Abstract
Purpose
We assessed factors associated with underutilization of lung protective ventilation (LPV) in patients with acute lung injury (ALI).
Methods
Secondary analysis of ARDSNet trial data, 1999-2005. Tidal volumes recorded prior to trial randomization were analyzed to determine receipt of LPV [tidal volume ≤ 6.5 cc/kg of predicted body weight (PBW)].
Results
430/1385 (31.2%) participants received LPV. Average tidal volume was 7.65±1.82 cc/kg PBW; measured tidal volumes were greater than “lung protective” tidal volumes predicted by 6.5cc/kg PBW (mean difference 67±108cc, p<0.0001). Multivariate predictors of LPV underutilization were older age [odds ratio (OR) per standard deviation (std) year 1.18 (95% confidence interval: 1.02-1.38)], white race [OR, 1.40 (1.05-1.88)], shorter stature [OR per std centimeter 0.55 (0.48-0.63)], lower Simplified Acute Physiology Score (SAPS)II [OR per std, 0.78 (0.67-0.92)], lower lung injury score [OR per std 0.83 (0.70-0.95)], decreased serum bicarbonate [OR per std mmol/l 0.83 (0.71-0.97)], shorter pre-enrollment ICU stay [OR per std day 0.84 (0.73-0.98)], and use of non-volume-controlled ventilation [OR 3.07 (1.78, 5.27)]. Setting tidal volumes to 450ml (men) or 350ml (women) would provide LPV to 80% of patients with ALI.
Conclusions
Simple interventions could substantially improve adherence with LPV among patients with ALI and warrant prospective study.
Keywords: acute lung injury, risk factors, quality improvement
INTRODUCTION
Although a decade has elapsed since publication of the landmark Acute Respiratory Distress Syndrome Clinical Trials Network (ARDSNet) “ARMA” trial in support of a lung protective ventilation (LPV) approach, LPV has been poorly adopted.[1] Previous studies have found that less than 50% of patients with acute lung injury (ALI) are ventilated with evidence-based 6cc/kg predicted body weight tidal volumes.[2-6] Two small studies have investigated specific factors associated with use of LPV. These showed that metabolic acidosis, lack of a formal ARDS protocol[5] higher lung compliance, and higher PaO2/FiO2[4] were associated with underuse of LPV. Others have hypothesized that under-recognition of ALI[7] and difficulties with calculating predicted body weight (PBW),[8-10] may be factors contributing to underuse of ALI.
Identification of barriers to implementation of evidence-based practice is a critical step towards improving patient outcomes. However, no adequately powered studies have investigated patient-level clinical and demographic factors associated with underuse of LPV. Baseline data from ARDSNet trial participants present a unique opportunity to study practice patterns across multiple centers. We sought to determine the factors associated with underuse of LPV prior to randomization of these trial participants with recognized ALI. We hypothesized that demographic (e.g., age, sex, and race), anthropometric, and severity of illness-associated factors would be predictive of LPV underutilization.
MATERIALS AND METHODS
Patients
We assembled the study cohort using open access de-identified data from participants previously enrolled in the two National Heart Lung and Blood Institute (NHLBI) ARDS Network trials conducted following release of ARMA trial[11] results that demonstrated improved outcomes with LPV. These included the Assessment of Low tidal Volume and End expiratory volume to Obviate Lung Injury (ALVEOLI) trial,[12] and the factorial Fluid and Catheter Treatment Trials (FACCT).[13,14] Data from the Late Steroid Rescue Study (LaSRS),[15] which began enrollment prior to release of ARMA findings,[15] were not used for the present study.
The ARDSNet is comprised of 42 hospitals across the United States; details regarding protocols of ARDSNet studies can be found at the ARDSNet.org website. All study procedures were approved by the Boston University School of Medicine Institutional Review Board as well as the NHLBI Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC).
Baseline demographics and clinical information were obtained prior to randomization of ARDSNet trial participants. This data was collected ideally from within 4 hours of study initiation, with allowance for up to 24 hours prior to randomization. This “baseline” data was used as the source data for the current research because it represents the care of patients after they have met American European Consensus ALI criteria,[16] but prior to initiation of mechanical ventilation per study protocols (which mandated use of LPV for all enrolled patients).
Outcome
Lung protective ventilation (LPV) was defined in the primary analysis as tidal volume of less than 6.5 cc/kg PBW. ARDSNet calculated PBW for each patient based on height and sex. The choice of the threshold of 6.5cc/kg to represent LPV is supported by literature precedent,[2,5] proximity to the mean tidal volumes (6.2-6.5 cc/kg PBW) actually delivered to the ARMA low tidal volume intervention group, and the fact that 6.5cc/kg PBW was selected by the ARDSNet investigators as the threshold for defining “on target” 6cc/kg ventilation for internal monitoring of the ARMA trial (B. Taylor Thompson, personal communication, 5/31/2011). Two sensitivity analyses to evaluate stability of predictors of LPV were performed: 1) using a tidal volume cutoff of less than or equal to 8cc/kg, which was recommended as the initial tidal volume in the ARDSNet ARMA Mechanical Ventilation Protocol and was allowed if a patient developed breath stacking or ventilator dys-synchrony to 6cc/kg PBW and plateau pressures remained less than 30 cm H2O (ARDSNet.org ventilator protocol), and 2) using cc/kg PBW as a continuous dependent variable.
Covariates
We compared pre-randomization characteristics of those participants who received LPV with those that did not. These characteristics included demographics and body morphology: [age, sex, race, body mass index (BMI), height, and weight], comorbidities [diabetes, acquired immune deficiency syndrome, end stage renal disease (dialysis requirement), and end stage liver disease], primary acute lung injury risk factor [characterized as direct lung injury (pneumonia, aspiration) or indirect lung injury (sepsis, trauma, transfusions)], number of quadrants affected by infiltrate on frontal chest radiograph (i.e., radiographic lung injury score), the duration of mechanical ventilation, and duration of intensive care unit stay prior to study enrollment. Baseline Simplified Acute Physiology II Scores (SAPS II)[17] and Brussels multiple organ dysfunction scores[18] were calculated for all participants. Because tidal volume setting may affect PaO2/FiO2,[11] airway pressures, lung compliance[19], PaCO2, and minute ventilation, as well as influence choice of positive end expiratory pressure (PEEP), these mechanical ventilation variables were not included as potential predictors of LPV.
Statistical Methods
In order to achieve 80% power to identify risk factors for use of LPV with an odds ratio of at least 1.50 at an alpha of 0.05, 1120 subjects were required.
Wilcoxon rank sum tests, t-tests and Fisher exact tests were used to determine differences in continuous and categorical variables, as applicable. In order to visually demonstrate differences in the distribution of actual and 6.5 cc/kg-predicted tidal volumes (calculated from PBW), we generated histograms of delivered tidal volumes and tidal volumes calculated from PBW, stratified by sex. Associations between LPV and clinical covariates were determined through multivariable logistic regression models. Associations between tidal volume as a continuous variable of cc/kg PBW and clinical covariates were determined through Analysis of Covariance. For all multivariable models, covariates with unadjusted p values less than 0.2 were entered into the model and remained in the model via backward selection if p was less than 0.05, the alpha threshold for statistical significance. SAS version 9.1 statistical software (Cary, NC) was used for all analyses.
RESULTS
A baseline tidal volume and PBW was recorded for 1385/1550 (89%) of trial participants. The study cohort had an average age of 50 ± 16 years and was 54% male. 430/1385 (31.2%) participants received lung protective ventilation, defined as less than or equal to 6.5cc/kg PBW. Figure 1 demonstrates the distribution of tidal volumes in all participants; the average tidal volume was 7.65±1.82 cc/kg PBW. Figure 2 demonstrates the distribution of observed (Figure 2A, Figure 2B) and 6.5 cc/kg PBW-calculated “lung protective” tidal volumes (cc) (Figure 2C, Figure 2D) for males and females, respectively. Delivered tidal volumes were significantly greater than “lung protective” tidal volumes predicted by 6.5 cc/kg PBW (mean difference 67 ± 108cc, p<0.0001). Histograms in Figure 2 show that the range of delivered tidal volumes is approximately two times the range of predicted “lung protective” tidal volumes.
Figure 1.
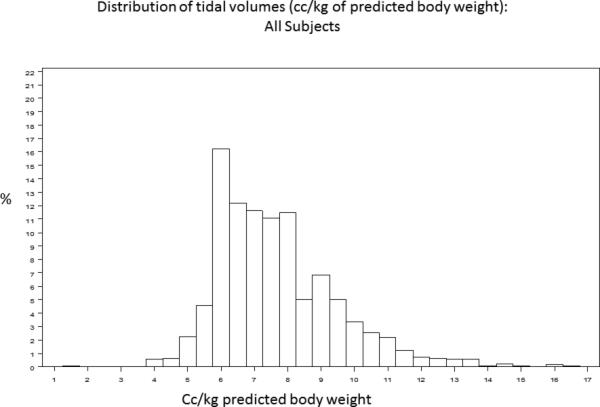
Distribution of tidal volumes (cc/kg predicted body weight) in all participants.
Figure 2.
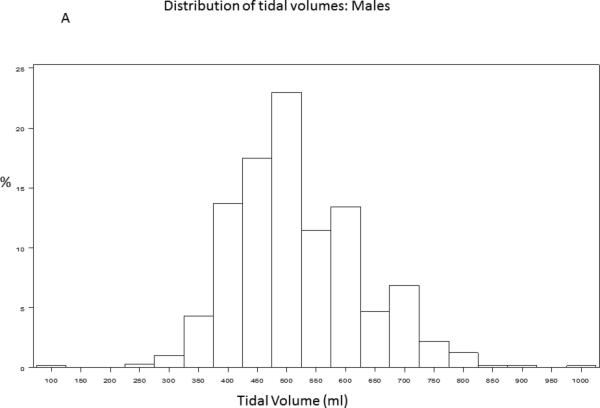
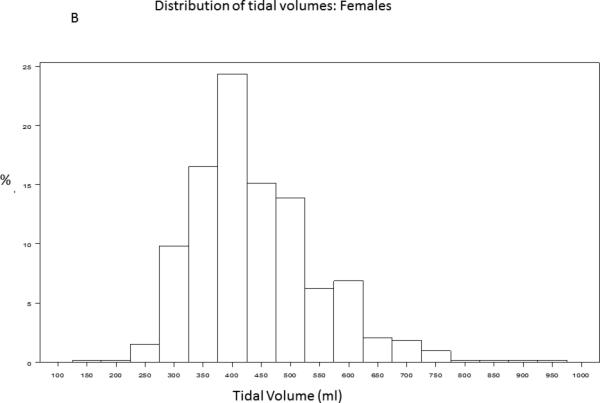
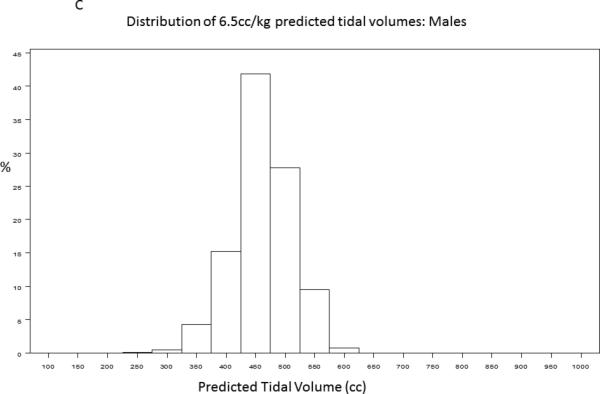
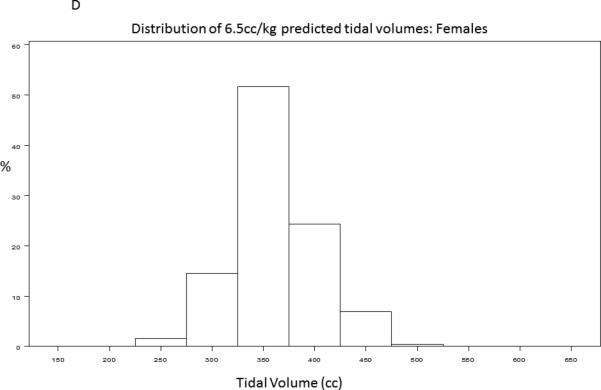
Distribution of observed (2A, 2B) and 6.5 cc/kg PBW-calculated “lung protective” (2C, 2D) tidal volumes (cc) for males and females, respectively.
Baseline subject characteristics stratified by LPV status are shown in Table 1.
Table 1.
Baseline variables
| Lung Protective Ventilation N=430 | No Lung protective Ventilation N=955 | p | |
|---|---|---|---|
| Age, years | 48.5 ± 16.5 | 50.7 ± 16.4 | 0.024 |
| Sex, male | 282 (65.6) | 462 (48.4) | 0.001 |
| Race | 0.011 | ||
| White | 276 (64.2) | 662 (69.3) | |
| Black | 103 (24.0) | 163 (17.1) | |
| Other | 51 (11.9) | 130 (13.6) | |
| Body Mass Index, (kg/m2) | 27.6 ± 7.2 | 28.4 ± 7.3 | 0.04 |
| Height (cm) | 173.7 ± 10.3 | 168.0 ± 10.1 | 0.001 |
| Weight (kg) | 83.5 ± 22.8 | 80.1 ± 22.0 | 0.015 |
| SAPS II | 50.0 ± 13.9 | 47.9 ± 14.2 | 0.03 |
| Brussels organ failures | 1.93 ± 0.98 | 1.99 ± 0.97 | 0.21 |
| Brussels non-pulmonary organ failures | 0.99 ± 0.91 | 1.0 ± 0.92 | 0.34 |
| ARDS (PaO2/FiO2 <200) | 383 (89) | 821 (86) | 0.067 |
| ALI Risk Factor | |||
| Direct lung injury | 272 (69.7) | 566 (63.7) | 0.04 |
| Pneumonia | 197 (45.8) | 432 (45.2) | 0.86 |
| Sepsis | 85 (19.8) | 233 (24.4) | 0.062 |
| Aspiration | 75 (17.4) | 134 (14.0) | 0.10 |
| Trauma | 22 (5.1) | 71 (7.4) | 0.13 |
| Transfusion | 11 (2.6) | 19 (2.0) | 0.55 |
| Other | 40 (9.3) | 66 (6.9) | 0.13 |
| Co-Morbidities | |||
| Malignancy | 19 (4.4) | 42 (4.4) | 1.0 |
| AIDS | 40 (9.7) | 60 (6.4) | 0.04 |
| Dialysis | 2 (0.48) | 17 (1.8) | 0.076 |
| Diabetes | 71 (17.2) | 157 (16.7) | 0.87 |
| Mechanical ventilation parameters | |||
| Volume assist control mode | 406 (94.4) | 804 (84.2) | 0.001 |
| PaO2/FiO2 | 122 ± 55 | 131 ± 60 | 0.017 |
| Pplat (cm H2O) | 25.7 ± 7.2 | 26.8 ± 6.8 | 0.01 |
| PaCO2 (mm Hg) | 43.2 ± 11.1 | 38.3 ± 8.9 | 0.001 |
| PEEP (cm H2O) | 10.2 ± 4.3 | 9.2 ± 3.9 | 0.001 |
| Static compliance (cc/cmH2O) | 29.8 ± 34 | 31.0 ± 33.8 | 0.001 |
| Minute ventilation (L/minute) | 12.3 ± 3.7 | 12.3 ± 4.0 | 0.51 |
| Arterial pH | 7.34 ± 0.096 | 7.37 ± 0.092 | 0.001 |
| Radiographic Lung Injury Score | 3.8 ± 0.50 | 3.70 ± 0.58 | 0.002 |
| Serum Bicarbonate (mmol/L) | 21.6 ± 5.3 | 21.1 ± 5.6 | 0.11 |
| Duration Intubation prior to enrollment (days) | 1.1 ± 1.0 | 1.1 ± 1.2 | 0.48 |
| Duration ICU stay prior to enrollment (days) | 1.8 ± 1.9 | 1.6 ± 2.1 | 0.03 |
Abbreviations: kg: kilogram, m: meter, cm: centimeter, SAPS: Simplified Acute Physiology Score, mmol: millimoles, L: liter, NS: not-significant and not included in final adjusted model, PaO2/FiO2: ratio of partial pressure of arterial oxygen to fraction of inspired oxygen, Pplat: static (plateau) airway pressure, PaCO2: partial pressure of arterial carbon dioxide, PEEP: positive end expiratory pressure, ICU: intensive care unit
Table 2 demonstrates the significant unadjusted and multivariable-adjusted predictors of LPV. Multivariable predictors of LPV underutilization were older age, white race, shorter height, less severe illness (as indicated by lower SAPSII and lower radiographic lung injury scores), lower serum bicarbonate levels, shorter duration of pre-enrollment ICU stay, and use of non-volume-targeted ventilation.
Table 2.
Unadjusted and multivariable-adjusted predictors of not receiving lung protective ventilation
| Variable | Unadjusted Odds Ratio (95% Confidence Interval), p | Adjusted Odds Ratio (95% Confidence Interval), p |
|---|---|---|
| Age, per std (16 years) | 1.14 (1.02-1.28), 0.024 | 1.18 (1.02-1.38), 0.028 |
| Sex, female | 2.03 (1.61-2.58), 0.0001 | NS |
| Race, white vs. non-white | 1.26 (0.99-1.60), 0.059 | 1.40 (1.05-1.88), 0.023 |
| Body Mass Index per std (7.3 kg/m2) | 1.12 (0.99-1.26), 0.076 | NS |
| Height, per std (10cm) | 0.56 (0.50-0.64), 0.0001 | 0.55 (0.48-0.63), 0.0001 |
| Weight, per std (22 kg) | 0.86 (0.77-0.97), 0.01 | NS |
| SAPS II, per std (14) | 0.86 (0.77-0.97), 0.01 | 0.78 (0.67-0.92), 0.003 |
| Direct lung injury | 0.76 (0.59-0.98), 0.035 | NS |
| Dialysis | 3.80 (0.87-16.5), 0.075 | NS |
| Acquired Immunodeficiency Syndrome | 0.64 (0.42-0.97), 0.036 | NS |
| Radiographic lung injury score per std (0.57) | 0.84 (0.74-0.95), 0.005 | 0.83 (0.70-0.95), 0.009 |
| Non-volume control ventilator mode | 3.18 (2.03-3.97), 0.0001 | 3.07 (1.78, 5.27), 0.0001 |
| Serum bicarbonate per std (5.5 mmol/L) | 0.92 (0.82-1.03), 0.14 | 0.83 (0.71-0.97), 0.017 |
| Duration of ICU stay prior to study enrollment per std (2 days) | 0.90 (0.80-1.01), 0.078 | 0.84 (0.73-0.98), 0.02 |
Hosmer-Lemeshow Goodness of fit for adjusted model p=0.33, c-statistic 0.712
Abbreviations: std: standard deviation, kg: kilogram, m: meter, cm: centimeter, SAPS: Simplified Acute Physiology Score, mmol: millimoles, L: liter, NS: not-significant and not included in final adjusted model.
In our sensitivity analysis, we found that 909/1385 (66%) of participants received less than or equal to 8cc/kg PBW. Using the cutoff of 8cc/kg PBW, the predictors of receiving LPV were similar to the 6.5cc/kg threshold model, except that age and duration of ICU stay were no longer significant, and lack of dialysis requirement [OR 4.57 (1.35, 15.4), p=0.014] and lower weight [OR per 10kg 0.91 (0.85, 0.97), p=0.003] were associated with LPV. In sensitivity analysis using cc/kg PBW as a continuous variable, significant predictors of tidal volume were identical to the 6.5cc/kg analysis, with the exception that increasing weight was an additional predictor of increasing tidal volumes (beta 0.01 per kg, p<0.0001).
DISCUSSION
Similar to previous studies, we found substantial underutilization of evidence-based LPV among patients with ALI - only 1 of 3 participants with ALI received LPV with tidal volumes less than or equal to 6.5cc/kg PBW prior to randomization in the ARDSNet trials.[2,5,10] Our study has identified novel patient characteristics associated with underuse of LPV that may be amenable to simple quality improvement interventions. Participants most at risk for LPV underutilization were older, of shorter stature and Caucasian race. Additional clinical risk factors for underuse of LPV included metabolic acidosis, lower illness severity, and use of non-volume targeted ventilator modes. Our findings were robust across a range of definitions of LPV. Results from this study suggest that suboptimal assessment of PBW and poor response to less severe lung injury are major factors affecting implementation of LPV.
Our study expands upon results of three prior studies that investigated reasons for underutilization of LPV. A survey of ARDSNet center nurses and respiratory therapists identified multiple factors felt to be contributing to underuse of LPV; these included under-recognition of ALI, difficulties in calculating PBW, and discomfort with the hypoxemia, hypercapnea and acidosis often associated with LPV.[9] The opinions documented by Rubenfeld et al. are in accordance with our data. Similar to our findings, an observational study by Umoh et al. (n=250) identified metabolic acidosis as an independent association with LPV.[5] A smaller study by Kalhan et al. (n=88) found an unadjusted association between poor lung compliance and oxygenation with use of LPV.[4] Although we found similar univariate relationships to Kalhan et al., we chose not to include respiratory variables in our models because low tidal volume ventilation can affect compliance and oxygenation.[11,19]
We identified multiple novel factors associated with underuse of LPV. Patients of shorter height were least likely to receive LPV. Thus, women were disproportionately affected by underuse of LPV. Past studies have identified that height is inaccurately estimated and recorded in the critically ill.[20,21] Therefore, increased attention to accurate height measurement as a “vital sign” in all mechanically ventilated patients, accompanied by accessible methods of translating height into PBW and tidal volumes, is essential for improving care of patients with ALI. In our institution, charts attached to ventilators translate height into 6cc/kg PBW tidal volumes. Others have shown such simple interventions, coupled with educational initiatives, to be effective for tidal volume reduction.[22]
Patients with longer ICU stay prior to study enrollment, less severe illness (as measured by SAPS II) and less obvious lung injury (lower radiographic lung injury score) were also at increased risk for not receiving LPV. The observation that larger tidal volumes were associated with longer ICU stay prior to enrollment is supported by data presented by Kallet showing that increased duration of mechanical ventilation in the absence of a protocol was associated with large variation in tidal volumes, with a trend towards increasing tidal volumes over time.[23] The association of LPV underutilization with less severe lung injury suggests under-recognition of ALI. Because outcomes are improved with LPV regardless of lung injury severity,[11] further development of innovative decision support interventions that facilitate ALI recognition[24,25] is especially important.
However, ALI recognition is only a part of the reason for LPV underutilization. In patients with recognized ALI, interventions to facilitate use of LPV are also warranted. For example, we identified that non-volume-targeted mechanical ventilation strategies were associated with increased tidal volumes. Because tidal volume during pressure-controlled modes is determined by respiratory system compliance and work of breathing, increases in compliance or work of breathing may result in high tidal volumes during pressure-controlled modes.[26] Thus, increased vigilance in monitoring tidal volumes during pressure targeted ventilation is warranted - lower tidal volumes are associated with improved outcomes regardless of plateau pressure.[27]
The association between older age and decreased use of LPV is consistent with the slower adoption of evidence-based treatment in elderly patients that has been demonstrated in other areas, such as beta-blockade after myocardial infarction.[28] In addition, underutilization of LPV in the elderly might be due to diagnostic uncertainty between cardiogenic and noncardiogenic pulmonary edema in older patients with greater cardiac comorbidity, or due to demographic variations by study site. Variations in study center demographics and practice patterns may also explain the association between white race and LPV underutilization; study center-level information was not available in our dataset. Further investigation of the demographic factors associated with LPV underutilization is warranted.
As shown in one prior study,[5] metabolic acidosis was associated with decreased use of LPV. Because of the respiratory acidosis that often accompanies LPV through “permissive hypercapnea”, a concomitant metabolic acidosis may induce significant acidemia and high respiratory drive potentially increasing ventilator dys-synchrony and difficulty implementing LPV. However, on average, metabolic acidosis was mild (mean bicarbonate: 21.1 mmol/L) and not associated with significant acidemia (mean pH 7.37). Greater adherence to the ARDSNet protocol (ardsnet.org) or other proposed strategies[29] for management of acidosis in ALI may decrease the proportion of patients who inappropriately receive potentially injurious tidal volumes to compensate for metabolic acidosis. While compensatory metabolic alkalosis due to permissive hypercapnea may develop in patients ventilated with LPV, it is unlikely to develop within the median 24 hours of mechanical ventilation prior to study enrollment and this “reverse causation” is unlikely to explain the decreased use of LPV in the presence of metabolic acidosis.
The systematic discrepancy between predicted and actual tidal volumes we observed suggests that patients are generally placed on a ‘default’ tidal volume at the time of intubation that is independent of measured height. The distribution of delivered tidal volumes in this study shows that male and female participants with ALI were most often placed on 500ml and 400ml tidal volumes, respectively (Figure 2A, Figure 2B). While accurate height measurement in all patients requiring mechanical ventilation is ideal practice, assessment of height may not occur until well after initial tidal volumes have been selected in a newly intubated patient, particularly if the patient is not clinically stable. These initial, non-lung-protective tidal volumes may then be propagated through inaction. Simple interventions that decrease the initial ‘default’ tidal volumes selected for patients with ALI risk factors would greatly increase both the proportion of ALI patients treated with LPV and the duration of time receiving LPV. For example, the histograms of tidal volumes predicted from calculation of PBW in this sample suggest that more than 80% of men and women with ALI would receive LPV if initial ‘default’ tidal volumes were lowered to 450ml and 350ml, respectively (Figure 2C, Figure 2D). Lowering the ‘default’ tidal volume may be advantageous for patients with ALI risk factors in light of recent studies demonstrating that use of LPV in patients without ALI may decrease incident ALI.[22,30] Future studies investigating strategies that combine early calculation of a Lung Injury Prediction Score, [31] (for example, in the emergency department) with lower default tidal volumes for patients with high ALI risk are warranted.
Our study has limitations. First it is unclear if risk factors for underuse of LPV identified in the ARDSNet centers are generalizable to other settings. However, ARDSNet centers do represent a range of hospital settings across the US and rates of LPV underutilization in the ARDSNet centers were not substantially different from rates reported in other centers. Second, it is unclear if identification of individual study centers may have changed our results. It is possible that practice variation by study center might account for many observed differences in LPV utilization; however this data was unavailable in the BioLincc data set. Third, tidal volumes were recorded at single time points prior to trial enrollment; identifying temporal patterns of tidal volume change was not possible with this trial data. It is possible that with longer duration of mechanical ventilation, tidal volumes may be lowered; however, this is not supported by other studies[23] and we did not observe an association between duration of mechanical ventilation and use of LPV to support this assertion. Finally, it is possible that we may have misclassified some patients as not having received LPV who were appropriately given tidal volumes between 6.5 and 8cc/kg PBW for severe dyspnea, breath stacking and plateau pressures less than 30 cm H2O. Unfortunately, an indicator of breath stacking or severe dyspnea was not available in the BioLincc dataset. However, predictors of LPV were robust across various definitions of LPV, including a sensitivity analysis defining LPV as less than or equal to 8cc/kg PBW.
In conclusion, we have identified multiple novel factors amenable to quality improvement interventions that may promote use of LPV. Patients of shorter stature, the elderly, and those with less severe lung injury, acidosis, or pressure-targeted ventilation modes are particularly at risk for not receiving LPV. A systematic reduction of the default tidal volumes in patients with ALI risk factors to 450ml in men and 350ml in women would likely provide LPV to 80% of patients at risk for ALI. Studies of initiatives aimed at decreasing default tidal volumes and increasing recognition of patients with the risk factors for LPV underutilization identified in this study are warranted.
Acknowledgements
Funding/Support: Dr. Wiener is supported by a career development award through the National Cancer Institute [K07 CA138772] and by the Department of Veterans Affairs.
We would like to acknowledge the work by the ARDSNet investigators, without which this work would not be possible.
Abbreviation List
- ARDS
Acute respiratory distress syndrome
- ALI
Acute lung injury
- ALVEOLI
Assessment of Low tidal Volume and End expiratory volume to Obviate Lung Injury Trial
- BioLINCC
Biologic Specimen and Data Repository Information Coordinating Center
- BMI
Body mass index
- FACCT
Fluid and Catheter Treatment Trials
- LaSRS
Late Steroid Rescue Study
- LPV
lung protective ventilation strategy
- PBW
predicted body weight
- PEEP
positive end expiratory pressure
- SAPS II
Simplified Acute Physiology II Scores
Footnotes
This study was performed at Boston University School of Medicine.
Dr. Walkey and Dr. Wiener report no conflicts of interest.
Disclaimer: The views expressed do not necessarily represent the views of the Department of Veterans Affairs, the US government, or the National Cancer Institute.
The authors have no conflicts of interest to declare.
References
- 1.Pronovost PJ, Murphy DJ, Needham DM. The science of translating research into practice in intensive care. Am J Respir Crit Care Med. 2010;182:1463–1464. doi: 10.1164/rccm.201008-1255ED. [DOI] [PubMed] [Google Scholar]
- 2.Checkley W, Brower R, Korpak A, et al. Effects of a clinical trial on mechanical ventilation practices in patients with acute lung injury. Am J Respir Crit Care Med. 2008;177:1215–1222. doi: 10.1164/rccm.200709-1424OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esteban A, Ferguson ND, Meade MO, et al. Evolution of mechanical ventilation in response to clinical research. Am J Respir Crit Care Med. 2008;177:170–7. doi: 10.1164/rccm.200706-893OC. [DOI] [PubMed] [Google Scholar]
- 4.Kalhan R, Mikkelsen M, Dedhiya P, et al. Underuse of lung protective ventilation: analysis of potential factors to explain physician behavior. Crit Care Med. 2006;34:300–306. doi: 10.1097/01.ccm.0000198328.83571.4a. [DOI] [PubMed] [Google Scholar]
- 5.Umoh NJ, Fan E, Mendez-Tellez PA, et al. Patient and intensive care unit organizational factors associated with low tidal volume ventilation in acute lung injury. Crit Care Med. 2008;36:1463–1468. doi: 10.1097/CCM.0b013e31816fc3d0. [DOI] [PubMed] [Google Scholar]
- 6.Weinert CR, Gross CR, Marinelli WA. Impact of randomized trial results on acute lung injury ventilator therapy in teaching hospitals. Am J Respir Crit Care Med. 2003;167:1304–1309. doi: 10.1164/rccm.200205-478OC. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson ND, Frutos-Vivar F, Esteban A, et al. Acute respiratory distress syndrome: underrecognition by clinicians and diagnostic accuracy of three clinical definitions. Crit Care Med. 2005;33:2228–2234. doi: 10.1097/01.ccm.0000181529.08630.49. [DOI] [PubMed] [Google Scholar]
- 8.Mikkelsen ME, Dedhiya PM, Kalhan R, et al. Potential reasons why physicians underuse lung-protective ventilation: a retrospective cohort study using physician documentation. Respir Care. 2008;53:455–461. [PubMed] [Google Scholar]
- 9.Rubenfeld GD, Cooper C, Carter G, et al. Barriers to providing lung-protective ventilation to patients with acute lung injury. Crit Care Med. 2004;32:1289–1293. doi: 10.1097/01.ccm.0000127266.39560.96. [DOI] [PubMed] [Google Scholar]
- 10.Young MP, Manning HL, Wilson DL, et al. Ventilation of patients with acute lung injury and acute respiratory distress syndrome: has new evidence changed clinical practice? Crit Care Med. 2004;32:1260–1265. doi: 10.1097/01.ccm.0000127784.54727.56. [DOI] [PubMed] [Google Scholar]
- 11.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 12.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–36. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 13.Wheeler AP, Bernard GR, Thompson BT, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–24. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 14.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–75. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 15.Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–84. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 16.Bernard GR, Artigas A, Brigham KL, et al. Report of the American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The Consensus Committee. Intensive Care Med. 1994;20:225–232. doi: 10.1007/BF01704707. [DOI] [PubMed] [Google Scholar]
- 17.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 18.Bernard GR. The Brussels Score. Sepsis. 1997;1:43–44. [Google Scholar]
- 19.Suter PM, Fairley HB, Isenberg MD. Effect of tidal volume and positive end-expiratory pressure on compliance during mechanical ventilation. Chest. 1978;73:158–162. doi: 10.1378/chest.73.2.158. [DOI] [PubMed] [Google Scholar]
- 20.Determann RM, Wolthuis EK, Spronk PE, et al. Reliability of height and weight estimates in patients acutely admitted to intensive care units. Crit Care Nurse. 2007;27:48–55. quiz 56. [PubMed] [Google Scholar]
- 21.Leary TS, Milner QJ, Niblett DJ. The accuracy of the estimation of body weight and height in the intensive care unit. Eur J Anaesthesiol. 2000;17:698–703. doi: 10.1046/j.1365-2346.2000.00751.x. [DOI] [PubMed] [Google Scholar]
- 22.Yilmaz M, Keegan MT, Iscimen R, et al. Toward the prevention of acute lung injury: protocol-guided limitation of large tidal volume ventilation and inappropriate transfusion. Crit Care Med. 2007;35:1660–6. doi: 10.1097/01.CCM.0000269037.66955.F0. quiz 1667. [DOI] [PubMed] [Google Scholar]
- 23.Kallet RH. What is the legacy of the National Institutes of Health Acute Respiratory Distress Syndrome Network? Respir Care. 2009;54:912–924. doi: 10.4187/002013209793800330. [DOI] [PubMed] [Google Scholar]
- 24.Herasevich V, Yilmaz M, Khan H, et al. Validation of an electronic surveillance system for acute lung injury. Intensive Care Med. 2009;35:1018–1023. doi: 10.1007/s00134-009-1460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herasevich V, Tsapenko M, Kojicic M, et al. Limiting ventilator-induced lung injury through individual electronic medical record surveillance. Crit Care Med. 2010 doi: 10.1097/CCM.0b013e3181fa4184. [DOI] [PubMed] [Google Scholar]
- 26.Kallet RH, Campbell AR, Dicker RA, et al. Work of breathing during lung-protective ventilation in patients with acute lung injury and acute respiratory distress syndrome: a comparison between volume and pressure-regulated breathing modes. Respir Care. 2005;50:1623–1631. [PubMed] [Google Scholar]
- 27.Hager DN, Krishnan JA, Hayden DL, et al. Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. Am J Respir Crit Care Med. 2005;172:1241–1245. doi: 10.1164/rccm.200501-048CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krumholz HM, Radford MJ, Wang Y, et al. National use and effectiveness of beta-blockers for the treatment of elderly patients after acute myocardial infarction: National Cooperative Cardiovascular Project. JAMA. 1998;280:623–629. doi: 10.1001/jama.280.7.623. [DOI] [PubMed] [Google Scholar]
- 29.Kallet RH, Liu K, Tang J. Management of acidosis during lung-protective ventilation in acute respiratory distress syndrome. Respir Care Clin N Am. 2003;9:437–456. doi: 10.1016/s1078-5337(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 30.Determann RM, Royakkers A, Wolthuis EK, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care. 2010;14:R1. doi: 10.1186/cc8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gajic O, Dabbagh O, Park PK, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011;183:462–470. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


