Abstract
Purpose
The ethnically-diverse Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study cohort provides a unique opportunity to explore associations among intraocular pressure (IOP), ethnicity, and refractive error while adjusting for potential confounding variables.
Methods
Mixed linear models were used to examine the effect of age, refractive error (cycloplegic autorefraction), ethnicity, sex, and measurement protocol on IOP (Tono-pen) in 3,777 children, aged 6-14 years at their first CLEERE visit (1995-2009). Children who became myopic during follow-up were used to examine the relationship between time since myopia onset and IOP. Clinically meaningful differences in IOP were preset at > 2 mm Hg.
Results
IOP differed among refractive error categories with higher IOP in children with low/moderate myopia than those with high hyperopia (differences < 1 mm Hg). There was a statistically significant relationship between age and IOP that depended on ethnicity (interaction p<0.0001) and measurement protocol (interaction p<0.0001). The relationship between sex and IOP depended on measurement protocol (interaction p=0.0004). For children who became myopic during follow-up, the adjusted mean IOP showed a significant decline for only Asian (p=0.024) and White children (p=0.004). As with other statistically significant results, these changes in mean adjusted IOPs from two years before to two years after myopia onset were < 2 mm Hg.
Conclusions
Small but significant differences in IOP by refractive error category were found in this ethnically diverse cohort of children. Relationships between IOP and age, ethnicity, sex, and measurement protocol were complicated by significant interactions between these parameters. Longitudinal analysis of children before and after myopia onset showed changes in IOP over time that varied by ethnicity. Higher IOPs before and at myopia onset were not present in all ethnic groups, with differences before and after onset too small to suggest a role for IOP in the onset of myopia.
Keywords: intraocular pressure, children, ethnicity, refractive error, Tono-Pen
An association between intraocular pressure (IOP) and refractive error has been reported for children1-3, young adults2,4, and presbyopic adults5-7. IOP has also long been hypothesized as a mechanism for the development of myopia1,8-10. If IOP plays a role in myopia development, then IOP should be higher before compared to after the onset of myopia; however, two small, longitudinal studies in young children have not provided support for this hypothesis. Edwards and Brown11 reported on 13 children who were not myopic at age 7 years but were myopic by age 9 years and found that the IOP was significantly higher at 9 years of age than at age 7. There was no change in IOP over the same age and time period in 82 non-myopic children. Goss and Caffey12 enrolled 87 children without myopia and followed them every 6 months for 3 years. There was no statistically significant difference in IOP in the group of “future” myopes (n=24) at the last study visit before the child became myopic when compared to the children who did not become myopic (n=53).
A recent study of intraocular pressure (IOP) in a subgroup of myopic children enrolled in the Correction of Myopia Evaluation Trial (COMET) reported differences in IOP between ethnic groups with significantly higher IOP in African-American children compared to Hispanic and White children13. The IOP of the COMET subgroup decreased over a 5-year follow-up period, but IOP was not associated with the amount of myopia at baseline or with myopic progression13.
The Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study is a large, ethnically-diverse, volunteer sample of children followed annually for up to eight years with standardized measures of cycloplegic refractive error, axial length, and IOP. The purpose of this report is to explore the relationships among IOP, refractive error, age, sex, and ethnicity while controlling for suspected confounding variables in this large sample.
Methods
Subjects
Potential subjects included children 6 to 16 years of age participating in the Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study examined between 1997 and 2009 and children participating in the Orinda Longitudinal Study of Myopia (OLSM) after 1995 when IOP measurement was added to the protocol. OLSM, a cohort study of ocular component development and risk factors for the onset of myopia in children, began in 1989 in Orinda, CA. At that time, the Orinda community was predominantly white. To improve generalizability with respect to ethnicity, four additional sites were added to recruit African-American children (Eutaw, AL); Asian children (Irvine, CA); Hispanic children (Houston, TX); and Native-American children (Tucson, AZ), and OLSM became the CLEERE study. Each affiliated university's institutional review board (The Ohio State University; University of California, Berkeley; University of Alabama at Birmingham; Southern California College of Optometry; University of Houston; and the University of Arizona) approved informed consent documents in accordance with the tenets of the Declaration of Helsinki. Parents provided consent and the children verbal assent prior to participation in the study. At each of the 5 centers, children were recruited through cooperation with a local school and tested at school during school hours with the exception of the 2001-2002 to 2005-2006 school years in Irvine, CA when children were tested at a site near their school. Children were enrolled provided the principal investigator at the site felt the child could complete the entire CLEERE study protocol. The pool of potential subjects for all analyses was 4,506 children with IOP on at least one visit who generated 19,812 visits between 1995 and 2009.
Ethnic group designation was determined by parent report on a medical history form where parents selected one of the following six ethnic designations (based on categories used by the National Institutes of Health in 1997): American Indian or Alaskan Native; Asian or Pacific Islander; Black, not of Hispanic origin; Hispanic; White, not of Hispanic origin; or other. When parents identified their child with more than one ethnicity, the child was classified by the ethnic group that was most prevalent at the site provided that the site's targeted ethnicity was one of those identified by the parent (1.7% of subjects). If more than one ethnic designation was provided but none of the ethnicities included the site's targeted recruitment, ethnicity was assigned to the non-white ethnicity. Any missing parent-reported ethnicity was assessed by the investigator (5.7%) by observation, or, in some cases, by questioning the child about their parents and/or grandparents. Agreement between parent-reported ethnicity and investigator-determined ethnicity has been previously reported to be excellent in this investigator group14. When parents classified their child as “other” without additional information the child was excluded from the data set reported here (167 visits of 47 subjects). Age was determined by the child's birthdate as reported by the parent on a medical history form. Because of the small number of children at each tail of the age distribution, only those subjects ages 6 to 14 years at their last birthday were included in the analysis (19,403 visits of 4,441 subjects).
Procedures
Intraocular pressure was measured in the right eye with a Tono-Pen XL (Mentor Ophthalmic). Children with iris color darker than grade 215 were measured immediately following one drop of 0.5% proparacaine and 1 drop each of 1% tropicamide and 1% cyclopentolate. The IOP of children with iris color of grade 1 or 2 was measured immediately following one drop of 0.5% proparacaine and 1 drop of 1% tropicamide. Five minutes later these children with light irises received a second drop of 1% tropicamide to obtain adequate cycloplegia for refractive error assessment.
The sites handled and reported abnormal IOP values differently. Some sites took and reported only one estimate of IOP (protocol 1). Other sites repeated IOP if the first measure was high (typically >20 mm Hg), the standard deviation of the estimate was large, and/or the child appeared apprehensive (holding breath, resistant to lid holding) and reported IOP as either the second estimate or the value that in his/her clinical judgment reflected the most accurate assessment of IOP (protocol 2). These site differences in measurement protocol were considered during the analysis.
Refractive error of the right eye was assessed by cycloplegic autorefraction, 25 to 30 minutes after initial drop instillation. Trained and certified examiners measured central refractive error with an open field auto refractor (Canon R1 between 1995 and 2000, [Canon USA, Lake Success, NY; no longer manufactured] or Grand Seiko autorefractor from 2001 to 2007, [WR 5100-K, Grand Seiko Co., Hiroshima, Japan]). The child fixated a row of 6/9 (20/30) letters on a near card viewed through a + 4.00 D Badal lens. The card was first placed at optical infinity and then moved to a position of maximum clarity. At least 10 readings of the right eye were obtained according to the standard CLEERE protocol for cycloplegic autorefraction16. Spurious readings were eliminated according to the protocol previously described16. Axial length was measured with an Allergan-Humphrey A-scan (model 820, San Leandro, CA) in semi-automatic mode.
Statistical Analysis
All statistical analyses were performed using SAS Version 9.2. Mixed linear models were used to examine the effect of age, refractive error, ethnicity, sex, and measurement protocol on IOP. Refractive error was based on the refractive error in the two principal meridians as described in Table 1. This classification eliminated visits where small amounts of simple hyperopic astigmatism, simple myopic astigmatism, and mixed astigmatism were present (7,115 visits) resulting in a sample of 3,777 children with 12,288 visits available for analysis. This categorical classification did not eliminate those with compound myopic or compound hyperopic astigmatism if both meridians fell within the categories specified in Table 1 and was employed to eliminate those visits where the refractive error was primarily astigmatic (e.g. low amounts of mixed astigmatism). One concern with using a classification other than spherical equivalent is the possibility of eliminating a disproportionate number of measures among the various ethnic groups. The refractive error based on each principal meridian reduced the sample relatively uniformly across ethnicity with elimination rates varying from 31% for African American visits up to 45% for Native Americans. A second analysis of refractive error and IOP was conducted using the spherical equivalent refractive error as a continuous variable that included all visits with a measurable refractive error (19,403 visits of 4,441 subjects).
Table 1.
Adjusteda mean IOP with 95% confidence interval (CI), by refractive error category.
| Refractive error category | # visits | Mean IOP (mm Hg) | 95% CI |
|---|---|---|---|
| High Myopia (≥ -4.00 D both meridians) | 324 | 17.46 | 17.03, 17.90 |
| Low/Moderate Myopia (≥ -0.75 D, < -4.00 D both meridians) | 2690 | 17.39 | 17.20, 17.57 |
| Emerging Myopia (≥ -0.25 D, < -0.75 D both meridians) | 518 | 17.16 | 16.86, 17.47 |
| Emmetropia (< -0.25 D, < +1.00 D both meridians) | 6629 | 17.26 | 17.14, 17.38 |
| Low Hyperopia (≥ +1.00 D, <+2.50 D both meridians) | 1877 | 17.00 | 16.79, 17.20 |
| High Hyperopia (≥ +2.50 D both meridians) | 250 | 16.59 | 16.07, 17.12 |
Adjusted for age, sex, protocol and ethnicity
To examine any potential changes in IOP with the onset of myopia, cases of incident myopia were identified. Incident myopia was defined as those participants with at least one study visit with -0.75 D or more myopia in both meridians, with at least one previous non-myopic visit. The visit at which myopia first presented (the onset visit) was identified (time=0), and data for two years prior and two years after were used in analysis. Six hundred ninety-six participants with 2,659 study visits were classified with incident myopia. Mixed linear models that included protocol, sex, ethnicity, and spherical equivalent refractive error were used to examine the relationship between time since myopia onset and IOP with the null hypothesis being that the mean IOP was the same at each time point. Additional analyses were also performed to compare the IOP measurements obtained from children with incident myopia during this time period to age-, sex-, protocol-, and ethnicity-matched children with stable emmetropia. Emmetropia was defined as refractive error in both meridians between -0.25 D and +1.00 D (exclusive) at all study visits.
Due to the large sample size, small differences in IOP may be statistically significant but not clinically relevant. Therefore, a priori, a clinically meaningful difference was set at 2 mm Hg. Although this criterion is arbitrary, the selection was based on a review of the literature17,18 and consultation with the study investigators; common methods used to base decisions concerning clinically meaningful differences. Similarly, the alpha level for statistical significance for post hoc pair-wise comparisons was set at a more conservative value of 0.01 (i.e., pair-wise comparisons are statistically significant if p-value < 0.01). As with the clinically relevant difference, there are a variety of methods to select a significant p-value for large samples and multiple comparisons. Some of these methods (e.g. Bonferroni correction) would require a different adjustment when the number of comparisons with a group differ (e.g., refractive error, ethnicity). Therefore, all p values are provided in the text to allow for other interpretations of the clinical meaningfulness and the statistical significance of the comparisons reported.
Results
IOP and Refractive Error
There was a statistically significant relationship between refractive error category and IOP (p=0.011). As can be seen in Table 1, individuals with high myopia had the highest IOP and those with high hyperopia the lowest IOP.
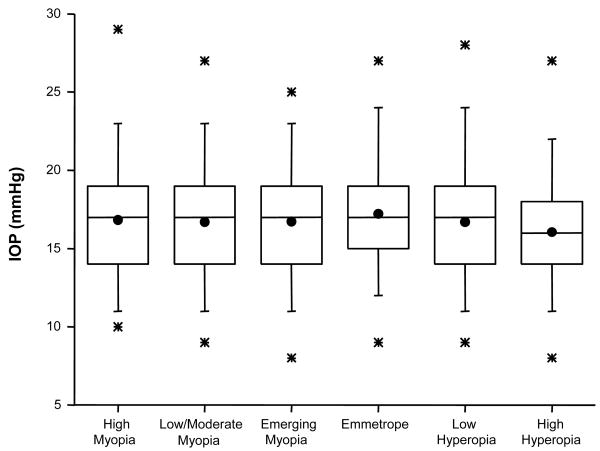
Pair-wise comparisons indicate that significant differences only existed between those with low/moderate myopia and those with either hyperopia (p=0.003) or high hyperopia (p=0.005). The largest difference (0.87 mm Hg), between individuals with high myopia and high hyperopia, fell just short of reaching statistical significance (p=0.012) due to the smaller sample sizes in each category and was far less than the preset 2-mm Hg difference considered to be clinically meaningful. These small differences are more clearly visualized in Figure 1, where box and whisker plots display the distribution of IOP values by the refractive error categories specified in Table 1.
Figure 1.
Box and whisker plots for IOP in mm Hg, by refractive error in the two principal meridians as specified in Table 1. The solid dot represents the mean while the box around it represents the 25th and 75th percentiles of the distribution. The line within the box corresponds to the median. The stars above and below the box are the 1st and 99th percentile.
Treating spherical equivalent refractive error as a continuous variable in a model that controlled for age, sex, measurement protocol, and ethnicity unveiled a weak, statistically significant but clinically insignificant relationship between IOP and spherical equivalent refractive error (beta = -0.059, p = 0.011). To reach a clinically meaningful difference in IOP of 2 mm Hg would require an improbable difference of 34 D in spherical equivalent refractive error.
Replacing spherical equivalent refractive error with axial length in a model that controlled for age, sex, measurement protocol, and ethnicity showed a weak relationship between IOP and axial length (beta = 0.081, p = 0.047). As with spherical equivalent refractive error, to achieve a meaningful difference of 2 mm Hg in IOP, an unrealistic 25 mm difference in axial length would be required.
IOP and Age
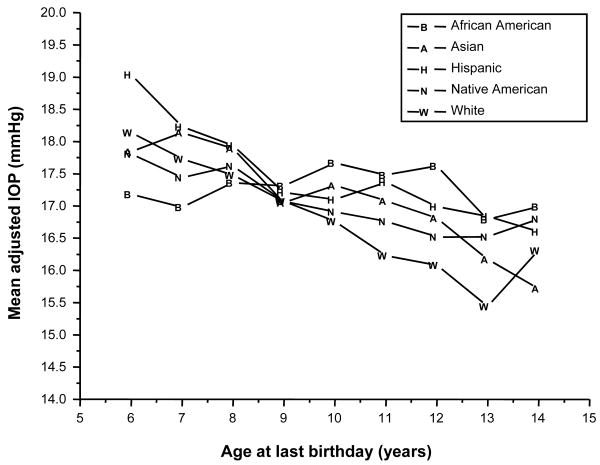
The model revealed a statistically significant relationship between age and IOP that depended on ethnicity (interaction p<0.0001) and the measurement protocol (interaction p<0.0001). The mean IOP values adjusted by age and ethnicity are shown in Table 2 and Figure 2. IOP decreased significantly with age for the African-American (p=0.006), Asian (p<0.0001), Hispanic (p<0.0001), and White (p<0.0001) children. There was no difference with age for the Native-American children (p=0.14).
Table 2.
Adjusteda mean IOP with 95% confidence interval (CI) and number of subjects, by ethnicity and age at last birthday.
| Age at last birthday | African American | Asian | Hispanic | Native American | White |
|---|---|---|---|---|---|
| 6 | 17.18 (16.55, 17.81) 218 |
17.84 (17.21, 8.48) 223 |
19.04 (18.38, 19.71) 239 |
17.81 (16.10, 19.53) 19 |
18.14 (17.64, 18.65) 358 |
| 7 | 16.98 (16.47, 17.48) 365 |
18.14 (17.56, 18.72) 285 |
18.23 (17.74, 18.72) 503 |
17.44 (16.56, 18.32) 98 |
17.73 (17.31, 18.14) 543 |
| 8 | 17.36 (16.90, 17.83) 394 |
17.89 (17.35, 18.43) 297 |
17.94 (17.49, 18.38) 582 |
17.62 (16.98, 18.26) 235 |
17.48 (17.11, 17.85) 679 |
| 9 | 17.31 (16.88, 17.75) 447 |
17.05 (16.57, 17.54) 365 |
17.21 (16.79, 17.62) 647 |
17.07 (16.52, 17.62) 345 |
17.07 (16.73, 17.42) 792 |
| 10 | 17.67 (17.27, 18.07) 505 |
17.32 (16.85, 17.79) 372 |
17.10 (16.71, 7.49) 689 |
16.91 (16.40, 17.41) 413 |
16.76 (16.41, 17.11) 792 |
| 11 | 17.48 (17.11, 17.84) 572 |
17.08 (16.61, 7.55) 352 |
17.37 (16.99, 17.75) 730 |
16.76 (16.25, 17.27) 398 |
16.23 (15.89, 16.57) 768 |
| 12 | 17.62 (17.24, 18.00) 530 |
16.81 (16.34, 17.28) 331 |
16.99 (16.60, 17.37) 707 |
16.52 (16.01, 17.04) 404 |
16.08 (15.74, 16.42) 738 |
| 13 | 16.79 (16.41, 17.16) 547 |
16.17 (15.66, 6.99) 269 |
16.84 (16.45, 17.24) 635 |
16.52 (15.96, 17.07) 350 |
15.44 (15.09, 15.79) 667 |
| 14 | 16.99 (16.48, 17.51) 260 |
15.72 (14.17, 17.27) 31 |
16.60 (16.03, 17.18) 332 |
16.80 (16.06, 17.55) 210 |
16.31 (15.65, 16.97) 167 |
Adjusted for sex, protocol and refractive error
Figure 2.
Mean IOP, by ethnic group and age at last birthday.
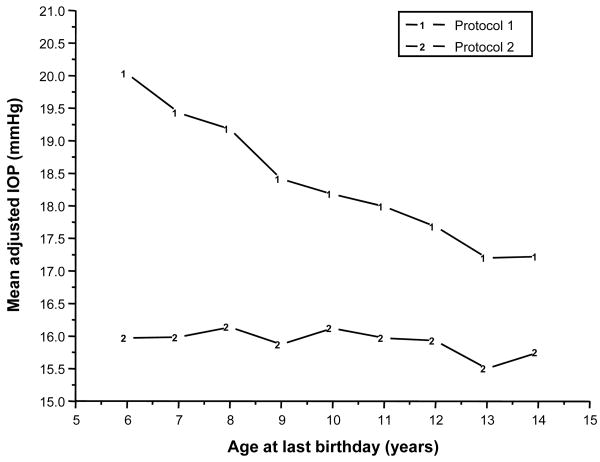
As shown in Figure 3, when a single IOP measurement was taken by Tono-Pen as in protocol 1, IOPs were lower with younger age (p<0.0001). In contrast, when the IOP measures were repeated, the effect of age was greatly reduced (protocol 2, p=0.012). Significantly higher mean IOP measurements were obtained using protocol 1 compared to protocol 2 at all ages (p<0.0001 at each age). From age 6 to 11 years, the differences between protocol 1 and 2 would be considered clinically meaningful (i.e., greater than 2 mm Hg).
Figure 3.
Mean IOP by protocol and age at last birthday.
IOP and Ethnicity
As might be anticipated from the results shown in Figure 2, ethnic differences in mean IOP varied across age (interaction p < 0.0001); however, even the largest difference between ethnic groups (1.87 mm Hg at age 6 years) was less than the pre-established clinically significant difference of 2 mm Hg. As shown in Table 3, there were significant differences among the ethnic groups in mean IOP at ages 6, 7, 10, 11, 12, and 13 years (p<0.01 at each age). As early as age 6 years, the mean IOP among African-American children was significantly lower than that of the Hispanic (p<0.001) and White (p=0.008) children. At age 7 years African-American children continued to have lower IOP than Hispanic children (0=0.002) but also had lower IOP than Asian children (p=0.004); however, from ages 10 to 13 years, African-American children had significantly higher IOP values compared to White children (p<0.001 at each age), and at age 12 years the mean IOP for African Americans was also significantly higher than Asians (p=0.009) and Native Americans (p=0.001). There were fewer differences between the other ethnic groups. At age 6 years Asian children had lower IOP than Hispanic children (p=0.004), but at age 11 they had higher IOP than White children (p<0.004). At ages 11, 12 and 13 Hispanic children had higher IOP than White children (p<0.001 at each age). At age 13 Native-American children had lower IOP values than White children (p=0.002).
Table 3.
Pair-wise comparisons of ethnic groups which are statistically significant (p<0.01), by age.
| Comparison | Difference | p-value | ||
|---|---|---|---|---|
| Age 6 | ||||
| African American | - | Hispanic | -1.87 | <0.001 |
| African American | - | White | -0.96 | 0.008 |
| Asian | - | Hispanic | -1.20 | 0.004 |
|
| ||||
| Age 7 | ||||
| African American | - | Asian | -1.16 | 0.004 |
| African American | - | Hispanic | -1.26 | 0.002 |
|
| ||||
| Age 10 | ||||
| African American | - | White | 0.91 | <0.001 |
|
| ||||
| Age 11 | ||||
| African American | - | White | 1.25 | <0.001 |
| Asian | - | White | 0.85 | 0.004 |
| Hispanic | - | White | 1.14 | <0.001 |
|
| ||||
| Age 12 | ||||
| African American | - | Asian | 0.81 | 0.009 |
| African American | - | Native American | 1.10 | 0.001 |
| African American | - | White | 1.54 | <0.001 |
| Hispanic | - | White | 0.91 | <0.001 |
|
| ||||
| Age 13 | ||||
| African American | - | White | 1.35 | <0.001 |
| Hispanic | - | White | 1.41 | <0.001 |
| Native American | - | White | -1.08 | 0.002 |
IOP and Sex
Interestingly, the relationship between sex and IOP depended on measurement protocol (interaction p=0.0004). Using protocol 1, the mean IOP in boys was 18.09 mm Hg, which was significantly lower than the mean IOP in girls (18.65 mm Hg, p<0.0001). While this difference was statistically significant, it is quite small (0.56 mm Hg) and not considered clinically meaningful. There was no difference in mean IOP between boys (15.92 mm Hg) and girls (15.91 mm Hg) for protocol 2 (p=0.90).
IOP and Myopia Onset
The longitudinal aspect of this data set was leveraged to examine the association between IOP and myopia onset by examining those children who became myopic during the observation period. Data from 696 incident myopes were available for analysis. Nearly one-third of the myopic children were Hispanic (n=214, 31%); 158 children (23%) were Asian; 147 children (21%) were White; 101 children (15%) were African American; and 76 children (11%) were Native American. IOP data at myopia onset were not available for eight Asian and nine White children. The mean age at onset of myopia ranged from 10.7 years (SD=1.8 years) among Asian children to 12.0 years (SD=2.0 years) among Native-American children. The mean spherical equivalent refractive error at onset ranged from -1.28 D (SD=0.40 years) in the African-American children to -1.60 D (SD=0.60 years) in the Asian children.
The mean IOP before onset, at onset, and after the onset of myopia are shown in Table 4 and plotted in Figure 4. These estimates are adjusted for measurement protocol and ethnicity. Sex (p=0.67) and spherical equivalent refractive error (p=0.69) were not included in the final models because neither was significantly related to IOP in this group of incident myopes.
Table 4.
Mean adjusteda IOP with 95% confidence interval and number of subjects, by time since onset of myopia and ethnicity.
| Time since onset of myopia (years) | |||||
|---|---|---|---|---|---|
| Ethnicity | -2 | -1 | 0 | +1 | +2 |
| African American | 17.31 (16.49, 18.12) 83 |
17.29 (16.54, 18.04) 98 |
17.85 (17.13, 18.58) 101 |
16.86 (16.12, 17.59) 82 |
16.93 (16.06, 17.80) 66 |
| Asian | 17.99 (17.30, 18.67) 113 |
17.84 (17.19, 18.49) 126 |
17.37 (16.77, 17.97) 150 |
17.03 (16.34, 17.71) 96 |
16.50 (15.69, 17.31) 75 |
| Hispanic | 17.84 (17.22, 18.46) 162 |
17.46 (16.89, 18.04) 199 |
17.72 (17.17, 18.28) 214 |
17.83 (17.25, 18.41) 166 |
17.92 (17.25, 18.59) 131 |
| Native American | 18.35 (17.29, 19.42) 46 |
17.48 (16.57, 18.39) 67 |
17.51 (16.66, 18.37) 76 |
17.54 (16.58, 18.50) 51 |
17.58 (16.44, 18.71) 40 |
| White | 17.48 (16.76, 18.20) 99 |
17.07 (16.41, 17.73) 119 |
16.17 (15.56, 16.78) 138 |
16.21 (15.53, 16.88) 96 |
16.09 (15.23, 16.96) 65 |
Adjusted for proto
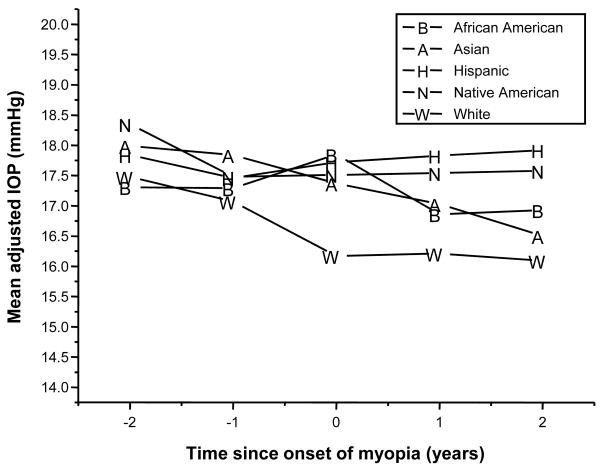
Figure 4.
Mean IOP in incident myopes before and after the onset of myopia, by ethnic group.
Statistical analysis confirmed what was visible in Figure 4 and Table 4. There was no significant change in the mean IOP across time for the African-American (p=0.24), the Native-American (p=0.61), or the Hispanic children (p=0.67) who became myopic during the course of the study. There was a significant change for both Asian (p=0.023) and White children (p=0.006). The largest change in IOP was seen in the Asian children, who showed a decline in the mean adjusted IOP of 1.49 mm Hg from two years prior to two years after myopia onset. The change in White children over the same time frame was similar to that of Asians at 1.39 mm Hg but both were less than the pre-established clinically significant difference of 2 mm Hg (Table 4).
Ethnic differences in mean IOP were apparent at the onset of myopia (p=0.001) and at both visits after the onset of myopia (p=0.013 and 0.009, respectively) but not at either pre-onset time point (p>0.50 at each time point). At onset, the mean IOP for White children was significantly lower than that observed for African-American (p=0.0004), Asian (p=0.007), and Hispanic (p<0.001) children. At both one and two years after onset of myopia, the mean IOP of Hispanics was significantly higher than the White children (p<0.001 and =0.001 respectively). In addition, two years after onset of myopia, Hispanics had a significantly higher mean IOP than the Asians (p=0.007). The largest difference between the ethnic groups occurred two years after the onset of myopia between Hispanic and White children, 1.83 mm Hg, a value that does not exceed the predetermined clinically significant difference of 2 mm Hg.
Additional analyses were performed to compare the observed changes in IOP before and after the onset of myopia to those of age-, sex-, protocol- and ethnicity-matched, stable emmetropes. The mean IOP of future myopes two years before onset was significantly greater (p<0.001) than that of emmetropes with a difference (0.57 mm Hg). No other significant differences were observed at subsequent visits (one year prior through two years post onset).
Discussion
IOP has been reported to be associated with a large number of ocular factors including refractive error1,2,4-7,19, age7,13,19-21, ethnicity13,22, sex20,21,23, and central corneal thickness22-28 however, not all investigations have found significant associations between IOP and refractive error12,29-31, age22,23,28,29,31 ethnicity32 sex4,13,19,22,29,31,33 or central corneal thickness32. It is difficult to sort out these relationships reported in the literature because of small sample sizes with limited ethnic diversity and/or age ranges. Interactions between ocular components can also complicate the relationships reported with IOP. For example, Shimmyo and coworkers22 found differences in IOP by ethnicity only after IOP was adjusted for differences in central corneal thickness that were measured between different ethnicities. Tomlinson and Phillips4 reported that IOP differed by sex, but when axial lengths of males were matched to those of the females in the sample the sex difference was no longer present. The role IOP may play in myopia has also been difficult to resolve due to small sample sizes11, limited long-term follow-up12, and minimal longitudinal data on ocular factors before and after the onset of myopia.
The data presented here from the CLEERE Study overcome a number of the limitations outlined above. The data meeting inclusion for this investigation comprise an ethnically diverse, large sample of children (4,441) that includes 696 incident myopes, of which 503 have data two years before myopia onset and 377 were measured two years after myopia onset. The range of refractive errors was large (-14.09 D to +12.63 D), and the ocular factors typically considered to influence IOP were measured and controlled for by the statistical analysis.
In this cohort, IOP varies by refractive error, with higher IOP in low/moderate myopes compared to low hyperopes and high hyperopes, when age, IOP measurement protocol, sex, and ethnicity are controlled for, but the differences are small, not clinically significant, and do not suggest a robust, meaningful association between IOP and refractive error. A weak but statistically significant relationship between IOP and spherical equivalent refractive error is also present when refractive error is treated as a continuous variable, but the relationship is clinically insignificant. These results are consistent with the literature that report small differences in IOP between refractive error categories or a correlation between refractive error and IOP in children of similar ages to the CLEERE cohort1-3,12,19. The relationship between IOP and age depends on both ethnicity and the measurement protocol. Younger children with a single estimate of IOP have higher IOPs than older children, suggesting the relationship between IOP and age is more related to the measurement protocol (perhaps reflecting apprehension to the procedure) than age over the age span of the cohort (6 to 14 years). IOP varies with ethnicity, but the differences depend on the age of the child. All the ethnic differences are small with even the largest difference still less than the pre-established clinically significant difference of 2 mm Hg. When only the myopic children are considered (Table 4 and Figure 4), the IOP of White children is lower than the other ethnic groups, but the differences are only statistically significant at the time of myopia onset and not clinically meaningful. This result is similar to that reported previously in a subgroup of the COMET cohort, a group of myopic children of similar age13. The differences in IOP between boys and girls are less than 1 mm Hg and vary with the protocol. The changes in IOP two years before, at, and two years after myopia onset vary with ethnicity. There are no changes in IOP in African-American, Hispanic or Native-American children who became myopic, but a small decrease in IOP after the onset of myopia was found in Asian and White children. Differences in IOP by ethnic group were found only at the onset of myopia. These results make it difficult to contemplate a significant role for IOP in the development of myopia. This lack of evidence for a significant role for IOP in myopia development is further supported by the results from a comparison of the mean changes in IOP before and after the onset of myopia to age-, sex-, protocol- and ethnicity-matched, stable emmetropes. A small, statistically significant, but clinically unimportant difference in the mean IOP between future myopes and emmetropes was only found two years before myopia onset with no other significant differences observed at any other visits (one year prior through two years post myopia onset), suggesting that the changes in myopic children were similar to matched emmetropes and do not support a role for IOP in myopia development.
The limitations of the current study include the use of the Tono-Pen rather than the current gold standard Goldmann tonometer to assess IOP and the lack of an assessment of central corneal thickness. The Tono-Pen was selected because of its portability and ease of use in young children. Recent studies have shown that the Tono-Pen has both reasonable repeatability18 and high testability31 in children. It should be noted than only 0.7% of all study visits were excluded from analysis due to an inability to obtain IOP measures. Another limitation is that the Tono-Pen protocol differed among clinical sites with some sites taking and recording only a single estimate of IOP while others repeated the measure if it was felt to be artificially high. This limitation was addressed through the statistical analysis but did demonstrate an interesting age effect and suggests that a single reading with the Tono-Pen in young children leads to higher estimates of IOP. Taking the average of several Tono-Pen measures may provide a better estimate of IOP, especially in young children. Although central corneal thickness was not assessed, the Tono-Pen has been reported to be less affected by variation in corneal thickness that the Goldmann tonometer25. Other limitations are that the CLEERE sample was comprised of volunteers rather than a population based study and that sites were selected to primarily target specific ethnicities creating the opportunity for confounding. These recruitment differences create the potential for site and ethnic interactions, so that if results differ among ethnic groups it is difficult to isolate intraocular pressure as the cause, when the difference could, instead, be related to the site's location (e.g. educational opportunities, socio-economic status).
Conclusions
There was a lack of clinically meaningful differences in IOP (greater than 2 mmHg) found in this large and ethnically diverse cohort despite statistical significance. Some of the largest differences found in this study were between the African-American and White children older than 10 years of age with African-American children showing higher IOPs. As African-American children are reported to have thinner corneas than White children28,32,34, the differences in the true IOP of myopic African-American children may be greater than reported here and thus may warrant additional study. Higher IOPs prior to and at the onset of myopia were not present in all ethnic groups, and the differences before and after onset were too small to suggest that IOP plays any significant role in the onset of myopia.
Acknowledgments
Supported by NIH/NEI grants U10-EY08893, the Ohio Lions Eye Research Foundation, and the E. F. Wildermuth Foundation, Columbus, OH.
The CLEERE Study Group
Clinical Centers
Franklin Primary Health Center, Inc.: Sandral Hullett, MD, MPH (Principal Investigator, 1997-2007); Robert N. Kleinstein, OD, MPH, PhD (Co-Investigator, 1997-2007); Janene Sims, OD (Optometrist, 1997-2001 and 2004-2007); Raphael Weeks, OD (Optometrist, 1999-2007); Sandra Williams (Study Coordinator, 1999-2007); LeeAndra Calvin (Study Coordinator, 1997-1999); Melvin D. Shipp, OD, MPH, DrPH (Co-Investigator, 1997-2004). Drs. Kleinstein and Sims are affiliated with the University of Alabama at Birmingham School of Optometry.
University of California, Berkeley School of Optometry, Berkeley, CA: Nina E. Friedman, OD, MS (Principal Investigator, 1999-2001); Pamela Qualley, MA (Study Coordinator, 1997-2001); Donald O. Mutti, OD, PhD (Principal Investigator, 1996-1999); Karla Zadnik, OD, PhD (Optometrist, 1996-2001).
University of Houston College of Optometry: Ruth E. Manny, OD, PhD (Principal Investigator, 1997-2007); Suzanne M. Wickum, OD (Optometrist, 1999-2007); Ailene Kim, OD (Optometrist, 2003-2007); Bronwen Mathis, OD (Optometrist, 2002-2007); Janice M. Wensveen, OD, PhD (Optometrist, 1997-2001); Connie J. Crossnoe, OD (Optometrist, 1997-2003); Stephanie L. Tom, OD (Optometrist, 1999-2002); Sally Henry (Study Coordinator, 1997-1998); Jennifer A. McLeod (Study Coordinator, 1998-2004); Mamie Batres (Study Coordinator, 2004-2007); Julio C. Quiralte (Study Coordinator, 1998-2005); Gaby Solis (Study Coordinator, 2005-2007).
Southern California College of Optometry, Fullerton, CA: Susan A. Cotter, OD, MS (Principal Investigator, 2004-2007, Optometrist, 1997-2004); Julie A. Yu, OD (Principal Investigator, 1997-2004; Optometrist 2005-2007); Raymond J. Chu, OD (Optometrist, 2001-2007); John Lee, OD (Optometrist, 2000-2003); Robert J. Lee, OD (Optometrist, 1997-2001); Raymond Maeda, OD (Optometrist, 1999-2003); Carmen N. Barnhardt, OD, MS (Optometrist 2004-2007); Jessica Chang, OD (Optometrist, 2005-2007); Kristine Huang, OD (Optometrist, 2005-2007); Connie Chu, OD (Optometrist, 2004-2005); Soonsi Kwon, OD (Optometrist, 1998-2004); Rachael Emerson (Study Coordinator, 1997-1999); Gen Lee (Study Coordinator, 1999-2003); Tracy Leonhardt (Study Coordinator, 2003-2004); Rebecca Bridgeford (Study Coordinator, 2005-2006).
University of Arizona, Department of Ophthalmology and Vision Science, Tucson, AZ: J. Daniel Twelker, OD, PhD (Principal Investigator, 2000-present); Dawn Messer, OD (Optometrist, 2000-present); Rita Bhakta, OD (Optometrist, 2000-2004); Katie Garvey, OD (Optometrist, 2006-present); Denise Flores (Study Coordinator, 2000-2007); Mabel Crescioni (2009-present).
Resource Centers and Executive Committee
Chairman's Office, The Ohio State University College of Optometry, Columbus, OH: Karla Zadnik, OD, PhD (Chairman, 1997-present); Jodi M. Malone, RN (Study Coordinator, 1997-present)
Optometry Coordinating Center, The Ohio State University College of Optometry, Columbus, OH: Lisa A. Jones-Jordan, PhD (Director, 1997-present); Linda Barrett (Data Entry Operator, 1997-2008); John Hayes, PhD (Biostatistician, 2001-2006); G. Lynn Mitchell, MAS (Biostatistician, 1998-present); Melvin L. Moeschberger, PhD (Consultant, 1997-present); Loraine Sinnott, PhD (Biostatistician, 2005-present); Pamela Wessel (Program Coordinator, 2000-present); Julie N. Swartzendruber, MA (Program Coordinator, 1998-2000).
Project Office, National Eye Institute, Rockville, MD: Donald F. Everett, MA.
Executive Committee: Karla Zadnik, OD, PhD (Chairman), Lisa A. Jones-Jordan, PhD, Robert N. Kleinstein, OD MPH PhD, Ruth E. Manny, OD PhD, Donald O. Mutti, OD PhD, J. Daniel Twelker, OD PhD, and Susan A. Cotter, OD, MS.
References
- 1.Quinn GE, Berlin JA, Young TL, Ziylan S, Stone RA. Association of intraocular pressure and myopia in children. Ophthalmology. 1995;102:180–5. doi: 10.1016/s0161-6420(95)31038-x. [DOI] [PubMed] [Google Scholar]
- 2.Abdalla MI, Hamdi M. Applanation ocular tension in myopia and emmetropia. Br J Ophthalmol. 1970;54:122–5. doi: 10.1136/bjo.54.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards MH, Brown B. Intraocular pressure in a selected sample of myopic and nonmyopic Chinese children. Optom Vis Sci. 1993;70:15–7. doi: 10.1097/00006324-199301000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Tomlinson A, Phillips CI. Applanation tension and axial length of the eyeball. Br J Ophthalmol. 1970;54:548–53. doi: 10.1136/bjo.54.8.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.David R, Zangwill LM, Tessler Z, Yassur Y. The correlation between intraocular pressure and refractive status. Arch Ophthalmol. 1985;103:1812–5. doi: 10.1001/archopht.1985.01050120046017. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell P, Hourihan F, Sandbach J, Wang JJ. The relationship between glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology. 1999;106:2010–5. doi: 10.1016/s0161-6420(99)90416-5. [DOI] [PubMed] [Google Scholar]
- 7.Nomura H, Ando F, Niino N, Shimokata H, Miyake Y. The relationship between intraocular pressure and refractive error adjusting for age and central corneal thickness. Ophthalmic Physiol Opt. 2004;24:41–5. doi: 10.1046/j.1475-1313.2003.00158.x. [DOI] [PubMed] [Google Scholar]
- 8.Young FA. The effect of atropine on the development of myopia in monkeys. Am J Optom Arch Am Acad Optom. 1965;42:439–49. doi: 10.1097/00006324-196508000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Pruett RC. Progressive myopia and intraocular pressure: what is the linkage? A literature review. Acta Ophthalmol Suppl. 1988;185:117–27. doi: 10.1111/j.1755-3768.1988.tb02685.x. [DOI] [PubMed] [Google Scholar]
- 10.Tokoro T, Funata M, Akazawa Y. Influence of intraocular pressure on axial elongation. J Ocul Pharmacol. 1990;6:285–91. doi: 10.1089/jop.1990.6.285. [DOI] [PubMed] [Google Scholar]
- 11.Edwards MH, Brown B. IOP in myopic children: the relationship between increases in IOP and the development of myopia. Ophthalmic Physiol Opt. 1996;16:243–6. [PubMed] [Google Scholar]
- 12.Goss DA, Caffey TW. Clinical findings before the onset of myopia in youth: 5. Intraocular pressure. Optom Vis Sci. 1999;76:286–91. doi: 10.1097/00006324-199905000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Manny RE, Deng L, Crossnoe C, Gwiazda J. IOP, myopic progression and axial length in a COMET subgroup. Optom Vis Sci. 2008;85:97–105. doi: 10.1097/OPX.0b013e3181622633. [DOI] [PubMed] [Google Scholar]
- 14.Jones LA, Mitchell GL, Zadnik K The CLEERE Study Group. Agreement between parent-reported and clinician-assessed race in the CLEERE Study. Control Clin Trials. 2001;22:98S. [Google Scholar]
- 15.Seddon JM, Sahagian CR, Glynn RJ, Sperduto RD, Gragoudas ES. Evaluation of an iris color classification system. The Eye Disorders Case-Control Study Group. Invest Ophthalmol Vis Sci. 1990;31:1592–8. [PubMed] [Google Scholar]
- 16.Zadnik K, Mutti DO, Friedman NE, Adams AJ. Initial cross-sectional results from the Orinda Longitudinal Study of Myopia. Optom Vis Sci. 1993;70:750–8. doi: 10.1097/00006324-199309000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Minckler DS, Baerveldt G, Heuer DK, Quillen-Thomas B, Walonker AF, Weiner J. Clinical evaluation of the Oculab Tono-Pen. Am J Ophthalmol. 1987;104:168–73. doi: 10.1016/0002-9394(87)90010-9. [DOI] [PubMed] [Google Scholar]
- 18.Khamees KM, Zadnik K. The interoccasion repeatability of intraocular pressure measurement using the Tono-Pen in a sample of school-aged children. Optom Vis Sci. 2001;78:580–3. doi: 10.1097/00006324-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Sihota R, Tuli D, Dada T, Gupta V, Sachdeva MM. Distribution and determinants of intraocular pressure in a normal pediatric population. J Pediatr Ophthalmol Strabismus. 2006;43:14–8. doi: 10.3928/01913913-20060101-01. quiz 36-7. [DOI] [PubMed] [Google Scholar]
- 20.Armaly MF. On the distribution of applanation pressure. I Statistical features and the effect of age, sex, and family history of glaucoma. Arch Ophthalmol. 1965;73:11–8. doi: 10.1001/archopht.1965.00970030013005. [DOI] [PubMed] [Google Scholar]
- 21.Pensiero S, Da Pozzo S, Perissutti P, Cavallini GM, Guerra R. Normal intraocular pressure in children. J Pediatr Ophthalmol Strabismus. 1992;29:79–84. doi: 10.3928/0191-3913-19920301-05. [DOI] [PubMed] [Google Scholar]
- 22.Shimmyo M, Ross AJ, Moy A, Mostafavi R. Intraocular pressure, Goldmann applanation tension, corneal thickness, and corneal curvature in Caucasians, Asians, Hispanics, and African Americans. Am J Ophthalmol. 2003;136:603–13. doi: 10.1016/s0002-9394(03)00424-0. [DOI] [PubMed] [Google Scholar]
- 23.Eysteinsson T, Jonasson F, Sasaki H, Arnarsson A, Sverrisson T, Sasaki K, Stefansson E. Central corneal thickness, radius of the corneal curvature and intraocular pressure in normal subjects using non-contact techniques: Reykjavik Eye Study. Acta Ophthalmol Scand. 2002;80:11–5. doi: 10.1034/j.1600-0420.2002.800103.x. [DOI] [PubMed] [Google Scholar]
- 24.Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: a review and meta-analysis approach. Surv Ophthalmol. 2000;44:367–408. doi: 10.1016/s0039-6257(00)00110-7. [DOI] [PubMed] [Google Scholar]
- 25.Bhan A, Browning AC, Shah S, Hamilton R, Dave D, Dua HS. Effect of corneal thickness on intraocular pressure measurements with the pneumotonometer, Goldmann applanation tonometer, and Tono-Pen. Invest Ophthalmol Vis Sci. 2002;43:1389–92. [PubMed] [Google Scholar]
- 26.Tong L, Saw SM, Siak JK, Gazzard G, Tan D. Corneal thickness determination and correlates in Singaporean schoolchildren. Invest Ophthalmol Vis Sci. 2004;45:4004–9. doi: 10.1167/iovs.04-0121. [DOI] [PubMed] [Google Scholar]
- 27.Kohlhaas M, Boehm AG, Spoerl E, Pursten A, Grein HJ, Pillunat LE. Effect of central corneal thickness, corneal curvature, and axial length on applanation tonometry. Arch Ophthalmol. 2006;124:471–6. doi: 10.1001/archopht.124.4.471. [DOI] [PubMed] [Google Scholar]
- 28.Muir KW, Duncan L, Enyedi LB, Freedman SF. Central corneal thickness in children: Racial differences (black vs. white) and correlation with measured intraocular pressure. J Glaucoma. 2006;15:520–3. doi: 10.1097/01.ijg.0000212284.78045.45. [DOI] [PubMed] [Google Scholar]
- 29.Puell-Marin MC, Romero-Martin M, Dominguez-Carmona M. Intraocular pressure in 528 university students: effect of refractive error [published erratum appears in J Am Optom Assoc 1997 Dec;68(12):756] J Am Optom Assoc. 1997;68:657–62. [PubMed] [Google Scholar]
- 30.Lee SM, Edwards MH. Intraocular pressure in anisometropic children. Optom Vis Sci. 2000;77:675–9. doi: 10.1097/00006324-200012000-00015. [DOI] [PubMed] [Google Scholar]
- 31.Lee AJ, Saw SM, Gazzard G, Cheng A, Tan DT. Intraocular pressure associations with refractive error and axial length in children. Br J Ophthalmol. 2004;88:5–7. doi: 10.1136/bjo.88.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haider KM, Mickler C, Oliver D, Moya FJ, Cruz OA, Davitt BV. Age and racial variation in central corneal thickness of preschool and school-aged children. J Pediatr Ophthalmol Strabismus. 2008;45:227–33. doi: 10.3928/01913913-20080701-07. [DOI] [PubMed] [Google Scholar]
- 33.Akinci A, Cetinkaya E, Aycan Z, Oner O. Relationship between intraocular pressure and obesity in children. J Glaucoma. 2007;16:627–30. doi: 10.1097/IJG.0b013e318057528a. [DOI] [PubMed] [Google Scholar]
- 34.Dai E, Gunderson CA. Pediatric central corneal thickness variation among major ethnic populations. J AAPOS. 2006;10:22–5. doi: 10.1016/j.jaapos.2005.12.007. [DOI] [PubMed] [Google Scholar]