homeostasis14, and development of ONJ from within the bone (“inside-out” theory). However, taken together with the growing amount of work indicating potential adverse effects of BPs on the soft tissues21–25, it seems likely that ONJ is a multifactorial disease, initiated and/or negatively affected by surgically-induced trauma.
CONCLUSIONS
Pamidronate inhibited cell viability, proliferation and wound healing, and induced apoptosis in primary human alveolar osteoblasts and iliac/long bone BMSC. In light of the high remodeling rates of alveolar bone, our data suggest that pamidronate treatment could excert negative effects if high concentrations (10−4 M or above) were reached in vivo for prolongued periods of time. Significant differences in proliferation of primary osteoblasts were observed during early passages in vitro, possibly reflecting higher remodeling activity of alveolar bone compared to iliac/long bone. The absence of anabolic effects of pamidronate, and induction of apoptosis in osteogenic cell populations could negatively affect bone balance, with implications to ONJ development and treatment. Future work is needed to further assess the molecular mechanisms of pamidronate toxicity in human alveolar osteoblasts, and to develop strategies for counteracting the inhibitory effects of pamidronate.
ACKNOWLEDGMENTS
We thank Srikala Raghavan, Warren Grayson and Mirjam Fröhlich for their help with experimental setup and discussions. This work was supported by the NIH funding (R21-DEO17164 to RL; EB002520 and DE016525 to GVN) and the New York Stem Cell Foundation (DM is NYSCF-Stanley and Fiona Druckenmiller fellow).
ABBREVIATIONS
- BPs
Bisphosphonates
- ONJ
Osteonecrosis of the jaw
- BMSCs
Bone marrow stromal cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Marx RE, Sawatari Y, Fortin M, et al. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005;63:1567–75. doi: 10.1016/j.joms.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Ruggiero SL, Dodson TB, Assael LA, et al. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws--2009 update. J Oral Maxillofac Surg. 2009;67:2–12. doi: 10.1016/j.joms.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–91. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 4.Kyle RA, Yee GC, Somerfield MR, et al. American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2007;25:2464–72. doi: 10.1200/JCO.2007.12.1269. [DOI] [PubMed] [Google Scholar]
- 5.Patel V, McLeod NM, Rogers SN, et al. Bisphosphonate osteonecrosis of the jaw-a literature review of UK policies versus international policies on bisphosphonates, risk factors and prevention. Br J Oral Maxillofac Surg. 2010 doi: 10.1016/j.bjoms.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Ruggiero SL, Dodson TB, Assael LA, et al. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaw - 2009 update. Aust Endod J. 2009;35:119–30. doi: 10.1111/j.1747-4477.2009.00213.x. [DOI] [PubMed] [Google Scholar]
- 7.Ruggiero SL, Fantasia J, Carlson E. Bisphosphonate-related osteonecrosis of the jaw: background and guidelines for diagnosis, staging and management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:433–41. doi: 10.1016/j.tripleo.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Yoneda T, Hagino H, Sugimoto T, et al. Bisphosphonate-related osteonecrosis of the jaw: position paper from the Allied Task Force Committee of Japanese Society for Bone and Mineral Research, Japan Osteoporosis Society, Japanese Society of Periodontology, Japanese Society for Oral and Maxillofacial Radiology, and Japanese Society of Oral and Maxillofacial Surgeons. J Bone Miner Metab. 2010;28:365–83. doi: 10.1007/s00774-010-0162-7. [DOI] [PubMed] [Google Scholar]
- 9.Lesclous P, Abi Najm S, Carrel JP, et al. Bisphosphonate-associated osteonecrosis of the jaw: a key role of inflammation? Bone. 2009;45:843–52. doi: 10.1016/j.bone.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Akintoye SO, Lam T, Shi S, et al. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 2006;38:758–68. doi: 10.1016/j.bone.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 11.Everts V, Korper W, Hoeben KA, et al. Osteoclastic bone degradation and the role of different cysteine proteinases and matrix metalloproteinases: differences between calvaria and long bone. J Bone Miner Res. 2006;21:1399–408. doi: 10.1359/jbmr.060614. [DOI] [PubMed] [Google Scholar]
- 12.Suttapreyasri S, Koontongkaew S, Phongdara A, et al. Expression of bone morphogenetic proteins in normal human intramembranous and endochondral bones. Int J Oral Maxillofac Surg. 2006;35:444–52. doi: 10.1016/j.ijom.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 13.van den Bos T, Speijer D, Bank RA, et al. Differences in matrix composition between calvaria and long bone in mice suggest differences in biomechanical properties and resorption: Special emphasis on collagen. Bone. 2008;43:459–68. doi: 10.1016/j.bone.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Stefanik D, Sarin J, Lam T, et al. Disparate osteogenic response of mandible and iliac crest bone marrow stromal cells to pamidronate. Oral Dis. 2008;14:465–71. doi: 10.1111/j.1601-0825.2007.01402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giuliani N, Pedrazzoni M, Negri G, et al. Bisphosphonates stimulate formation of osteoblast precursors and mineralized nodules in murine and human bone marrow cultures in vitro and promote early osteoblastogenesis in young and aged mice in vivo. Bone. 1998;22:455–61. doi: 10.1016/s8756-3282(98)00033-7. [DOI] [PubMed] [Google Scholar]
- 16.Im GI, Qureshi SA, Kenney J, et al. Osteoblast proliferation and maturation by bisphosphonates. Biomaterials. 2004;25:4105–15. doi: 10.1016/j.biomaterials.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 17.Reinholz GG, Getz B, Pederson L, et al. Bisphosphonates directly regulate cell proliferation, differentiation, and gene expression in human osteoblasts. Cancer Res. 2000;60:6001–7. [PubMed] [Google Scholar]
- 18.von Knoch F, Jaquiery C, Kowalsky M, et al. Effects of bisphosphonates on proliferation and osteoblast differentiation of human bone marrow stromal cells. Biomaterials. 2005;26:6941–9. doi: 10.1016/j.biomaterials.2005.04.059. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Moreno C, Serrano S, Nacher M, et al. Effect of alendronate on cultured normal human osteoblasts. Bone. 1998;22:233–9. doi: 10.1016/s8756-3282(97)00270-6. [DOI] [PubMed] [Google Scholar]
- 20.Kellinsalmi M, Monkkonen H, Monkkonen J, et al. In vitro comparison of clodronate, pamidronate and zoledronic acid effects on rat osteoclasts and human stem cell-derived osteoblasts. Basic Clin Pharmacol Toxicol. 2005;97:382–91. doi: 10.1111/j.1742-7843.2005.pto_176.x. [DOI] [PubMed] [Google Scholar]
- 21.Walter C, Klein MO, Pabst A, et al. Influence of bisphosphonates on endothelial cells, fibroblasts, and osteogenic cells. Clin Oral Investig. 2010;14:35–41. doi: 10.1007/s00784-009-0266-4. [DOI] [PubMed] [Google Scholar]
- 22.Cozin M, Pinker BM, Solemani K, et al. Novel Therapy To Reverse The Cellular Effects of Bisphosphonates on Primary Human Oral Fibroblasts. J Oral Maxillofac Surg. 2011 doi: 10.1016/j.joms.2011.03.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim RH, Lee RS, Williams D, et al. Bisphosphonates Induce Senescence in Normal Human Oral Keratinocytes. J Dent Res. 2011 doi: 10.1177/0022034511402995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landesberg R, Cozin M, Cremers S, et al. Inhibition of oral mucosal cell wound healing by bisphosphonates. J Oral Maxillofac Surg. 2008;66:839–47. doi: 10.1016/j.joms.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walter C, Pabst A, Ziebart T, et al. Bisphosphonates affect migration ability and cell viability of HUVEC, fibroblasts and osteoblasts in vitro. Oral Dis. 2010 doi: 10.1111/j.1601-0825.2010.01720.x. [DOI] [PubMed] [Google Scholar]
- 26.Ziebart T, Pabst A, Klein MO, et al. Bisphosphonates: restrictions for vasculogenesis and angiogenesis: inhibition of cell function of endothelial progenitor cells and mature endothelial cells in vitro. Clin Oral Investig. 2011;15:105–11. doi: 10.1007/s00784-009-0365-2. [DOI] [PubMed] [Google Scholar]
- 27.Cremers SC, Papapoulos SE, Gelderblom H, et al. Skeletal retention of bisphosphonate (pamidronate) and its relation to the rate of bone resorption in patients with breast cancer and bone metastases. J Bone Miner Res. 2005;20:1543–7. doi: 10.1359/JBMR.050522. [DOI] [PubMed] [Google Scholar]
- 28.Hoff AO, Toth BB, Altundag K, et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23:826–36. doi: 10.1359/JBMR.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Idris AI, Rojas J, Greig IR, et al. Aminobisphosphonates cause osteoblast apoptosis and inhibit bone nodule formation in vitro. Calcif Tissue Int. 2008;82:191–201. doi: 10.1007/s00223-008-9104-y. [DOI] [PubMed] [Google Scholar]
- 30.Suri S, Monkkonen J, Taskinen M, et al. Nitrogen-containing bisphosphonates induce apoptosis of Caco-2 cells in vitro by inhibiting the mevalonate pathway: a model of bisphosphonate-induced gastrointestinal toxicity. Bone. 2001;29:336–43. doi: 10.1016/s8756-3282(01)00589-0. [DOI] [PubMed] [Google Scholar]
- 31.van Beek ER, Cohen LH, Leroy IM, et al. Differentiating the mechanisms of antiresorptive action of nitrogen containing bisphosphonates. Bone. 2003;33:805–11. doi: 10.1016/j.bone.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Marolt D, Augst A, Freed LE, et al. Bone and cartilage tissue constructs grown using human bone marrow stromal cells, silk scaffolds and rotating bioreactors. Biomaterials. 2006;27:6138–49. doi: 10.1016/j.biomaterials.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 33.Malicev E, Marolt D, Kregar Velikonja N, et al. Growth and differentiation of alveolar bone cells in tissue-engineered constructs and monolayer cultures. Biotechnol Bioeng. 2008;100:773–81. doi: 10.1002/bit.21815. [DOI] [PubMed] [Google Scholar]
- 34.Siggelkow H, Rebenstorff K, Kurre W, et al. Development of the osteoblast phenotype in primary human osteoblasts in culture: comparison with rat calvarial cells in osteoblast differentiation. J Cell Biochem. 1999;75:22–35. [PubMed] [Google Scholar]
- 35.Kidd VJ, Lahti JM, Teitz T. Proteolytic regulation of apoptosis. Semin Cell Dev Biol. 2000;11:191–201. doi: 10.1006/scdb.2000.0165. [DOI] [PubMed] [Google Scholar]
- 36.Silverman SL, Landesberg R. Osteonecrosis of the jaw and the role of bisphosphonates: a critical review. Am J Med. 2009;122:S33–45. doi: 10.1016/j.amjmed.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Scheller EL, Krebsbach PH, Kohn DH. Tissue engineering: state of the art in oral rehabilitation. J Oral Rehabil. 2009;36:368–89. doi: 10.1111/j.1365-2842.2009.01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berenson JR, Rosen L, Vescio R, et al. Pharmacokinetics of pamidronate disodium in patients with cancer with normal or impaired renal function. J Clin Pharmacol. 1997;37:285–90. doi: 10.1002/j.1552-4604.1997.tb04304.x. [DOI] [PubMed] [Google Scholar]
- 39.Plotkin LI, Manolagas SC, Bellido T. Dissociation of the pro-apoptotic effects of bisphosphonates on osteoclasts from their anti-apoptotic effects on osteoblasts/osteocytes with novel analogs. Bone. 2006;39:443–52. doi: 10.1016/j.bone.2006.02.060. [DOI] [PubMed] [Google Scholar]
- 40.Hoggarth CR, Bennett R, Daley-Yates PT. The pharmacokinetics and distribution of pamidronate for a range of doses in the mouse. Calcif Tissue Int. 1991;49:416–20. doi: 10.1007/BF02555853. [DOI] [PubMed] [Google Scholar]
- 41.Leyvraz S, Hess U, Flesch G, et al. Pharmacokinetics of pamidronate in patients with bone metastases. J Natl Cancer Inst. 1992;84:788–92. doi: 10.1093/jnci/84.10.788. [DOI] [PubMed] [Google Scholar]
- 42.Masarachia P, Weinreb M, Balena R, et al. Comparison of the distribution of 3H-alendronate and 3H-etidronate in rat and mouse bones. Bone. 1996;19:281–90. doi: 10.1016/8756-3282(96)00182-2. [DOI] [PubMed] [Google Scholar]
- 43.Roelofs AJ, Coxon FP, Ebetino FH, et al. Fluorescent Risedronate Analogs Reveal Bisphosphonate Uptake by Bone Marrow Monocytes and Localization Around Osteocytes In Vivo. J Bone Miner Res. 2009 doi: 10.1359/jbmr.091009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coxon FP, Thompson K, Roelofs AJ, et al. Visualizing mineral binding and uptake of bisphosphonate by osteoclasts and non-resorbing cells. Bone. 2008;42:848–60. doi: 10.1016/j.bone.2007.12.225. [DOI] [PubMed] [Google Scholar]
- 45.Declercq H, Van den Vreken N, De Maeyer E, et al. Isolation, proliferation and differentiation of osteoblastic cells to study cell/biomaterial interactions: comparison of different isolation techniques and source. Biomaterials. 2004;25:757–68. doi: 10.1016/s0142-9612(03)00580-5. [DOI] [PubMed] [Google Scholar]
- 46.Russell RG, Watts NB, Ebetino FH, et al. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19:733–59. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- 47.Twiss IM, Pas O, Ramp-Koopmanschap W, et al. The effects of nitrogen-containing bisphosphonates on human epithelial (Caco-2) cells, an in vitro model for intestinal epithelium. J Bone Miner Res. 1999;14:784–91. doi: 10.1359/jbmr.1999.14.5.784. [DOI] [PubMed] [Google Scholar]